Abstract
Dendritic cells (DCs) that capture apoptotic cells (ACs) in the steady state mediate peripheral tolerance to self-antigens. ACs are recognized by an array of receptors on DCs, the redundancy of which is not completely defined. We made use of an AC surrogate system to address the individual roles of the αvβ5 and complement receptors (CRs) in the phagocytosis and induction of immunity. CR3 and CR4, while substantially less efficient than αvβ5 in internalizing ACs, initiate signals that render DCs tolerogenic. Responding T cells show impaired proliferation and IFNγ production and subsequently die by apoptosis. While tolerogenic DCs are not induced via αvβ5, coligation of CR3 and αvβ5 maintains the DC's tolerogenic profile. This immunomodulatory role, however, is countered by a significant inflammatory stimulus such as bacterial infection. Overall, our data suggest that under steady-state conditions, signaling via CRs predominates to render DCs tolerogenic.
Introduction
In the steady state, dendritic cells (DCs) capture self-antigens and maintain low expression of costimulatory molecules but nevertheless migrate to lymph nodes where they tolerize self-reactive T cells.1-7 Physiologically arising apoptotic cells (ACs) are one significant source of self-antigens for DCs.5,7 However, following their encounter with ACs, DCs can be rendered immunologically inert, immunosuppressive, or immunostimulatory.8-15 These contradictory findings have been difficult to resolve due to the complexity of receptors on DCs and the model systems used (eg, ex vivo versus in vivo or rodent versus human sources of ACs).
ACs are recognized and captured by human DCs via an array of receptors, including LOX-1, CD36, αvβ3, αvβ5, and the complement receptors (CRs) CR3 and CR4.16 The precise contribution of individual receptors in the binding/uptake of ACs, the initiation of downstream signaling pathways, and cross-presentation of cell-associated antigens remains undefined. Blocking antibodies targeting individual receptors inhibit no more than 50% of the association with ACs, and CD36–/– mice have no obvious defects in phagocytosis or in cross-presentation of antigens encoded within ACs, indicating a redundancy in the system and/or the incomplete characterization of AC receptors on DCs.17-20
More recent studies have begun to evaluate the ability of specific AC receptors to modulate DC function. DCs exposed to ACs opsonized with C3bi fragments are inhibited from maturing upon stimulation with LPS or CD40L. This effect is presumably mediated through CR3 and CR4, the receptors for C3bi.1-1,21 Studies of the scavenger receptor CD36 and the αv-integrin receptor CD51 have led to similar conclusions regarding their ability to modulate DC function.12,22 However, it is not known whether such function extends to other members or components of the αv-integrin receptor family, particularly the well-established phagocytic receptor αvβ5.17,23 Nor is it clear whether ligation of AC receptors that interfere with DC maturation consequently alters their T-cell–stimulating potential.
To gain a better understanding of the phagocytic and immunomodulatory role of the various AC receptors on human DCs, we made use of an AC surrogate system24 that permitted us to evaluate the function of individual AC receptors. We focused on αvβ5 and the CRs, especially CR3. We established that these different AC receptors are not equivalent in function, as might be presumed from published studies, but that there is a distinct division of labor, at least with respect to phagocytosis and tolerance induction potential. While αvβ5 mediates efficient phagocytosis, it does not interfere with the DC's capacity to undergo maturation or stimulate T cells. In contrast, engagement of CR3 (or CR4) inhibits the ability of DCs to undergo maturation, produce proinflammatory cytokines or chemokines, and activate T cells. Finally, coligation of these receptors reveals for the first time the predominance of CR3 over αvβ5.
Materials and methods
Culture medium
RPMI 1640 (Cellgro, Herndon, VA) supplemented with 1 mM Hepes (Gibco, Rockville, MD) and 5% pooled human serum (PHS; ValleyBiomedical, Winchester, VA) was used for infection of DCs with Listeria. For monocyte adherence, priming, mixed lymphocyte reaction (MLR), and enzyme-linked immunospot (ELISPOT) assay, media were supplemented with 20 μg/mL gentamicin (Gibco). For HEK293-cell culture, RPMI was supplemented with 10% FCS (Gibco) was used instead of PHS. For generation of DCs, apoptotic cell surrogate (ApoS) uptake, and DC maturation, we used serumfree media X-VIVO 15 (BioWhittaker, Walkersville, MD) supplemented with 100 IU/mL recombinant human (rHu) GM-CSF (Immunex, Seattle, WA) and 300 IU/mL rHu IL4 (R&D Systems, Minneapolis, MN).
DC generation and cell culture
DCs were generated from buffy coats (New York Blood Center, New York, NY) or leukaphereses (BRL, Baltimore, MD) as described previously25 in X-VIVO 15 supplemented with GM-CSF and IL4.
Antibodies, ApoS preparation, and ApoS-DCs coculture
To prepare ApoS conjugates, we used a modification of a published protocol.24 Human red blood cells (RBCs) obtained from buffy coats were biotinylated and conjugated via streptavidin to selected biotinylated antibodies (1 μg/mL final concentration) (Figure 1A). The biotinylated antibodies included CD32 (clone 7.3), CD18 (clone IB4), and CD11c (clone 3.9) from IDLabs (London, ON, Canada), CD11b (clone ICRF44) (Pharmingen, San Diego, CA), irrelevant IgG (BioSource, Camarillo, CA), and αvβ5 (clone P1F6) (Chemicon, Temecula, CA). The same antibodies were used for staining of HEK293 cells followed by staining with streptavidin-PE (Pharmingen). In selected experiments RBCs were dyed with PKH67 (Sigma, St Louis, MO) prior to biotinylation according to the manufacturer's instructions. On day 5 of culture DCs were harvested, incubated with mouse serum–derived IgG (Sigma) to prevent unspecific binding, and exposed to ApoS for 2 hours at 37°C. In some experiments noninternalized ApoS was lysed with ACK buffer (BioWhittaker). DCs were incubated in X-VIVO 15 media with GM-CSF and IL4. To selected groups, MCM-mimic,25 LPS (Sigma; at a final concentration of 100 ng/mL), or LPS and 1000 IU/mL IFNγ (R&D Systems) were added for 40 hours.
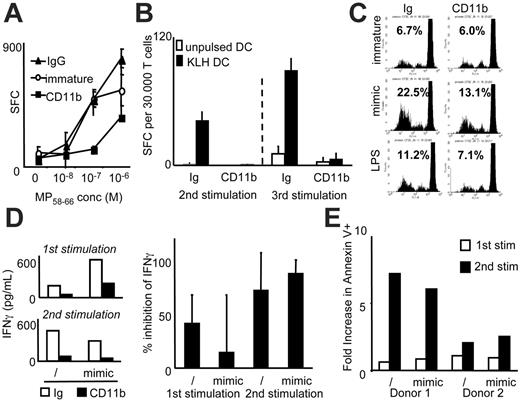
CR3 and CR4 ligation interferes with DC maturation. (A) Schematic representation of ApoS consisting of biotinylated human erythrocytes conjugated via streptavidin to biotinylated antibody targeting a chosen apoptotic cell receptor. (B) DCs were incubated with CD11b-, CD11c-, CD32-, or Ig-ApoS for 2 hours, after which noninternalized ApoS were lysed and MCM-mimic (left) or LPS (right) was added to the cultures. After 40 hours, DCs were analyzed for expression of MHC I, MHC II, CD80, CD86, CD40, and CD83 molecules. Mean fluorescence intensity and increase over immature DCs (gray histograms) is shown for each sample. Culture supernatants of DCs stimulated with LPS with or without IFNγ (1000 IU/mL) for 40 hours were tested in an ELISA assay for the indicated cytokines (C, D) and chemokines (F). Representative experiments of at least 3 are shown (B-D, F). In panel E, the average percent of inhibition of cytokine production by CR3-ligated compared with control DCs (Ig) was calculated (number of averaged experiments is shown above each bar; SD is represented with the error bar). In these experiments the DCs were stimulated with LPS and IFNγ (for detection of IL12) or LPS (the rest of the cytokines).
CR3 and CR4 ligation interferes with DC maturation. (A) Schematic representation of ApoS consisting of biotinylated human erythrocytes conjugated via streptavidin to biotinylated antibody targeting a chosen apoptotic cell receptor. (B) DCs were incubated with CD11b-, CD11c-, CD32-, or Ig-ApoS for 2 hours, after which noninternalized ApoS were lysed and MCM-mimic (left) or LPS (right) was added to the cultures. After 40 hours, DCs were analyzed for expression of MHC I, MHC II, CD80, CD86, CD40, and CD83 molecules. Mean fluorescence intensity and increase over immature DCs (gray histograms) is shown for each sample. Culture supernatants of DCs stimulated with LPS with or without IFNγ (1000 IU/mL) for 40 hours were tested in an ELISA assay for the indicated cytokines (C, D) and chemokines (F). Representative experiments of at least 3 are shown (B-D, F). In panel E, the average percent of inhibition of cytokine production by CR3-ligated compared with control DCs (Ig) was calculated (number of averaged experiments is shown above each bar; SD is represented with the error bar). In these experiments the DCs were stimulated with LPS and IFNγ (for detection of IL12) or LPS (the rest of the cytokines).
Assessment of DC maturation
After the 40-hour culture, DCs were double stained with FITC-conjugated HLA-ABC (PharMingen) and one of the following PE-conjugated Abs: αHLA-DR, αCD80, αCD83, αCD86, αCD40 (Pharmingen), or α CCR7 (R&D Systems). DCs were gated by forward scatter/side scatter (FSC/SSC) and expression of HLA-ABC and analyzed for the expression of the maturation markers on a FACSCalibur (BD, San Diego, CA).
ELISA
Supernatants from DC cultures were collected and tested for presence of the following cytokines: IL1β, IL6, IL10, TGFβ (R&D Systems), IL12p70, and TNFα (BD); or chemokines IP10, MCP3, and MCP2 (R&D Systems) by enzyme-linked immunosorbent assay (ELISA). IFNγ (R&D Systems) was similarly measured in supernatants of T-cell–DC cocultures 6 days after the primary or secondary stimulation of T cells with DCs.
Electron microscopy
After incubation with ApoS the DC pellet was fixed with glutaraldehyde fixation buffer. Ultrathin sections were prepared (Reichert Ultracut E ultramicrotome; Leica, Wetzlar, Germany), cells were stained with uranyl acetate and lead citrate (Electron Microscopy Sciences, Fort Washington, PA), embedded in EPON (Ted Pella, Redding, CA) and photographs were taken (Kodak electron microscopy film; Kodak, Rochester, NY) after electron microscopy (EM) analysis (Philips CM 10 transmission electron microscope at 80 kV; FEI, Mahwah, NJ).
Endocytic capacity
DCs were preexposed to ApoS and cultured a further 40 hours with or without maturation stimuli. Subsequently, FITC-labeled dextran beads were added. After 1.5 hours, DCs were extensively washed and analyzed by flow cytometry for association with the dextran beads. DCs were gated by FSC/SSC and expression of HLA-DR–PE. To monitor the interaction of DCs with different ApoS, RBCs were labeled with PKH67. DCs and ApoS were cocultured for 2 hours at 37°C or 4°C. DCs were then extensively washed and analyzed for association with PKH67-labeled ApoS by fluorescence-activated cell sorting (FACS).
KLH priming and allogeneic MLR
DCs were preincubated with ApoS and cultured with 10 μg/mL KLH protein and MCM-mimic.25 After 40 hours DCs were harvested, irradiated, and incubated with naive CD4+ T cells obtained by negative magnetic selection with CD8, CD19, CD14, CD56, and CD45RO microbeads (Miltenyi Biotec, Auburn, CA). On days 10 and 20, frozen aliquots of ApoS-treated DCs were thawed, irradiated, and used to restimulate T-cell cultures. On days 20 and 30, the generated T cells were stimulated with mature DCs pulsed or unpulsed with KLH. Cytokine production was determined in an IFNγ, IL4, or IL10 ELISPOT assay. Naive allogeneic T cells (a proportion of which were labeled with CFSE) were cocultured at a 1:30 ratio with ApoS-treated DCs that were cultured with or without maturation stimuli. After 6 days of culture, labeled T cells were tested for proliferation. Remaining nonlabeled T cells were washed, stained with CFSE, and restimulated with allogeneic ApoS-treated DCs for another 6 days in the presence of 150 IU/mL human rIL2 (R&D Systems). In some experiments T cells were stained at the indicated days with annexin V (FITC; Pharmingen) to determine the extent of cell death.
ELISPOT assay
ELISPOT assay was performed as previously described26 (antibodies from Mabtech, Nacka Strand, Sweden; plates from Millipore, Bedford, MA). DCs were fixed with glutaraldehyde before incubation with a matrix protein (MP)–specific CD8 T-cell clone27 or seeded into plates when cocultured with KLH-specific CD4 T cells. Spots were counted using an automated ELISPOT reader system (Aid, Strassberg, Germany).
Infection with Listeria monocytogenes
Listeria monocytogenes strain DP-L4056 was kindly provided by Cerus (Concord, CA) and stored at –80°C until use. DCs were infected at MOI 10 in the absence of antibiotics for 1 hour. They were then washed and recultured in the presence of gentamicin for 40 hours.
Statistics
The statistical analyses were performed in the Excel program. Average values and SD were calculated. Where indicated, values were compared using paired Student t test. Inhibition index was calculated as (1 – CD11b/IgG) × 100.
Further discussion of materials and methods is provided in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results
Complement receptor ligation interferes with DC maturation
It was previously shown that C3bi-opsonized ACs inhibit human DC maturation when the latter are subsequently exposed to LPS21 ; however, cooperation with other receptors could not be definitively excluded. We recapitulated the contribution of CR3 and CR4 to the impairment of DC maturation by using an AC surrogate (ApoS) system that can stimulate one receptor at a time. ApoS consist of biotinylated human erythrocytes that are coupled through streptavidin to a biotinylated antireceptor antibody (Figure 1A). DCs and macrophages are efficient phagocytes that can internalize large particles, including whole cells. This characteristic made possible the successful usage of ApoS to analyze the contribution of individual macrophage AC receptors.24
We used the ratio of 20:1 of ApoS to immature human DCs (iDCs), which permitted us to adequately monitor ApoS binding, phagocytosis, and immunomodulatory effects on DCs. ApoS was conjugated to antibodies targeting CD11b (the α chain of the CR3) or an isotype control IgG. To construct CD11b-ApoS we chose antibody clone ICFR44 (formerly 44) that targets the I domain of CD11b molecule, the same site that binds C3bi.28 Upon binding to monocytes these antibodies activate NFκB and transcription of selected chemokines in a manner similar to the natural ligands of CR3.29
Exposure of iDCs to CD11b-ApoS or Ig-ApoS did not alter their phenotype or induce the expression of DC maturation markers over 48 hours (not shown). When DCs were exposed to conventional maturation signals such as a combination of IL1β, TNFα, IL6, and PGE2 (a mimic of monocyte-conditioned medium [MCM-mimic]) or LPS (Figure 1B) following preexposure to ApoS, their ability to up-regulate CD83, CD40, major histocompatibility complex (MHC) I and MHC II molecules, and costimulatory molecules was significantly impaired in response to CD11b-ApoS. A similar arrest was observed when we preexposed DCs to CD11c-ApoS (Figure 1B; CD11c refers to the α chain of CR4) or CD18-ApoS (targeting the β chain shared by CR3 and CR4; not shown). In contrast, control Fc receptor targeting CD32-ApoS or irrelevant Ig-conjugated ApoS did not alter the response of iDCs. CD11b-ApoS–exposed DCs retained better ability to phagocytose dextran beads (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) compared with controls, further supporting a maturation-inhibitory role of CR3. However expression of CCR7, a chemokine receptor that enables homing to lymph nodes, was not affected by exposure to CD11b-ApoS and maturation stimuli. Moreover, ligation of CR3 in the absence of maturation stimuli was sufficient to induce expression of CCR7 and migration to its cognate ligand, albeit at lower levels than with the maturation stimuli (Document S1 and Figure S2).
These results specifically identify CR3 and CR4 as receptors involved in the inhibition of DC maturation, confirm studies suggesting their involvement in mediating the immunosuppressive effects of ACs,21 and verify the use of the ApoS system to examine the consequences of ligating specific AC receptors. Importantly, this effect is unlikely to be due to siglec receptors (eg, CD169), which may bind RBCs and down-modulate DC function.30 This receptor is significantly expressed only 1 to 2 days after stimulation of monocyte-derived cells,31 by far exceeding the 2-hour presence of RBCs in our system. The specificity of the ApoS system is further confirmed by using ApoS conjugated to control antibodies, by comparable results obtained using natural ligands of CR3,21 as well as our preliminary experiments using biotinylated latex beads in place of RBCs (not shown).
CR3 ligation of DCs triggers production of TGFβ and alters secretion of proinflammatory cytokines and chemokines upon stimulation with LPS or MCM-mimic
Previous studies showed that DCs cocultured with ACs opsonized with complement fragments down-regulate IL12 production in response to LPS.11 We therefore tested whether specific CR3 ligation alters the inflammatory profile of DCs by examining secretion of various cytokines and chemokines. Ligation of CR3 itself did not induce secretion of proinflammatory cytokines (Figure 1C-D). Upon exposure to LPS, the production of IL1β, IL6, and TNFα was significantly impaired when DCs were pretreated with CD11b-ApoS but not control surrogates (Figure 1C).
IL12 is an immunostimulatory cytokine that promotes the development of T-helper 1 (Th1) responses.32 TGFβ, on the other hand, is an immune-regulating cytokine that suppresses effector T cells and is associated with induction of regulatory T cells.33 Biologically active IL12 (p70) production was impaired by CD11b-ApoS–ligated and LPS–stimulated DCs. The addition of IFNγ enhanced IL12 production in CD11b-ApoS–targeted DCs but to less than 50% of levels induced by control-ApoS. Significantly, the inhibition of IL12 production was coupled to the induction of TGFβ1, an effect not observed with either Ig-ApoS or CD32-ApoS (Figure 1D). CR3-dependent production of IL10, another T-cell–regulatory cytokine, was not detected at either protein or RNA levels (not shown).
DCs secrete numerous chemokines to recruit and engage other cell types such as natural killer (NK) cells, NK T cells (NKT cells), or memory Th1 or Th2 cells. These in turn influence DC maturation (eg, via CD40 ligation) and consequently the T-cell stimulatory capability.34 Interestingly, ligation of CD11b decreased production of IP10, MCP2, and MCP3 (Figure 1F). Because these chemokines recruit cells participating in development of delayed-type hypersensitivity (DTH) and Th1 responses, their reduction may contribute to reduced inflammation in the local environment. Indeed, antigen-pulsed, CR3-ligated antigen-presenting cells (APCs) injected into footpads of mice are diminished in their ability to elicit potent DTH responses.35
CR3 ligation on DCs interferes with activation of memory T cells and priming of naive CD4+ T cells
It is not known how the negative impact of CR3 ligation on DC maturation influences the T-cell stimulatory capacity of DCs. Therefore, we evaluated the ability of DCs to activate T cells following CR3 engagement. In these studies we chose to preferentially study CR3 versus CR4, because it consistently mediates greater inhibitory effects on DC maturation in several parameters tested (Figures 1B and S3).
DCs preexposed to CD11b-ApoS were treated with MCM-mimic and loaded with influenza HLA-A2.1–restricted MP peptide. They were subsequently fixed with glutaraldehyde and cocultured with an MP-specific CD8+ T-cell clone.27 CD11b-ApoS–treated DCs were impaired in their ability to stimulate the MP58-66-specific CD8+ T-cell clone (Figure 2A). In fact, these DCs induced even lower responses than immature DCs. These data are consistent with reduced levels of MHC class I and costimulatory molecules on CD11b-ApoS–treated DCs (Figure 1B).
We next studied the impact of CR3 ligation on DCs cocultured with allogeneic naive T cells. CD45RO–, CD4+ T cells were isolated, stained with CFSE, and mixed with allogeneic CD11b-ApoS– or Ig-ApoS–exposed DCs. The DCs were either pretreated with maturation stimuli or cultured in media alone. After 6 days, proliferation of T cells was measured by CFSE dilution. T cells stimulated by CD11b-ApoS DCs showed reduced proliferation compared with their control Ig-ApoS–exposed counterparts (Figure 2C). Furthermore, there was a profound inhibition of the induction of Th1 cells compared with the control group that was most apparent after 2 rounds of stimulation (Figure 2D). Th2 cytokines IL5, IL13, and IL4 were not detected in either group (data not shown). The loss of IFNγ-producing cells could be attributed to the reduced viability of responding T cells that underwent apoptosis as measured by the binding of annexin V to exposed phosphatidylserine (PS) (Figure 2E). After a second stimulation with immature DCs, about 20% of T cells (range, 12.1% to 30.9%; n = 3) expressed PS on the outer surface of membranes when treated with CR3-ligated DCs as opposed to 5.3% (range, 4.3% to 5.9%; n = 3) in control groups.
Because the allogeneic MLR represents the response of T cells to several antigens, we sought a better-defined antigen-specific system to test the functional capacity of CR3-ligated DCs. CD11b-ApoS– or Ig-ApoS–preexposed DCs were cultured with keyhole limpet hemocyanin (KLH) protein in the presence of MCM-mimic and then with naive CD4+ T cells. Mature DCs efficiently process and present KLH protein to induce a Th1 response.25 Ten days after a second and third stimulation with CD11b-ApoS– or Ig-ApoS–treated DCs, the expanding T cells were restimulated with fully mature KLH-loaded DCs. While antigen-specific responses increased in the control group, coincident with the number of restimulations, few IFNγ–producing T cells (and no IL4- or IL10-producing cells) were found in groups stimulated with CD11b-ApoS–treated DCs (Figure 2B). The decrease in the number of IFNγ responders could not be attributed to a defect of antigen presentation by KLH-pulsed, CD11b-ApoS–treated DCs, because they were able to activate CD4+-, Th1-, KLH-specific T-cell lines (62 spot-forming cells per 20 000 T cells seeded versus 48 in the control Ig-ApoS–stimulated group). Therefore, despite the ability of CR3-ligated DCs to process and present exogenous antigens, they are nevertheless impaired in their capacity to activate and prime resting T cells. Failure to differentiate into effector cells and cell death was observed instead. T cells did not express FoxP3 or produce IL10, indicating that typical T regulatory cells were not generated in these cultures (not shown).
CR3 ligation impairs T-cell priming capacity of DCs. DCs were treated with the indicated ApoS. (A) DCs were further cultured in media alone (○) or exposed to MCM-mimic (▴, ▪). After 40 hours, DCs were loaded with titrated concentrations of MP peptide, washed, fixed, and added to an MP-specific CD8+ T-cell clone. The number of IFNγ spot-forming cells (SFCs) detected by ELISPOT assay is shown. (B) KLH and MCM-mimic were added to DC cultures after treatment with the indicated ApoS. After 40 hours, DCs were washed, irradiated, and added to naive CD4+ T cells. At 10-day intervals, T cells were restimulated with the same DC subsets for a second and third time. Ten days after each restimulation (ie, days 20 and 30, respectively), T cells were added to KLH-pulsed, MCM-mimic–matured DCs, and IFNγ production was measured by ELISPOT assay. Number of responding cells per 30 000 viable seeded T cells is shown. In panels A and B, error bars represent standard deviation (SD) of triplicate wells in ELISPOT plate. (C) After exposure to ApoS, DCs were stimulated for 40 hours with MCM-mimic or LPS and then cultured with allogeneic CFSE-labeled naive CD4+T cells. Proliferation of T cells was measured after 6 days by dilution of CFSE dye by FACS. Numbers denote percent of proliferating cells. (D) In similar cultures as in panel C, supernatants were collected 6 days after the first or second stimulation with immature (/) or mimic-exposed DCs (mimic), and IFNγ content was measured by ELISA. The left panel shows the results of an individual experiment and the right the average inhibition with SD of multiple experiments. The production of IFNγ by control (Ig-ApoS–ligated) DCs was considered the 100% value, and percent inhibition in the CD11b-ligated DC groups (with or without mimic) was calculated. (E) Similar cultures as in panel D were monitored for the binding of annexin V to T cells by FACS. The fold increase over the control Ig-ApoS group is shown. Representative experiments from at least 2 or 3 donors are shown in all panels.
CR3 ligation impairs T-cell priming capacity of DCs. DCs were treated with the indicated ApoS. (A) DCs were further cultured in media alone (○) or exposed to MCM-mimic (▴, ▪). After 40 hours, DCs were loaded with titrated concentrations of MP peptide, washed, fixed, and added to an MP-specific CD8+ T-cell clone. The number of IFNγ spot-forming cells (SFCs) detected by ELISPOT assay is shown. (B) KLH and MCM-mimic were added to DC cultures after treatment with the indicated ApoS. After 40 hours, DCs were washed, irradiated, and added to naive CD4+ T cells. At 10-day intervals, T cells were restimulated with the same DC subsets for a second and third time. Ten days after each restimulation (ie, days 20 and 30, respectively), T cells were added to KLH-pulsed, MCM-mimic–matured DCs, and IFNγ production was measured by ELISPOT assay. Number of responding cells per 30 000 viable seeded T cells is shown. In panels A and B, error bars represent standard deviation (SD) of triplicate wells in ELISPOT plate. (C) After exposure to ApoS, DCs were stimulated for 40 hours with MCM-mimic or LPS and then cultured with allogeneic CFSE-labeled naive CD4+T cells. Proliferation of T cells was measured after 6 days by dilution of CFSE dye by FACS. Numbers denote percent of proliferating cells. (D) In similar cultures as in panel C, supernatants were collected 6 days after the first or second stimulation with immature (/) or mimic-exposed DCs (mimic), and IFNγ content was measured by ELISA. The left panel shows the results of an individual experiment and the right the average inhibition with SD of multiple experiments. The production of IFNγ by control (Ig-ApoS–ligated) DCs was considered the 100% value, and percent inhibition in the CD11b-ligated DC groups (with or without mimic) was calculated. (E) Similar cultures as in panel D were monitored for the binding of annexin V to T cells by FACS. The fold increase over the control Ig-ApoS group is shown. Representative experiments from at least 2 or 3 donors are shown in all panels.
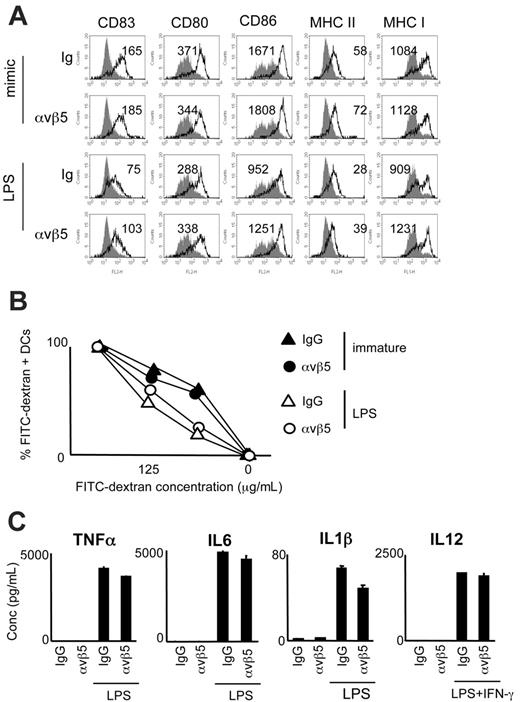
Ligation of αvβ5 does not interfere with DC maturation
A major role for αvβ5 has been implied in the cross-presentation of antigens within ACs by DCs,17 while other studies have indicated that ligating αv integrins with monoclonal antibodies inhibits DC immunostimulatory function.22 However, using the surrogate AC system, we found that exposure to αvβ5-ApoS did not significantly interfere with the DC's ability to mature in response to stimulation with either LPS or MCM-mimic (Figure 3A). Furthermore, αvβ5-ApoS–pretreated DCs did not differ from control (Ig-ApoS) DCs in their ability to internalize dextran beads (Figure 3B). Finally, ligation of αvβ5 and stimulation with LPS or with LPS and IFNγ did not affect TNFα, IL6, IL1β, or IL12 production to the same extent as ligation of CD11b (Figure 3C). Therefore, we show here for the first time that unlike CR3 ligation, αvβ5 engagement does not overtly alter the capacity of DCs to respond to an inflammatory stimulus.
Impact of αvβ5 ligation on DC maturation. DCs were incubated with αvβ5- or Ig-ApoS, after which noninternalized Apo-S was lysed and MCM-mimic, LPS, or LPS and IFNγ were added to the cultures for 40 hours. (A) Expression of MHC I, MHC II, CD80, CD86, and CD83 molecules on DCs. Mean fluorescence intensity and increase over immature DCs (gray histograms) are shown for each sample. (B) Following preexposure to ApoS, DCs were exposed to LPS for 40 hours or left immature and then cocultured for 1.5 hours with increasing concentrations of FITC-dextran beads. Uptake of beads by αvβ5-ApoS–exposed versus control DCs was measured by FACS. Percentage of dextran-FITC–positive, MHC I (PE)–gated DCs is shown for immature (•, ▴) versus LPS-matured DCs (○, ▵). (C) Culture supernatants were collected and analyzed for the presence of IL12, TNFα, IL1β, and IL6. Error bars represent SD of duplicate wells in ELISA plate. Representative experiments of at least 3 performed are shown.
Impact of αvβ5 ligation on DC maturation. DCs were incubated with αvβ5- or Ig-ApoS, after which noninternalized Apo-S was lysed and MCM-mimic, LPS, or LPS and IFNγ were added to the cultures for 40 hours. (A) Expression of MHC I, MHC II, CD80, CD86, and CD83 molecules on DCs. Mean fluorescence intensity and increase over immature DCs (gray histograms) are shown for each sample. (B) Following preexposure to ApoS, DCs were exposed to LPS for 40 hours or left immature and then cocultured for 1.5 hours with increasing concentrations of FITC-dextran beads. Uptake of beads by αvβ5-ApoS–exposed versus control DCs was measured by FACS. Percentage of dextran-FITC–positive, MHC I (PE)–gated DCs is shown for immature (•, ▴) versus LPS-matured DCs (○, ▵). (C) Culture supernatants were collected and analyzed for the presence of IL12, TNFα, IL1β, and IL6. Error bars represent SD of duplicate wells in ELISA plate. Representative experiments of at least 3 performed are shown.
Hybrid apoptotic-cell surrogates specific for αvβ5 and CR3 retain a regulatory effect on DC maturation
The redundancy of AC receptors has been widely touted in the literature, but our data suggest that at least 2 of the receptors diverge in their overall effects when they encounter ACs. To gain a better understanding of the effects incurred on DCs when more than one receptor is ligated, we constructed “hybrid” AC surrogates that target 2 receptors at a time, namely CD11b and αvβ5. Control experiments were undertaken to assure that hybrid ApoS can bind individual receptors to a similar extent as the “single” surrogates. We took advantage of HEK293 cells that express αvβ5 but not CD11b (Figure 4A, first 3 panels) and showed that hybrid αvβ5/Ig or αvβ5/CD11b-ApoS bound to HEK293 cells to a similar extent as did αvβ5-ApoS (Figure 4A, fourth panel).
In functional assays the hybrid αvβ5/CD11b-ApoS behaved similarly to the CD11b-ApoS (ie, inhibiting the expression of DC maturation markers [Figure 4B] and significantly impairing the production of the proinflammatory cytokines [Figure 4C and Table 1]). Similarly, αvβ5/CD11b-ApoS impaired the priming of Th1 T cells. Inhibition of T-cell activation by the hybrid αvβ5/CD11b-ApoS–treated DCs was similar to that seen with CD11b-ApoS–treated DCs. We observed a small decrease in the numbers of proliferating T cells in coculture with αvβ5-ApoS–treated, MCM-mimic–stimulated DCs, but the impairment was significantly less than seen in other groups (Figure 4D). The differences between cocultures of T cells with Ig-, CD11b-, αvβ5/CD11b-, or αvβ5-targeted DCs were less pronounced when DCs were exposed to maturation stimuli, although the inhibition mediated by CR3 ligation was still evident (Figure 4D). Thus, CR3 is a major modulator of DC function following exposure to ACs. Significantly, it negatively governs the APC's effector function even in the presence of exceptionally phagocytic AC receptor that by itself does not exert tolerogenic effects.
Inhibition of proinflammatory cytokines by ligation of AC receptors
. | CD11b . | Avβ5 . | CD11b and αvβ5 . |
|---|---|---|---|
| TNF-α | 42.7 ± 25.9 (11) | 8.9 ± 5.5 (9) | 39.2 ± 27.3 (6) |
| IL-6 | 44.9 ± 27.1 (12) | 18.1 ± 13.1 (5) | 41.4 ± 36.1 (3) |
| IL-1β | 61.1 ± 21.6 (9) | 16.6 ± 10.5 (3) | 51.5 ± 16.9 (3) |
. | CD11b . | Avβ5 . | CD11b and αvβ5 . |
|---|---|---|---|
| TNF-α | 42.7 ± 25.9 (11) | 8.9 ± 5.5 (9) | 39.2 ± 27.3 (6) |
| IL-6 | 44.9 ± 27.1 (12) | 18.1 ± 13.1 (5) | 41.4 ± 36.1 (3) |
| IL-1β | 61.1 ± 21.6 (9) | 16.6 ± 10.5 (3) | 51.5 ± 16.9 (3) |
Percent inhibition in cytokine production by CD11b-, αvβ5-, or αvβ5/CD11b-ApoS and LPS stimulated, compared to control (Ig), DCs, was calculated for all donors tested. Data are expressed as the average ± SD, with the number of averaged values shown in parentheses.
Evaluation of DCs that have been ligated by hybrid ApoS targeting both CR3 and αvβ5. (A) To evaluate the ability of hybrid αvβ5/CD11b-ApoS to bind individual receptors to the same extent as “single” ApoS, we cocultured HEK293 cells that express αvβ5, but not CD11b (as assessed by FACS staining [left]), with single or hybrid ApoS for 2 hour at 4°C at different HEK293/ApoS ratios. Importantly, the hybrid αvβ5/CD11b-ApoS bound HEK293 cells to same extent as the “single” αvβ5-ApoS (right). (B,C) DCs were exposed to either αvβ5-, CD11b-, Ig-, or αvβ5/CD11b-ApoS for 2 hours and then cultured either in media alone or in the presence of LPS for 40 hours. (B) Maturation of DCs was assessed by determining the expression of CD83, CD80, and CD86 by FACS. (C) Supernatants of DC cultures were analyzed by ELISA for the presence of TNFα, IL1β, and IL6. A representative experiment is shown. Error bars represent SD of duplicate test wells. (D) ApoS-pretreated DCs were cocultured with naive CFSE-labeled CD4+ T cells at a 1:30 ratio. After 6 days the proliferation of T cells was assessed by analyzing the dilution of CFSE dye in T-cell membranes by FACS. (Left) Immature ApoS-exposed DCs. (Right) MCM-mimic–treated ApoS-exposed DCs. Averages and SDs of 3 experiments are shown. Stars represent P < .05 in a paired Student t test.
Evaluation of DCs that have been ligated by hybrid ApoS targeting both CR3 and αvβ5. (A) To evaluate the ability of hybrid αvβ5/CD11b-ApoS to bind individual receptors to the same extent as “single” ApoS, we cocultured HEK293 cells that express αvβ5, but not CD11b (as assessed by FACS staining [left]), with single or hybrid ApoS for 2 hour at 4°C at different HEK293/ApoS ratios. Importantly, the hybrid αvβ5/CD11b-ApoS bound HEK293 cells to same extent as the “single” αvβ5-ApoS (right). (B,C) DCs were exposed to either αvβ5-, CD11b-, Ig-, or αvβ5/CD11b-ApoS for 2 hours and then cultured either in media alone or in the presence of LPS for 40 hours. (B) Maturation of DCs was assessed by determining the expression of CD83, CD80, and CD86 by FACS. (C) Supernatants of DC cultures were analyzed by ELISA for the presence of TNFα, IL1β, and IL6. A representative experiment is shown. Error bars represent SD of duplicate test wells. (D) ApoS-pretreated DCs were cocultured with naive CFSE-labeled CD4+ T cells at a 1:30 ratio. After 6 days the proliferation of T cells was assessed by analyzing the dilution of CFSE dye in T-cell membranes by FACS. (Left) Immature ApoS-exposed DCs. (Right) MCM-mimic–treated ApoS-exposed DCs. Averages and SDs of 3 experiments are shown. Stars represent P < .05 in a paired Student t test.
Phagocytosis via CR3 and αvβ5
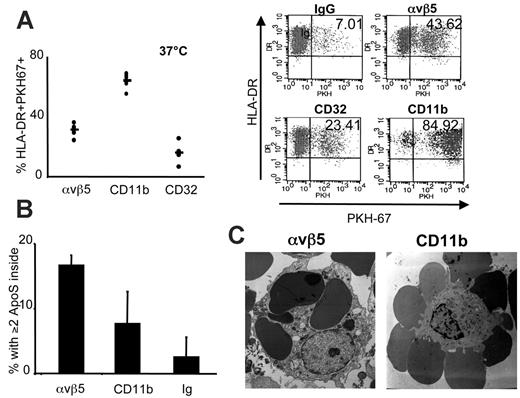
The iDCs constitutively acquire antigens through a variety of mechanisms (eg, receptor-mediated endocytosis and phagocytosis). Ligation of CR3 rendered DCs poorly able to prime T cells, but these DCs could still acquire antigens independently of CR as shown by their capacity to internalize dextran particles and to present KLH to T-cell lines. To understand if CR3 ligation affects antigen uptake similarly to αvβ5, a receptor that is known to mediate the efficient internalization of bound ACs,17 we studied the interaction of CD11b-ApoS with CR3 on DCs. We compared the behavior of PKH 67-stained CD11b-ApoS with control IgG-ApoS and with αvβ5-ApoS (antibody that binds the same epitope of αvβ5 as the ACs was used to prepare ApoS) by flow cytometry. CD11b-ApoS bound to a vast number of DCs via CR3 (more than 64%) while, on average, 31.08% of DCs associated with αvβ5-ApoS and 16.03% with CD32-ApoS (Figure 5A).
Flow cytometry does not permit discrimination between opsonized and internalized ApoS. Therefore, we used electron microscopy to investigate whether CR3 can signal the internalization of CD11b-ApoS (Figure 5B). Of an average of 64% of DCs that interacted with CD11b-ApoS, only 7.67% also ingested 2 or more of the docked ApoS after a 2-hour coculture (corresponding to less than 10% of initially docked surrogates after subtraction of the background). In contrast, αvβ5 was a much more efficient uptake receptor, leading to the internalization of about 50% of the docked ApoS. Similar data were obtained by confocal microscopy (Figure S4) where αvβ5-ApoSs or CD32-ApoSs were localized to the interior of DCs, while CD11b-ApoSs were observed preferentially at the surface. This confirms αvβ5 to be a highly efficient and rapid phagocytic receptor17 while uptake via CR3, at least in the absence of a second signal, does not result in swift uptake.36-38 These results further indicate that among AC receptors on human DCs, αvβ5 and CR3 have distinct biologic effects.
Internalization of ApoS by CR3 and αvβ5. (A) ApoS were stained with PKH67 and cocultured with DCs at 37°C for 2 hours. DCs were gated by FSC/SSC and expression of MHC II and analyzed for association with PKH-labeled ApoS. Each dot represents the value for DCs of a healthy donor after the background value (association of DCs with IgG-ApoS) was subtracted (left), and the line represents the averaged value of all donors. A representative dot plot is shown on the right. (B) DCs were cocultured with CD11b-, αvβ5-, or control ApoS for 2 hours and then fixed for preparation of EM slides. The data represent an average from 3 donors tested; error bars represent SD. For each donor, DCs that contained 2 or more ApoS were counted in at least 5 EM slides at low magnification (magnification × enlargement factor was 4500 for αvβ5 and 2100 for CD11b). (C) EM photos are shown of DCs interacting with αvβ5-ApoS (left) or CD11b-ApoS (right).
Internalization of ApoS by CR3 and αvβ5. (A) ApoS were stained with PKH67 and cocultured with DCs at 37°C for 2 hours. DCs were gated by FSC/SSC and expression of MHC II and analyzed for association with PKH-labeled ApoS. Each dot represents the value for DCs of a healthy donor after the background value (association of DCs with IgG-ApoS) was subtracted (left), and the line represents the averaged value of all donors. A representative dot plot is shown on the right. (B) DCs were cocultured with CD11b-, αvβ5-, or control ApoS for 2 hours and then fixed for preparation of EM slides. The data represent an average from 3 donors tested; error bars represent SD. For each donor, DCs that contained 2 or more ApoS were counted in at least 5 EM slides at low magnification (magnification × enlargement factor was 4500 for αvβ5 and 2100 for CD11b). (C) EM photos are shown of DCs interacting with αvβ5-ApoS (left) or CD11b-ApoS (right).
Infection with L monocytogenes overturns the inhibitory impact of CR3. DCs preexposed to CD11b- or control Ig-ApoS for 2 hours were incubated in the presence of L monocytogenes, MCM-mimic, or media alone for the remaining 40-hour culture. (A) Maturation of DCs (CD80, MHC I) was assessed by flow cytometry. Listeria-infected DCs were compared with those exposed to MCM-mimic. Inhibition was calculated as the percentage decrease in mean fluorescence in CD11b-ApoS–exposed DCs compared with control, Ig-ApoS–exposed DCs. The left panel shows the average of 6 experiments with P values (paired Student t test) and the right panel a representative experiment. (B) DCs preincubated with CD11b- or control Ig-ApoS were infected with Listeria or exposed to MCM-mimic. After 24 hours, DCs were washed and cocultured with naive allogeneic CD4+ T cells for 6 days. Proliferation of T cells was assessed by determining dilution of CFSE dye in T-cell membranes, and amount of secreted IFNγ was determined by ELISA assay. The percentages above each column denote the difference in value of control Ig-ApoS over CD11b-ApoS. Results from 2 experiments are shown. Error bars represent SD of duplicate test wells.
Infection with L monocytogenes overturns the inhibitory impact of CR3. DCs preexposed to CD11b- or control Ig-ApoS for 2 hours were incubated in the presence of L monocytogenes, MCM-mimic, or media alone for the remaining 40-hour culture. (A) Maturation of DCs (CD80, MHC I) was assessed by flow cytometry. Listeria-infected DCs were compared with those exposed to MCM-mimic. Inhibition was calculated as the percentage decrease in mean fluorescence in CD11b-ApoS–exposed DCs compared with control, Ig-ApoS–exposed DCs. The left panel shows the average of 6 experiments with P values (paired Student t test) and the right panel a representative experiment. (B) DCs preincubated with CD11b- or control Ig-ApoS were infected with Listeria or exposed to MCM-mimic. After 24 hours, DCs were washed and cocultured with naive allogeneic CD4+ T cells for 6 days. Proliferation of T cells was assessed by determining dilution of CFSE dye in T-cell membranes, and amount of secreted IFNγ was determined by ELISA assay. The percentages above each column denote the difference in value of control Ig-ApoS over CD11b-ApoS. Results from 2 experiments are shown. Error bars represent SD of duplicate test wells.
Simultaneous exposure of DCs to L monocytogenes prevents CR3-mediated inhibition
In the steady state, ACs likely induce tolerance to encoded self-antigens by the mechanism of cross-tolerance. However, ACs also arise in the setting of infection, as a result of pathogen-induced apoptosis. In such situations, cross-priming to the pathogen-encoded antigens appears to remain intact39-41 although the basis for this is largely unexplained. Because inflammatory stimuli such as LPS or a cocktail of inflammatory cytokines were unable to reverse the arrest of DC maturation elicited by CR3 ligation, we tested whether simultaneous infection with L monocytogenes (a potent activator of DCs)42 and ligation of CR3 would have a similar effect. In contrast to LPS or MCM-mimic, infection substantially overturned the inhibitory signals mediated through CR3. Inhibition via CR3 was reduced from 32.5% to 18% for expression of CD80 and from 38.2% to 10.8% for MHC I (Figure 6A). Moreover, DCs that were infected with Listeria were significantly (P = .043; 3 donors) superior to MCM-mimic–matured DCs in stimulating proliferation of naive allogeneic T cells and in inducing IFNγ production (Figure 6B). Therefore, in the presence of a significant infection or inflammatory stimulus, the inhibitory signals relayed by CRs can be overturned to a substantial degree.
Discussion
Under certain circumstances, the injection of ACs, or of DCs that have ingested them, can induce tolerance to antigens expressed within the dead cells.4,43 Previous studies have implied an immunosuppressive role of CRs, CD36, and the αv-integrin receptors in mediating this effect.11,12,21,35 In most cases, however, the observations were made using whole ACs (opsonized with complement fragments) or ligating DCs with unconjugated antireceptor antibodies (CD36, CD51). Because several of these receptors participate in signaling partnerships,38,44-46 the individual contribution of each in inducing tolerogenic signals has been difficult to decode. AC-based mechanisms of tolerance can be better evaluated using an AC surrogate system that permits the controlled targeting of specific AC receptors. Using this approach, we show for the first time that integrin AC receptors differ in their biologic roles. While αvβ5 individually induces the efficient phagocytosis of ApoS without adversely affecting DC function, the CRs are poor in phagocytosis and in contrast convey distinct negative immunomodulatory effects that dominate the signals imparted by αvβ5. These effects finally result in impaired Th1 priming and induction of deletional tolerance in vitro.
DCs that are involved in tolerance induction typically have low expression of maturation markers.47-49 Consistent with this, CR3-ApoS or CR4-ApoS do not alter the expression of MHC or costimulatory molecules on human iDCs. In fact, DCs are refractory to the up-regulation of these molecules upon LPS or MCM-mimic exposure. This indicates that ligation of CRs constitutes an active mechanism of interference, corroborating similar observations made by Verbovetski et al21 using ACs opsonized by C3bi.
It was shown previously that ligation of CR3/CR4 on APCs leads to tolerance in vivo and failure to evoke DTH.35 While this study indicated that triggering CR3 on DCs could result in tolerance of responding T cells, no mechanism for reduced DTH responses was identified. Besides costimulation, further signals in the form of cytokines or contact with TLR agonists are essential for the full activation of T cells. We show that CR3 or CR4 ligation is accompanied by a lack of secretion of the Th1-promoting cytokine IL12 in addition to IL1β, TNFα, and IL6.
The possibility that DCs themselves can directly deliver the inhibitory signal to T cells is supported by several lines of evidence. The provision of signal through the TCR without the signal provided by costimulatory molecules induces antigen-specific unresponsiveness of T cells.50 In addition, iDCs, when injected into healthy donors, induce regulatory rather than effector T cells.51 In our defined priming system involving solely CR3-ligated DCs and naive CD4 T cells, we showed that lack of costimulation coupled to reduced amounts of pro-Th1 and proinflammatory cytokines translates into impaired priming of KLH- or alloantigen-specific Th1 T cells and ultimately cell death. We could not detect FoxP3 in T cells cocultured with CR3 ligated DCs, indicating that T regulatory cells are not induced in these circumstances, but noted increased amounts of annexin V binding, suggesting that deletional tolerance is the predominant mechanism. Therefore, CR3-ligated DCs have a different impact on T cells than do nonmanipulated iDCs, where T regulatory cells are generated under similar in vitro conditions.52 CR3-ApoS induced the production of TGFβ by DCs that possibly further compromised their ability to activate T cells.
Altogether, this study is consistent with the concept that ACs opsonized with C3bi induce tolerance to self-antigens in the steady state and further implies that signaling via CRs imparts multiple signals to DCs that collectively down-regulate T-cell activation. Importantly, down-regulation of DC maturation via CRs does not seem to impair exogenous antigen uptake, processing, or presentation. Although Bordetella pertussis adenylate cyclase that binds CR3 can deliver antigens for presentation by MHC molecules,53 experiments to address whether cellular antigens are presented in a similar manner are the subject of separate studies. Interestingly, our studies indicate that while CRs efficiently tether apoptotic cells when they are individually ligated, their phagocytic capacity is less proficient. Tethering of ApoS delivers early signals that culminate in the down-modulation of DC function. Cytochalasin D, an inhibitor of actin cytoskeletal rearrangements, inhibits these suppressive effects, implicating the cytoskeleton in the early events of CR3/CR4-mediated signaling (not shown). Subsequent studies will be required to determine which signaling pathways are triggered by CRs, but likely Rho GTPases are involved, given the involvement of CRs in type II “sinking” phagocytosis.
Nonefficient phagocytosis by CRs led to the hypothesis that ACs use a separate receptor to supplement CRs. The integrin αvβ5 is an efficient receptor for cross-presentation of cellular antigens,17 and its ligation rapidly activates Rac1 and phagosome formation.23 Consistently, αvβ5-ApoS was rapidly internalized and observed within membrane-bound vesicles. However, αvβ5 is not a self-sufficient receptor to present AC antigens and render DCs tolerogenic because no or a very limited impairment of DC function was seen when the receptor was ligated alone. To mimic ACs more closely we constructed hybrid ApoS, simultaneously targeting CR3 and αvβ5. The hybrid surrogates preserved the function of both receptors, indicating that a division of labor takes place among AC receptors.
Besides CR3, other AC receptors have been described to convey negative signals to DCs upon ligation such as CD36, which functions in concert with the αv integrins.9,12 Further studies using the ApoS or equivalent system will be necessary to confirm the inhibitory role and to determine how these receptors function together with CRs. With respect to αvβ5, our studies are consistent with observations by Morelli et al,54 who show that binding of exosomes by αvβ3 and possibly αvβ5 does not prevent DC maturation.54
For DCs to tolerize naive T cells to peripherally acquired antigens, they must navigate draining lymphatics to access lymph nodes. Apoptotic cells induce functional expression of CCR7,13,21 responsiveness of DCs to CCL19, and migration to T-cell areas of lymph nodes.5,7 By using the AC surrogate system, we show for the first time that exclusive ligation of CR3 is sufficient for this and provide an explanation of how iDCs, which have acquired ACs peripherally, can access T cells in draining lymph nodes.
In certain circumstances (eg, infection39,40 or autoimmune diseases13 ), apoptosis is not necessarily associated with impairment of immune responses. As shown previously, DCs pulsed with infected ACs can stimulate potent T-cell responses in vitro and in vivo.55 Significantly, we found that infection of DCs with L monocytogenes could to some extent overturn inhibitory signals mediated through CR3. In these cases, cross-presentation leading to immunity versus tolerance may be due to (a) the production of high levels of several inflammatory cytokines, (b) the delivery of microbial TLR ligands to endosomal TLRs,56 and/or (c) opsonization of ACs with antibodies, which may circumvent the tolerogenic effects of ACs. Ultimately, CR3 may act as a “sensor,” determining the final outcome of DC function through the ligands recognized (because the portion of its receptor that is ligated—the I domain—versus the lectin like domain can differentially activate APCs57,58 ) and through cooperation with other receptors such as FcR.
In summary, we have conclusively established that in steady-state conditions, the CR3/CR4 pathway delivers a dominant negative signal to human DCs. Our findings present the opportunity to potentially test the effectiveness of individual ligation of CR3/CR4 in order to induce tolerance in patients with autoimmune diseases.
Prepublished online as Blood First Edition Paper, April 13, 2006; DOI 10.1182/blood-2005-12-4812.
Supported by grants from the National Cancer Institute (GM61031 [P.M.H.], CA84512 [N.B.]), the National Institute of Allergy and Infectious Diseases (AI44628), the Mary Kirkland Foundation, the Cancer Research Institute, and the Burroughs Welcome Foundation (N.B.). N.B. is a Doris Duke Distinguished Scientist and an Elizabeth Glaser Scientist.
N.B. is a member of Scientific Advisory Board at Cerus Corp. Listeria, which was used in some experiments in the present article, is studied as a potential vaccine vector by Cerus Corp.
M.Š. conceptualized, designed, and performed experiments; acquired, analyzed, and interpreted data; drafted and revised the manuscript; and created all figures; S.S. conceptualized, designed, and performed selected experiments involving phagocytosis of ApoS and assisted in acquisition and analysis of data; W.A. contributed technical expertise to experiments (effect of αvβ5 on DC maturation) and assisted in acquisition and analysis of data; K.P. contributed technical expertise to experiments (uptake of latex beads—ApoS) and assisted in acquisition and analysis of data; T.T. contributed technical expertise to PCR experiments; P.M.H. provided key technology and contributed to experimental design; and N.B. conceptualized and designed experiments; interpreted and analyzed data; and drafted and revised the manuscript.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Alice Yewdall for technical assistance and Olivier Manches for helpful comments. We acknowledge the generous gift of Listeria stock by Cerus.


![Figure 4. Evaluation of DCs that have been ligated by hybrid ApoS targeting both CR3 and αvβ5. (A) To evaluate the ability of hybrid αvβ5/CD11b-ApoS to bind individual receptors to the same extent as “single” ApoS, we cocultured HEK293 cells that express αvβ5, but not CD11b (as assessed by FACS staining [left]), with single or hybrid ApoS for 2 hour at 4°C at different HEK293/ApoS ratios. Importantly, the hybrid αvβ5/CD11b-ApoS bound HEK293 cells to same extent as the “single” αvβ5-ApoS (right). (B,C) DCs were exposed to either αvβ5-, CD11b-, Ig-, or αvβ5/CD11b-ApoS for 2 hours and then cultured either in media alone or in the presence of LPS for 40 hours. (B) Maturation of DCs was assessed by determining the expression of CD83, CD80, and CD86 by FACS. (C) Supernatants of DC cultures were analyzed by ELISA for the presence of TNFα, IL1β, and IL6. A representative experiment is shown. Error bars represent SD of duplicate test wells. (D) ApoS-pretreated DCs were cocultured with naive CFSE-labeled CD4+ T cells at a 1:30 ratio. After 6 days the proliferation of T cells was assessed by analyzing the dilution of CFSE dye in T-cell membranes by FACS. (Left) Immature ApoS-exposed DCs. (Right) MCM-mimic–treated ApoS-exposed DCs. Averages and SDs of 3 experiments are shown. Stars represent P < .05 in a paired Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/3/10.1182_blood-2005-12-4812/4/m_zh80150699140004.jpeg?Expires=1765885323&Signature=S~iczZVK2ulA3xM9qkPjRJ23T2zIyE0M-EUx26qE4FQo2YN64PYghbo0qre7H7u6Ztc6df0oZA3ZLULbawV3lz0EwZrTAK2AgWQhioQteC0C2k9fs9LV5Fm9g~kruED7yxED2ipNaqekK006DXtB5-~Ditv9EBNS~3pPlwiyFZZnDI2z~~lFYJ31~4esvwLORjKz4DGG4L3qSYq5spfRAH8kZE3HjeKV9aI6X5uWI3FWydVBj~kh4EoIgFBwmp0kow7dy~E8~seGy9~FF~rurTGKJTgB9dTHhOY7ZJQEB90727EpA0Ap-IqtqE1OH63Y2NzzdWSM0SJqN66fqrrjgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal