Abstract
Following bone marrow transplantation, delayed donor leukocyte infusions (DLIs) can induce graft-versus-leukemia (GVL) effects without graft-versus-host disease (GVHD). These antitumor responses are maximized by the presence of host hematopoietic antigen-presenting cells (APCs) at the time of DLI. Using a tumor-protection model, we demonstrate here that GVL activity following administration of DLIs to established mixed chimeras is dependent primarily on reactivity to allogeneic MHC antigens rather than minor histocompatibility or tumor-associated antigens. CD8+ T-cell–dependent GVL responses against an MHC class II–negative tumor following delayed DLI require CD4+ T-cell help and are reduced significantly when host APCs lack MHC class II expression. CD4+ T cells primed by host APCs were required for maximal expansion of graft-versus-host reactive CD8+ T cells but not their synthesis of IFN-γ. In contrast, the GVL requirement for CD4+ T-cell help was bypassed almost completely when DLI was administered to freshly irradiated recipients, indicating that the host environment is a major factor influencing the cellular mechanisms of GVL.
Introduction
Following allogeneic bone marrow transplantation (BMT), donor T-cell alloreactivity can be co-opted to generate powerful antitumor activity, an effect termed the graft-versus-leukemia (GVL) response.1,2 The GVL effect is associated with the presence of graft-versus-host disease (GVHD) and is linked to the degree of major MHC disparity and the presence of T cells within the graft, indicating that graft-versus-host (GVH) alloreactive donor T cells are important for this effect.3 We have previously shown that administration of delayed donor leukocyte infusion (DLI) to established mixed chimeras (MCs; in which hematopoietic elements from both the donor and recipient are present) produces dramatically improved GVL effects compared with those seen following delayed DLI to full chimeras (FCs).4 Host hematopoietic antigen-presenting cells (APCs) expressing MHC class I molecules are necessary for this optimization of GVL effects in MCs.4 The importance of host APCs in inducing GVL has recently been confirmed in freshly irradiated mice,5 and previous studies have shown their importance in inducing GVHD under such conditions.6,7
The marked overlap of GVL and GVHD limit the wider application of allogeneic BMT, especially in those individuals who lack an HLA-identical donor. However, GVL can be achieved without GVHD by administration of DLI to established MCs that lack proinflammatory stimuli from recent conditioning.4,8 A precise definition of the mechanisms that underlie the GVL effect of DLI in MCs will be important for the rational development of this strategy for achieving maximal GVL effects without GVHD in humans. Using a tumor protection model, we demonstrate here that GVL responses of DLI are due to alloresponses against recipient MHC antigens. We also demonstrate a requirement for CD4+ T-cell help in generating maximal CD8+ T-cell–mediated GVL activity against MHC class II–negative tumors. CD4+ T-cell help is dependent on the presence of host APCs that express MHC class II. Moreover, the requirement for CD4+ T-cell help is completely bypassed when DLIs are transferred to freshly irradiated recipients. These findings have major implications for the design of transplantation protocols that aim to maximize GVL responses in the clinical setting.
Materials and methods
Animals
Animals were used under a protocol approved by our institutional Subcommittee on Research Animal Care, and experiments were performed in accordance with NIH guidelines. Female BALB/cJ (H-2d), B10.A (H2a), C57BL/6 (H-2b), and B6.SJL (CD45.1) mice were purchased from the Frederick Cancer Research Facility (Frederick, MD). Female donor MHC class II–/– B6.129-H2-Ab1tm1GlmN12 mice were purchased from Taconic (Germantown, NY). 2C T-cell–receptor transgenic mice (H2b on C57BL/6 background)9 were kindly provided by Dr Dennis Loh (Washington University School of Medicine, St Louis, MO). B10.MBR (H-2bq1) and C3.SW-H-2b/Sn5 mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Donor mice were aged 6 to 13 weeks, and recipient mice were aged 11 to 12 weeks at the time of transplantation.
Bone marrow transplantation
Mixed or full donor hematopoietic chimeras were established by reconstitution of lethally irradiated recipients with a mixture of T-cell–depleted (TCD) donor (15 × 106) and recipient (5 × 106) bone marrow cells (BMCs) or TCD donor (10 × 106–15 × 106) BMCs alone, respectively. Recipient mice were lethally irradiated (B6, 10.25 Gy; BALB/c, 8 Gy; 137Cs source, 0.8 Gy/min), and TCD BMCs were injected intravenously 4 hours later. T-cell depletion was performed using anti-CD4 (GK1.5) plus anti-CD8 (2.43) monoclonal antibodies (mAbs) and rabbit complement as previously described10 or through immunomagnetic depletion using anti-CD4 or anti-CD8 microbeads (Miltenyi Biotec, Auburn, CA).
Donor leukocyte infusion
Splenocyte (SC) suspensions were prepared as described.11 In GVL experiments, DLIs (3 × 106–3 × 107 donor SCs) were administered to recipient mice. For T-cell subset–depleted DLIs, CD4+, CD8+, or both subsets were depleted from the spleens using mAb and complement as previously described.12 When 1 subset was depleted, administered SC numbers were adjusted to contain the same number of CD4+ or CD8+ T cells as the nondepleted control DLI. Flow cytometric analysis showed that depletion of each subset was at least 96%. When required, donor CD4+ and CD8+ T cells were isolated (purity > 93%) by immunomagnetic selection using anti-CD4 or anti-CD8 microbeads (Miltenyi Biotec). In experiments to track proliferation of transferred donor T cells, labeling was performed with CFSE as previously described.13
Flow cytometry
The following mAbs were used: anti–IFN-γ–PE (XMG1.2), anti–34-2-12–FITC (anti–H2-Dd), anti–I-Ab–FITC (25-9-17), anti–CD62L-FITC (MEL-14), anti–CD44-FITC (IM7), anti–CD45.2-FITC (104), anti–CD4-PE (RM4-5), anti–CD8β-PE (H35-17.2), anti–B220-PE (RA3-6B2), anti–Mac-1–PE (M1/70), anti–CD45.1-biotin (A20), anti–Vβ3.1-biotin (KJ25), anti–Vβ8.1/2-biotin (MR5.2), and the appropriate isotype controls (PharMingen, San Diego, CA). CD8+ T cells bearing the 2C T-cell receptor (TCR) were identified via the clonotype-specific mAb, 1B214 and anti– mouse IgG1–APC (X56; PharMingen). Intracellular staining of cytokines after brief ex vivo stimulation with PMA (10 ng/mL) and ionomycin (1 μg/mL) was performed as previously described.15 Flow cytometry was performed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA).
EL4 and C1498 cell culture and administration
EL4 and C1498 cells were obtained from American Type Culture Collection (Manassas, VA) and cultured as previously described.16
Statistical analysis
Survival data were analyzed using the log-rank test. Otherwise statistical analyses were performed using the student t test (2-tailed).
Results
GVL effects of delayed DLIs are dependent on MHC alloreactivity
We have previously shown that GVL effects of DLI against EL4, a T-cell lymphoma, are superior in MCs compared with FCs because of the presence of professional host APCs expressing MHC class I.4 To confirm that the requirement of host APCs for maximal GVL activity was not related to a specific tumor type, we examined GVL effects against a myeloid leukemia cell line, C1498. Like EL4, C1498 is of B6 origin and expresses MHC class I but not MHC class II.16 We created MCs (B10.A + B6 → B6) or FCs (B10.A → B6) across a full MHC barrier and after 8 weeks transferred 3 × 107 B10.A SCs by intravenous injection. One week later, 5 × 104 C1498 cells were injected intravenously. As for EL4, injection of C1498 was performed after administration of DLI because of the very rapid kinetics of tumor growth, which precluded an evaluation of the GVL activity against tumor administered prior to or at the time of DLI (data not shown). Both MCs and FCs rapidly succumbed to tumor in the absence of DLI. DLI mediated minimal GVL activity in FCs (median survival time [MST] 36 days in FCs receiving DLI and C1498, versus 28 days in FCs receiving C1498 alone; P < .008). As we have shown for the EL4 tumor,4 the presence of host APCs maximized the antitumor effects following delayed DLI (Figure 1A-B). Thus, the MST was more than 90 days in MC recipients receiving DLI versus an MST of 36 days in FC recipients receiving DLI (P < .001). None of the surviving mice showed any clinical evidence of tumor or GVHD.
GVL following DLI may conceivably be directed against MHC alloantigens, minor H antigens, or TAA. In the experiments described in Figure 1 and in our previously published study,4 the mouse strains used were matched for minor H antigens but fully MHC mismatched. In the absence of MHC class I sharing by the donor and recipient, cytotoxic T lymphocytes (CTLs) recognizing TAA-derived peptides presented by MHC class I on donor APCs would not encounter that MHC molecule on tumor cells. If cross-presented TAAs contribute to the GVL effect of DLI in this model, we reasoned that sharing of MHC class I between donor and recipient might permit detection of measurable GVL effects in FCs. Thus, we tested GVL effects of DLI using minor H antigen–matched, partially MHC-mismatched recipient and donor strain combinations in which the Kb class I antigen was shared (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). However, despite the capacity for cross-presentation of TAA in this model, no GVL activity of delayed DLI was observed in FCs (Figure S1). We extended these initial findings by assessing the potential contribution of both minor H antigen disparities and TAA to the GVL effects of DLI in the MHC-matched, minor H antigen–mismatched C3.SW (CD45.2) → B6.SJL (CD45.1) combination. In FCs, mice receiving EL4 with or without DLI showed rapid tumor mortality (MST, 26 and 25 days) with no measurable GVL effect of DLI. Similarly, DLI had no measurable GVL effect in MCs, because recipients of EL4 with or without DLI both showed rapid tumor mortality (MST, 29 and 26 days). Compared with FCs receiving EL4 and DLI, MCs receiving EL4 and DLI showed no improvement in survival (Figure 1C-D; P = .66). These results demonstrate that in contrast to models that involve MHC alloreactivity, anti–minor H antigen or anti-TAA–specific immune responses are insufficient to generate measurable GVL activity when delayed DLI is administered prior to the tumor.
GVL effects of delayed DLI. (A) B10.A → B6 FCs received 3 × 107 B10.A DLI alone (▴, n = 7) on day 56 after BMT, C1498 myeloid leukemia cells (5 × 104) alone (*, n = 8) on day 63, or both (□, n = 8). (B) In the same experiment, B10.A + B6 → B6 MCs received DLI alone (•, n = 6) on day 56, C1498 cells alone (○, n = 7) on day 63, or both (▾, n = 7). MCs receiving DLI and C1498 (▾, n = 7) showed markedly improved survival compared with FCs receiving DLI and C1498 (panel A: □, n = 8; P < .01). (C) C3.SW → B6 FCs received 1 × 103 EL4 cells (*, n = 7) on day 63 or 3 × 107 C3.SW SCs as DLI on day 56 after BMT plus 1 × 103 EL4 on day 63 (□, n = 11). (D) C3.SW + B6 → B6 MCs also received EL4 cells alone (○, n = 7) on day 63, or both DLI and EL4 cells (▾, n = 10). MCs receiving DLI and EL4 showed similar survival compared with full chimeras receiving DLI and EL4.
GVL effects of delayed DLI. (A) B10.A → B6 FCs received 3 × 107 B10.A DLI alone (▴, n = 7) on day 56 after BMT, C1498 myeloid leukemia cells (5 × 104) alone (*, n = 8) on day 63, or both (□, n = 8). (B) In the same experiment, B10.A + B6 → B6 MCs received DLI alone (•, n = 6) on day 56, C1498 cells alone (○, n = 7) on day 63, or both (▾, n = 7). MCs receiving DLI and C1498 (▾, n = 7) showed markedly improved survival compared with FCs receiving DLI and C1498 (panel A: □, n = 8; P < .01). (C) C3.SW → B6 FCs received 1 × 103 EL4 cells (*, n = 7) on day 63 or 3 × 107 C3.SW SCs as DLI on day 56 after BMT plus 1 × 103 EL4 on day 63 (□, n = 11). (D) C3.SW + B6 → B6 MCs also received EL4 cells alone (○, n = 7) on day 63, or both DLI and EL4 cells (▾, n = 10). MCs receiving DLI and EL4 showed similar survival compared with full chimeras receiving DLI and EL4.
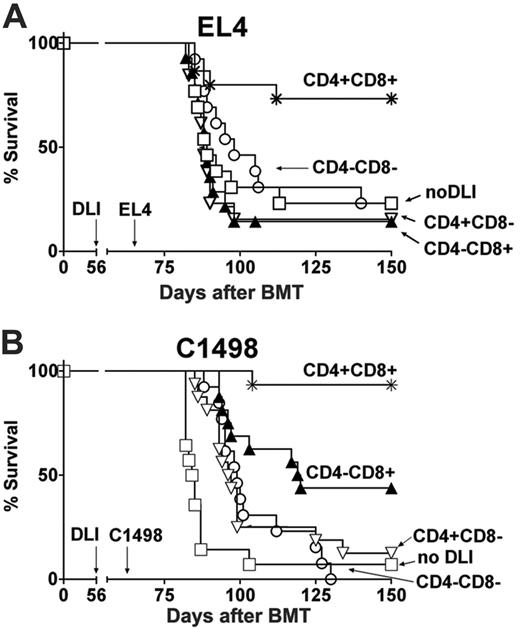
Both CD4+ and CD8+ T cells are required for maximal GVL effects of delayed DLI in MCs. Data were pooled from 2 independent experiments. (A) B10.A + B6 → B6 MCs received 3 × 107 nondepleted CD4+CD8+ DLIs (*, n = 15; inoculum contained a total of 5.2 × 106 CD4+ plus 2.5 × 106 CD8+ T cells), CD4–CD8+ DLI (▴, n = 14; inoculum contained 2.5 × 106 CD8+ T cells), CD4+CD8– DLI (▿, n = 13; inoculum contained 5.2 × 106 CD4+ T cells), CD4–CD8– DLI (○, n = 13), or no DLI (□, n = 13) on day 56 after BMT. All mice received 1 × 103 EL4 T leukemia cells on day 63. (B) MCs received 3 × 107 B10.A nondepleted CD4+CD8+ DLIs (*, n = 16; inoculum contained a total of 5.7 × 106 CD4+ plus 2.3 × 106 CD8+ T cells), CD4–CD8+ DLI (▴, n = 16; inoculum contained 2.3 × 106 CD8+ T cells), CD4+CD8– DLI (▿, n = 16; inoculum contained 5.8 × 106 CD4+ T cells), CD4–CD8– DLI (○, n = 13), or no DLI (□, n = 14) on day 56 after BMT followed by 5 × 104 C1498 myeloid leukemia cells on day 63.
Both CD4+ and CD8+ T cells are required for maximal GVL effects of delayed DLI in MCs. Data were pooled from 2 independent experiments. (A) B10.A + B6 → B6 MCs received 3 × 107 nondepleted CD4+CD8+ DLIs (*, n = 15; inoculum contained a total of 5.2 × 106 CD4+ plus 2.5 × 106 CD8+ T cells), CD4–CD8+ DLI (▴, n = 14; inoculum contained 2.5 × 106 CD8+ T cells), CD4+CD8– DLI (▿, n = 13; inoculum contained 5.2 × 106 CD4+ T cells), CD4–CD8– DLI (○, n = 13), or no DLI (□, n = 13) on day 56 after BMT. All mice received 1 × 103 EL4 T leukemia cells on day 63. (B) MCs received 3 × 107 B10.A nondepleted CD4+CD8+ DLIs (*, n = 16; inoculum contained a total of 5.7 × 106 CD4+ plus 2.3 × 106 CD8+ T cells), CD4–CD8+ DLI (▴, n = 16; inoculum contained 2.3 × 106 CD8+ T cells), CD4+CD8– DLI (▿, n = 16; inoculum contained 5.8 × 106 CD4+ T cells), CD4–CD8– DLI (○, n = 13), or no DLI (□, n = 14) on day 56 after BMT followed by 5 × 104 C1498 myeloid leukemia cells on day 63.
MHC class II expression by bone marrow–derived APCs is required for maximal GVL effects in MCs. (A) Peripheral blood chimerism analysis was performed in various cell lineages by flow cytometry 6 weeks after BMT. Representative contour plots show B10.A donor MHC class I (34-2-12, H2-Dd) and B6 recipient MHC class II (H2-Ab) expression in B cells of MCs generated by reconstitution with B10.A and either B6 MHC class II+/+ or B6 MHC class II–/– bone marrow. (B) MCs received either 3 × 107 B10.A DLIs on day 56 after BMT, EL4 on day 63, or both. B6 MHC class II+/+ plus B10.A MCs: no DLI (▪, n = 4), DLI (•, n = 7), DLI + EL4 (▾, n = 18), EL4 (○, n = 8). B6 MHC class II–/– plus B10.A MCs (CII– MCs): no DLI (⋄, n = 3), DLI (▿, n = 5), DLI + EL4 (▵, n = 25), EL4 (*, n = 11). Data were pooled from 2 similar, independent experiments. (C) Comparisons of GVL activities of DLI in MCs versus CII– MCs versus FCs. MCs: DLI (• n = 7), DLI + EL4 (▾, n = 11). CII– MCs: DLI (▿, n = 2), DLI + EL4 (▵, n = 14). FCs: DLI (♦, n = 6), DLI + EL4 (□, n = 8).
MHC class II expression by bone marrow–derived APCs is required for maximal GVL effects in MCs. (A) Peripheral blood chimerism analysis was performed in various cell lineages by flow cytometry 6 weeks after BMT. Representative contour plots show B10.A donor MHC class I (34-2-12, H2-Dd) and B6 recipient MHC class II (H2-Ab) expression in B cells of MCs generated by reconstitution with B10.A and either B6 MHC class II+/+ or B6 MHC class II–/– bone marrow. (B) MCs received either 3 × 107 B10.A DLIs on day 56 after BMT, EL4 on day 63, or both. B6 MHC class II+/+ plus B10.A MCs: no DLI (▪, n = 4), DLI (•, n = 7), DLI + EL4 (▾, n = 18), EL4 (○, n = 8). B6 MHC class II–/– plus B10.A MCs (CII– MCs): no DLI (⋄, n = 3), DLI (▿, n = 5), DLI + EL4 (▵, n = 25), EL4 (*, n = 11). Data were pooled from 2 similar, independent experiments. (C) Comparisons of GVL activities of DLI in MCs versus CII– MCs versus FCs. MCs: DLI (• n = 7), DLI + EL4 (▾, n = 11). CII– MCs: DLI (▿, n = 2), DLI + EL4 (▵, n = 14). FCs: DLI (♦, n = 6), DLI + EL4 (□, n = 8).
GVL effects of delayed DLI are dependent on cooperative interactions between donor CD4+ and CD8+ T cells
To determine which T-cell subsets were involved in the powerful GVL effect following DLI to MCs established across a full MHC mismatch, we evaluated the effects of depleting CD4+ and CD8+ T-cell subsets from DLIs. Thus, we compared GVL activity against EL4 in MCs that received nondepleted, CD4+ T-cell–depleted, CD8+ T-cell–depleted, both CD4+ and CD8+ T-cell–depleted DLI, or no DLI. In 2 independent experiments (pooled data are shown in Figure 2A), mice receiving EL4 alone had the worst survival (MST, 29 days), and mice receiving EL4 plus nondepleted control DLI containing both CD4+ and CD8+ T cells had the best survival (MST, > 90 days; P < .05). There was no detectable GVL effect in mice receiving DLI depleted of both CD4+ and CD8+ T-cell subsets (MST, 33 days). Depletion of CD4+ or CD8+ T cells alone from DLI also led to complete loss of the GVL effect (MST, 26 and 25 days; P < .005, P < .005, respectively, versus nondepleted DLI). Thus, both CD4+ and CD8+ T cells were required for the achievement of GVL effects from DLI in established MCs.
Similar studies were performed using the C1498 tumor model (Figure 2B). MCs receiving C1498 without DLI showed rapid tumor mortality (MST, 21 days), whereas excellent protection was observed in mice receiving C1498 with nondepleted DLI (Figure 3B; MST, > 90 days; P < .001). Depletion of CD4+ T cells, CD8+ T cells, or both CD4+ and CD8+ T cells from DLI increased tumor mortality (MST, 57, 33, and 36 days; P < .005, P < .001, P < .001, respectively, versus nondepleted DLI). However, in these experiments, the requirement for CD4+ T cells was incomplete (MST CD4+ T-cell–depleted DLI 57 days compared with MST 33 days following CD8+ T-cell–depleted DLI; P < .05). Taken together these data suggest that maximal GVL effects of DLI against C1498 are dependent on both CD4+ and CD8+ T cells in established MCs.
MHC class II expression on host APCs is required to induce maximal GVL effects of DLI in MCs
Because both EL4 (data not shown) and C149816 lack expression of MHC class II, we reasoned that the primary requirement for CD4+ T cells was to provide help for CD8+ T-cell–mediated GVL activity. We have previously shown that the GVL response requires expression of MHC class I on host APCs,4 and we hypothesized that provision of T-cell help would also require MHC class II expression by host APCs. We therefore evaluated whether expression of MHC class II on host APCs contributes to the improved GVL effects of DLI in mixed compared with full allogeneic chimeras. B10.A + B6 MHCII–/– → B6 MCs (CII– MCs) were generated which had normal MHC class II expression on nonhematopoietic tissues but showed only minimal (< 1%) recipient MHC class II (I-Ab) expression on peripheral blood B cells and myeloid cells (Figure 3A; data not shown). Levels of chimerism in peripheral blood T, B, and myeloid cells at the time of DLI were equivalent in CII– MCs and control MCs (data not shown). B10.A SCs (3 × 107) were transferred to established CII– MCs and control MCs or to established B10.A → B6 FCs. EL4 cells were injected intravenously 1 week later. As shown in Figure 3B-C, all recipients of DLI without EL4 survived more than 100 days with good health. FCs receiving DLI and EL4 showed the shortest survival (MST, 24 days), whereas control MCs receiving DLI and EL4 had excellent survival (MST, > 100 days; P < .001). In contrast, CII– MCs receiving DLI and EL4 showed intermediate survival (MST, > 100 days), with better survival than FCs (P < .001) but worse survival than control MCs (P = .05). These data suggest that direct recognition of MHC class II alloantigen on host APCs is important for priming helper T-cell responses that drive CD8+ T-cell–dependent GVL activity.
Graft-versus-host reactive T-cell expansion following delayed DLI requires host APCs
To establish the mechanism underlying the requirement for host APCs in inducing GVL following DLI, we tracked donor T-cell responses following transfer to MCs or FCs. Thus, we generated B6 + BALB/c → BALB/c MCs and B6 → BALB/c FCs, and, after a 10-week interval and recovery of peripheral blood lymphocytes (data not shown), we transferred 10 × 107 CFSE-labeled B6 CD45.1 donor SCs and 1 × 106 purified CD8+ T cells derived from 2C transgenic mice. CD8+ T cells from 2C mice express a high-affinity T-cell receptor recognizing the Ld class I molecule expressed by BALB/c recipients.9 As previously reported,4,17,18 transfer of DLIs to established MCs was associated with marked increases in the levels of donor chimerism (data not shown). Despite a robust lymphohematopoietic graft-versus-host reaction, no GVHD was observed (data not shown).
The proliferation of polyclonal CD45.2–CD4+ T cells and CD8+ T cells derived from the DLI was evaluated by flow cytometric measurement of CFSE dilution at intervals following transfer (Figure 4A). By day +6 following DLI, the majority of polyclonal CD4+ T cells (86% ± 2%, mean ± SEM) and polyclonal CD8+ T cells (61% ± 5%) isolated from recipient spleens had undergone more than 7 divisions in established MCs. In contrast, polyclonal CD4+ (21% ± 3% > 7 divisions, P < .001, versus MCs) and CD8+ (25% ± 3%, P = .003) T-cell proliferation was markedly reduced following transfer to FCs, in which host-derived APCs are largely absent (Figure 4A). Thus, initial proliferation of donor CD4+ and CD8+ T-cell populations is highly dependent on the presence of host APCs at the time of transfer. The presence or absence of host APCs directly influenced the degree to which polyclonal DLI-derived CD4+ and CD8+ T cells accumulated within recipient spleens. By day +6, the absolute numbers of CD45.2–CD4+ T cells in recipient spleens were 3.4 × 106 ± 0.1 × 106 and 1.0 × 106 ± 0.5 × 106 in MCs and FCs, respectively (P = .009). At this time point, the absolute numbers of CD45.2–CD8+ T cells were 4.3 × 106 ± 1.6 × 106 and 1.0 × 106 ± 0.4 × 106 in MCs and FCs, respectively (P = NS). By day +12 following transfer (Figure 4B), the absolute numbers of DLI-derived CD4+ T cells in MCs were 5.6-fold greater than in FCs (2.1 × 106 ± 0.7 × 106 versus 0.4 × 106 ± 0.03 × 106; P = .001), and the absolute numbers of DLI-derived CD8+ T cells in MCs were 6-fold greater than in FCs (5.0 × 106 ± 1.0 × 106 versus 1.0 × 106 ± 0.8 × 106; P = .002).
Donor CD4+ and CD8+ T-cell expansion requires the presence of host-derived hematopoietic APCs. B6 CD45.1 SCs (1 × 107) and 2C CD8+ T cells (1 × 106) were CFSE-labeled and then transferred to B6 CD45.2 + BALB/c → BALB/c MCs or B6 CD45.2 → BALB/c FCs 10 weeks following BMT. (A) Histograms show log CFSE staining of gated CD4+CD45.2– and CD8+CD45.2– T-cell populations within the spleen on day +6 following DLI. Results are representative of 3 independent experiments. (B) Absolute numbers of DLI-derived CD4+ and CD8+ T cells in recipient spleens of MCs and FCs on day +12 following DLI (n = 6 each group). Data are shown as mean ± SEM.
Donor CD4+ and CD8+ T-cell expansion requires the presence of host-derived hematopoietic APCs. B6 CD45.1 SCs (1 × 107) and 2C CD8+ T cells (1 × 106) were CFSE-labeled and then transferred to B6 CD45.2 + BALB/c → BALB/c MCs or B6 CD45.2 → BALB/c FCs 10 weeks following BMT. (A) Histograms show log CFSE staining of gated CD4+CD45.2– and CD8+CD45.2– T-cell populations within the spleen on day +6 following DLI. Results are representative of 3 independent experiments. (B) Absolute numbers of DLI-derived CD4+ and CD8+ T cells in recipient spleens of MCs and FCs on day +12 following DLI (n = 6 each group). Data are shown as mean ± SEM.
We also evaluated the numbers and phenotype of known graft-versus-host reactive CD4+ and CD8+ T-cell populations derived from DLIs. As observed for polyclonal CD8+ T cells, maximal proliferation of host-specific 2C CD8+ T cells required the presence of host APCs (Figure 5A). By day +6 following DLI, 80% ± 2% of 2C CD8+ T cells isolated from recipient spleens had undergone more than 7 divisions in established MCs compared with 63% ± 4% in FCs (P = .003). Although 2C CD8+ T cells underwent substantial proliferation even in FCs, they failed to accumulate, suggesting that the dividing antihost T cells had a marked survival disadvantage in the absence of host APCs (Figure 5A-B; Figure S2). No accumulations of 2C CD8+ T cells in lymph nodes, bone marrow, liver, lung, and the intestine of recipient FCs were observed, ruling out altered distribution of this population (data not shown). Interestingly, the majority of 2C CD8+ T cells in both MCs and FCs demonstrated a CD44highCD62L– memory/effector phenotype (Figure 5C), presumably as a result of significant proliferation of this cell population in both contexts. Similarly, the percentage of 2C CD8+ cells expressing IFN-γ or TNF-α after brief ex vivo stimulation was similar in MCs and FCs (Figure 5D; data not shown).
CD45.2–CD4+Vβ3+ T cells were evaluated as representative of graft-versus-host–reactive CD4+ T-cell populations because they recognize an endogenous superantigen that is expressed in the host but not the donor.19 As a control population, we evaluated the numbers of CD45.2–CD4+Vβ8.1/2+ T cells that do not recognize endogenous superantigens in this model system. Following transfer of DLI, we observed that the absolute numbers of graft-versus-host–reactive CD45.2–CD4+Vβ3+ T cells in the spleen were 6.4 ± 2.1-fold greater in recipient MCs than FCs (Figure 5E; Figure S3; P < .05). In contrast, the absolute numbers of the control CD4+Vβ8.1/2+ T-cell population in the spleen were only 1.7 ± 0.3-fold greater in MCs than FCs (Figure 5E; Figure S3; P = NS). Thus, host APCs prime graft-versus-host reactive CD4+ T-cell populations selectively. Consistent with this concept, CD4+Vβ3+ T cells expressed a CD44highCD62L– memory/effector phenotype in MCs, whereas CD4+Vβ8.1/2+ T cells expressed a phenotype that was closer to that of naive T cells (Figure 5F). In contrast, there was no significant difference in the expression of memory/effector markers between the 2 CD4+ T-cell populations in FCs (Figure 5F). Down-regulation of Vβ3+ and Vβ8.1/2+ TCRs among CD4+ T cells following ex vivo stimulation made evaluation of cytokine synthesis by these populations in MCs and FCs difficult. We therefore evaluated intracellular IFN-γ and TNF-α in DLI-derived polyclonal CD45.2–CD4+ T cells in MCs and FCs on day +12. No differences in the percentage of CD45.2–CD4+ T cells that expressed IFN-γ and TNF-α were noted (Figure S4; data not shown).
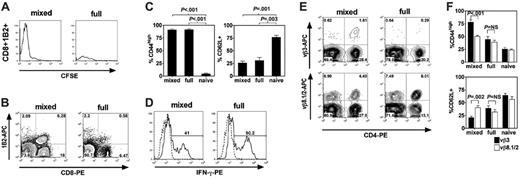
Host hematopoietic APCs prime host-specific donor T-cell expansion. B6 CD45.1 SCs (1 × 107) and 2C CD8+ T cells (1 × 106) were CFSE-labeled and then transferred to B6 CD45.2 + BALB/c → BALB/c MCs or B6 CD45.2 → BALB/c FCs 10 weeks following BMT. (A) Histograms show log CFSE staining of gated CD8+1B2+ T-cell populations within the spleen on day +6 following transfer. 1B2 is a clonotypic marker that identifies cells bearing the 2C TCR. Data are representative of 3 independent experiments. (B) Representative flow cytometric contour plots of recipient spleens of MCs and FCs on day +12 following DLI (x-axis, CD8-PE; y-axis, 1B2-APC). Data are representative of 3 independent experiments. (C) Percentage of gated CD8+1B2+ T cells that were CD44high (left) or CD62L+ (right) in recipient spleens of MCs and FCs on day +12 following DLI (naive controls shown for comparison, n = 3 each group). Data shown represent 1 of 2 independent experiments. (D) Representative flow cytometric plots of intracellular staining of IFN-γ in gated CD8+1B2+ T cells in recipient spleens of MCs and FCs on day +12 following DLI after brief ex vivo stimulation. Percentages of gated CD8+1B2+ cells that were IFN-γ+ were 40% ± 9% in MCs versus 37% ± 8% in FCs (P = NS; mean ± SEM; n = 4 each group). (E) Representative flow cytometric contour plots of recipient spleens of MCs and FCs on day +12 following DLI on gated CD45.2– cells (x-axis, CD4-PE; y-axis, Vβ3-APC or Vβ8.1/2-APC). (F) Percentage of gated CD4+Vβ3+ or CD4+Vβ8.1/2+ T cells that were CD44high (top) or CD62L+ (bottom) in recipient spleens of MCs and FCs on day +12 following DLI (naive phenotype shown for comparison, n = 3 each group). (C,F) Data are shown as mean ± SEM.
Host hematopoietic APCs prime host-specific donor T-cell expansion. B6 CD45.1 SCs (1 × 107) and 2C CD8+ T cells (1 × 106) were CFSE-labeled and then transferred to B6 CD45.2 + BALB/c → BALB/c MCs or B6 CD45.2 → BALB/c FCs 10 weeks following BMT. (A) Histograms show log CFSE staining of gated CD8+1B2+ T-cell populations within the spleen on day +6 following transfer. 1B2 is a clonotypic marker that identifies cells bearing the 2C TCR. Data are representative of 3 independent experiments. (B) Representative flow cytometric contour plots of recipient spleens of MCs and FCs on day +12 following DLI (x-axis, CD8-PE; y-axis, 1B2-APC). Data are representative of 3 independent experiments. (C) Percentage of gated CD8+1B2+ T cells that were CD44high (left) or CD62L+ (right) in recipient spleens of MCs and FCs on day +12 following DLI (naive controls shown for comparison, n = 3 each group). Data shown represent 1 of 2 independent experiments. (D) Representative flow cytometric plots of intracellular staining of IFN-γ in gated CD8+1B2+ T cells in recipient spleens of MCs and FCs on day +12 following DLI after brief ex vivo stimulation. Percentages of gated CD8+1B2+ cells that were IFN-γ+ were 40% ± 9% in MCs versus 37% ± 8% in FCs (P = NS; mean ± SEM; n = 4 each group). (E) Representative flow cytometric contour plots of recipient spleens of MCs and FCs on day +12 following DLI on gated CD45.2– cells (x-axis, CD4-PE; y-axis, Vβ3-APC or Vβ8.1/2-APC). (F) Percentage of gated CD4+Vβ3+ or CD4+Vβ8.1/2+ T cells that were CD44high (top) or CD62L+ (bottom) in recipient spleens of MCs and FCs on day +12 following DLI (naive phenotype shown for comparison, n = 3 each group). (C,F) Data are shown as mean ± SEM.
Graft-versus-host reactive CD8+ T-cell expansion following delayed DLI is dependent on CD4+ T-cell help
To test the extent to which cooperative interactions between CD4+ and CD8+ T cells are relevant to the effects of delayed DLIs, we transferred donor CD8+ T cells to MCs or FCs, either alone or in combination with donor CD4+ T cells. As shown in Figure 4, when donor CD4+ and CD8+ T cells were cotransferred, accumulation occurred only in the presence of host APCs (Figure 6A-B). Significantly, when CD8+ T cells were transferred alone, they failed to accumulate not only in FCs but also in MCs (Figure 6B). Instead, full donor CD8+ T-cell expansion required the presence of both host APCs and donor CD4+ T cells. Like polyclonal CD8+ T cells, 2C CD8+ T-cell accumulation also required the presence of both host APCs and CD4+ T cells (Figure 6C). Although donor CD8+ T-cell expansion was dependent on CD4+ T-cell help in MCs, expression of IFN-γ or TNF-α in gated CD45.2–CD8+ and 2C CD8+ T cells was completely CD4+ T cell independent (data not shown).
Taken together, these data indicate that maximal proliferation and accumulation of graft-versus-host–reactive T cells following DLI requires the presence of host APCs. Graft-versus-host–reactive CD8+ T-cell expansion is dependent on the presence of both host APCs and CD4+ T-cell help. Although host APCs maximize the expansion of graft-versus-host–reactive T-cell populations, they have no effect on their effector cytokine synthesis on a per cell basis.
CD4+ T cells do not contribute to the GVL effect of DLI in freshly irradiated BMT recipients
To determine whether CD8+ GVL activity required T-cell help when donor T cells are transferred without delay following allogeneic transplantation, freshly irradiated B6 mice received mixed donor and recipient TCD BM together with 3 × 106 nondepleted, CD4+ T-cell–depleted, CD8+ T-cell–depleted, CD4+ and CD8+ T-cell–depleted B10.A DLI, or no DLI and then received EL4 1 week later (Figure 7A-B). T-cell depletions were at least 96% effective. Mortality because of tumor was distinguished from GVHD mortality by clinical features, such as paraplegia, and autopsy findings, including splenic enlargement, hepatomegaly, and nephromegaly, all of which are specific for tumor infiltration and not GVHD in this model.9 Control mice receiving mixed donor and recipient TCD BM without DLI survived and became MCs (data not shown). Although nondepleted DLI led to some death from GVHD in freshly irradiated mice (Figure 7A), those receiving EL4 with nondepleted DLI showed prolonged overall survival (Figure 7A) and tumor-free survival (Figure 7B; MST, > 90 days and 31 days; P < .005) compared with those receiving EL4 alone. As expected, depletion of CD8+ T cells or both CD4+ and CD8+ T cells from DLI markedly reduced tumor-free survival (Figure 7B; MST, 25 and 30 days; P < .001 and P < .001, respectively versus nondepleted DLI). Strikingly, DLI CD4+ T-cell depletion did not diminish tumor-free survival (MST, > 90 days), indicating that CD4+ T cells do not contribute to GVL effects of DLI in freshly irradiated BMT recipients.
Graft-versus-host reactive donor CD8+ T-cell expansion following delayed DLI is dependent on CD4+ T-cell help. B6 CD45.1 CD8+ T cells (1 × 106) and 2C CD8+ T cells (1 × 106) with or without 2 × 106 B6 CD45.1 CD4+ T cells were transferred to B6 CD45.2 + BALB/c → BALB/c MCs or B6 CD45.2 → BALB/c FCs 10 weeks following BMT. (A-C) Histograms show absolute numbers of CD4+CD45.2–, CD8+CD45.2–, and CD8+1B2+ populations within the spleen on day 12 following transfer (n = 3 each group). Open bars indicate CD8+ T cells transferred without CD4+ T cells; closed bars, CD4+ and CD8+ T cells cotransferred. Note that in Figure 6A, only closed bars are shown (CD4+ and CD8+ T cells cotransferred). Data are shown as mean ± SEM.
Graft-versus-host reactive donor CD8+ T-cell expansion following delayed DLI is dependent on CD4+ T-cell help. B6 CD45.1 CD8+ T cells (1 × 106) and 2C CD8+ T cells (1 × 106) with or without 2 × 106 B6 CD45.1 CD4+ T cells were transferred to B6 CD45.2 + BALB/c → BALB/c MCs or B6 CD45.2 → BALB/c FCs 10 weeks following BMT. (A-C) Histograms show absolute numbers of CD4+CD45.2–, CD8+CD45.2–, and CD8+1B2+ populations within the spleen on day 12 following transfer (n = 3 each group). Open bars indicate CD8+ T cells transferred without CD4+ T cells; closed bars, CD4+ and CD8+ T cells cotransferred. Note that in Figure 6A, only closed bars are shown (CD4+ and CD8+ T cells cotransferred). Data are shown as mean ± SEM.
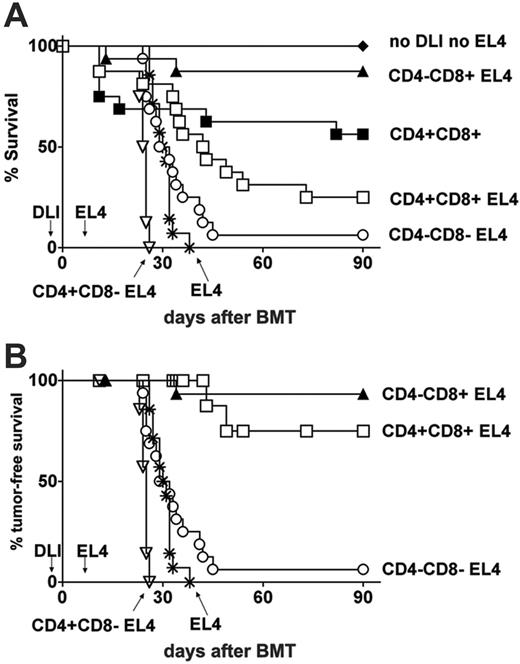
CD4+ T cells do not contribute to GVL effects of DLI in freshly irradiated BMT recipients. Lethally irradiated B6 mice were reconstituted with a mixture of TCD B10.A and B6 bone marrow together with nondepleted DLI (□, n = 8; inoculum contained a total of 5.3 × 105 CD4+ plus 2.1 × 105 CD8+ T cells), CD4–CD8+ DLI (▴, n = 8; inoculum contained 2.0 × 105 CD8+ T cells), CD4+CD8– DLI (▿, n = 7; inoculum contained 5.4 × 105 CD4+ T cells), CD4–CD8– DLI (○, n = 8), or no DLI (*, n = 4) on day 0 followed by 1 × 103 EL4 cells on day 7. Additional groups received the same bone marrow inoculum and nondepleted DLI (▪, n = 8) or no DLI (♦, n = 4) but did not receive EL4 tumor. Graphs show (A) overall survival and (B) tumor-free survival in which nontumor deaths (as assessed at autopsy) were censored at the time of death.
CD4+ T cells do not contribute to GVL effects of DLI in freshly irradiated BMT recipients. Lethally irradiated B6 mice were reconstituted with a mixture of TCD B10.A and B6 bone marrow together with nondepleted DLI (□, n = 8; inoculum contained a total of 5.3 × 105 CD4+ plus 2.1 × 105 CD8+ T cells), CD4–CD8+ DLI (▴, n = 8; inoculum contained 2.0 × 105 CD8+ T cells), CD4+CD8– DLI (▿, n = 7; inoculum contained 5.4 × 105 CD4+ T cells), CD4–CD8– DLI (○, n = 8), or no DLI (*, n = 4) on day 0 followed by 1 × 103 EL4 cells on day 7. Additional groups received the same bone marrow inoculum and nondepleted DLI (▪, n = 8) or no DLI (♦, n = 4) but did not receive EL4 tumor. Graphs show (A) overall survival and (B) tumor-free survival in which nontumor deaths (as assessed at autopsy) were censored at the time of death.
Discussion
In this study, we have examined CD8+ T-cell–dependent GVL effects against highly lethal and rapidly growing tumors that express MHC class I but not MHC lass II. Both MHC disparity and host professional APCs were required for optimal GVL effects following DLI. Under the specific conditions of this model in which DLI is administered prior to tumor, reactivity against minor H antigens or cross-presented TAA is insufficient to protect against tumor-induced lethality. Although GVL directed against TAA20 or minor H antigens5,20,21 has been demonstrated in other murine models involving freshly conditioned hosts, such responses are not elicited as readily when tumor bulk is great5 or when donor T cells are transferred after a significant delay.22 In the outbred human population, GVL responses may be observed following delayed DLI to recipients of HLA-identical transplants.23 However, few patients with rapidly growing tumors respond to such an approach,23 and we believe the requirement for MHC disparity for GVL effects in our studies reflects the rapid growth and lethality of the tumor cell lines used in our models. Other studies in mice have shown that GVL responses following MHC-mismatched transplantation to freshly irradiated recipients are superior to those following MHC-matched transplantation, but these benefits are offset by a markedly increased risk of GVHD.8,24 However, MHC alloreactivity and GVL are separable from GVHD when donor T cells are administered as delayed DLI.4,25,26 In this study, we have demonstrated that host APCs expressing MHC alloantigens are required to prime maximal GVL effects in MCs receiving delayed DLI. These findings provide added impetus to the development of strategies that permit HLA-mismatched hematopoietic stem cell transplantation and the induction of mixed chimerism as a means of maximizing GVL in patients with advanced hematologic malignancies.27,28 Protocols incorporating extensive T-cell depletion may potentially reduce the risk of acute GVHD and thus may permit subsequent administration of delayed DLI.
The relative lack of GVL-priming activity by donor APCs in FCs or of recipient APCs in MHC-matched MCs, combined with the dependence of GVL on class I4 and class II MHC on recipient APCs in MHC-mismatched MCs, shows that direct GVH alloreactivity against host MHC antigens is necessary for GVL in these aggressive tumor models. Minimal GVL activity against both EL4 and C1498 was evident in FCs, and this most likely represents priming by residual host APCs present at the time of T-cell transfer. The failure to achieve GVL in FCs might reflect either a low precursor frequency of donor T cells specific for indirectly presented host alloantigens or low expression of alloantigen-derived peptides in the context of donor MHC. Similarly, the failure to observe GVL activity against EL4 in the MHC-matched, minor H antigen–mismatched strain combination might also reflect the low precursor frequency of T cells reactive against immunodominant peptides in the strain combination tested.29 Because responses against EL4 TAA are not measurable in unprimed mice,21,29 it is not unexpected that such reactivity failed to contribute to GVL activity in the models evaluated here. The necessity of administering DLI before the tumor to detect measurable protection reflects the aggressiveness of the tumor models used, and it is therefore not surprising that cross-presentation of TAA to donor T cells following tumor administration could not contribute significant antitumor effects.
Consistent with the critical role of direct host MHC alloantigen presentation in inducing GVH reactivity leading to GVL, donor DLI-derived CD4+ and CD8+ T-cell expansion was significantly reduced in FCs compared with MCs, reflecting a failure of these cells to undergo proliferation or to survive in the absence of host APCs. Strikingly, 2C CD8+ T cells that recognize the MHC class I Ld alloantigen only when it is presented directly failed to accumulate in the spleens or other tissues of FCs, despite undergoing significant activation and proliferation. This suggests that, although nonhematopoietic cells or residual host APCs expressing Ld alloantigen may induce 2C CD8+ T-cell proliferation, the survival of these cells is severely impaired in the absence of greater numbers of host APCs. Of note, in this model, we could detect no influence of host APCs on activation causing conversion to the “memory” phenotype or effector differentiation, as evaluated by cytokine synthesis of surviving graft-versus-host reactive CD4+ and CD8+ T cells. Thus, although the absolute numbers of IFN-γ–producing graft-versus-host reactive T cells are markedly higher in MCs, host APCs appear to maximize expansion (through effects on proliferation or survival) rather than influence effector functions on a per cell basis. Strategies (eg, administration of IL-15) aimed at maximizing the proliferation or survival of such effector/memory cells30 in FCs might therefore permit enhancement of GVL activity even in the absence of host APCs. Similarly, such approaches might also be pertinent to preserving durable CD8+ GVL responses following the initial graft-versus-host reaction which leads to conversion to full donor chimerism and the loss of host hematopoietic APCs.
The absence or loss of MHC class II expression is common in many human tumors31 and may be associated with a poor prognosis.32 In this study, we demonstrated that GVL responses against 2 independent MHC class II–negative tumors were dependent on the presence of donor CD4+ T cells when DLI were administered to MCs. Although CD4+ T cells might have additional effector functions, including the recruitment of activated macrophages31 or generation of cytokines, it seems unlikely that such functions have any role in GVL, because no antitumor activity following CD8+ T-cell–depleted DLI was observed. Instead, our data are consistent with a model in which donor CD4+ T cells are required to provide exogenous help, perhaps by “licensing” host APCs to prime the maximal expansion and survival of graft-versus-host reactive CD8+ T cells and CD8+ T-cell–dependent GVL. Consistent with this concept, the presence of host APCs expressing MHC class II alloantigen was required for maximal GVL activity following delayed transfer of donor CD4+ and CD8+ T cells. Note, however, that the GVL effect was still significant in MCs despite the absence of host APC MHC class II expression. The greater GVL effects of DLI in these mice compared with FCs argues against the possibility that residual host APCs or class II expressed on nonhematopoietic cells may have triggered GVL effects in these mice. One possibility is that expression of I-AαI-Eβ hybrid class II molecules by APCs from I-A –/–β mice33 may be sufficient to provide some degree of CD4 help for GVL responses. In humans, CD4+ T-cell–mediated GVL activity is reported to occur following delayed CD8+ T-cell–depleted DLI.34,35 In this case, it is possible that CD4+ T cells provide help for graft-versus-host reactive CD8+ T cells pre-existing in the recipient at the time of transfer. Alternatively, such CD4+ T cells may have direct cytolytic activity against MHC class II+ tumors.
Although donor CD4+ T cells are powerful mediators of GVHD when administered immediately following MHC-mismatched BMT,36 this effect is absent when they are transferred as a component of delayed DLI. Thus, the precise role of CD4+ T cells is critically dependent on the host environment. This is consistent with our finding that the need for CD4+ T cells for CD8+ T-cell–mediated GVL effects is overridden completely in freshly irradiated recipients. In this case, helper independence might result from the enhancement of CD8+ T-cell proliferation or functions following transfer to a lymphopenic environment.13,37-39 Alternatively, the levels of APC activation might be radically altered in the immediate aftermath of lethal irradiation. For example, radiation-induced injury to the gut leads to the translocation of microbial products into the peripheral circulation that may trigger TLRs on APCs,40 enhancing their activation and their capacity to promote effective CD8+ T-cell–mediated immunity in the absence of CD4+ T cells. Depletion of host regulatory populations or TLR-induced inhibition of their functions41 might also reduce the dependence of CD8+ CTLs on helper responses. Finally, natural killer-to-dendritic cell interactions in the early phase following conditioning might also contribute to CD4+ T-cell–independent CTL induction.42
In summary, we have demonstrated that MHC alloreactivity can be coopted to generate strong GVL responses without GVHD following delayed DLI and that priming of this response requires host APCs. Importantly, in the absence of conditioning-induced inflammation, CD8+ T-cell–mediated GVL activity is dependent on CD4+ T-cell help and the expression of MHC class II alloantigens on host APCs. These studies provide a powerful rationale for the development of nonmyeloablative protocols that permit, first, the establishment of mixed chimerism across partial MHC-mismatched barriers, and, second, the induction of GVL against poorly immunogenic tumors following delayed DLI.
Prepublished online as Blood First Edition Paper, June 6, 2006; DOI 10.1182/blood-2006-03-007427.
Supported by the National Institutes of Health (grant RO1 CA79989), by a Senior Research Award from the Multiple Myeloma Foundation, and by a Bennett Senior Fellowship in Experimental Haematology from the Leukemia Research Fund, United Kingdom.
R.C. and H.-S.E. performed, designed, and interpreted the research and cowrote the paper; J.S. performed and designed the research; J.B., P.C., R.H., and G.Z. performed research; M.S. designed and interpreted the research and oversaw and contributed to the writing of the paper.
R.C. and H.-S.E. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mr Orlando Moreno for technical assistance and expert oversight of animal care, Dr Yong-Guang Yang for helpful review of the manuscript, and Ms Luisa Raleza for excellent assistance with the manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal