Abstract
Lck-interacting adaptor protein/Rlk/Itk-binding protein (Lad/RIBP) was previously identified as an adaptor protein involved in TCR-mediated T-cell activation. To elucidate the functions of Lad further, we here performed yeast 2-hybrid screening using Lad as bait and discovered that the G protein β subunit (Gβ) is a Lad-binding partner. Since the most well-known G protein–coupled receptor in T cells is the chemokine receptor, we investigated whether Lad is involved in chemokine signaling. We found that, upon chemokine treatment, Lad associated with Gβ in Jurkat T cells. Furthermore, ectopic expression of dominant-negative Lad or the reduction of endogenous Lad expression by siRNA impaired the chemokine-induced migration of T cells, indicating that Lad is required for chemokine-induced T-cell migration. Subsequent investigation of the signaling pathways revealed that, in response to chemokine, Lad associated with the tyrosine kinases Lck and Zap-70 and that Lad was essential for the activation of Zap-70. Moreover, Lad was required for the chemokine-dependent tyrosine phosphorylation of focal adhesion molecules that included Pyk2 and paxillin. Taken together, these data show that, upon chemokine stimulation, Lad acts as an adaptor protein that links the G protein β subunit to the tyrosine kinases Lck and Zap-70, thereby mediating T-cell migration.
Introduction
Lymphocytes circulate continually in the blood and secondary lymphoid organs, and migrate into the tissues to sites of infection. Chemokines, which are small proteins with molecular weights in the range of 8 to 12 kDa, play important roles in these migratory processes. To date, more than 50 chemokines and 20 receptors in the human chemokine system have been characterized.1,2 Chemokines and their receptors have also been divided in terms of their functions into the homeostatic and inflammatory families.1–3 In general, homeostatic chemokine receptors are constitutively expressed and act to guide the traffic of lymphocytes under normal conditions. In contrast, inflammatory chemokine receptors are inducibly expressed upon antigen exposure and regulate the migration of lymphocytes in response to inflammatory signals. For example, the constitutive chemokine receptors CXCR4 and CCR7 regulate naive or memory T-cell homing upon the binding of SDF-1α (CXCL12),4,5 while inducible chemokine receptors such as CCR5 regulate the movement of effector T cells to inflammatory sites upon the binding of RANTES (CCL5).4,5
The chemokine receptors are members of a family of 7-transmembrane–spanning, G protein–coupled receptors.3,6 Upon engagement of a chemokine with their receptor, the receptor-associated heterotrimeric G protein dissociates into the Gα and Gβγ subunits. While it is not clear yet how the Gαi subunit contributes to chemokine-dependent migration,7 it is known that the Gβγ subunit recruits effector molecules such as phospholipase C-β (PLC-β) and phosphatidylinositol 3-kinase (PI3K) and transmits the chemokine signal.8 Moreover, PLC-β2 and PLC-β3 directly associate with Gβγ, which activates their enzymatic activities.8,9 However, a study with mice revealed that the absence of PLC-β2 and PLC-β3 had no effect on the chemokine-dependent migration of leukocytes despite the impairment of chemoattractant-induced Ca++ efflux.8,10 This suggests that PLC-β is not directly involved in chemotaxis. With regard to PI3Kγ, which also directly associates with Gβγ and becomes activated,11,12 its activation stimulates the production of phosphatidylinositol 3,4,5-triphosphate (PtdIns(3,4,5)P3) and thereby recruits signaling molecules containing the PH domain into the plasma membrane. However, the migration of T cells is not particularly affected by the inhibition of the PI3K pathway.13,14 Instead, the DOCK2-dependent, PI3K-independent pathway seems to play an important role in the migration of T cells.15,16
Another important aspect of chemokine signaling is the activation of membrane-proximal tyrosine kinases such as Lck, Zap-70, and Itk. Upon chemokine treatment, Lck and Zap-70 are phosphorylated.17–20 Furthermore, Jurkat T cells lacking Lck or Zap-70 exhibit impaired migration, while the introduction of Lck or Zap-70 restores the migration of these cells in response to SDF-1α (CXCL12).17–20 This indicates that chemokine and TCR signaling share the same components. In addition to Lck and Zap-70, the Tec family kinases Itk and Rlk have been shown to be required for chemokine-dependent migration. Itk and Rlk are phosphorylated upon chemokine treatment, and T cells from Itk−/−Rlk−/− mice exhibit defective migration in response to multiple chemokines.21,22 Itk regulates chemokine-dependent migration by activating Rac and Cdc42, thereby altering the actin cytoskeleton.21 However, despite the requirement of these tyrosine kinases in chemokine-dependent migration, it is not known how these tyrosine kinases are recruited to the chemokine receptor complex and activated.
Another important aspect of chemokine signaling is the activation of the focal adhesion complex. The chemokine signal results in the tyrosine phosphorylation and activation of focal adhesion components in various cell types such as FAK/Pyk2 tyrosine kinase, paxillin, and Crk.23–26 The upstream signaling molecules of focal adhesions have been suggested to be Lck, Zap-70,26,27 Rap,28,29 protein kinase C and PI3K,23 and Jak2.30 In particular, Zap-70 has been shown to associate with FAK/Pyk2 in response to chemokine stimulation.26,27
Lck-interacting adaptor protein (Lad)/RIBP was previously identified as an adaptor protein that binds to Lck, Itk/Rlk, or MEKK-2.31–33 The human counterpart of Lad was identified as T-cell–specific adaptor protein (TSAd),34 which is referred to as human Lad (hLad) in this report. Lad contains protein-protein interaction domains including an SH2 domain, a proline-rich SH3-binding motif, and 4 phosphotyrosine sites.31 The expression of hLad/TSAd is significantly elevated upon T-cell activation,32,34 indicating the possible involvement of Lad in the late stage of T-cell activation or in effector T cells. Upon T-cell activation, Lad is tyrosine phosphorylated and redistributed from the cytoplasm to the plasma membrane, and plays an essential role in T-cell activation that leads to IL-2 promoter activation.31 In Lad/RIBP-deficient mice, TCR-mediated T-cell proliferation, the production of IL-2 and IFN-γ, and Th1 differentiation are significantly impaired.32 In addition, Lad-deficient mice show defective activation-induced T-cell death and as a result develop autoimmune disease.35
In this report, we found through yeast 2-hybrid screening using Lad as bait that Lad interacts with the G protein β subunit. Since the most well-known G protein–coupled receptors in T cells are chemokine receptors, we investigated the role Lad plays in chemokine signaling. These analyses revealed that Lad is essential for the chemokine-mediated signaling events that lead to T-cell migration.
Materials and methods
Yeast 2-hybrid screening
Yeast 2-hybrid screening was performed using the method described previously.31,36 Briefly, Lad cDNA was cloned in-frame with the GAL4 DNA-binding domain of pGBT9 and the resulting plasmid was used as the bait in the screening of 2 × 106 colonies of a murine T-cell lymphoma cDNA library (Clontech, Palo Alto, CA). Approximately 40 positives grew in the absence of histidine and showed detectable β-galactosidase staining within 2 hours of incubation. The sequencing of the positives revealed that 3 clones contained portions of the same cDNA encoding the G protein β4 subunit. All other yeast 2-hybrid assay methods used were performed according to the manufacturer's protocol (Stratagene, La Jolla, CA).
Plasmids
As the prey for other yeast 2-hybrid assays, the full open reading frames of Gβ1 and Gβ5 were amplified by polymerase chain reaction (PCR) by using a 5′ primer bearing an EcoRI site and a 3′ primer carrying a SalI site extension. The PCR products were then digested with EcoRI/SalI and ligated in-frame with the transcriptional activation domain of GAL4 into the pGAD424 vector. As the baits for these yeast 2-hybrid assays, cDNA fragments corresponding to the full-length and deletion mutants of Lad were subcloned into the pGBT9 vector using the same method.
The expression plasmids encoding FLAG-tagged Lad (pcDNA 3.1-Lad) or the SH2 domain of Lad (pcDNA 3.1-Lad-SH2) have been described previously.36 The expression plasmids for Gβ1 (pcDM 8.1-Gβ1) and Gβ5 (pcDNA1-Amp-Gβ5) were kindly provided by Dr M. Simon (University of California, CA).37 The expression constructs for the Gγ2 subunit (pcDM 8.1-Gγ2) and CCR5 (pEF-BOS-CCR5-flag)38 were provided by Dr S. Gutkind (National Institutes of Health, Bethesda, MD) and Dr M. Oppermann (Geirg-August University, Gottingen, Germany), respectively.
Chemokines and antibodies
Recombinant human SDF-1α and RANTES were purchased from BD Biosciences (San Jose, CA). AntiLad and antiLck antisera were generated by immunizing rabbits and have been described previously.31 Other antibodies were obtained from commercial sources. These include the anti-CD3ϵ (BD Biosciences), anti-phosphotyrosine (4G10; Upstate, Lake Placid, NY), anti-Flag M2 (Sigma, St Louis, MO), anti-Gβ (SantaCruz, Santa Cruz, CA), anti-Pyk2 (Upstate), anti-ERK (Cell Signaling, Beverly, MA), anti-phospho-ERK (Cell Signaling), anti-phosphoZap-70 (Tyr493) (Cell Signaling), anti-Zap-70 (Upstate), and anti-paxillin (BD Biosciences) antibodies.
Establishment of Jurkat clones stably expressing Lad, Lad-SH2, or CCR5-Jurkat T cells were transfected with 5 μg pcDNA3.1, pcDNA3.1-Lad, or pcDNA3.1-Lad-SH239 by using the Superfect reagent according to the manufacturer's protocol (Qiagen, Valencia, CA). For establishing Jurkat clones expressing CCR5, cells were transfected with pEF-BOS-CCR5-flag by electroporation. After incubation for 48 hours, the cells were placed in growth medium containing 1 mg/mL geneticin (G418, Gibco, Grand Island, NY) to select for the transfectants. After selection for a week, the cells were subjected to limiting dilution in 96-well microtiter plates and individual clones were established.
siRNA-expressing plasmids and transfection
The pSUPER plasmid was a kind gift from Dr Agami (Netherlands Cancer Institutes, Amsterdam, Netherlands)40 and was used to construct the small interfering RNA (siRNA)–expressing plasmids. In brief, 2 19–base pair sequences corresponding to nucleotides 194 to 212 (siA) and 871 to 889 (siB) in the coding sequence of human Lad were selected for the construction of siRNA plasmids according to the previously described method.40 The target sequence for siA was 5′-CCTGGGCTACACTGCGGCA-3′, while that for siB was 5′-TCCCTGTTCCACGACACCG-3′. For the control siRNA expression, 3 underlined nucleotides in the target sequence of siA were changed to generate 5′-CCTGCCCTACACTGCCGCA-3′. For the knockdown assays using these siRNA plasmids, Jurkat T cells were electroporated with 20 μg of the indicated siRNA plasmids (240V, 25 ms, 1 pulse; BTX BT820; Harvard Apparatus, Hollistone, MA) and subsequently incubated in growth medium for 48 to 60 hours before further processing. The transfection efficiency was estimated by cotransfection of pEGFP plasmids (Clontech) and counting the EGFP-expressing cells by fluorescent microscopy. We routinely obtained a transfection efficiency of 50% to 70%.
Cell stimulation, immunoprecipitation, and Western analysis
Cells were activated by being resuspended at a concentration of 5 × 107/mL and incubated with 50 ng/mL SDF-1α or RANTES at 4°C for 20 minutes, followed by activation at 37°C for indicated time periods. Subsequent immunoprecipitation and Western analysis were performed as described previously.31
Chemotaxis assay
Chemotaxis assays were basically performed as described previously.41 Prior to starting the assay, cells were resuspended in RPMI-1640 medium containing 0.1% BSA (BSA medium). Subsequently, 5 × 105 cells in 100 μL BSA medium were loaded onto the top chamber of 6.5-mm diameter 5-μm pore polycarbonate Transwell inserts (Costar, Cambridge, MA). The Transwell chambers were then inserted into wells filled with BSA medium containing 25 ng/mL or 50 ng/mL SDF-1α and incubated for 2 hours at 37°C. Cells migrating to the bottom chamber were counted via hematocytometer 4 times and the numbers were averaged. The experiments were performed at least 3 times in duplicate and the results were expressed as means ± standard deviation (SD)
Results
Identification of the G protein β subunit as a Lad-binding partner
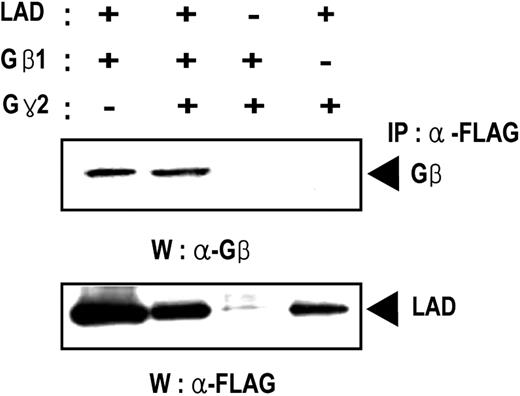
To identify proteins that bind to Lad, we performed yeast 2-hybrid screening using full-length Lad as the bait. The screening of 2 × 106 independent colonies from a murine T-cell lymphoma library yielded about 40 strong positives. Partial nucleotide sequencing of these cDNA fragments revealed that 3 clones encoded a portion of the G protein β4 subunit (data not shown). This raised the possibility that Lad is involved in Gβ-mediated signaling events. We then used the yeast 2-hybrid system again to assess whether the interaction between Lad and Gβ is Gβ-subtype specific. Of the different Gβ subtypes that have been cloned to date, we tested the abilities of Gβ1, Gβ4, and Gβ5 to interact with Lad. Lad could interact with all of the Gβ subtypes tested (data not shown), which suggests that Lad interacts with Gβ regardless of subtype. In addition, as shown in Figure 1, Lad coimmunoprecipitated with Gβ in Cos-7 cells that coexpressed FLAG-tagged Lad and Gβ. The latter experiment also revealed that coexpressing the G protein β subunit did not affect the binding between Gβ and Lad.
Lad associates with Gβ. Cos7 cells were transfected with the indicated combinations of expression plasmids encoding Lad, Gβ, and/or Gγ. After incubation for 48 hours, the cell lysates were subjected to immunoprecipitation with the antiFLAG antibody to precipitate FLAG-tagged Lad. This was followed by Western blotting with antiGβ (upper panel) or antiFLAG (lower panel) antibody.
Lad associates with Gβ. Cos7 cells were transfected with the indicated combinations of expression plasmids encoding Lad, Gβ, and/or Gγ. After incubation for 48 hours, the cell lysates were subjected to immunoprecipitation with the antiFLAG antibody to precipitate FLAG-tagged Lad. This was followed by Western blotting with antiGβ (upper panel) or antiFLAG (lower panel) antibody.
Lad associates with Gβ in response to chemokine
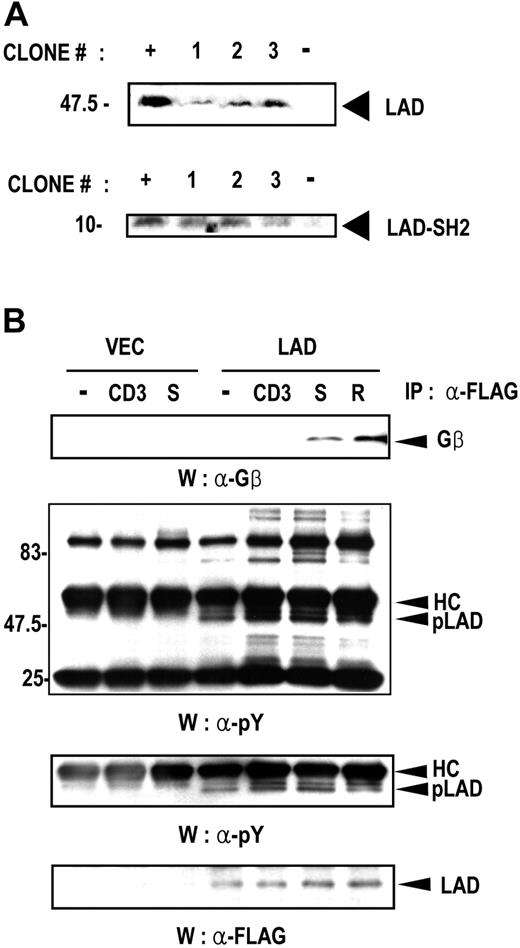
The observation that Lad and Gβ interact suggests that Lad may be involved in G protein–coupled receptor (GPCR)–mediated signaling. As the most well-known GPCR in T cells is the chemokine receptor, we tested the possibility that Lad participates in chemokine-mediated signaling. As the first step, we generated stable Jurkat T-cell clones expressing FLAG-tagged Lad or the SH2 domain of Lad. The SH2 domain of Lad was previously found to act as the dominant-negative form of Lad since it can impair TCR-mediated IL-2 promoter activation.31 The expression of the transfected genes was confirmed by Western analysis of 3 independent clones with anti-FLAG antibody (Figure 2A). These stable Lad-expressing clones were then used to examine the interaction between Gβ and Lad in T cells upon chemokine treatment. SDF-1α (CXCL12) and RANTES (CCL5) were selected as the chemokines since Jurkat T cells have been reported to bear the receptors for these chemokines.42,43 Thus, the Lad-expressing Jurkat stable cells were treated with anti-CD3 Ab, SDF-1α, or RANTES for 10 minutes, after which the cell lysates were subjected to immunoprecipitation with anti-FLAG antibody to precipitate Lad. Western analysis of the Lad immunoprecipitates for the presence of Gβ showed that Lad interacted with Gβ in cells treated with SDF-1α or RANTES but not with anti-CD3 antibody (Figure 2B upper panel). Thus, Lad specifically associates with Gβ in response to chemokine treatment, while TCR stimulation does not have this effect. In addition, analysis of the same precipitates by Western blotting with anti-phosphotyrosine antibody showed that the intensity of the tyrosine-phosphorylated band corresponding to the size of Lad was significantly enhanced in cells that had been stimulated with SDF-1α, RANTES, or antiCD3 antibody compared to control (Figure 2B middle panels). This suggests that Lad is tyrosine phosphorylated in response to chemokine stimulation. The levels of precipitated Lad, which were assayed as a control, were equal throughout the lanes (Figure 2B lower panel).
Lad associates with Gβ in T cells upon chemokine treatment. (A) Establishment of Jurkat T-cell clones that stably express FLAG-tagged full-length Lad (Lad) or Lad-SH2 domain (Lad-SH2). Three independent clones were selected for each transfectant, and the expression of the transfected genes was assayed by Western blotting with anti-FLAG antibody. The positive control (+) is the lysate of Cos7 cells transfected with expression plasmids encoding Lad or Lad-SH2 domain. The negative control (−) is the lysate of untransfected COS7 cells. (B) Lad associates with Gβ in T cells upon chemokine treatment. Jurkat T cells that stably express full-length Lad were activated with anti-CD3ϵ antibody (CD3), SDF-1α (S), or RANTES (R) for 10 minutes. The cell lysates were then subjected to immunoprecipitation with anti-FLAG antibody and Western blotting with anti-Gβ antibody (upper panel). The same precipitates were also analyzed by Western blotting with anti-phosphotyrosine (α-pY) antibody (middle panels) or anti-Flag antibody (lower panel). To visualize the phosphorylated Lad band more clearly, another panel from a shorter exposure of the same blot around the phosphorylated Lad band is shown below middle panel. pLAD indicates phosphorylated Lad; HC, IgG heavy chain
Lad associates with Gβ in T cells upon chemokine treatment. (A) Establishment of Jurkat T-cell clones that stably express FLAG-tagged full-length Lad (Lad) or Lad-SH2 domain (Lad-SH2). Three independent clones were selected for each transfectant, and the expression of the transfected genes was assayed by Western blotting with anti-FLAG antibody. The positive control (+) is the lysate of Cos7 cells transfected with expression plasmids encoding Lad or Lad-SH2 domain. The negative control (−) is the lysate of untransfected COS7 cells. (B) Lad associates with Gβ in T cells upon chemokine treatment. Jurkat T cells that stably express full-length Lad were activated with anti-CD3ϵ antibody (CD3), SDF-1α (S), or RANTES (R) for 10 minutes. The cell lysates were then subjected to immunoprecipitation with anti-FLAG antibody and Western blotting with anti-Gβ antibody (upper panel). The same precipitates were also analyzed by Western blotting with anti-phosphotyrosine (α-pY) antibody (middle panels) or anti-Flag antibody (lower panel). To visualize the phosphorylated Lad band more clearly, another panel from a shorter exposure of the same blot around the phosphorylated Lad band is shown below middle panel. pLAD indicates phosphorylated Lad; HC, IgG heavy chain
Lad mediates chemokine-dependent T-cell migration
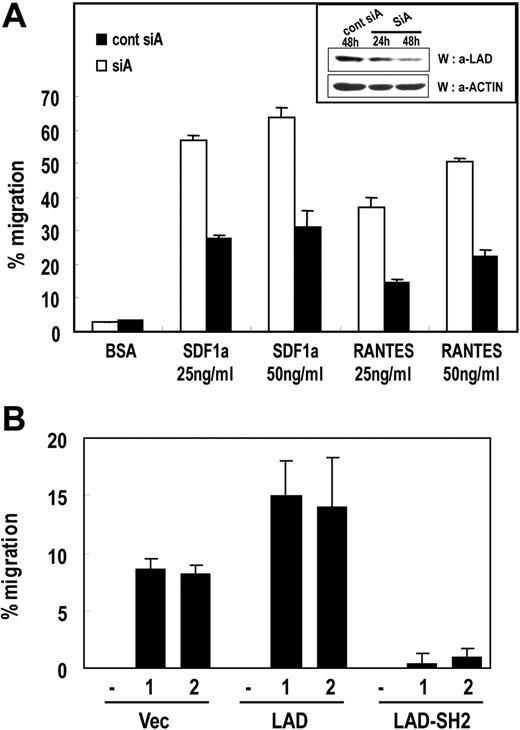
We then tested whether Lad mediates the chemokine-dependent migration of T cells by introducing siRNAs and examining the effect on T-cell migration. For the migration assay, Jurkat T cells that stably express CCR5 were transfected with the pSUPER plasmid encoding control siA or siA, transferred to the upper chamber of Transwell inserts immersed in 25 or 50 ng/mL SDF-1α or RANTES and incubated for 2 hours. The numbers of cells that migrated to the lower chamber were then counted. As shown in Figure 3A, the SDF-1α–induced migration of T cells was reduced significantly by Lad siRNA expression. This indicates that Lad is required for the chemokine-dependent migration of T cells. As a control, we measured the protein levels of endogenous Lad by Western blotting and confirmed that the expression of hLad-specific siRNAs resulted in the significant reduction of Lad protein levels (Figure 3A inset). In addition, fluorescent-activated cell sorting (FACS) analysis revealed that the surface levels of the SDF-1α receptor (CXCR4) were not affected by the siRNA expression (data not shown).
Lad is required for chemokine-induced T-cell migration. (A) Suppression of endogenous Lad expression by siRNA inhibits chemokine-dependent T-cell migration. Jurkat T cell clones that stably express CCR5 were transfected with pSUPER plasmid expressing control siA or siA. Subsequently, 5 × 105 cells were loaded into the upper part of a Transwell chamber inserted into medium containing 25 ng/mL or 50 ng/mL SDF-1α or RANTES. After incubation for 2 hours, cells that had migrated into the bottom chamber were counted. The experiments were performed 3 times in duplicate, and the results were expressed as mean ± SD. Error bars represent the standard deviation. As a control, protein level of endogenous Lad was analyzed by Western blotting with antiLad antibody (inset). (B) Jurkat T-cell clones that stably express Lad (Lad) or the SH2 domain of Lad (Lad-SH2) were subjected to the chemotaxis assay described in panel A. Two independent clones were tested for each transfectant.
Lad is required for chemokine-induced T-cell migration. (A) Suppression of endogenous Lad expression by siRNA inhibits chemokine-dependent T-cell migration. Jurkat T cell clones that stably express CCR5 were transfected with pSUPER plasmid expressing control siA or siA. Subsequently, 5 × 105 cells were loaded into the upper part of a Transwell chamber inserted into medium containing 25 ng/mL or 50 ng/mL SDF-1α or RANTES. After incubation for 2 hours, cells that had migrated into the bottom chamber were counted. The experiments were performed 3 times in duplicate, and the results were expressed as mean ± SD. Error bars represent the standard deviation. As a control, protein level of endogenous Lad was analyzed by Western blotting with antiLad antibody (inset). (B) Jurkat T-cell clones that stably express Lad (Lad) or the SH2 domain of Lad (Lad-SH2) were subjected to the chemotaxis assay described in panel A. Two independent clones were tested for each transfectant.
Next, to confirm that Lad participates in chemokine-induced T-cell migration, we assayed the migration of Jurkat T-cell clones that express Lad or the SH2 domain of Lad. As shown in Figure 3B, Lad-expressing cells migrated approximately 2-fold more frequently than the empty vector–bearing cells, whereas the cells expressing the Lad-SH2 domain showed almost complete impairment of migration. Again, the surface levels of CXCR4 were found to be equal between the cell clones (data not shown). These data confirm that Lad is essential for chemokine-induced T-cell migration.
Lck and Zap-70 are recruited to Lad in response to chemokine
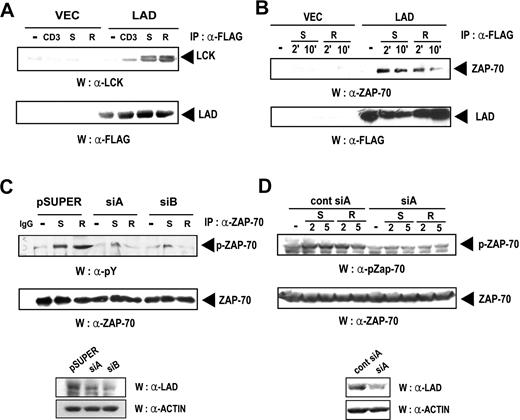
Src-type kinases are known to be tyrosine-phosphorylated and activated by chemokines, although the precise mechanism remains to be established.17 Moreover, we have previously demonstrated that Lad and Lck interact upon TCR stimulation.31 Based on these previous findings, we tested whether Lad and Lck also interact upon chemokine treatment. Thus, Jurkat clones stably expressing Lad were stimulated with anti-CD3 Ab, SDF-1α, or RANTES and the cell lysates were subjected to immunoprecipitation with anti-FLAG antibody and subsequent immunoblotting with antiLck antibody. As shown in Figure 4A (upper panel), Lck was coprecipitated with Lad when the cells had been stimulated with antiCD3 Ab, SDF-1α, or RANTES, but not when the cells had not been stimulated. The amounts of precipitated Lad, which were analyzed as a control, were approximately equal throughout the lanes (Figure 4A lower panel). These results, combined with those in Figure 2, show that Lad acts as an adaptor that bridges Lck to Gβ in response to chemokine.
Lad associates with Lck and Zap-70 in response to chemokine treatment. (A) Lck associates with Lad upon chemokine treatment. Jurkat T cells that stably express Lad-FLAG (Lad) or were transfected with empty vector (Vec) were activated with anti-CD3 antibody (CD3), SDF-1α (S), or RANTES (R) for 10 minutes. The cell lysates were then subjected to immunoprecipitation with 5 μg antiFLAG antibody, and the precipitates were analyzed by immunoblotting with antiLck antibody (upper panel). The membrane was reprobed with the anti-FLAG Ab (bottom panel). (B) Zap-70 associates with Lad upon chemokine treatment. Jurkat T cells were transfected with pcDNA3.1 or the expression plasmid encoding Lad-FLAG. These cells were then stimulated for the indicated periods with SDF-1α (S) or RANTES (R), after which the cell lysates were subjected to immunoprecipitation with an anti-FLAG antibody and subsequent immunoblotting with anti–Zap-70 antibody (top panel). The membrane was reprobed with the anti-FLAG Ab (bottom panel). (C) The chemokine-dependent phosphorylation of Zap-70 is repressed by expressing hLad siRNA. Jurkat T cells were transfected with empty pSUPER or pSUPER plasmids expressing SiA or SiB and stimulated with SDF-1α (S) or RANTES (R). The cell lysates were then analyzed by immunoprecipitation with anti–Zap-70 antibody and subsequent immunoblotting with anti-phosphotyrosine antibody (4G10) (top panel). The blot was also probed with the anti–Zap-70 antibody (bottom panel). (D) Jurkat T-cell clones that stably express CCR5 were transfected with pSUPER plasmids expressing control siA or siA and stimulated with SDF-1α or RANTES. The cell lysates were analyzed by Western with anti–phosphoZap-70 (Tyr493) or anti–Zap-70 Abs.
Lad associates with Lck and Zap-70 in response to chemokine treatment. (A) Lck associates with Lad upon chemokine treatment. Jurkat T cells that stably express Lad-FLAG (Lad) or were transfected with empty vector (Vec) were activated with anti-CD3 antibody (CD3), SDF-1α (S), or RANTES (R) for 10 minutes. The cell lysates were then subjected to immunoprecipitation with 5 μg antiFLAG antibody, and the precipitates were analyzed by immunoblotting with antiLck antibody (upper panel). The membrane was reprobed with the anti-FLAG Ab (bottom panel). (B) Zap-70 associates with Lad upon chemokine treatment. Jurkat T cells were transfected with pcDNA3.1 or the expression plasmid encoding Lad-FLAG. These cells were then stimulated for the indicated periods with SDF-1α (S) or RANTES (R), after which the cell lysates were subjected to immunoprecipitation with an anti-FLAG antibody and subsequent immunoblotting with anti–Zap-70 antibody (top panel). The membrane was reprobed with the anti-FLAG Ab (bottom panel). (C) The chemokine-dependent phosphorylation of Zap-70 is repressed by expressing hLad siRNA. Jurkat T cells were transfected with empty pSUPER or pSUPER plasmids expressing SiA or SiB and stimulated with SDF-1α (S) or RANTES (R). The cell lysates were then analyzed by immunoprecipitation with anti–Zap-70 antibody and subsequent immunoblotting with anti-phosphotyrosine antibody (4G10) (top panel). The blot was also probed with the anti–Zap-70 antibody (bottom panel). (D) Jurkat T-cell clones that stably express CCR5 were transfected with pSUPER plasmids expressing control siA or siA and stimulated with SDF-1α or RANTES. The cell lysates were analyzed by Western with anti–phosphoZap-70 (Tyr493) or anti–Zap-70 Abs.
Like Lck, another tyrosine kinase, Zap-70, has been shown to become activated in response to chemokine.18–20,26 Therefore, we tested whether Lad also interacts with Zap-70 in T cells upon chemokine treatment. Thus, Jurkat T cells transfected with the plasmid encoding FLAG-tagged hLad were stimulated with SDF-1α or RANTES (Figure 4B). Cell lysates were then subjected to immunoprecipitation with anti-FLAG antibody and subsequent Western blotting with anti–Zap-70 antibody. As shown in Figure 4B, upon SDF-1α or RANTES treatment, Zap-70 coimmunoprecipitated with Lad (upper panel). The amounts of precipitated hLad, which were assayed as a control, were found to be equal in each lane (Figure 4B lower panel). These results show that Lad interacts with Zap-70 in T cells in response to chemokine.
Having found that Lad and Zap-70 interact, we then tested whether Lad is involved in the chemokine-mediated activation of Zap-70. Thus, Jurkat T cells were transfected with empty pSUPER or pSUPER plasmids expressing SiA or SiB and then stimulated with SDF-1α or RANTES. The cell lysates were analyzed by immunoprecipitation with anti–Zap-70 antibody and subsequent Western blotting with antiphosphotyrosine antibody (4G10). As shown in Figure 4C, the SDF-1α– or RANTES-induced phosphorylation of Zap-70 is abolished by the introduction of hLad siRNA. The levels of endogenous Lad, which were assayed as a control, were significantly reduced by the introduction of the siRNAs (Figure 4C lower panel). In addition, analysis of Zap-70 phosphorylation by Western blotting with anti–phosphoZap-70 (Tyr493) Ab revealed that chemokine-induced Zap-70 phosphorylation was significantly reduced by the introduction of siRNAs. These results show that, in response to chemokine, Lad acts as a linker between Gβ and the 2 tyrosine kinases Lck and Zap-70. Moreover, we showed that when brought into close proximity to Zap-70 by its association with Lad, Lck may phosphorylate and activate Zap-70.
Lad mediates the chemokine-dependent activation of p42/44 ERK
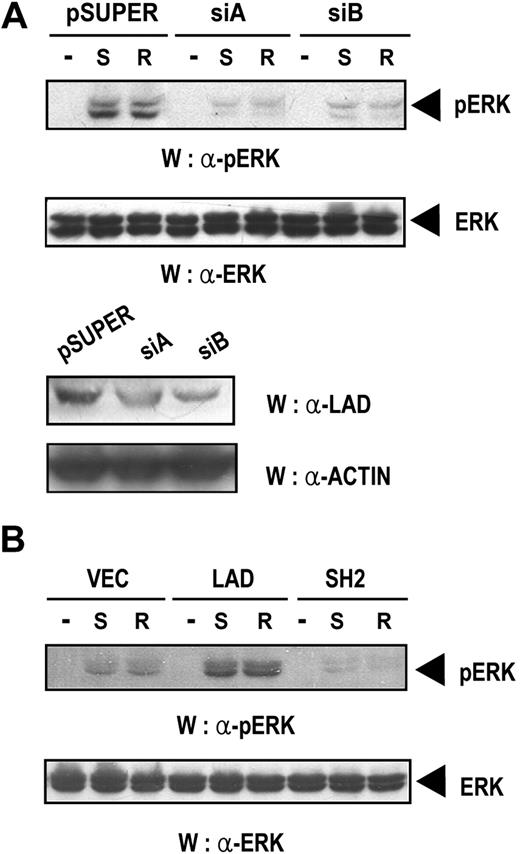
p42/44 ERK is known to be an important signaling molecule that is activated by chemokine,44 although how it is activated remains unclear. Therefore, we were interested in determining whether Lad is involved in the chemokine-mediated activation of ERK. Thus, Jurkat T cells transfected with empty pSUPER or pSUPER plasmids expressing SiA or SiB were stimulated with SDF-1α or RANTES. Subsequently, the cell lysates were analyzed by Western blotting with anti–phospho ERK antibody. As shown in Figure 5A (upper panel), the phosphorylation of ERK observed in SDF-1α– or RANTES-treated samples was significantly down-regulated by hLad siRNA expression, indicating that Lad is required for the chemokine-induced activation of ERK. The levels of ERK, which were assayed as control, were equal throughout the lanes (Figure 5A middle panel). In addition, the levels of endogenous Lad, which were also assayed as a control, were significantly reduced by the introduction of the Lad siRNAs (Figure 5A bottom panels). We also found that the phosphorylation of ERK was dramatically up-regulated in Jurkat T-cell clones that express Lad but was abrogated in clones expressing the SH2 domain of Lad (Figure 5B), which confirms that Lad is essential in chemokine-induced ERK activation.
Lad is required for the chemokine-induced activation of ERK. (A) Jurkat T cells transfected with empty pSUPER or pSUPER plasmids expressing SiA or SiB were treated with SDF-1α (S) or RANTES (R) for 10 minutes, and the cell lysates were analyzed by Western blotting with anti–phospho p42/44 ERK antibody (upper panel). The same blot was reprobed with anti–p42/44 ERK antibody to control for the level of ERK (middle panel). The same lysates were also analyzed for the level of endogenous Lad by Western blotting with antiLad antibody (bottom panel). (B) Jurkat T-cell clones that stably expressed Lad or the SH2 domain of Lad were activated and processed as described in panel A.
Lad is required for the chemokine-induced activation of ERK. (A) Jurkat T cells transfected with empty pSUPER or pSUPER plasmids expressing SiA or SiB were treated with SDF-1α (S) or RANTES (R) for 10 minutes, and the cell lysates were analyzed by Western blotting with anti–phospho p42/44 ERK antibody (upper panel). The same blot was reprobed with anti–p42/44 ERK antibody to control for the level of ERK (middle panel). The same lysates were also analyzed for the level of endogenous Lad by Western blotting with antiLad antibody (bottom panel). (B) Jurkat T-cell clones that stably expressed Lad or the SH2 domain of Lad were activated and processed as described in panel A.
Lad is essential for the chemokine-dependent phosphorylation of focal adhesion molecules such as Pyk2 and paxillin
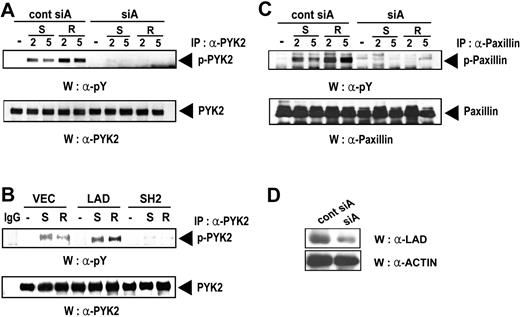
Prior studies have demonstrated that tyrosine phosphorylation of the focal adhesion molecules Pyk2 and paxillin and their association with each other play important roles in chemokine-induced migration of hematopoietic cells by changing the cytoskeleton.24 Based on this information, we examined whether Lad is involved in the activation of Pyk2. Thus, Jurkat T cells that express CCR5 were transfected with pSUPER plasmids expressing control siA or siA and stimulated with SDF-1α or RANTES. Subsequently, the cell lysates were assayed for Pyk2 activity by immunoprecipitation with antiPyk2 Ab and subsequent Western blotting with antiphosphotyrosine Ab. As shown in Figure 6A, the phosphorylation of Pyk2 induced by SDF-1α or RANTES treatment was almost completely abolished by hLad siRNA, indicating that Lad is required for chemokine-induced Pyk2 activation. The amounts of precipitated Pyk2, which were assayed as a control, were found to be equal (Figure 6A middle panel). In addition, the tyrosine phosphorylation of Pyk2 induced by SDF-1α or RANTES treatment was enhanced in Jurkat clones expressing Lad and significantly reduced in Jurkat clones expressing the SH2 domain of Lad (Figure 6B). This confirms that Lad is essential for chemokine-induced Pyk2 activation.
Lad is required for the chemokine-induced activation of focal adhesion molecules such as Pyk2 and paxillin. (A) Jurkat T-cell clones expressing CCR5 were transfected with pSUPER plasmids expressing control siA or siA and treated with SDF-1α (S) or RANTES (R) for 2 or 5 minutes. Pyk2 was immunoprecipitated and the immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine or anti-Pyk2 antibody. IgG was used as a negative control. (B) Jurkat T cells stably expressing Lad or the SH2 domain of Lad were treated with SDF-1α or RANTES and processed as described in panel A. (C) The cell lysates prepared as described in panel A were analyzed by immunoprecipitation with anti-paxillin antibody and subsequent immunoblotting with anti-phosphotyrosine antibody (4G10) (upper panel). As a protein-loading control, the blots were reprobed with anti-paxillin antibody (lower panel). (D) The same cell lysates used in panel A were analyzed for the Lad levels by Western blotting with anti-Lad antibody (upper panel) and with anti-actin antibody (lower panel).
Lad is required for the chemokine-induced activation of focal adhesion molecules such as Pyk2 and paxillin. (A) Jurkat T-cell clones expressing CCR5 were transfected with pSUPER plasmids expressing control siA or siA and treated with SDF-1α (S) or RANTES (R) for 2 or 5 minutes. Pyk2 was immunoprecipitated and the immunoprecipitates were analyzed by immunoblotting with anti-phosphotyrosine or anti-Pyk2 antibody. IgG was used as a negative control. (B) Jurkat T cells stably expressing Lad or the SH2 domain of Lad were treated with SDF-1α or RANTES and processed as described in panel A. (C) The cell lysates prepared as described in panel A were analyzed by immunoprecipitation with anti-paxillin antibody and subsequent immunoblotting with anti-phosphotyrosine antibody (4G10) (upper panel). As a protein-loading control, the blots were reprobed with anti-paxillin antibody (lower panel). (D) The same cell lysates used in panel A were analyzed for the Lad levels by Western blotting with anti-Lad antibody (upper panel) and with anti-actin antibody (lower panel).
Analysis of the cell lysates prepared as described in Figure 6A by immunoprecipitation with anti-paxillin antibody and subsequent Western blotting with anti-phosphotyrosine antibody also showed that the chemokine-induced tyrosine phosphorylation of paxillin was blocked when Lad expression was reduced by siRNA (Figure 6C). These results indicate that Lad is required for the chemokine-induced tyrosine phosphorylation and activation of focal adhesion complexes.
Discussion
To date, we know that Lad is involved in T-cell activation, Th1 differentiation, and activation-induced T-cell death.31,32,34,35 Here, we provide evidence supporting a novel function of Lad, namely, in chemokine-induced T-cell migration. We showed that in response to chemokine, Lad associates with Gβ and recruits the tyrosine kinases Lck and Zap-70 to the Gβ-mediated signaling complex. Furthermore, we found that Lad is required for the activation of Zap-70 and the focal adhesion complex as well as the chemokine-dependent migration of T cells.
In response to chemokine, the heterotrimeric G proteins dissociate into their Gα and Gβγ subunits and the Gβγ subunit then mediates the signals that lead to migration.45,46 Regarding the effectors of Gβγ, PI3Kγ and PLC-β have been characterized and found to associate directly with Gβ.8,9,11,12 As far as we know, Lad is the third effector molecule that directly binds to Gβ in response to chemokine.
Previous studies have established that tyrosine kinases such as Lck, Zap-70, and Itk that are involved in T-cell receptor–mediated signaling are also activated by chemokine stimulation.17–22 While the activation of these kinases has been shown to be required for chemokine-dependent lymphocyte migration, the mechanisms responsible for their activation have been unclear to date. Our observation described in this report, namely, that Lad has an adaptor function in linking Gβγ to the tyrosine kinases Lck and Zap-70, reveals the mechanism that leads to the chemokine-induced activation of these tyrosine kinases. Thus, upon binding to Lad, Lck and Zap-70 may be brought into close proximity with each other; Lck may then be able to phosphorylate and activate Zap-70, as has also been observed in TCR-induced signaling events.47
In addition to Lck and Zap-70, the Tec family tyrosine kinases Itk/Rlk have also been shown to be activated by chemokines and to mediate the chemokine-induced activation of Rac and Cdc42, thereby regulating the migration of lymphocytes.21,22 In TCR signaling, Itk becomes activated by its Lck-mediated phosphorylation.48 In addition, 2 different groups have identified Lad as a binding partner of Itk/Rlk32 and Lck.31 By analogy to TCR signaling, therefore, it is reasonable to hypothesize that chemokine-induced Lck activation leads to the phosphorylation and activation of Itk and the subsequent activation of Rac and Cdc42 that leads to cytoskeletal structure change.
In this report, we studied the function of Lad in chemokine signaling in T cells. At present, the role of Lad in other types of migrating cells, such as B cells, neutrophils, and macrophages, is unclear. However, although initially thought of as a T-cell–specific protein,34 Lad is expressed in other cell types,39,49 which raises the possibility that Lad may also mediate the chemokine-dependent migration of cells other than T cells.
In this report, we used the Jurkat T-cell line to show that Lad is required for the signaling that is stimulated by both the homeostatic chemokine SDF-1α and the inflammatory chemokine RANTES. Since Lad expression is induced upon T-cell activation,32,34 one could speculate that Lad function in vivo may be restricted to signaling events in response to inflammatory chemokines. However, considerable amounts of Lad protein are detected in even resting T cells,32,33 the amount of which may be sufficient for involvement in homeostatic chemokine-dependent cell migration. Therefore, we do not exclude the possibility that Lad is involved in both homeostatic and inflammatory chemokine-mediated migration. In addition, Lad expression was found in both CD4+ and CD8+ T cells32,34 in response to T-cell activation as well as in Th1 and Th2 clones,32 suggesting that Lad may regulate migration of these broad subsets of T cells in response to various chemokines.
In summary, we here describe a previously unidentified mechanism for chemokine signaling wherein the adaptor protein Lad provides the scaffold onto which Gβ and the tyrosine kinases Lck and Zap-70 are recruited. Future studies will focus on whether Lad is also involved in other chemokine-dependent signaling pathways, such as those containing PI3K, DOCK2, and Rap.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from KBRDG initiative research program (2006-08367), National Research Laboratory program (2004-20858), and the NCRC program (through the Center for Cell Signaling and Drug discovery research at Ewha Woman's University) from the Korean Ministry of Science & Technology/KOSEF. I.P. and D.L. were supported by BK21 project.
We thank Drs Simon, Gutkind, Agami, and Oppermann for the Gβ1 and Gβ5 expression plasmids, the Gγ2 subunit expression construct, the pSUPER plasmid, and the CCR5 expression plasmid, respectively. We appreciate Dr J. Kim for critical reading of the paper.
Authorship
Contribution: D.P., I.P., D.L., and Y.B.C. designed and performed research, collected data, and analyzed data; H.L. and Y.Y. analyzed data and wrote the paper.
D.P. and I.P. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yungdae Yun, Ewha Woman's University, 11-1, Daehyundong, Seodaemungu, Seoul, 120-750, Korea; e-mail: yunyung@ewha.ac.kr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal