Abstract
Interactions between UCN-01 and HMG-CoA reductase inhibitors (ie, statins) have been examined in human leukemia and myeloma cells. Exposure of U937 and U266 cells to minimally toxic concentrations of UCN-01 and various statins (eg, lovastatin, simvastatin, or fluvastatin) dramatically increased mitochondrial dysfunction, caspase activation, and apoptosis. Comparable effects were observed in other leukemia and myeloma cell lines as well as in primary acute myeloid leukemia (AML) blasts but not in normal hematopoietic cells. Potentiation of UCN-01 lethality by lovastatin was associated with disruption of Ras prenylation and activation. These events were significantly attenuated by farnesyl pyrophosphate (FPP) but not by geranylgeranyl pyrophosphate (GGPP), implicating perturbations in farnesylation rather than geranylgeranylation in synergistic interactions. Coexposure to statins and UCN-01 resulted in inactivation of ERK1/2 and Akt, accompanied by JNK activation. U266 cells ectopically expressing JNK1-APF, a dominant negative JNK1 mutant, displayed significantly reduced susceptibility to lovastatin/UCN-01–mediated lethality. Moreover, transfection of U266 cells with constitutively activated H-Ras (Q61L) attenuated ERK1/2 inactivation and dramatically diminished the lethality of this regimen. Collectively, these findings indicate that HMG-CoA reductase inhibitors act through a Ras farnesylation-associated mechanism to induce signaling perturbations, particularly prevention of Ras and ERK1/2 activation, in UCN-01–treated cells, resulting in the synergistic induction of cell death.
Introduction
HMG-CoA reductase inhibitors (also known as statins) are anticholesterolemic agents that act by interfering with the mevalonate pathway.1 These compounds inhibit the activity of 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase, which catalyzes the rate-limiting step in mevalonate biosynthesis, a key intermediate in cholesterol metabolism. However, the mevalonate pathway is also critically involved in several signal transduction cascades, particularly those related to Ras activation.2,3 Ras is a small GTP-binding protein that transduces mitogenic signals emanating from growth factors and cytokines as well as from activation of other oncogenes.4 The Ras family is composed of H-Ras, N-Ras, and K-Ras and is frequently activated, generally by point mutations, in diverse human cancers including those of hematopoietic origin.5 Activation of Ras requires translocation to the plasma membrane, a process that is mediated by the attachment of isoprenyl moieties generated from farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) through the actions of farnesyltransferases (FTases) and geranylgeranyltransferases (GGTases), respectively.5,6 By interrupting the biosynthesis of mevalonate, a precursor of both FPP and GGPP, statins oppose activation of Ras and downstream signaling cascades, including the Raf/MEK/ERK and PI3K/Akt among others, which play critical roles in regulation of cell survival and proliferation.2,5,7 Statins also interfere with prenylation of other proteins, including other members of the small GTP-binding protein superfamily such as Rho.8,9 In malignant hematopoietic cells such as leukemia and myeloma, statins exert antiproliferative and proapoptotic effects10–12 and potentiate the lethal actions of more conventional cytotoxic agents.8,13,14 Accordingly, the addition of statins to the therapeutic armamentarium in human cancers including hematologic malignancies has been proposed,15–18 particularly in combination with conventional or more novel anticancer agents, given their uncertain activity as monotherapy.19
UCN-01 (7-hydroxystaurosporine) was originally developed as a specific protein kinase C (PKC) inhibitor and also acts as an inhibitor of cyclin-dependent kinases (CDKs).20 More recently, the ability of UCN-01 to inhibit checkpoint kinase 1 (Chk1)21 and abrogate checkpoint regulation in tumor cells exposed to DNA-damaging agents has been described.22 UCN-01 lethality has also been associated with perturbations in various survival signaling cascades. For example, UCN-01–mediated inhibition of the PDK1/Akt pathway has been implicated in the cytotoxic actions of this agent.23,24 In addition, exposure of human leukemia and myeloma cells to UCN-01 results in activation of MEK1/2 (mitogen-activated protein kinase kinase 1/2)/ERK1/2 (extracellular signal-regulated kinase 1/2)25,26 through a mechanism yet to be elucidated. The latter actions appear to be cytoprotective in view of evidence that interruption of the MEK/ERK pathway by pharmacologic inhibitors (eg, PD184352) leads to a dramatic increase in apoptosis in both malignant hematopoietic and nonhematopoietic cells.25–27 Such findings raise the possibility that in addition to synergistic interactions with conventional cytotoxic DNA-damaging drugs,28 strategies combining UCN-01 with other novel signal transduction inhibitors warrant investigation.
While the mechanism underlying the lethality of statins toward tumor cells remains to be determined, it is tempting to invoke disruption of Ras superfamily protein-mediated signaling pathway, including Raf/MEK/ERK and PI3K/Akt.2,5 Recent studies indicate that pharmacologic MEK1/2 inhibitors markedly lower the threshold for lovastatin-induced lethality.29 Furthermore, agents that block Akt signaling, including perifosine and the Hsp90 antagonist 17-AAG, lower the threshold for UCN-01–mediated apoptosis.30,31 Recently, we reported that FTase inhibitors interact synergistically with UCN-01 to induce apoptosis in human leukemia and myeloma cells.32,33 Because statins target both farnesylation and geranylgeranylation of Ras superfamily proteins and disrupt downstream pathways (eg, MEK/ERK and PI3K/Akt), the possibility arose that such agents might be particularly effective in promoting the anticancer activity of UCN-01. To test this possibility, we examined interactions between clinically relevant statins and UCN-01 in human leukemia and myeloma cells. Here we report that statins interact in a highly synergistic manner with UCN-01 to induce apoptosis in association with perturbations in prenylation of Ras family proteins, leading to inactivation of Ras, ERK, and Akt as well as the reciprocal activation of SAPK/JNK. Moreover, enhanced lethality of these regimens critically depends upon disruption of Ras farnesylation rather than geranylgeranlyation. Collectively, these findings suggest that regimens combining statins with UCN-01 deserve further investigation as a novel antileukemic and antimyeloma strategy.
Materials and methods
Cells and reagents
Human leukemia (U937, HL-60, Jurkat, and Raji) and myeloma (U266, RPMI8226) cells were purchased from American Type Culture Collection (ATCC; Manassas, VA). Dexamethasone-sensitive (MM.1S) and -resistant (MM.1R) human myeloma cell lines were provided by Dr Steven Rosen (Northwestern University, Chicago, IL).34 Cells were maintained in RPMI 1640 medium containing 10% FBS as previously reported.25,26 U266 cells were transfected with a construct encoding activated form of Ras (Q61L; Upstate Biotechnology, Lake Placid, NY),35 and clones were selected by G418. U266 cells stably expressing JNK1-APF, a dominant negative form of JNK1 in which the phosphorylation site Thr166 and Tyr165 are replaced with Ala and Phe, respectively), were kindly provided by the laboratory of Dr Stanley Korsmeyer (Dana-Farber Cancer Institute, Boston, MA).36 All experiments used logarithmically growing cells (3 × 105 to 5 × 105/mL).
Peripheral blood samples were obtained with informed consent from 3 patients with acute myeloid leukemia (AML) undergoing routine diagnostic aspirations with approval from the institutional review board of Virginia Commonwealth University. Informed consent was provided in accordance with the Declaration of Helsinki. AML blasts were isolated as previously reported.32 Mononuclear cells were also obtained with informed consent from the bone marrow of 2 patients with nonmalignant hematopoietic disorders in which the white blood cell series was uninvolved (eg, iron deficiency anemia, immune thrombocytopenia) as well as from peripheral blood donated by 1 healthy volunteer.
UCN-01, provided by the Cancer Treatment and Evaluation Program, National Cancer Institute (NCI), was prepared as previously reported.25 Lovastatin (Calbiochem, San Diego, CA) and fluvastatin (LKT, St. Paul, MN) were dissolved in DMSO and stored at −80°C. Simvastatin (LKT) was dissolved at 50 mg/mL in ethanol with adding 0.813 mL 1N NaOH, stored at −80°C in aliquots, and subsequently neutralized to pH 7.2 with 1N HCl before use. The selective FTase inhibitor FTI-277, the selective GGTase I inhibitor GGTI-2147, and the JNK inhibitor SP600125 were purchased form Calbiochem (San Diego, CA), dissolved in sterile DMSO, and stored at −20°C. FPP and GGPP in solution were obtained from Sigma (St Louis, MO). In all experiments, the final concentration of DMSO did not exceed 0.1%.
Analysis of apoptosis and clonogenicity
The extent of apoptosis was evaluated by assessing Wright-Giemsa–stained cytospin slides under light microscopy and by flow cytometry with annexin V–FITC and propidium iodide (PI) staining as described previously.32 To evaluate colony-forming ability following drug treatment, a soft agar cloning assay was performed as described previously.25,26 Colonies consisting of more than 50 cells were scored under inverted microscopy and colony formation for each condition calculated in relation to values obtained for untreated control cells.
Western blot analysis
Samples from whole-cell pellets were prepared, and 30 μg protein for each condition was subjected to Western blot analysis following procedures previously described in detail.25 Alternatively, S-100 cytosolic samples for Western blot analysis were prepared using digitonin lysis buffer as previously reported.25 Where indicated, blots were reprobed with an antibody against β-actin (Transduction Laboratories, Lexington, KY) or α-tubulin (Calbiochem) to ensure equal loading and transfer of proteins. The following primary antibodies were used: caspase-8 (Alexis, San Diego, CA); caspase-3 (BD, San Diego, CA); H-Ras, RhoB, Rap1A, phospho-JNK (Thr183/Tyr185), phospho-Akt (Ser473), Akt1/2/3, JNK1, cytochrome c, AIF (Santa Cruz Biotechtechnology, Santa Cruz, CA); phospho-p44/42 MAP kinase (ERK1/2, Thr202/Tyr204), p44/42 MAP kinase, SAPK/JNK, cleaved caspase-3 (Cell Signaling Technology, Beverly, MA); Smac/DIABLO (Upstate Biotechnology); caspase-9 (BD); and PARP (Biomol, Plymouth Meeting, PA).
Ras activation assay
Ras activity was detected using a Ras Activation Assay Kit (Upstate Biotechnology) as per the manufacturer's instructions. Briefly, after drug treatment, 107 cells were lysed on ice. To load GTPγS (positive control) or GDP (negative control), cell extracts (400 μg protein) from untreated cells were incubated with either 100 μM GTPγS or 1 mM GDP for 30 minutes at 30°C with agitation, and the reactions were stopped by adding 60 mM MgCl2 on ice. For Ras pull-down, Raf-1 Ras binding domain (RBD) agarose was added in cell extract from each condition, including GTPγS- or GDP-loaded controls, and incubated for 45 minutes at 4°C with gentle agitation. After being washed 3 times, the agarose beads were resuspended in 2× sample buffer with addition of 50 mM dithiothreitol prior to boiling and boiled for 5 minutes. Pulled-down Ras protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and detected by an anti-Ras antibody (clone RAS10) that recognizes H-, K- and N-Ras.
Statistical analysis
For analysis of apoptosis and clonogenicity, values represent the means ± SD for at least 3 separate experiments performed in triplicate. The significance of differences between experimental variables was determined using the Student t test. Analysis of synergism was performed according to median dose effect analysis37 using the software program Calcusyn (Biosoft, Ferguson, MO).
Results
Statins synergistically potentiate UCN-01 lethality in human leukemia and myeloma cells
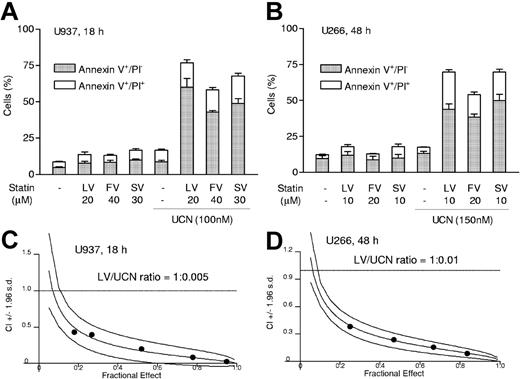
To determine what effect coadministration of statins might have on the response of human leukemia and myeloma cells to UCN-01, U937 myelomonocytic and U266 myeloma cells were exposed for 18 hours or 48 hours to the indicated concentrations of UCN-01 with or without lovastatin, simvastatin, or fluvastatin, after which the extent of apoptosis was assessed by annexin V–FITC/PI analysis. As shown in Figure 1A-B, drugs administered alone exerted relatively little toxicity. However, when UCN-01 was combined with each of the statins, there was a dramatic increase in early (annexin V+/PI−) and late (annexin V+/PI+) apoptosis. Median dose effect analysis of drug interactions administered at a fixed dose ratio yielded combination index (CI) values considerably less than 1.0 for both U937 and U266 cells, signifying a highly synergistic interaction (Figure 1C-D).
Statins interact synergistically with UCN-01 to induce apoptosis in human malignant hematopoietic cells. (A) Human myelomonoyctic leukemia U937 cells were exposed for 18 hours to 100 nM UCN-01 (UCN) with or without 20 μM lovastatin (LV), 40 μM fluvastatin (FV), or 30 μM simvastatin (SV), respectively, after which the percentage of annexin V+ cells exhibiting either annexin V+/PI− (early apoptosis) or annexin V+/PI+ (late apoptosis) was determined by annexin V–FITC/PI staining and flow cytometry as described in “Analysis of apoptosis and clonogenicity.” (B) Human myeloma U266 cells were exposed for 48 hours to 150 nM UCN-01 with or without 10 μM lovastatin, 20 μM fluvastatin (FV), or 10 μM simvastatin (SV), respectively, after which the percentage of apoptotic cells was determined as described in panel A. For panels A and B, results represent the means ± SD for 3 separate experiments performed in triplicate. (C-D) U937 and U266 cells were exposed to a range of lovastatin (5 to 25 μM) and UCN-01 (50 to 250 nM) concentrations alone and in combination at a fixed ratio (eg, U937, 1:0.005; U266, 1:0.01) for 18 hours (U937) or 48 hours (U266). At the end of the exposure intervals, the percentage of annexin V+ cells was determined for each condition; fractional effect values were determined by comparing results with those of untreated controls, and median dose effect analysis was employed to characterize the nature of the interaction between lovastatin and UCN-01. CI values less than 1.0 (horizontal line) denote a synergistic interaction. The results of representative experiments are shown; 2 additional studies yielded equivalent results.
Statins interact synergistically with UCN-01 to induce apoptosis in human malignant hematopoietic cells. (A) Human myelomonoyctic leukemia U937 cells were exposed for 18 hours to 100 nM UCN-01 (UCN) with or without 20 μM lovastatin (LV), 40 μM fluvastatin (FV), or 30 μM simvastatin (SV), respectively, after which the percentage of annexin V+ cells exhibiting either annexin V+/PI− (early apoptosis) or annexin V+/PI+ (late apoptosis) was determined by annexin V–FITC/PI staining and flow cytometry as described in “Analysis of apoptosis and clonogenicity.” (B) Human myeloma U266 cells were exposed for 48 hours to 150 nM UCN-01 with or without 10 μM lovastatin, 20 μM fluvastatin (FV), or 10 μM simvastatin (SV), respectively, after which the percentage of apoptotic cells was determined as described in panel A. For panels A and B, results represent the means ± SD for 3 separate experiments performed in triplicate. (C-D) U937 and U266 cells were exposed to a range of lovastatin (5 to 25 μM) and UCN-01 (50 to 250 nM) concentrations alone and in combination at a fixed ratio (eg, U937, 1:0.005; U266, 1:0.01) for 18 hours (U937) or 48 hours (U266). At the end of the exposure intervals, the percentage of annexin V+ cells was determined for each condition; fractional effect values were determined by comparing results with those of untreated controls, and median dose effect analysis was employed to characterize the nature of the interaction between lovastatin and UCN-01. CI values less than 1.0 (horizontal line) denote a synergistic interaction. The results of representative experiments are shown; 2 additional studies yielded equivalent results.
Parallel results were obtained in several other leukemia and myeloma cell lines, including HL-60, Jurkat, Raji, RPMI8266, as well as MM.1S and MM.1R myeloma cells (Figure 2A). In addition, coadministration of minimally toxic concentrations of lovastatin and UCN-01 also resulted in a clear increase in apoptosis in primary leukemic blasts obtained from 3 patients with AML (French-American-British [FAB] classification M2; Figure 2B). In contrast, combined exposure to these agents resulted in minimal toxicity in normal peripheral blood or bone marrow mononuclear cells. Notably, when exposure intervals were increased (eg, to 48 hours or 72 hours), similar interactions were observed in U937 cells with considerably lower concentrations of lovastatin (eg, 1 μM) and UCN-01 (eg, 50 nM; Figure 2C). Clonogenic assays revealed that combined treatment of U937 or U266 cells with lovastatin/UCN-01 dramatically reduced clonogenic survival (eg, by more than 90%; Figure 2D).
The statin/UCN-01 regimen induces apoptosis in multiple leukemia and myeloma cell lines as well as primary AML cells but is minimally toxic to normal hematopoietic cells. (A) Human leukemia (Jurkat, HL-60, and Raji) and myeloma (RPMI8226 [8226], MM.1S, and MM.1R) cells were exposed to UCN-01 ([UCN] 100 nM for HL-60, RPMI8226, MM.1S, and MM.1R; 150 nM for Jurkat and Raji) with or without lovastatin ([LV] 20 μM for all leukemia cell lines; 10 μM for all myeloma cell lines) for 18 hours (leukemia cells) or 24 hours (myeloma cells), after which the percentage of annexin V+ cells was determined by flow cytometry. (B) AML blasts were isolated from peripheral blood samples derived from 3 patients with AML (FAB classification M2) as described in “Cells and reagents.” Mononuclear cells were isolated from the bone marrow (BM/MC) of 2 patients with nonmalignant, nonmyeloid hematopoietic disorders and the peripheral blood (PB/MC) from 1 healthy volunteer. AML blasts and normal cells were treated with 150 nM UCN-01 with or without 20 μM lovastatin for 18 hours, after which the percentage of apoptotic cells was determined by evaluating Giemsa-Wright–stained cytospin preparations. (C) U937 cells were incubated with UCN-01 (50 to 100 nM) with or without low doses of lovastatin (1 to 5 μM) for 48 hours or 72 hours, after which the percentage of annexin V+ cells, including annexin V+/PI− and annexin V+/PI+, was determined by flow cytometry. (D) U937 and U266 cells were treated with UCN-01 (U937, 100 nM; U266, 150 nM) with or without lovastatin (U937, 20 μM; U266, 10 μM) for 18 hours (U937) or 48 hours (U266), after which cells were washed free of drug and plated in soft agar as described in “Analysis of apoptosis and clonogenicity.” After incubation for 12 days, colonies (more than 50 cells) were scored, and colony formation for each condition expressed relative to untreated control cells. For all panels, results represent the means ± SD for 3 separate experiments performed in triplicate.
The statin/UCN-01 regimen induces apoptosis in multiple leukemia and myeloma cell lines as well as primary AML cells but is minimally toxic to normal hematopoietic cells. (A) Human leukemia (Jurkat, HL-60, and Raji) and myeloma (RPMI8226 [8226], MM.1S, and MM.1R) cells were exposed to UCN-01 ([UCN] 100 nM for HL-60, RPMI8226, MM.1S, and MM.1R; 150 nM for Jurkat and Raji) with or without lovastatin ([LV] 20 μM for all leukemia cell lines; 10 μM for all myeloma cell lines) for 18 hours (leukemia cells) or 24 hours (myeloma cells), after which the percentage of annexin V+ cells was determined by flow cytometry. (B) AML blasts were isolated from peripheral blood samples derived from 3 patients with AML (FAB classification M2) as described in “Cells and reagents.” Mononuclear cells were isolated from the bone marrow (BM/MC) of 2 patients with nonmalignant, nonmyeloid hematopoietic disorders and the peripheral blood (PB/MC) from 1 healthy volunteer. AML blasts and normal cells were treated with 150 nM UCN-01 with or without 20 μM lovastatin for 18 hours, after which the percentage of apoptotic cells was determined by evaluating Giemsa-Wright–stained cytospin preparations. (C) U937 cells were incubated with UCN-01 (50 to 100 nM) with or without low doses of lovastatin (1 to 5 μM) for 48 hours or 72 hours, after which the percentage of annexin V+ cells, including annexin V+/PI− and annexin V+/PI+, was determined by flow cytometry. (D) U937 and U266 cells were treated with UCN-01 (U937, 100 nM; U266, 150 nM) with or without lovastatin (U937, 20 μM; U266, 10 μM) for 18 hours (U937) or 48 hours (U266), after which cells were washed free of drug and plated in soft agar as described in “Analysis of apoptosis and clonogenicity.” After incubation for 12 days, colonies (more than 50 cells) were scored, and colony formation for each condition expressed relative to untreated control cells. For all panels, results represent the means ± SD for 3 separate experiments performed in triplicate.
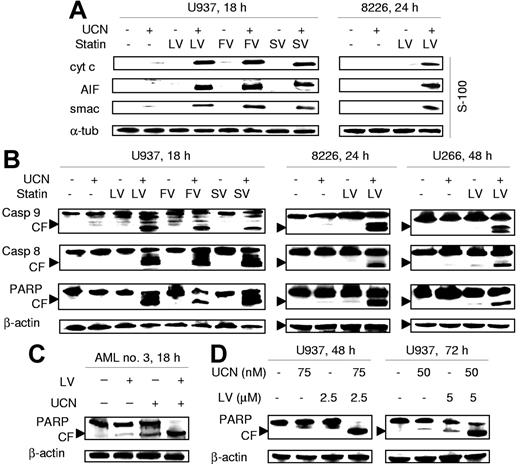
Analysis of cytosolic redistribution of mitochondrial proteins revealed that coexposure of U937 leukemia cells or RPMI8226 myeloma cells to UCN-01 plus each statin resulted in a marked release of the proapoptotic proteins cytochrome c, AIF, or Smac/DIABLO (Figure 3A). Western blot analysis revealed that combined treatment also resulted in a pronounced increase in cleavage of caspase-9, caspase-8, and PARP in U937 as well as RPMI8226 and U266 cells (Figure 3B). Markedly increased PARP degradation was also observed in primary AML cells following combined lovastatin/UCN-01 exposure (Figure 3C). Lastly, enhanced PARP cleavage was noted at later intervals (eg, 48 to 72 hours) after combined exposure of U937 cells to low concentrations of UCN-01 and lovastatin (Figure 3D). Collectively, these findings indicate that cotreatment with statins and UCN-01 results in a marked increase in mitochondrial dysfunction and apoptosis in human leukemia and myeloma cell lines as well as in primary AML blasts.
Statins potentiate mitochondrial dysfunction and activation of caspase cascades mediated by UCN-01 in cultured leukemia and myeloma cells as well as in primary AML blasts. (A) U937 cells were exposed for 18 hours to 100 nM UCN-01 (UCN) with or without 20 μM lovastatin (LV), 40 μM fluvastatin (FV), or 30 μM simvastatin (SV) (left panels), and RPMI8226 cells (8226) for 24 hours to 100 nM UCN-01 with or without 10 μM lovastatin (right panels). At the end of the incubation period, expression of cytochrome c, Smac/DIABLO, and AIF in cytosolic fractions (S-100) was evaluated by Western blot analysis as described in “Western blot analysis.” (B) U937 (left panels) and RPMI8226 cells (middle panels) were treated as described in panel A. U266 cells were exposed for 48 hours to 150 nM UCN-01 with or without 10 μM lovastatin (right panels). Cells were then lysed and subjected to Western blot analysis to assess cleavage of caspases and degradation of PARP using the indicated primary antibodies. (C) Blasts from AML patient no. 3 were treated with 150 nM UCN-01 with or without 20 μM lovastatin for 18 hours, after which Western blot analysis was performed to assess PARP degradation. (D) U937 cells were incubated with low concentrations of UCN-01 (50 to 75 nM) with or without lovastatin (2.5 to 5μM) for either 48 hours or 72 hours, after which PARP degradation was monitored by Western blot analysis. For all panels, each lane was loaded with 30 μg protein; blots were subsequently stripped and reprobed for expression of α-tubulin or β-actin to ensure equivalent loading and transfer of protein. The results of a representative experiment are shown; an additional study yielded equivalent results. CF indicates cleavage fragment.
Statins potentiate mitochondrial dysfunction and activation of caspase cascades mediated by UCN-01 in cultured leukemia and myeloma cells as well as in primary AML blasts. (A) U937 cells were exposed for 18 hours to 100 nM UCN-01 (UCN) with or without 20 μM lovastatin (LV), 40 μM fluvastatin (FV), or 30 μM simvastatin (SV) (left panels), and RPMI8226 cells (8226) for 24 hours to 100 nM UCN-01 with or without 10 μM lovastatin (right panels). At the end of the incubation period, expression of cytochrome c, Smac/DIABLO, and AIF in cytosolic fractions (S-100) was evaluated by Western blot analysis as described in “Western blot analysis.” (B) U937 (left panels) and RPMI8226 cells (middle panels) were treated as described in panel A. U266 cells were exposed for 48 hours to 150 nM UCN-01 with or without 10 μM lovastatin (right panels). Cells were then lysed and subjected to Western blot analysis to assess cleavage of caspases and degradation of PARP using the indicated primary antibodies. (C) Blasts from AML patient no. 3 were treated with 150 nM UCN-01 with or without 20 μM lovastatin for 18 hours, after which Western blot analysis was performed to assess PARP degradation. (D) U937 cells were incubated with low concentrations of UCN-01 (50 to 75 nM) with or without lovastatin (2.5 to 5μM) for either 48 hours or 72 hours, after which PARP degradation was monitored by Western blot analysis. For all panels, each lane was loaded with 30 μg protein; blots were subsequently stripped and reprobed for expression of α-tubulin or β-actin to ensure equivalent loading and transfer of protein. The results of a representative experiment are shown; an additional study yielded equivalent results. CF indicates cleavage fragment.
Statins disrupt prenylation of Ras superfamily proteins and inhibit UCN-01–mediated Ras activation
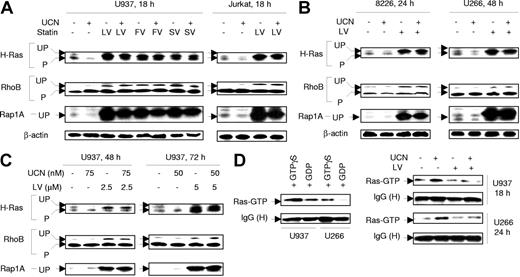
Effects of statins on the prenylation status of Ras as well as Ras-related proteins (eg, Rap1A) and other small GTP-binding proteins (eg, RhoB) were then examined in UCN-01–treated leukemia and myeloma cells. Of these proteins, H-Ras is generally processed through farnesylation,38,39 Rap1A through geranylgeranylation,40 and RhoB through both.41 Administration of statins (ie, lovastatin, fluvastatin, or simvastatin), either alone or in combination with UCN-01, inhibited prenylation of H-Ras and RhoB as well as Rap1A in U937 and Jurkat leukemia cells, reflected by an increase in slower-migrating bands corresponding to unprenylated (unprocessed) forms (Figure 4A). 42 Interestingly, UCN-01 (100 nM) slightly decreased levels of unprenylated H-Ras and Rap1A in cells with or without statin treatment. Essentially identical responses were obtained in RPMI8226 and U266 myeloma cells (Figure 4B). Lastly, comparable results were observed when U937 cells were exposed to low concentrations of UCN-01 and lovastatin for longer intervals (eg, 48 to 72 hours; Figure 4C). Thus, statins inhibited both farnesylation and geranylgeranylation of Ras superfamily proteins in human leukemia and myeloma cells, effects that were not substantially altered by UCN-01.
Statins, administered alone or in combination with UCN-01, induce perturbations in protein prenylation. (A) U937 cells (left panels) were exposed for 18 hours to 100 nM UCN-01 (UCN) with or without 20 μM lovastatin (LV), 40 μM fluvastatin (FV), or 30 μM simvastatin (SV), respectively; alternatively, Jurkat cells (right panels) were treated with 150 nM UCN-01 with or without 20 μM lovastatin for 18 hours. (B) RPMI8226 cells (8226) were exposed to 100 nM UCN-01 with or without 10 μM lovastatin for 24 hours and U266 to 150 nM UCN-01 with or without 10 μM lovastatin for 48 hours. (C) U937 cells were incubated with low concentrations of UCN-01 (50 to 75 nM) with or without 2.5 to 5 μM lovastatin for 48 hours or 72 hours, respectively. For panels A-C, after treatment, cells were lysed and prenylation status of H-Ras, RhoB, and Rap1A was determined by Western blot analysis. Each lane was loaded with 30 μg protein; blots were subsequently stripped and reprobed for expression of β-actin to ensure equivalent loading and transfer of protein. The results of a representative experiment are shown; an additional study yielded equivalent results. UP indicates unprenylated (corresponding to slower-migrating bands); P, prenylated. (D) U937 cells were exposed to 100 nM UCN-01 with or without 20 μM lovastatin for 18 hours and U266 cells to 150 nM UCN-01 with or without 10 μM lovastatin for 24 hours. At the end of the incubation period, cells were lysed and subjected (400 μg protein per condition) to a Ras activation assay as described in “Ras activation assay” (right panels). In parallel, untreated U937 and U266 cells were lysed, and cell extracts incubated/loaded with 100 μM GTPγS or 1 mM GDP for 30 minutes at 30°C for positive and negative controls, respectively (left panels). Ras activity is reflected by the amount of Ras (Ras-GTP) pulled down by Raf-1 RBD agarose beads. IgG (H) indicates IgG heavy chain. The results are representative of 3 separate experiments.
Statins, administered alone or in combination with UCN-01, induce perturbations in protein prenylation. (A) U937 cells (left panels) were exposed for 18 hours to 100 nM UCN-01 (UCN) with or without 20 μM lovastatin (LV), 40 μM fluvastatin (FV), or 30 μM simvastatin (SV), respectively; alternatively, Jurkat cells (right panels) were treated with 150 nM UCN-01 with or without 20 μM lovastatin for 18 hours. (B) RPMI8226 cells (8226) were exposed to 100 nM UCN-01 with or without 10 μM lovastatin for 24 hours and U266 to 150 nM UCN-01 with or without 10 μM lovastatin for 48 hours. (C) U937 cells were incubated with low concentrations of UCN-01 (50 to 75 nM) with or without 2.5 to 5 μM lovastatin for 48 hours or 72 hours, respectively. For panels A-C, after treatment, cells were lysed and prenylation status of H-Ras, RhoB, and Rap1A was determined by Western blot analysis. Each lane was loaded with 30 μg protein; blots were subsequently stripped and reprobed for expression of β-actin to ensure equivalent loading and transfer of protein. The results of a representative experiment are shown; an additional study yielded equivalent results. UP indicates unprenylated (corresponding to slower-migrating bands); P, prenylated. (D) U937 cells were exposed to 100 nM UCN-01 with or without 20 μM lovastatin for 18 hours and U266 cells to 150 nM UCN-01 with or without 10 μM lovastatin for 24 hours. At the end of the incubation period, cells were lysed and subjected (400 μg protein per condition) to a Ras activation assay as described in “Ras activation assay” (right panels). In parallel, untreated U937 and U266 cells were lysed, and cell extracts incubated/loaded with 100 μM GTPγS or 1 mM GDP for 30 minutes at 30°C for positive and negative controls, respectively (left panels). Ras activity is reflected by the amount of Ras (Ras-GTP) pulled down by Raf-1 RBD agarose beads. IgG (H) indicates IgG heavy chain. The results are representative of 3 separate experiments.
To assess the functional significance of statin-mediated alterations in protein prenylation in UCN-01–treated cells, a Ras activation assay was employed that is based on the binding of only active GTP-bound Ras to Raf.43 As shown in Figure 4D (left panels), in vitro loading of extracts of untreated U937 and U266 cells with GTP markedly increased Ras binding to Raf-1 pulled down by Raf-1 RBD agarose beads, compared with those loaded with GDP. UCN-01 treatment of both U937 and U266 cells induced a clear increase in GTP-bound (active) Ras (Figure 4D, right panels). Moreover, coadminstration of lovastatin diminished the UCN-01–mediated increase in Ras activation in both cell types. These findings indicate, for the first time, that UCN-01 activates Ras. They also suggest that statin-mediated perturbations in Ras prenylation are responsible for disruption of Ras activation in UCN-01–treated cells.
Statins block ERK1/2 activation and enhance Akt inactivation in UCN-01–treated cells while reciprocally promoting activation of the stress-related JNK pathway
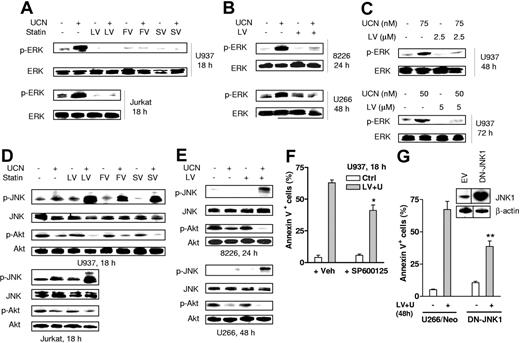
The combined effects of statins and UCN-01 were then examined in relation to perturbations in cytoprotective and stress-related signaling pathways linked to interruption of Ras function. We have previously observed that UCN-01 induces activation of MEK/ERK, one of the major Ras downstream signaling pathways, in both human leukemia and myeloma cells.25,26 As shown in Figure 5A, exposure of U937 leukemia cells to 100 nM UCN-01 resulted in a marked increase in phosphorylation/activation of ERK1/2 (Figure 5A; upper panels). Notably, coadministration of statins almost completely blocked this effect. Comparable results were observed in Jurkat cells (Figure 5A, lower panels). Similarly, cotreatment with lovastatin largely prevented UCN-01–induced ERK1/2 phosphorylation in RPMI8226 and U266 myeloma cells (Figure 5B). Furthermore, similar response patterns were observed in U937 cells exposed to low concentrations of lovastatin and UCN-01 for longer intervals (48 to 72 hours; Figure 5C).
Statins diminish UCN-01–induced ERK1/2 activation and enhance Akt inactivation while reciprocally promoting JNK activation. (A) U937 cells (upper panels) were exposed for 18 hours to 100 nM UCN-01 (UCN) with or without 20 μM lovastatin (LV), 40 μM fluvastatin (FV), or 30 μM simvastatin (SV), respectively; alternatively, Jurkat cells (lower panels) were exposed to 150 nM UCN-01 with or without 20 μM lovastatin, after which Western blot analysis was performed to monitor the phosphorylation status of ERK1/2. (B) RPMI8226 cells (8226) were treated with 100 nM UCN-01 with or without 10 μM lovastatin for 24 hours, and U266 cells were exposed to 150 nM UCN-01 with or without 10 μM lovastatin for 48 hours, after which ERK1/2 phosphorylation was assessed by Western blot analysis. (C) U937 cells were incubated with lower concentrations of UCN-01 (50 to 75 nM) with or without 2.5 to 5μM lovastatin for 48 hours or 72 hours, after which cells were lysed and subjected to Western blot analysis to monitor ERK1/2 phosphorylation. (D) U937 (upper panels) and Jurkat cells (lower panels) were treated as described in panel A, after which phosphorylation/activation of JNK and Akt was monitored by Western blot analysis. (E) RPMI8226 (upper panels) and U266 cells (lower panels) were treated as described in panel B, after which phosphorylation of JNK and Akt was assessed by Western blot analysis. For panels A-E, 30 μg protein was loaded in each lane. The results are representative of 3 separate experiments. (F) U937 cells were exposed (18 hours) to 100 nM UCN-01 + 20 μM lovastatin in the presence or absence of 10 μM SP600125, after which the percentage of annexin V+ cells was determined by flow cytometry. The results represent the means ± SD for 3 separate experiments performed in triplicate. *Significantly lower than values for treatment without SP600125 (P < .05). Veh indicates vehicle. (G) U266 cells ectopically expressing JNK1-APF (inset, Western blot), a dominant negative form of JNK1, or its corresponding empty vector were exposed to 150 nM UCN-01 + 10 μM lovastatin for 48 hours, after which the percentage of apoptotic cells was assessed by annexin V staining and flow cytometry. The results represent the means ± SD for 3 separate experiments performed in triplicate. **Significantly lower than values for empty vector controls (U266/neo) (P < .02). The blots shown (inset) were obtained from same films, and vertical lines indicate where they were cut.
Statins diminish UCN-01–induced ERK1/2 activation and enhance Akt inactivation while reciprocally promoting JNK activation. (A) U937 cells (upper panels) were exposed for 18 hours to 100 nM UCN-01 (UCN) with or without 20 μM lovastatin (LV), 40 μM fluvastatin (FV), or 30 μM simvastatin (SV), respectively; alternatively, Jurkat cells (lower panels) were exposed to 150 nM UCN-01 with or without 20 μM lovastatin, after which Western blot analysis was performed to monitor the phosphorylation status of ERK1/2. (B) RPMI8226 cells (8226) were treated with 100 nM UCN-01 with or without 10 μM lovastatin for 24 hours, and U266 cells were exposed to 150 nM UCN-01 with or without 10 μM lovastatin for 48 hours, after which ERK1/2 phosphorylation was assessed by Western blot analysis. (C) U937 cells were incubated with lower concentrations of UCN-01 (50 to 75 nM) with or without 2.5 to 5μM lovastatin for 48 hours or 72 hours, after which cells were lysed and subjected to Western blot analysis to monitor ERK1/2 phosphorylation. (D) U937 (upper panels) and Jurkat cells (lower panels) were treated as described in panel A, after which phosphorylation/activation of JNK and Akt was monitored by Western blot analysis. (E) RPMI8226 (upper panels) and U266 cells (lower panels) were treated as described in panel B, after which phosphorylation of JNK and Akt was assessed by Western blot analysis. For panels A-E, 30 μg protein was loaded in each lane. The results are representative of 3 separate experiments. (F) U937 cells were exposed (18 hours) to 100 nM UCN-01 + 20 μM lovastatin in the presence or absence of 10 μM SP600125, after which the percentage of annexin V+ cells was determined by flow cytometry. The results represent the means ± SD for 3 separate experiments performed in triplicate. *Significantly lower than values for treatment without SP600125 (P < .05). Veh indicates vehicle. (G) U266 cells ectopically expressing JNK1-APF (inset, Western blot), a dominant negative form of JNK1, or its corresponding empty vector were exposed to 150 nM UCN-01 + 10 μM lovastatin for 48 hours, after which the percentage of apoptotic cells was assessed by annexin V staining and flow cytometry. The results represent the means ± SD for 3 separate experiments performed in triplicate. **Significantly lower than values for empty vector controls (U266/neo) (P < .02). The blots shown (inset) were obtained from same films, and vertical lines indicate where they were cut.
UCN-01 is known to inhibit Akt by targeting PKD1, events implicated in lethality.23 Moreover, the PI3K/Akt pathway lies downstream of Ras signaling.44 Consistent with previous results,23,32 exposure to subtoxic concentrations (eg, 100 to 150 nM) of UCN-01 modestly reduced levels of phospho-Akt in both leukemia (Figure 5D) and particularly myeloma cells (Figure 5E). Notably, while statins alone also slightly decreased Akt phosphorylation (Ser473),44 combined treatment induced a further reduction in Akt phosphorylation/activation in both cell types compared with UCN-01 alone. Moreover, U937 cells ectopically expressing myristoylated (active) Akt displayed significant resistance to UCN-01/lovastatin-induced apoptosis compared with empty vector controls (P < .02, data not shown).
Lastly, in view of evidence that MEK/ERK and JNK activation reciprocally regulate cell survival,45 as well as reports of JNK activation in leukemia and myeloma cells exposed to UCN-01 and FTase inhibitors,32,33 the effects of UCN-01 and statins were examined in relation to this stress-related pathway. Whereas individual administration of UCN-01 and statins minimally affected JNK phosphorylation/activation, combined treatment led to pronounced JNK activation in both leukemia and myeloma cells (Figure 5D-E). In contrast, UCN-01 by itself or in combination with each of the statins failed to activate p38 MAPK (data not shown). To assess the functional significance of enhanced JNK activation in UCN-01/lovastatin-treated cells, studies were performed employing either the selective JNK inhibitor SP600125 46 or stable transfection of cells with a dominant negative JNK1-APF construct.36 As shown in Figure 5F, coadministration of SP600125 significantly reduced the lethality of lovastatin/UCN-01 regimen in U937 cells (P < .05). Moreover, U266 cells ectopically expressing JNK1-APF (Figure 5G, inset) markedly increased resistance to lovastatin/UCN-01 lethality (P < .02, Figure 5G). Both results suggest a functional role for the JNK-related stress signaling pathway in these events. Collectively, these findings indicate that coexposure of human leukemia and myeloma cells to UCN-01 and statins results in inactivation of MEK/ERK and Akt cytoprotective signaling pathways, accompanied by reciprocal activation of the stress-related JNK pathway.
Potentiation of UCN-01–induced apoptosis by statins is functionally associated with interruption of protein farnesylation rather than geranylgeranylation
Because statins were able to inhibit both farnesylation and geranylgeranylation of Ras and Ras-related proteins,2,18 add-back experiments29,47 were then performed to investigate the functional significance of interruption of specific prenylation pathways. Addition of FPP markedly reduced accumulation of unprenylated H-Ras in UCN-01/lovastatin-treated U937 cells but did not modify Rap1A prenylation (Figure 6A). In contrast, GGPP but not FPP completely blocked the accumulation of unprenylated Rap1A in U937 cells coexposed to lovastatin/UCN-01 but had little effect on H-Ras prenylation status. Moreover, addition of GGPP did not further modify FPP effects on H-Ras prenylation. These results indicate that H-Ras is a farnesylation while Rap1A is a geranylgeranylation target and that lovastatin blocks both events. Moreover, FPP alone and coadministration of FPP with GGPP, but not GGPP alone, partially restored ERK1/2 inactivation in U937 cells cotreated with lovastatin and UCN-01. Consistent with these results, addition of FPP partially but significantly diminished the lethality of the UCN-01/lovastatin regimen (P < .05; Figure 6B) while addition of GGPP alone had no significant effect (P > .05).
Statin-mediated disruption of farnesylation but not geranylation contributes to potentiation of UCN-01–induced apoptosis. (A) U937 cells were incubated for 18 hours with 100 nM UCN-01 (UCN) + 20 μM lovastatin (LV) in either the presence or absence of 10 μM GGPP, 10 μM FPP, or both, after which Western blot analysis was performed to assess the prenylation status of H-Ras and Rap1A as well as phosphorylation of ERK1/2. (B) Alternatively, the percentage of cells exhibiting apoptotic morphology was determined by evaluating Wright-Giemsa–stained cytospin preparations. Veh indicates vehicle. (C) U937 cells were treated for 18 hours with 100 nM UCN-01 in the presence of either 20 μM FTI-277 or 20 μM GGTI-2147, after which Western blot analysis was performed to monitor prenylation of H-Ras and Rap1A as well as phosphorylation of ERK1/2. (D) U937 cells were treated with 100 nM UCN-01 in the presence of the indicated concentrations (0 to 30 μM) of either GGTI-2147or FTI-277 for 18 hours, after which the percentage of apoptotic cells was determined by evaluating Wright-Giemsa–stained cytospin preparations. For panels A and C, each lane was loaded with 30 μg protein; blots were subsequently stripped and reprobed for expression of β-actin to ensure equivalent loading and transfer of protein. To detect changes in phosphorylation status of ERK1/2 under the conditions shown in panel A, 100 μg protein was loaded onto each lane, and total ERK1/2 was monitored in parallel in the same gel. Two additional studies yielded equivalent results. UP indicates unprenylated; P, prenylated. For panels B and D, results represent the means ± SD for 3 separate experiments performed in triplicate. *Significantly lower than values for cells cotreated with UCN-01 + lovastatin in the absence of FPP or FPP/GGPP ([B] *P < .05) and FTI-277 treatment alone ([D] **P < .01).
Statin-mediated disruption of farnesylation but not geranylation contributes to potentiation of UCN-01–induced apoptosis. (A) U937 cells were incubated for 18 hours with 100 nM UCN-01 (UCN) + 20 μM lovastatin (LV) in either the presence or absence of 10 μM GGPP, 10 μM FPP, or both, after which Western blot analysis was performed to assess the prenylation status of H-Ras and Rap1A as well as phosphorylation of ERK1/2. (B) Alternatively, the percentage of cells exhibiting apoptotic morphology was determined by evaluating Wright-Giemsa–stained cytospin preparations. Veh indicates vehicle. (C) U937 cells were treated for 18 hours with 100 nM UCN-01 in the presence of either 20 μM FTI-277 or 20 μM GGTI-2147, after which Western blot analysis was performed to monitor prenylation of H-Ras and Rap1A as well as phosphorylation of ERK1/2. (D) U937 cells were treated with 100 nM UCN-01 in the presence of the indicated concentrations (0 to 30 μM) of either GGTI-2147or FTI-277 for 18 hours, after which the percentage of apoptotic cells was determined by evaluating Wright-Giemsa–stained cytospin preparations. For panels A and C, each lane was loaded with 30 μg protein; blots were subsequently stripped and reprobed for expression of β-actin to ensure equivalent loading and transfer of protein. To detect changes in phosphorylation status of ERK1/2 under the conditions shown in panel A, 100 μg protein was loaded onto each lane, and total ERK1/2 was monitored in parallel in the same gel. Two additional studies yielded equivalent results. UP indicates unprenylated; P, prenylated. For panels B and D, results represent the means ± SD for 3 separate experiments performed in triplicate. *Significantly lower than values for cells cotreated with UCN-01 + lovastatin in the absence of FPP or FPP/GGPP ([B] *P < .05) and FTI-277 treatment alone ([D] **P < .01).
Comparison studies were then performed with an FTase inhibitor and a GGTase inhibitor. FTI-277, either alone or in the presence of UCN-01, increased unprenylated H-Ras but had no effect on Rap1A (Figure 6C). Conversely, GGTI-2147 (with or without UCN-01) increased levels of unprenylated Rap1A but had little effect on H-Ras prenylation status. Furthermore, coadministration of GGTI-2147 failed to block ERK1/2 activation induced by UCN-01 (Figure 6C); nor did it increase the lethality of UCN-01 in U937 cells (Figure 6D). In contrast, cotreatment with FTI-277 significantly prevented ERK1/2 activation (Figure 6C) and enhanced UCN-01–induced apoptosis (P < .01 at each FTI-277 concentration; Figure 6D), consistent with our previous findings involving the FTase inhibitor L778534.32 Collectively, these findings suggest that statins block UCN-01–induced ERK1/2 activation and potentiate UCN-01–mediated lethality by interfering with protein farnesylation rather than geranylgeranylation.
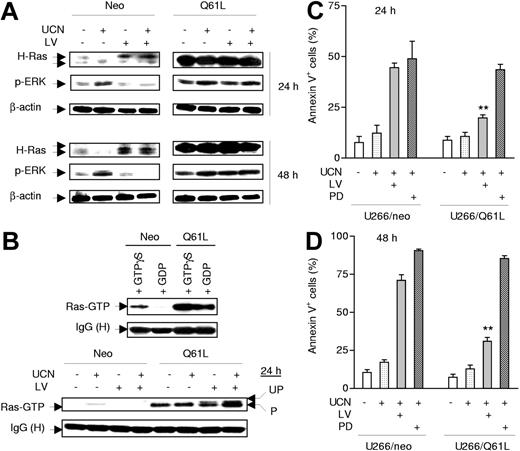
Ectopic expression of constitutively activated Ras (Q61L) diminishes lovastatin-mediated ERK1/2 inactivation and potentiation of UCN-01 lethality
To assess further the functional role of perturbations in Ras prenylation/activation in synergistic interaction between UCN-01 and statins, U266 cells were stably transfected with an activated H-Ras construct encoding a mutant Ras (Q61L) that constitutively remains in a GTP-bound form because of lack of intrinsic GTPase activity stemming from the inability to interact with GTPase activating proteins (GAPs).48 As shown in Figure 7A, ectopic expression of Q61L Ras markedly increased basal ERK1/2 phosphorylation/activation, consistent with previous reports.49 Notably, the ability of lovastatin to abrogate UCN-01–mediated ERK1/2 phosphorylation was substantially diminished in Q61L Ras mutants (Figure 7A). Ras activation assays demonstrated that constitutively active Ras (Q61L) was bound to Raf-1 RBD 43 regardless of whether U266/Q61L cell lysates were loaded in vitro with GDP or GTP (Figure 7B, upper panels).50 Notably, it was observed that in lovastatin-treated cells, both prenylated and unprenylated forms of Q61L Ras were able to bind in vitro to Raf-1 RBD, while both bindings, particularly that involving the prenylated form of the mutant protein with Raf-1, were increased in lysates from cells cotreated with UCN-01 (Figure 7B, lower panels). These results indicate that Q61L Ras proteins bind to Raf independently of their prenylation status, which is known to control Ras membrane translocation. Finally, as shown in Figure 7C-D, ectopic expression of Q61L Ras dramatically blocked the capacity of lovastatin to potentiate UCN-01 lethality (P < .01 in each case at 24 hours and 48 hours, compared with empty vector-transfected U266/neo cells). In marked contrast, the Q61L Ras mutant cells remained equally sensitive to a regimen combining UCN-01 and the MEK1/2 inhibitor PD184352, which acts downstream of Ras (P > .05). Together, these findings demonstrate that statin-mediated disruption of Ras signaling plays a significant functional role in blocking MEK/ERK activation and the resulting potentiation of apoptosis in cells exposed to UCN-01. They also raise the possibility that the mutational status of Ras may affect the relative efficacy of statins versus MEK1/2 inhibitors in potentiating UCN-01 lethality.
Expression of constitutively activated Ras (Q61L) prevents lovastatin from interrupting UCN-01–mediated ERK1/2 activation and significantly attenuates apoptosis induced by the regimen. (A) U266 cells were stably transfected with constructs encoding a constitutively activated mutant (Q61L) form of H-Ras or its empty vector control (neo). Cells were then treated with 150 nM UCN-01 (UCN) with or without 10 μM lovastatin (LV) for 24 hours (upper panels) or 48 hours (lower panels), after which cells were lysed and subjected to Western blot analysis to monitor expression of H-Ras and phosphorylated ERK1/2; 30 μg protein was loaded in each lane, and blots were subsequently stripped and reprobed for expression of β-actin to ensure equivalent loading and transfer of protein. Results are representative of 3 separate experiments. (B) U266/neo (neo) and U266/Q61L (Q61L) cells were incubated with 150 nM UCN-01 with or without 10 μM lovastatin for 24 hours, after which cell lysates (100 μg protein per condition) were subjected to a Ras activation assay (lower panels) as described in Figure 4D. In parallel, lysates from untreated cells were incubated with 100 μM GTPγS or 1 mM GDP for 30 minutes at 30°C for positive and negative controls, respectively (upper panels). Two additional studies yielded equivalent results. (C-D) U266/Q61L and neo cells were exposed to 150 nM UCN-01 in the presence or absence of either 10 μM lovastatin or 5 μM PD184352 (PD) for 24 hours (C) or 48 hours (D), after which the percentage of apoptotic cells was determined by annexin V–FITC/flow cytometry. The results represent the means ± SD for 3 separate experiments performed in triplicate. **Significantly lower than values for U266/neo cells (P < .01).
Expression of constitutively activated Ras (Q61L) prevents lovastatin from interrupting UCN-01–mediated ERK1/2 activation and significantly attenuates apoptosis induced by the regimen. (A) U266 cells were stably transfected with constructs encoding a constitutively activated mutant (Q61L) form of H-Ras or its empty vector control (neo). Cells were then treated with 150 nM UCN-01 (UCN) with or without 10 μM lovastatin (LV) for 24 hours (upper panels) or 48 hours (lower panels), after which cells were lysed and subjected to Western blot analysis to monitor expression of H-Ras and phosphorylated ERK1/2; 30 μg protein was loaded in each lane, and blots were subsequently stripped and reprobed for expression of β-actin to ensure equivalent loading and transfer of protein. Results are representative of 3 separate experiments. (B) U266/neo (neo) and U266/Q61L (Q61L) cells were incubated with 150 nM UCN-01 with or without 10 μM lovastatin for 24 hours, after which cell lysates (100 μg protein per condition) were subjected to a Ras activation assay (lower panels) as described in Figure 4D. In parallel, lysates from untreated cells were incubated with 100 μM GTPγS or 1 mM GDP for 30 minutes at 30°C for positive and negative controls, respectively (upper panels). Two additional studies yielded equivalent results. (C-D) U266/Q61L and neo cells were exposed to 150 nM UCN-01 in the presence or absence of either 10 μM lovastatin or 5 μM PD184352 (PD) for 24 hours (C) or 48 hours (D), after which the percentage of apoptotic cells was determined by annexin V–FITC/flow cytometry. The results represent the means ± SD for 3 separate experiments performed in triplicate. **Significantly lower than values for U266/neo cells (P < .01).
Discussion
Accumulating evidence suggests that neoplastic cells, particularly those of hematopoietic origin, are ill-equipped to survive simultaneous disruption of cell cycle and survival signaling pathways. For example, the Chk1 inhibitor UCN-01 lethality is dramatically enhanced by multiple classes of signal transduction inhibitors, including pharmacologic MEK1/2 inhibitors,25,26 the Hsp90 antagonist 17-AAG,31 NF-κB inhibitors (eg, Bay 11-7082)46 and, most recently, FTase inhibitors.32,33 The present studies indicate that clinically relevant statins also strikingly potentiate UCN-01–mediated lethality in human leukemia and myeloma cells. In each case, coadministration of these signaling inhibitors with UCN-01 results in a net shift away from survival signal transduction pathways (eg, Raf/MEK/ERK and PI3K/Akt) and toward stress-related pathways (eg, JNK), a shift that strongly favors cell death over survival.45 One caveat is that while UCN-01, by virtue of its ability to inhibit Chk1, is a potent abrogator of the G2M and G1S checkpoints, it also exerts pleiotropic actions, including inhibition of CDKs, PKC, and PDK1/Akt.20,22 Consequently, the possibility that one or more of these actions contributes to synergistic interactions with statins cannot be excluded. In this context, the ability of statins to potentiate UCN-01–mediated Akt inactivation may be particularly relevant.
The present findings provide the first evidence that that induction of MEK/ERK by UCN-01 in human leukemia and myeloma cells involves activation of Ras and that disruption of this process plays a significant functional role in synergism between UCN-01 and statins. Ras family members (ie, H-Ras, N-Ras, and K-Ras) are frequently mutated and activated in human cancers, including hematopoietic malignancies, although there is considerable variation with respect to Ras mutation and tumor type.4–6 For example, N-Ras mutations account for most cases of Ras mutation in human myeloid leukemias.5 Ras mutations (N-Ras > K-Ras) occur in about 40% of newly diagnosed patients with multiple myeloma.5 In addition to mutations, Ras can be constitutively activated due to dysregulation of other protooncogenes (eg, activating mutations of FLT3 in myeloid leukemias)51 or stimulation by cytokines and growth factors (eg, IL-6 in multiple myeloma).52,53 Nevertheless, regardless of whether a specific Ras mutation or a specific Ras activation stimulus is present or contributes to transformation, Ras-related pathways play key roles in the transduction of diverse survival signals.4,5 In this context, the Raf/MEK/ERK pathway is known to transmit critical prosurvival signals downstream of Ras in diverse neoplastic cell types.4,5
Following the initial observation that UCN-01 induced MEK/ERK activation in human leukemia and myeloma cells, it was postulated that MEK/ERK activation reflected a compensatory response to inappropriate activation of cdc2/CDK1, a potent proapoptotic stimulus.25 However, recent findings indicate that cdc2 activation induced by UCN-01 does not contribute functionally to MEK/ERK activation.54 Furthermore, the present observation that UCN-01 exposure triggers Ras activation and that disruption of this process (eg, by statins) attenuates the resulting ERK1/2 activation argues strongly for a functional role for Ras in this UCN-01 action. Thus, in addition to other UCN-01 activities associated with cytotoxicity, including inhibition of PKC, Chk1, PDK1, and CDKs,20,22 involvement of Ras-related signaling events upstream of MEK/ERK represents a previously unreported self-protective response, at least in malignant hematopoietic cells. The question of how Ras and/or Ras-related proteins are involved in this process remains to be determined, and studies addressing this question are currently underway.
The bulk of evidence suggests that potentiation of UCN-01 lethality by statins reflects interference with farnesylation rather than geranylgeranylation of Ras family proteins. By inhibiting HMG-CoA reductase, lovastatin and related agents interfere with the biosynthesis of substrates (eg, FPP and GGPP) necessary for both farnesylation and geranylgeranylation reactions.1,2 However, the relative contribution of these distinct actions in the lethality of statins may vary significantly. For example, in myeloid leukemia55 and multiple myeloma cells,8,12 apoptosis mediated by lovastatin results primarily from interference with protein geranylgeranylation, based upon the observations that lovastatin toxicity can be reversed by addition of GGPP but not FPP and mimicked by GGTase inhibitors. However, potentiation of UCN-01–mediated lethality by statins appears primarily related to inhibition of Ras farnesylation rather than geranylgeranylation. Specifically, UCN-01/lovastatin-induced apoptosis was partially but significantly attenuated by FPP but not by GGPP. Moreover, only FPP, but not GGPP, blocked UCN-01/lovastatin-associated interference with H-Ras prenylation and partially reversed ERK1/2 inactivation. In marked contrast, GGPP but not FPP abolished the ability of lovastatin to interrupt the prenylation of Rap1A, a well-described target of GGTase,40 but failed to reverse ERK1/2 inactivation. Thus, the inability of GGPP to rescue cells exposed to lovastatin/UCN-01 cannot be attributed to failure to reverse the blockade of GGTase. Furthermore, the FTase inhibitor FTI-277, but not the GGTase inhibitor GGTI-2147, blocked UCN-01–induced ERK1/2 activation and dramatically increased UCN-01 lethality. These findings are consistent with recent evidence that the FTase inhibitor L774832 markedly lowers the threshold for UCN-01–induced mitochondrial dysfunction and apoptosis in human leukemia and myeloma cells.32,33 Notably, statins, like FTase inhibitors in general,6,41 may interfere with the farnesylation of substrates other than Ras proteins,2 and such actions may also contribute to synergistic lethality. In addition, coadministration of FPP only partially protected cells from UCN-01/lovastatin-mediated cell killing, suggesting that other consequences of HMG-CoA reductase inhibition are involved in the marked increase in UCN-01–induced apoptosis.
It is noteworthy that expression of an activated form of Ras (Q61L) prevented lovastatin from blocking ERK1/2 activation in UCN-01–treated cells and substantially protected them from the lethality of this combination regimen. It has been found that, in cells expressing active Ras (eg, V12Ras), these mutant Ras proteins colocalize with Raf at the plasma membrane,56 leading to activation of downstream signal pathways. In the present work, it was noted that active mutant Ras (Q61L) proteins bind to Raf, at least in vitro, independently of GTP loading,50 as well as their prenylation status following lovastatin treatment. Collectively, these findings may provide an explanation for the observation that lovastatin failed to inhibit the capacity of these mutant proteins to activate the downstream target ERK (Figure 7A). Moreover, UCN-01 treatment enhanced, by a mechanism yet to be defined, the ability of both prenylated and unprenylated Q61L Ras to bind to Raf. This phenomenon is consistent with the observation that UCN-01 activated ERK in U266/Q61L cells with or without coadministration of lovastatin. Interestingly, the MEK1/2 inhibitor PD184352 continued to potentiate UCN-01 lethality in cells ectopically expressing Q61L Ras, presumably because its target lies downstream of Ras. Such findings raise the possibility that MEK1/2 inhibitors may be more appropriate potentiators of UCN-01 action in hematologic malignancies associated with certain Ras mutations. Further studies will be required to define the genetic background that might favor the use of statins over MEK1/2 inhibitors in potentiating UCN-01 activity. However, given evidence that MEK/ERK and PI3K/Akt cooperate to promote the survival of malignant hematopoietic cells,32 the ability of statins to block MEK/ERK activation as well as to promote UCN-01–mediated Akt inactivation could prove advantageous in certain settings.
As recently observed in the case of FTase inhibitors,32,33 combined exposure to statins and UCN-01 resulted in a shift toward the relative outputs of the proapoptotic stress-related JNK from the antiapoptotic MEK/ERK cascade. The balance between these pathways has previously been shown to be an important determinant of cell death.45 The mechanism of JNK-related cell killing may involve a direct role in cytochrome c release57 or, more indirectly, activation of the extrinsic apoptotic pathway,58 among others. On the other hand, MEK/ERK activation may oppose cell death by multiple mechanisms, including direct phosphorylation/inactivation of various proapoptotic factors, including Bim, Bad, and caspase-9, among others.59 The net effect of the reciprocal changes in stress- and survival-related pathways could account for the marked increase in mitochondrial dysfunction and apoptosis experienced by cells exposed to the UCN-01/statin regimen.
The finding that statins such as lovastatin potentiate apoptosis induced by UCN-01 in malignant hematopoietic cells in association with perturbations in survival signaling pathways has implications for the development of novel antileukemic and antimyeloma strategies. From a practical standpoint, while extensive binding of UCN-01 to α1-acidic glycoprotein in plasma limits levels of free UCN-01 and has hindered development of this agent,60 a new 3-hour infusion schedule has recently been described that yields sustained free salivary UCN-01 concentrations of 434 to 1425 nM.61 Furthermore, in a recent phase 1 trial of lovastatin administered every 6 hours for 96 hours, peak lovastatin plasma concentrations of 12 μM were achieved and no dose-limiting toxicities experienced,62 raising the possibility that even higher plasma concentrations may be possible. Notably, in the present study, low, pharmacologically achievable concentrations of lovastatin (eg, 1 to 5μM) significantly increased UCN-01 lethality, particularly after longer exposure intervals (eg, 48 to 72 hours). In this context, lovastatin has been shown to potentiate the antileukemic activity of conventional cytotoxic agents13,14 and to circumvent certain forms of drug resistance.8 Similarly, a marked potentiation of lovastatin-related antileukemic activity by the MEK1/2 inhibitor PD98059 has recently been described,29 and simvastatin has been found to sensitize human colon cancer cells to TRAIL-induced apoptosis.63 To the best of our knowledge, this is the first report demonstrating that UCN-01 induces Ras activation in human malignant hematopoietic cells and that statins block this process as well as resulting ERK1/2 activation, leading to a dramatic increase in cell death. While the in vivo relevance of these findings remains to be determined, it is encouraging that the UCN-01/statin regimen appears to be active against at least some primary AML blast samples, is relatively nontoxic to certain normal human hematopoietic cells, and occurs at lovastatin and UCN-01 concentrations that are pharmacologically achievable. Given the relative lack of toxicity of statins in the clinic, this strategy may warrant further examination in leukemia and multiple myeloma as well as nonhematologic malignancies.
Authorship
Contribution: Y.D. designed and performed the research, analyzed the data, and wrote the paper; P.K., S.C., and X.-Y.P. performed the research; P.D. helped to design the research; and S.G. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Grant, Division of Hematology/Oncology, Virginia Commonwealth University/Medical College of Virginia, MCV Station Box 230, Richmond, VA 23298; e-mail: stgrant@hsc.vcu.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by awards CA63753, CA93738, CA100866, CA88906, and CA72955 from the National Institutes of Health (NIH); by a Translational Research Award from the Leukemia and Lymphoma Society of America (6045-03); by an award from the Department of Defense (DAMD-17-03-1-0209); by an award from the V Foundation; and by the Universal Professorship (P.D.).

![Figure 2. The statin/UCN-01 regimen induces apoptosis in multiple leukemia and myeloma cell lines as well as primary AML cells but is minimally toxic to normal hematopoietic cells. (A) Human leukemia (Jurkat, HL-60, and Raji) and myeloma (RPMI8226 [8226], MM.1S, and MM.1R) cells were exposed to UCN-01 ([UCN] 100 nM for HL-60, RPMI8226, MM.1S, and MM.1R; 150 nM for Jurkat and Raji) with or without lovastatin ([LV] 20 μM for all leukemia cell lines; 10 μM for all myeloma cell lines) for 18 hours (leukemia cells) or 24 hours (myeloma cells), after which the percentage of annexin V+ cells was determined by flow cytometry. (B) AML blasts were isolated from peripheral blood samples derived from 3 patients with AML (FAB classification M2) as described in “Cells and reagents.” Mononuclear cells were isolated from the bone marrow (BM/MC) of 2 patients with nonmalignant, nonmyeloid hematopoietic disorders and the peripheral blood (PB/MC) from 1 healthy volunteer. AML blasts and normal cells were treated with 150 nM UCN-01 with or without 20 μM lovastatin for 18 hours, after which the percentage of apoptotic cells was determined by evaluating Giemsa-Wright–stained cytospin preparations. (C) U937 cells were incubated with UCN-01 (50 to 100 nM) with or without low doses of lovastatin (1 to 5 μM) for 48 hours or 72 hours, after which the percentage of annexin V+ cells, including annexin V+/PI− and annexin V+/PI+, was determined by flow cytometry. (D) U937 and U266 cells were treated with UCN-01 (U937, 100 nM; U266, 150 nM) with or without lovastatin (U937, 20 μM; U266, 10 μM) for 18 hours (U937) or 48 hours (U266), after which cells were washed free of drug and plated in soft agar as described in “Analysis of apoptosis and clonogenicity.” After incubation for 12 days, colonies (more than 50 cells) were scored, and colony formation for each condition expressed relative to untreated control cells. For all panels, results represent the means ± SD for 3 separate experiments performed in triplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-09-047076/4/m_zh80100701100002.jpeg?Expires=1765930445&Signature=Ztqv1oGAJIVIPRuW4BqEYGfY8z6B-gPSNTbjRUEt6xxQrrWPRQqI-VaJaHf7AHjxmy2lE9iW08vwtJ-YUsycol525uOcJmSt4BQ0OFTZNUSTEY6eTV1A~xXBrDtJqCW7HVB9N24niWZz99CgUd1QmqmOTapMupCAZrRXI7FSbXioyEioUHRrI2cEVDTUvw4J4js~j~zJtLtZloXouPn3qLqEu55oFDsyj0uTQR0~~~IWlPUQAim3fzzTObUf1Ri7hKMIYU-xjKvRjKh~T776ai5IKCKDgsg-R5BVWAN3YdKp-Z5EAk8oUJ-FZEC8fVf569Qd8-fEYgTqDOrv5vGDWg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Statin-mediated disruption of farnesylation but not geranylation contributes to potentiation of UCN-01–induced apoptosis. (A) U937 cells were incubated for 18 hours with 100 nM UCN-01 (UCN) + 20 μM lovastatin (LV) in either the presence or absence of 10 μM GGPP, 10 μM FPP, or both, after which Western blot analysis was performed to assess the prenylation status of H-Ras and Rap1A as well as phosphorylation of ERK1/2. (B) Alternatively, the percentage of cells exhibiting apoptotic morphology was determined by evaluating Wright-Giemsa–stained cytospin preparations. Veh indicates vehicle. (C) U937 cells were treated for 18 hours with 100 nM UCN-01 in the presence of either 20 μM FTI-277 or 20 μM GGTI-2147, after which Western blot analysis was performed to monitor prenylation of H-Ras and Rap1A as well as phosphorylation of ERK1/2. (D) U937 cells were treated with 100 nM UCN-01 in the presence of the indicated concentrations (0 to 30 μM) of either GGTI-2147or FTI-277 for 18 hours, after which the percentage of apoptotic cells was determined by evaluating Wright-Giemsa–stained cytospin preparations. For panels A and C, each lane was loaded with 30 μg protein; blots were subsequently stripped and reprobed for expression of β-actin to ensure equivalent loading and transfer of protein. To detect changes in phosphorylation status of ERK1/2 under the conditions shown in panel A, 100 μg protein was loaded onto each lane, and total ERK1/2 was monitored in parallel in the same gel. Two additional studies yielded equivalent results. UP indicates unprenylated; P, prenylated. For panels B and D, results represent the means ± SD for 3 separate experiments performed in triplicate. *Significantly lower than values for cells cotreated with UCN-01 + lovastatin in the absence of FPP or FPP/GGPP ([B] *P < .05) and FTI-277 treatment alone ([D] **P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-09-047076/4/m_zh80100701100006.jpeg?Expires=1765930445&Signature=LbaB8Hz4mwQoZmdFOED1ut0zu5kWnMI-vKCSps90BGAR6k3QeHputLigVUMycdg9FbkbhFab5PIGvMSj-C6Wk4mM18pVA0Cg77iak-r7M1Sc7fBgZzzTdU7IRKra49jhJaMqGWcDo5TDC5KpixMvCEhxrHGiy~g2pd7~gm8xKQeipS8A7oNQfKRRjVPPXjXPSvXJuRRRGPktU0wIqUDjEIz65977BdAl1IN-zexVEjIb6K8BeLCjuZAYQfqyrirI6DgNJVM3RS521hbQk-Iq6u8PUWFdZlsBzSHER02LZ0O6t5KmYNt85EZbepVhYVEhBHxpB89Zkpj~TCnaeKYk5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal