Abstract
Intravenous immunoglobulin G (IVIG) is used to treat idiopathic thrombocytopenic purpura (ITP). Although many patients benefit from IVIG, some are refractory to this therapy. ITP is characterized by platelet clearance mediated primarily by antiplatelet antibodies against GPIIbIIIa and/or the GPIbα complex. These 2 groups of antibodies may induce ITP through different mechanisms. We tested the hypothesis that IVIG may not be equally effective in preventing ITP caused by anti-GPIIbIIIa versus anti-GPIbα antibodies in mice. Thrombocytopenia was induced in BALB/c mice using monoclonal antibodies against either mouse GPIIbIIIa (JON1, JON2, and JON3) or GPIbα (p0p3, p0p4, p0p5, p0p9, and p0p11). Pretreatment with IVIG significantly ameliorated ITP in all anti-GPIIbIIIa–injected animals. Conversely, IVIG failed to prevent ITP in all anti-GPIbα–treated mice, except for p0p4. These results were repeated in C57BL/6 mice, and with different IVIG preparations. These data in mice suggest that patients with ITP mediated by anti-GPIbα antibodies may be less responsive to IVIG treatment.
Introduction
Idiopathic thrombocytopenic purpura (ITP) is an autoimmune disease caused by autoantibodies generated against a patients' own platelets, leading to decreased platelet counts and a bleeding diathesis.1-3 Platelet surface glycoproteins IIbIIIa (GPIIbIIIa, αIIbβ3 integrin) and Ibα (GPIbα) are the 2 most common antigenic targets in ITP.4-6 Approximately 70% to 80% of patients have autoantibodies to GPIIbIIIa, 20% to 40% to the GPIb complex, and a significant number of patients simultaneously have antibodies to both or to other glycoproteins (eg, GPIV and Ia/IIa).2,6,7 Reports that anti-GPIbα may cause thrombocytopenia through a different mechanism (Fc-independent pathway) from anti-GPIIbIIIa (Fc-dependent pathway)8 are intriguing, though the mechanism of Fc-independent platelet clearance is unknown. It is not clear whether pathogenesis and therapeutic responses are different for ITP induced by these 2 groups of antibodies.
IVIG is used to treat several autoimmune diseases, including ITP.9-11 However, the mechanism of action of IVIG is not fully understood.12 Although IVIG effectively ameliorates thrombocytopenia in many patients with ITP, a significant number (15%-25%) are refractory to this therapy.13 It is unknown whether refractory cases result from IVIG being less efficacious in ITP caused by certain antibody specificities (ie, anti-GPIbα). To test this hypothesis, we induced thrombocytopenia in a murine model14 using well-characterized rat monoclonal antibodies against either mouse GPIIbIIIa or GPIbα, and tested the ability of IVIG to attenuate ITP induced by these antibodies in this model.
Study design
Animals
BALB/c and C57BL/6 mice, 6 to 8 weeks of age, were purchased from Charles River Canada (Montreal, QC, Canada), and maintained in the local research vivarium.
Platelet preparation and counting
Whole blood (10 μL) was obtained from the saphenous vein and immediately diluted 1:100 in 990 μL of 1% (vol/vol) EDTA/PBS, pH 7.4, then further to a final dilution of 1:12 000 in PBS. Platelets were enumerated in a flow rate–calibrated FACScan flow cytometer (Becton Dickinson, Mountain View, CA) as previously described.14 Reference samples were incubated with fluorescein isothiocyanate (FITC)–conjugated anti-CD61 antibody (BD Pharmingen, Mississauga, ON, Canada) to identify the platelet population. Red blood cells were enumerated as internal controls.14
Induction and treatment of ITP
On day 1 of each experiment, platelet counts were enumerated for each mouse. These initial platelet counts (700 × 109/L-1100 × 109/L) were considered 100% and subsequent counts (until day 5) were normalized to this value. Different doses of IVIG (Gamimune or Gamunex; Bayer, Elkhart, IN, or Gammagard; Baxter, Westlake Village, CA), or human serum albumin (HSA; Bayer, Toronto, ON, Canada), were then injected intraperitoneally into mice.14 Twenty-four hours later (day 2), thrombocytopenia was induced by intravenous injection of antiplatelet antibodies (7 μg/mouse) in 200 μL PBS. IVIG-, HSA-, and PBS-alone controls were performed with each experiment (data not shown).
The rat anti–mouse GPIIbIIIa and GPIbα used have been described previously.8 The isotypes of JON1, JON2, JON3 are IgG2b, IgG2a, and IgG1, and p0p3, p0p4, p0p5, p0p9, p0p11 are IgG2a, IgG2b, IgG1, IgG2a, and IgG2a, respectively. The antibodies p0p3 and p0p4 are able to induce thrombocytopenia in an Fc-independent manner. In contrast, JON1 and JON2 require the Fc-portion of the antibody to induce thrombocytopenia (this information is not available for JON3 and p0p5 due to inability of pepsin to digest rat IgG1).8
Examination of anti-idiotype activity in IVIG
The amount of antibodies, HSA, and IVIG for in vitro experiments was calculated based on the amounts used in vivo. JON and p0p antibodies (0.35 μg/50 μL) were incubated with either HSA or IVIG (2 mg/50 μL) for one hour at room temperature. Wild-type BALB/c platelets were added (1 × 106/50 μL) and incubated for another hour. After washing with PBS, platelets were incubated with FITC-conjugated goat anti–rat IgG (1 μg/100 μL; Caltag Laboratories, Burlingame, CA), and binding of antiplatelet antibodies was detected by flow cytometry. Sheep anti–rat IgG-Fab fragment antiserum (Bethyl Laboratories, Montgomery, TX) was used as a control.
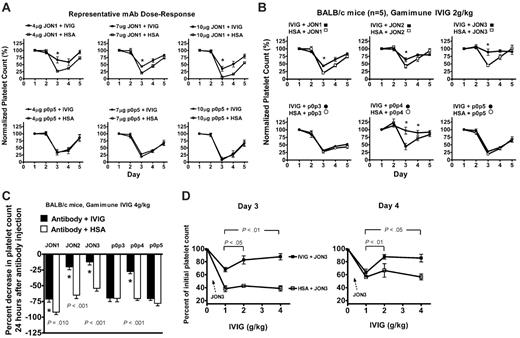
Investigation of dose-dependent responses of monoclonal antibodies and IVIG. (A) Dose-dependent response experiments were performed with each antibody using 4 μg, 7 μg, and 10 μg per mouse, and 2 g/kg IVIG or control HSA. The data of a representative experiment from each group of antibodies (ie, JON1 and p0p5) are shown. The severity of thrombocytopenia increases following the escalating doses of both antibodies. Platelet counts are expressed as a percentage of the basal platelet count (700 × 109/L-1100 × 109/L) on day 1. n = 5; *P < .05. (B) Groups of BALB/c mice were injected with either IVIG (▪, •; 2g/kg) or HSA (□, ○) on day 1 and received 7 μg of either anti-GPIIbIIIa (JON1, JON2, JON3) or anti-GPIbα (p0p3, p0p4, p0p5) monoclonal antibody on day 2. n = 5; *P < .05. (C) BALB/c mice were injected with either IVIG (▪; 4g/kg) or HSA (□) on day 1 and received 7 μg of either anti-GPIIbIIIa (JON1, JON2, JON3) or anti-GPIbα (p0p3, p0p4, p0p5) monoclonal antibody on day 2. Percent decreases in platelet counts 24 hours after antiplatelet antibody injection were compared. n = 5; *P < .05. (D) IVIG-treated mice injected with JON3 mAb showed significant improvement in the attenuation of thrombocytopenia with escalating doses of IVIG (1 g/kg, 2 g/kg, and 4 g/kg). Platelet counts on both day 3 and day 4 at each dose of IVIG are compared with normal platelet counts (100%) on day 2, prior to JON3 injection. n = 5.
Investigation of dose-dependent responses of monoclonal antibodies and IVIG. (A) Dose-dependent response experiments were performed with each antibody using 4 μg, 7 μg, and 10 μg per mouse, and 2 g/kg IVIG or control HSA. The data of a representative experiment from each group of antibodies (ie, JON1 and p0p5) are shown. The severity of thrombocytopenia increases following the escalating doses of both antibodies. Platelet counts are expressed as a percentage of the basal platelet count (700 × 109/L-1100 × 109/L) on day 1. n = 5; *P < .05. (B) Groups of BALB/c mice were injected with either IVIG (▪, •; 2g/kg) or HSA (□, ○) on day 1 and received 7 μg of either anti-GPIIbIIIa (JON1, JON2, JON3) or anti-GPIbα (p0p3, p0p4, p0p5) monoclonal antibody on day 2. n = 5; *P < .05. (C) BALB/c mice were injected with either IVIG (▪; 4g/kg) or HSA (□) on day 1 and received 7 μg of either anti-GPIIbIIIa (JON1, JON2, JON3) or anti-GPIbα (p0p3, p0p4, p0p5) monoclonal antibody on day 2. Percent decreases in platelet counts 24 hours after antiplatelet antibody injection were compared. n = 5; *P < .05. (D) IVIG-treated mice injected with JON3 mAb showed significant improvement in the attenuation of thrombocytopenia with escalating doses of IVIG (1 g/kg, 2 g/kg, and 4 g/kg). Platelet counts on both day 3 and day 4 at each dose of IVIG are compared with normal platelet counts (100%) on day 2, prior to JON3 injection. n = 5.
Statistical analysis
Data are presented as mean plus or minus standard error of the mean (SEM). Statistical significance was assessed by unpaired Student t test.
Results and discussion
We first studied antibody dose-response in this murine ITP model using a broad range of doses of both anti-GPIIbIIIa and anti-GPIbα antibodies. A 7-μg mAb/mouse dose was chosen for subsequent experiments. Representative dose-response curves for JON1 and p0p5 are shown in Figure 1A. IVIG effectively ameliorated thrombocytopenia at all doses of JON1, but not with p0p5.
As shown in Figure 1B-C, Gamimune IVIG significantly ameliorated the decrease in platelet count on day 3 (24 hours after mAb injection) in JON1-, JON2-, and JON3-treated BALB/c mice (P < .05) compared with HSA-treated controls. This is consistent with previous results that thrombocytopenia induced by other anti-GPIIbIIIa mAbs can be rescued by IVIG.14 In contrast, IVIG was completely ineffective at attenuating thrombocytopenia induced by p0p3 and p0p5 antibodies, but was effective with p0p4 (P < .05). In the 2 g/kg IVIG-treated groups, platelet counts were maintained at approximately 90% of normal levels after JON3 injection (Figure 1B). There was enhanced amelioration of the JON3-induced thrombocytopenia with increasing doses of IVIG (P < .05; Figure 1D) on both day 3 and day 4; although this was also seen to a lesser extent with JON2, it was not evident in the other groups. At all doses tested, IVIG was unable to attenuate thrombocytopenia induced by p0p3 and p0p5 (Figure 1B-C). Similar results were observed with 2 g/kg IVIG in C57BL/6 mice (Figure 2A), and with other preparations of IVIG (Gamunex, Figure 2B; Gammagard, data not shown) in BALB/c mice.
Effect of IVIG treatment using different mice, IVIG preparations, and antibodies. (A) C57BL/6 mice were treated with 2 g/kg Gamimune IVIG. Results were similar to those seen with BALB/c mice in Figure 1B-C. n = 3; *P < .05. (B) Results in BALB/c mice pretreated with 2 g/kg of another preparation of IVIG (Gamunex) were also comparable to those treated with Gamimune IVIG in Figure 1B-C. n = 3; *P < .05. (C) Two additional anti-GPIbα monoclonal antibodies were tested in BALB/c mice. Mice were pretreated with 2 g/kg Gamimune IVIG on day 1, and given 7 μg of either p0p9 or p0p11 antibody on day 2. IVIG pretreatment was ineffective at attenuating thrombocytopenia caused by these 2 antibodies; n = 3. (D) No anti-idiotype effect of Gamimune IVIG on these monoclonal antibodies was found. Incubation of IVIG with each mAb did not affect their ability to bind to platelets in vitro. Also, no IVIG was detected bound to platelets, or bound to platelets preincubated with JON or p0p antibody (data not shown); n = 3. In the control group, sheep anti–rat IgG-Fab fragment antiserum significantly decreased the ability of JON1 to bind to platelets. n = 3; *P < .005.
Effect of IVIG treatment using different mice, IVIG preparations, and antibodies. (A) C57BL/6 mice were treated with 2 g/kg Gamimune IVIG. Results were similar to those seen with BALB/c mice in Figure 1B-C. n = 3; *P < .05. (B) Results in BALB/c mice pretreated with 2 g/kg of another preparation of IVIG (Gamunex) were also comparable to those treated with Gamimune IVIG in Figure 1B-C. n = 3; *P < .05. (C) Two additional anti-GPIbα monoclonal antibodies were tested in BALB/c mice. Mice were pretreated with 2 g/kg Gamimune IVIG on day 1, and given 7 μg of either p0p9 or p0p11 antibody on day 2. IVIG pretreatment was ineffective at attenuating thrombocytopenia caused by these 2 antibodies; n = 3. (D) No anti-idiotype effect of Gamimune IVIG on these monoclonal antibodies was found. Incubation of IVIG with each mAb did not affect their ability to bind to platelets in vitro. Also, no IVIG was detected bound to platelets, or bound to platelets preincubated with JON or p0p antibody (data not shown); n = 3. In the control group, sheep anti–rat IgG-Fab fragment antiserum significantly decreased the ability of JON1 to bind to platelets. n = 3; *P < .005.
To further confirm whether thrombocytopenia induced by anti-GPIbα antibodies is less responsive to IVIG, we tested 2 additional mAbs, p0p9 and p0p11. Consistent with p0p3 and p0p5, IVIG failed to attenuate thrombocytopenia induced by either of these antibodies (Figure 2C). Thus, IVIG was effective in only 1 of 5 of the anti-GPIbα mAbs.
To investigate whether anti-idiotype antibodies in IVIG were responsible for the ameliorating effect, we preincubated Gamimune IVIG with p0p3-5 and JON1-3 antibodies, and observed that this did not decrease the binding of mAbs to platelets (Figure 2D). This demonstrates that the ability of IVIG to attenuate thrombocytopenia induced by p0p4 or the JON mAbs is not due to anti-idiotype antibodies present in IVIG. These results were repeated with p0p9 and p0p11, and with Gamunex IVIG (data not shown).
It has been reported that ITP caused by anti-GPIbα antibodies may occur via an Fc-independent pathway.8 Our preliminary experiments also demonstrated that 3 mAbs (p0p3, p0p4, p0p5) were able to induce thrombocytopenia in FcRγ-chain–deficient mice, whereas JON2, which is an Fc-dependent mAb, did not. Since the prevailing view of the mechanism of action of IVIG in ITP is Fc-receptor blockade, it may be expected that IVIG would have little or no effect in ITP caused by Fc-independent anti-GPIbα antibodies. Our results in this study may explain some ITP cases that are refractory to IVIG therapy. This is consistent with several case reports of patients with anti-GPIbα antibodies who were resistant to a variety of treatments.15-19
The ability of IVIG to ameliorate thrombocytopenia induced by p0p4 suggests that Fc-independent mechanism(s) of platelet clearance may not be identical for all anti-GPIbα antibodies. These mechanisms are currently unknown; however, it has been reported that the sera of some patients with ITP activated platelets and enhanced platelet aggregation.20-22 Thus, it is possible that these GPIb-specific antibodies used in our murine model may induce different signaling events in platelets, which could contribute to this heterogeneity.23
IVIG is a costly therapy, and annual increases in IVIG utilization and limitations in the available volume of humansource plasma may cause IVIG shortages. There is also potential for patients to develop serious side effects to IVIG, such as renal failure24 and thrombosis.25 Taking these factors into consideration, excluding IVIG nonresponders from IVIG treatment would be beneficial to the patients' safety (decreasing the possibility of side effects and time required to select another effective therapy to control bleeding), and would decrease unnecessary IVIG consumption.
The results presented here, to our knowledge, are the first to distinguish the therapeutic effects of IVIG between these 2 major pathogenic antibodies (ie, anti-GPIIbIIIa and GPIb complex) in this mouse model of ITP. Further studies in ITP patients with antibodies against either or both of these 2 antigens, or other platelet glycoproteins, could provide insight into the relationship between antibody specificity and the efficacy of IVIG treatment.
Prepublished online as Blood First Edition Paper, April 6, 2006; DOI 10.1182/blood-2005-06-009761.
Supported by grants from the Canadian Blood Services and Canadian Institutes of Health Research (CIHR), and the Bayer Canadian IVIG Steering Committee. E.S. is a fellow of the Keenan foundation at St Michael's Hospital, Toronto, ON, Canada.
M.L.W. and E.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Dr Bernard Chiasson for his valuable comments and support during the experiments.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal