Abstract
Neutrophils are key mediators of the innate immune response and are required to function at sites of low oxygenation. We have shown that in hypoxia neutrophils are protected from apoptosis via a mechanism dependent on prolyl hydroxylase domain/hypoxia-inducible factor 1α (PHD/HIF-1α). This response would be predicted to involve the von Hippel Lindau protein (pVHL)–dependent ubiquitination and degradation of HIF-1α. Patients with VHL disease inherit a mutation in one VHL allele, which allows us to study the effects of heterozygous VHL expression in human neutrophils. Neutrophils exhibited a striking “partial hypoxic” pheno-type, with delayed rates of apoptosis and enhanced bacterial phagocytosis under normoxic conditions and preserved responses to low levels of oxygen. This provides direct evidence that the HIF-1α/VHL pathway regulates the innate immune response in humans. It also establishes that heterozygous VHL defects are sufficient to perturb normal responses and illustrates the potential to use this to address the role of HIF and VHL in human biology.
Introduction
The regulation of neutrophil activation and longevity is critical to the resolution of acute inflammation. Apoptosis results in the functional “shut down” of the neutrophil1 and initiates its recognition, engulfment, and removal from an inflamed site.2 In contrast to its effects in many other cell types, hypoxia causes a profound inhibition of neutrophil apoptosis.3 This effect appears to be directly dependent on hypoxia-inducible factor 1α (HIF-1α) because neutrophils from mice lacking this transcription factor display a marked reduction in survival under hypoxic conditions.4
HIF is a heterodimer composed of a constitutively expressed β subunit and a hydroxylase-regulated oxygen sensitive α subunit.5,6 In the presence of dioxygen the N-terminal domain of the α subunit is hydroxylated by prolyl hydroxylase domain (PHD)–containing enzymes, enabling its high-affinity binding to the von Hippel Lindau (VHL) E3 ubiquitin ligase complex and allowing subsequent ubiquitination and rapid proteasomal degradation.7,8 The VHL tumor syndrome affects 1:36 000 of the population with its manifestations (retinal angioma, cerebellar hemangioblastoma, renal-cell carcinoma, and pheochromocytoma) arising only after a second, somatic mutation.9 Approximately 70% of germline mutations of VHL are inactivating (frameshift, nonsense, deletions) with the remainder being missense mutations; a close correlation between genotype and tumor predisposition has also been described in families with VHL disease. To establish the importance of the PHD/HIF/VHL oxygen-sensing pathway in regulating both neutrophil longevity and function we have used neutrophils derived from patients with heterozygous germline mutations in the VHL gene. Here we show that such neutrophils exhibit delayed apoptosis, increased sensitivity to hydroxylase inhibition, and enhanced rates of bacterial phagocytosis. This is the first demonstration of an effect of hypoxia on neutrophil phagocytosis and the first to show a partial “hypoxic phenotype” in human cells with heterozygous VHL mutations.
Study design
Neutrophil isolation and culture
Neutrophils were purified from patients with VHL (Table 1) and healthy controls in parallel using discontinuous plasma-Percoll gradients.10 Cells were cultured (5 × 106 cells/mL) in Iscove modified DMEM with 10% autologous serum11 with or without 10 nM to 1 mM dimethyloxaloylglycine (DMOG) under predefined normoxic (19 kPa), hypoxic (3 kPa), or anoxic (0 kPa) environments as previously detailed.3,4
Individual patient genotypes
VHL patient no. . | Mutation . | Type . |
|---|---|---|
| 1 | Whole gene deletion | Deletion |
| 2 | c.430C>T(G73ter) | Nonsense |
| 3 | c.713G>A(R167Q) | Missense |
| 4 | Deletion | Deletion |
| 5 | c.713G>A(R167W) | Missense |
| 6 | c.713G>A(R167W) | Missense |
| 7 | c.667insA | Frameshift |
| 8 | c.712C>T(R167W) | Missense |
| 9 | c.555insG | Frameshift |
| 10 | Deletion exon 1 and 2 | Deletion |
| 11 | c.427insGCCC | Frameshift |
| 12 | c.719T>C(L169P) | Missense |
| 13 | Deletion | Deletion |
| 14 | c.665T>C(I151T) | Missense |
| 15 | c.713G>A(R167Q) | Missense |
VHL patient no. . | Mutation . | Type . |
|---|---|---|
| 1 | Whole gene deletion | Deletion |
| 2 | c.430C>T(G73ter) | Nonsense |
| 3 | c.713G>A(R167Q) | Missense |
| 4 | Deletion | Deletion |
| 5 | c.713G>A(R167W) | Missense |
| 6 | c.713G>A(R167W) | Missense |
| 7 | c.667insA | Frameshift |
| 8 | c.712C>T(R167W) | Missense |
| 9 | c.555insG | Frameshift |
| 10 | Deletion exon 1 and 2 | Deletion |
| 11 | c.427insGCCC | Frameshift |
| 12 | c.719T>C(L169P) | Missense |
| 13 | Deletion | Deletion |
| 14 | c.665T>C(I151T) | Missense |
| 15 | c.713G>A(R167Q) | Missense |
Assessment of neutrophil apoptosis
Neutrophils were harvested at 6 and 20 hours, cytocentrifuged, fixed in methanol, stained with May-Grünwald-Giemsa, and examined by light microscopy.2 Apoptotic neutrophils were defined as those with darkly stained pyknotic nuclei. Apoptosis was also assessed by flow cytometry with FITC-labeled recombinant human annexin V and propidium iodide (PI) staining.11
Phagocytosis assays
Streptococcus pneumoniae type 14 organisms were cultured to log phase in Todd-Hewitt broth containing 0.5% yeast extract (Oxoid, Basingstoke, Hampshire, United Kingdom), heat inactivated at 60°C for 1 hour, and labeled with FITC as previously detailed.12 Neutrophils were allowed to equilibrate at the appropriate oxygen tensions and temperature (37°C or 4°C) with or without 1 mM DMOG for 1 hour prior to the addition of the pneumococci for 1 hour. The neutrophils were then washed and analyzed by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA). The percentage of FITC+ neutrophils and the geometric mean fluorescence of the FITC+ cells were used as a measure of phagocytosis, with uptake at 4°C used to control for bacterial adhesion.
PCR assays
Neutrophils were lysed with TRIzol, RNA extracted using BCP phase partitioning and isopropanol, and PHD1-3 mRNAs detected by reverse transcription-polymerase chain reaction (RT-PCR; Figure 2).
Data presentation and statistical analysis
All data are expressed as mean ± SEM of (n) separate experiments performed in triplicate with significance determined by one-way ANOVA and a post-test Tukey (P < .05). The study was approved by the Cambridge Research Ethics Committee and all participants gave written and informed consent, in accordance with the Declaration of Helsinki.
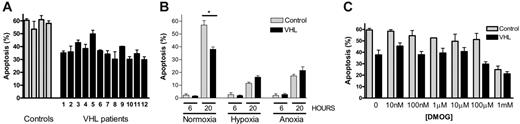
Neutrophils from patients with VHL disease show modified rates of constitutive apoptosis. Peripheral-blood neutrophils were obtained from patients with genotyped VHL (▪) or matched healthy controls (▦) and cultured in either normoxia (19 kPa; A), normoxia, hypoxia (3 kPa), or anoxia (0 kPa; B) or in normoxia in the presence of the hydroxylase inhibitor DMOG (1 μM to 1 mM; C). Cells were assessed for apoptosis by morphologic appearance at 20 hours unless otherwise stated. Data in panel A represent the mean ± SEM of triplicate determinations for 4 control and 12 VHL patients. Data in panels B and C represent the mean ± SEM for 4 separate experiments each performed in triplicate (* P < .001).
Neutrophils from patients with VHL disease show modified rates of constitutive apoptosis. Peripheral-blood neutrophils were obtained from patients with genotyped VHL (▪) or matched healthy controls (▦) and cultured in either normoxia (19 kPa; A), normoxia, hypoxia (3 kPa), or anoxia (0 kPa; B) or in normoxia in the presence of the hydroxylase inhibitor DMOG (1 μM to 1 mM; C). Cells were assessed for apoptosis by morphologic appearance at 20 hours unless otherwise stated. Data in panel A represent the mean ± SEM of triplicate determinations for 4 control and 12 VHL patients. Data in panels B and C represent the mean ± SEM for 4 separate experiments each performed in triplicate (* P < .001).
Results and discussion
Heterozygous germline mutations in VHL modify the extent of neutrophil apoptosis
Neutrophils isolated from patients with VHL disease and cultured under standard normoxic conditions showed a significant reduction in constitutive apoptosis at 20 hours compared to neutrophils from matched healthy controls (Figure 1A-B; mean percent apoptosis: controls, 56.9% ± 3.4%; VHL, 38.1% ± 1.8%, P < .001). Within the same series of experiments hypoxia (3 kPa) imparted an additional survival effect on VHL neutrophils (16.3% ± 1.1%; P < .001); identical rates of apoptosis were apparent under hypoxia in both VHL and control cells (Figure 1B). These results were independently verified by flow cytometry analysis of annexin V/PI staining (data not shown). VHL neutrophils also displayed an increased sensitivity to the hydroxylase inhibitor DMOG, with an IC50 for the inhibition of neutrophil apoptosis in VHL patients of 110 μM compared to 290 μM in healthy controls (P < .05, n = 4; Figure 1C).
Hypoxia and VHL protein expression regulate neutrophil phagocytosis
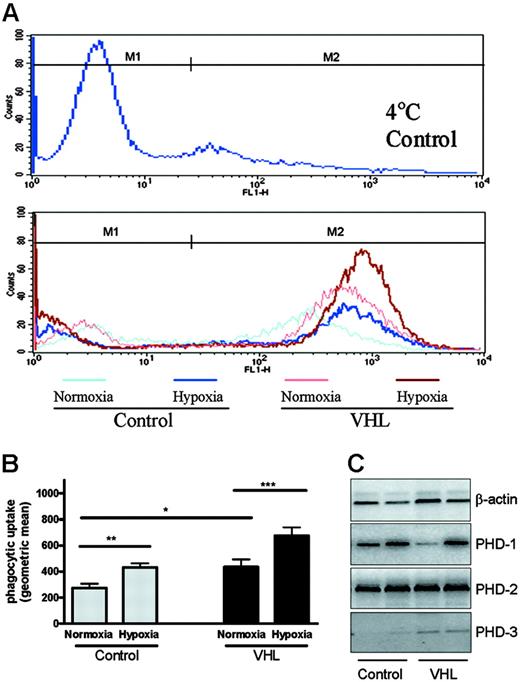
Representative histograms showing phagocytosis of FITC-labeled streptococci in VHL and control cells under normoxia or hypoxia are shown in Figure 2A. Incubation of peripheral-blood neutrophils from healthy controls under hypoxic conditions caused a significant increase in the uptake of heat-inactivated streptococci at 1 hour (Figure 2B, geometric mean fluorescence: normoxia, 262 ± 23; hypoxia, 442 ± 27, P < .03, n = 4). VHL neutrophils displayed enhanced uptake of FITC-labeled streptococci under normoxic conditions (Figure 2B) with the phagocytic index of VHL cells matching that observed in hypoxic control cells (geometric mean, 436 ± 40). Exposure of the VHL cells to hypoxia caused a further increase in their phagocytic capacity (geometric mean, 667 ± 47). These data reveal for the first time the ability of physiologically relevant levels of hypoxia to enhance the phagocytic capacity of neutrophils.
To confirm a partial hypoxic phenotype in the VHL neutrophils, we looked for direct evidence of HIF activation. Freshly isolated neutrophils derived from patients with VHL but not controls showed detectable PHD3, a known HIF target gene (Figure 2C).13,14
Clinical mutations in VHL (missense substitutions or truncations) could influence the pathway through dominant-negative effects, for example, by sequestering other components of the ubiquitin ligase complex or interacting with hydroxylated HIF-α subunits but not achieving their destruction. In our study data from patients 1, 4, 10, and 13 directly address this question because the VHL allele cannot encode a functional protein. This argues strongly that the effect on neutrophil apoptosis is due to haploinsufficiency of VHL. The findings therefore imply that, at least in neutrophils, reduced levels of VHL are unable to ubiquitinylate the HIF that is produced and hydroxylated under standard conditions.
Hypoxia and VHL expression regulate neutrophil phagocytosis. Following a 1-hour preincubation in normoxia (19 kPa), hypoxia (3 kPa), or normoxia at 4°C (binding control) neutrophils from healthy controls or patients with genotyped VHL disease were cocultured with heat-inactivated FITC-labeled streptococci and the uptake of FITC by neutrophils assessed by flow cytometry at 1 hour. (A) Representative histograms of uptake of FITC-labeled streptococci from VHL and control neutrophils in normoxia (pink line VHL, pale blue line control) and hypoxia (red line VHL, dark blue line control), with M2 indicating the region used to calculate the mean fluorescence. (B) Mean ± SEM of the geometric mean fluorescence values following ingestion of streptococci (* P < .05; ** P < .03; *** P < .001; n = 4). Detection of PHD1-3 mRNAs was performed on freshly isolated neutrophils by RT-PCR, using RNA isolated from patients 13 and 14 and 2 healthy controls (C). The following primer sequences were used: PHD1, forward 5′ AGAGAACCAGGAGGCAGAGC 3′, reverse 5′AAATGAGCAACCGGTCAAAG 3′; PHD2, forward 5′ GAGAAGGCGAACCTGTACCC 3′, reverse 5′ GCTCGTGCTCTCTCATCTGC 3′; PHD3, forward 5′ GCTTCCTCCTGTCCCTCATC 3′, reverse 5′ CAGAGCACGGTCAGTCTTCA 3′; β-actin, forward 5′ CTACAATGAGCTGCGTGTGG 3′, reverse 5′ GCACTCTTCCAGCCTTCCTT 3′.
Hypoxia and VHL expression regulate neutrophil phagocytosis. Following a 1-hour preincubation in normoxia (19 kPa), hypoxia (3 kPa), or normoxia at 4°C (binding control) neutrophils from healthy controls or patients with genotyped VHL disease were cocultured with heat-inactivated FITC-labeled streptococci and the uptake of FITC by neutrophils assessed by flow cytometry at 1 hour. (A) Representative histograms of uptake of FITC-labeled streptococci from VHL and control neutrophils in normoxia (pink line VHL, pale blue line control) and hypoxia (red line VHL, dark blue line control), with M2 indicating the region used to calculate the mean fluorescence. (B) Mean ± SEM of the geometric mean fluorescence values following ingestion of streptococci (* P < .05; ** P < .03; *** P < .001; n = 4). Detection of PHD1-3 mRNAs was performed on freshly isolated neutrophils by RT-PCR, using RNA isolated from patients 13 and 14 and 2 healthy controls (C). The following primer sequences were used: PHD1, forward 5′ AGAGAACCAGGAGGCAGAGC 3′, reverse 5′AAATGAGCAACCGGTCAAAG 3′; PHD2, forward 5′ GAGAAGGCGAACCTGTACCC 3′, reverse 5′ GCTCGTGCTCTCTCATCTGC 3′; PHD3, forward 5′ GCTTCCTCCTGTCCCTCATC 3′, reverse 5′ CAGAGCACGGTCAGTCTTCA 3′; β-actin, forward 5′ CTACAATGAGCTGCGTGTGG 3′, reverse 5′ GCACTCTTCCAGCCTTCCTT 3′.
Work using myeloid-targeted VHL and HIF-1α knockout murine models and myelocytic cell lines has revealed a direct role for the VHL/HIF oxygen-sensing pathway in the regulation of myeloid-cell functions.15-17 Interestingly, Peyssonnaux et al17 have recently described the ability of bacterial infection alone to induce HIF-1α expression with subsequent bacterial killing. The current study reveals the capacity of hypoxia to stimulate the phagocytic efficacy of human neutrophils and this, together with the antiapoptotic effect of hypoxia, provides further support for the view that HIF-1α controls several key effector functions in the neutrophil. Hence, the neutrophil appears to be remarkably well adapted to survive and function under oxygen-deplete environments. The cell's capacity to rely almost exclusively on glycolysis for the generation of ATP18 is further testimony to this adaptive state.
In summary, this study demonstrates that hypoxia enhances the phagocytic capacity of human neutrophils and that neutrophils from patients with VHL display functional abnormalities consistent with aberrant HIF-1α signaling. The direct regulation of neutrophil function and longevity by the HIF/VHL oxygensensing pathway further highlights the close integration of oxygen sensing and inflammatory pathways in myeloid cells.
Prepublished online as Blood First Edition Paper, June 29, 2006; DOI 10.1182/blood-2006-04-018796.
Supported by the Medical Research Council, Wellcome Trust, Issac Newton Trust, British Lung Foundation, and a Raymond and Beverly Sackler Studentship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank C. Pugh for providing the DMOG, S. Downing for help with patient recruitment, and K. Smith for helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal