Abstract
Chronic graft-vs-host disease (GVHD) is a major cause of morbidity and mortality of long-term survivors of allogeneic hemato-poietic cell transplantation (HCT). Chronic GVHD can have features of an autoimmune collagen vascular disease with clinical manifestations similar to autoimmune scleroderma and systemic lupus erythematosus (SLE). However, the pathogenesis of chronic GVHD is poorly understood. It is unclear how autoreactive T and B cells are generated in chronic GVHD recipients. We have recently developed a new chronic GVHD model by transplantation of donor DBA/2 (H-2d) spleen cells into major histocompatibility complex (MHC)-matched but minor antigen-mismatched sublethally irradiated BALB/c (H-2d) recipients as well as athymic BALB/cnu/nu and adult-thymectomized BALB/c recipients. Both euthymic and athymic BALB/c recipients developed high levels of serum IgG autoantibodies, sclerodermatous skin damage, and glomerulonephritis. Disease induction required both donor CD25-CD4+ T and B cells in transplants. In contrast, donor CD25+CD4+ T regulatory (Treg) cells prevented the disease induction. These results indicate that host thymus is not required for induction of chronic GVHD and that quiescent autoreactive T and B cells in transplants from nonautoimmune donors may be activated and expanded to cause chronic GVHD with autoimmune manifestations in allogeneic recipients, and donor Treg cells can suppress this process.
Introduction
Chronic graft-versus-host disease (GVHD) is a serious and common long-term complication of allogeneic hematopoietic cell transplantation (HCT) occurring in 20% to 70% of patients surviving more than 100 days after HCT.1-4 Despite improvements in the practice of allogeneic HCT over the last 25 years, there has been little change in the incidence, morbidity, and mortality of this complication.3 One of the difficulties in combating chronic GVHD is the poor understanding of its pathogenesis.
Chronic GVHD differs from acute GVHD in many aspects. First, the onset of acute GVHD is usually at 1 to 2 months following transplantation but the onset of chronic GVHD is usually delayed until 4 to 6 months after transplantation.3 Second, although the target organ tissues of chronic GVHD are significantly overlapped with that of acute GVHD (ie, skin, gut, liver, and lung), the histopathology is distinguishably different.3 While acute GVHD shows donor lymphocyte infiltration and host tissue-cell apoptosis and necrosis in target organs, chronic GVHD is featured by a marked increase in collagen deposition and a lack of T-lymphocyte infiltration in the target organ tissues.3,4 Third, up to 70% of chronic GVHD patients have elevated levels of serum autoantibodies (eg, antinuclear, anti-dsDNA, and anti-smooth-muscle antibodies),1,5,6 and depletion of B cells ameliorates refractory chronic GVHD in some patients.3,7,8 Therefore, chronic GVHD has features similar to autoimmune collagen vascular disease such as scleroderma and systemic lupus erythematosus (SLE).1,9 However, it is unclear how the autoimmune responses develop in chronic GVHD.
Several murine HCT models have been used to study the pathogenesis of chronic GVHD. The first type of model is transplantation of parental lymphocytes into nonirradiated major histocompatibility complex (MHC)-mismatched F1 recipients.10,11 In those models, the F1 recipients developed high levels of serum anti-dsDNA and glomerulonephritis, and the production of autoantibodies is a result of a cognate interaction between donor CD4+ T cells and host B cells.10-15 However, it is not clear whether the mechanisms revealed in those models reflect the pathogenesis of chronic GVHD in the irradiated HC transplant recipients.
The second type of model is transplantation of donor lymphocytes into MHC-matched but minor antigen-mismatched irradiated recipients. In one model, donor LP/J (H-2b) bone marrow and spleen cells were transplanted into lethally irradiated C57BL/6 (H-2b) recipients and the recipients developed acute and chronic forms of GVHD.16 Clonal analysis of T cells from the acute and chronic GVHD C57BL/6 recipients indicated that acute GVHD was due primarily to recipient-specific cytotoxic donor T lymphocytes, whereas chronic GVHD was due to autoreactive CD4+ T lymphocytes.16 However, it was unknown how the autoreactive CD4+ T cells were generated.
In another model, B10D2 (H-2d) donor spleen cells were transplanted into lethally irradiated BALB/c recipients and the recipients developed sclerodermatous skin damage.17-19 Donor CD4+ T cells were required for the initiation of the disease,20 but it was unclear how donor CD4+ T cells induced the disease.
In the current study, we have developed a new model of chronic GVHD with autoimmune manifestations in which donor DBA/2 (H-2d) spleen cells were transplanted into sublethally irradiated, MHC-matched but minor antigen-mismatched, euthymic BALB/c (H-2d) recipients as well as athymic BALB/cnu/nu and adult-thymectomized BALB/c recipients. The recipients developed an autoimmune lupus and scleroderma overlap syndrome manifested with high levels of serum autoantibodies, sclerodermatous skin damage, proteinuria, and glomerulonephritis. Disease induction required both donor conventional CD25-CD4+ T and B cells in transplants. In contrast, donor CD4+CD25+ T regulatory (Treg) cells in transplants suppressed the disease induction. Our studies demonstrate that host thymus is not required for induction of chronic GVHD and that both donor CD4+ T and B cells in transplants from nonautoimmune donors are required for the disease induction in this model.
Materials and methods
Mice
Male DBA/2(H-2d), B10D2/nSnJ (H-2d), BALB/c (H-2d), and BALB/cnu/nu and adult-thymectomized (aged 6 weeks) BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in a pathogen-free room at City of Hope Research Animal Facilities (Duarte, CA). Mice aged 8 to 12 weeks were used in the current studies.
Monoclonal antibodies, flow cytometric analysis, and cell sorting
The FITC-, phycoerythrin-, APC-, and Cy7-APC-conjugated monoclonal antibodies (mAbs) to mouse T-cell receptor (TCR), B220, CD4, CD8, and CD25 were all purchased from BD Pharmingen (San Diego, CA). Multiple-color fluorescence-activated cell sorter (FACS) analysis and sorting were performed at City of Hope FACS facility using a 4-laser MOFLO immunocytometry system (Dako Cytomation, Fort Collins, CO), and data was analyzed using FLOWJO software (Tree Star, San Carlos, CA).21,22 The FITC-, APC-conjugated mAbs to mouse IL-2, IL-4, and IFN-γ were all purchased from BD Pharmingen. CD8+ and CD4+ T cells were purified with a magnetic purification system from Miltenyi Biotec (Auburn, CA) as described previously.21 The purity of positively selected CD8+ or CD4+ T cells was greater than 96%. Depletion of CD4+, CD8+, and B220+ cells was performed with magnetic microbeads and Miltenyi depletion columns. The residual target cells were about 1% of total mononuclear cells.
Proliferation assays
Purified CD25-CD4+ T cells (1 × 105) together with 2 × 105 irradiated syngeneic T-cell-depleted spleen cells were cultured in a U-bottomed 96-well plate for 72 hours in the presence of 0.5 μg/mL anti-CD3 mAb. 3H-TdR (1 μCi/mL [0.037 MBq/mL]) was added 8 hours before harvest. Results are expressed as the mean of triplicate cultures.
Histopathology and immunofluorescence microscopy
Hematoxylin and eosin (HE) staining of skin tissues and immunofluorescent staining of IgG immune complex in skin and kidney tissues of BALB/c recipients were described previously.21,23,24 For collagen I staining, cryosections were first incubated with rabbit anti-mouse collagen I at 1:200 dilution (Chemicon International, Temecula, CA) and then with Texas red-conjugated donkey anti-rabbit IgG (Jackson Immunoresearch Laboratories). After extensive washes, the samples were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Sigma, St Louis, MO) and embedded in Vectashield (Vector Laboratories, Burlingame, CA). Visualization and image preparation procedures were described previously.21 Briefly, the HE staining slides and immunofluorescent staining slides were examined at 400 × magnification. The samples were visualized with an Olympus BX51 fluorescent microscope equipped with Olympus 20 ×/0.70 NA and 40 ×/0.90 Plan Apo objectives (Olympus America, Melville, NY) and a Pixera (600CL) cooled CCD camera (Pixera, Los Gatos, CA). Fluorescent images relative to each marker were collected using a corresponding filter set and Pixera viewfinder acquisition software 3.0. Color composite images were generated using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Measurement of cytokines and antibodies in serum and culture supernatants
Cytokines were measured using the Luminex LabMap system and enzyme-linked immunosorbent assay (ELISA) kits (Biosource International, Camarillo, CA) as described previously.21,23 Total IgG and anti-dsDNA IgG were measured with ELISA as described previously.24,25 For determination of the allotype of IgG2a anti-dsDNA, plates coated with calf thymus ds-DNA were used to capture anti-dsDNA, then biotin-conjugated anti-mouse IgG2aa mAb (BD Pharmingen clone 8.3) was applied to the plates, and streptavidin-conjugated HRP (BD Pharmingen) was used to develop the ELISA.
Statistical analysis
Proteinuria incidences and survival in different groups were compared using the log-rank test with GraphPad Prism Version 3.0 (Graph Pad Software, San Diego, CA). Comparison of 2 means was analyzed using unpaired 2-tailed Student t test.
Results
DBA/2 spleen cells induced GVHD in sublethally irradiated BALB/c recipients in a dose-dependent manner
Since parental donor DBA/2 alloreactive CD4+ T cells induced lupuslike GVHD in nonirradiated (DBA/2 x C57BL/6) F1 recipients via activation of host B cells and DBA/2 donor CD8+ T cells were unable to induce GVHD,12,26 we tested whether transplantation of DBA/2 (H-2d) spleen cells into sublethally irradiated BALB/c (H-2d) recipients induced GVHD with autoimmune manifestations. Accordingly, graded numbers (3.125 × 106 to 100 × 106) of DBA/2 spleen cells were injected intravenously into sublethally irradiated (6.5 Gy [650 rad]) BALB/c recipients. The recipients were monitored for clinical signs of GVHD such as diarrhea, weight loss, hair loss, and death and were also checked for proteinuria twice a week. As shown in Figure 1A, all the recipients given irradiation alone or syngeneic BALB/c spleen cells (100 × 106) showed no signs of GVHD and survived for more than 50 days. In contrast, the recipients injected with DBA/2 spleen cells showed mild acute GVHD for the first 2 weeks and then showed chronic GVHD with proteinuria and hair loss in a donor-cell dose-dependent manner. The recipients given 100 × 106 spleen cells all developed proteinuria (4+), ascites, and hair loss by day 22 and all died by day 42 after cell injection, but none of the recipients given 3.125 × 106 cells developed proteinuria or hair loss. There was no new onset of proteinuria, hair loss, or death after day 50. The clinical signs of hair loss and proteinuria in the survival recipients given 50 × 106 donor cells lasted for more than 100 days, but those clinical signs in a majority of the recipients given fewer than 50 × 106 donor cells disappeared around 80 days after transplantation (data not shown).
DBA/2 spleen cells induced sclerodoma-like chronic GVHD in a dose-dependent manner. (A) Sublethally irradiated (6.5 Gy [650 rad]) BALB/c recipients were injected with graded numbers (3.125 × 106 to 100 × 106) of DBA/2 spleen cells. The left panel shows percentage of mice without proteinuria and the right panel shows the percentage of survival. There were 10 mice in each group combined from 2 replicate experiments. (B) Tissue sections of skin and kidney from GVHD BALB/c recipients injected 30 days earlier with DBA/2 or B10D2 spleen cells (50 × 106) and control recipients without injection. The top row shows hematoxylin and eosin (HE) staining of skin tissues. The second row shows anti-collagen I staining of skin tissue. The third row shows immunofluorescent staining of IgG immune complex deposition in frozen skin tissues. The bottom row shows immunofluorescent staining of IgG immune complex deposition in frozen kidney tissues. Arrows show IgG immune complex deposition in glomerular capillary loops. One representative is shown of 4 examined recipients in each group.
DBA/2 spleen cells induced sclerodoma-like chronic GVHD in a dose-dependent manner. (A) Sublethally irradiated (6.5 Gy [650 rad]) BALB/c recipients were injected with graded numbers (3.125 × 106 to 100 × 106) of DBA/2 spleen cells. The left panel shows percentage of mice without proteinuria and the right panel shows the percentage of survival. There were 10 mice in each group combined from 2 replicate experiments. (B) Tissue sections of skin and kidney from GVHD BALB/c recipients injected 30 days earlier with DBA/2 or B10D2 spleen cells (50 × 106) and control recipients without injection. The top row shows hematoxylin and eosin (HE) staining of skin tissues. The second row shows anti-collagen I staining of skin tissue. The third row shows immunofluorescent staining of IgG immune complex deposition in frozen skin tissues. The bottom row shows immunofluorescent staining of IgG immune complex deposition in frozen kidney tissues. Arrows show IgG immune complex deposition in glomerular capillary loops. One representative is shown of 4 examined recipients in each group.
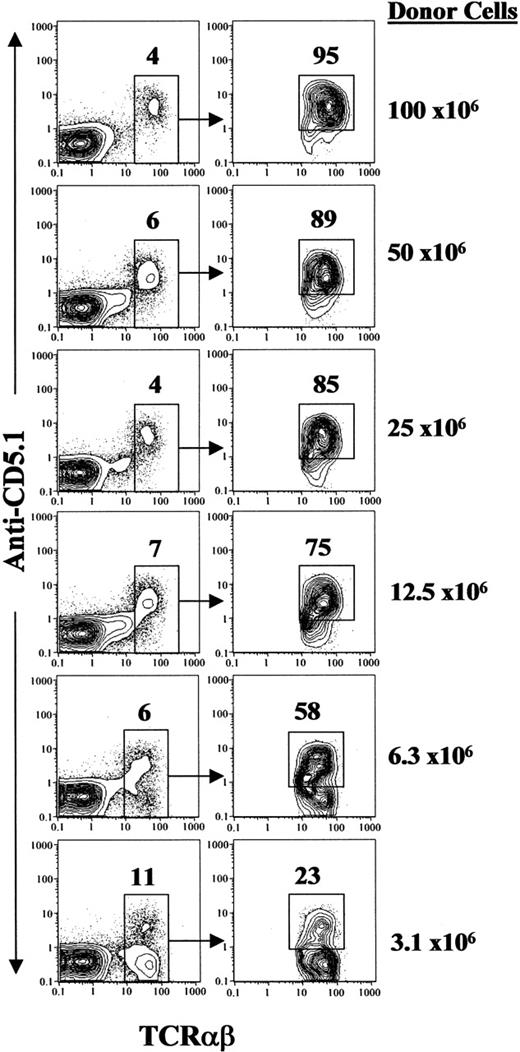
The presence of DBA/2 donor T cells in the BALB/c recipients was measured by anti-CD5.1 mAb 15 days after donor-cell injection, since DBA/2 T cells express CD5.1 and BALB/c T cells express CD5.2.27 As shown in Figure 2, there were 4% to 11% of TCRαβ+ cells among blood mononuclear cells. The percentage of donor T cells among all TCRαβ+ T cells in BALB/c recipients was dependent on donor spleen-cell dose. It was greater than 95% in the recipients given 100 × 106 donor cells and about 23% in the recipients given 3.125 × 106 donor cells. The incidence of chronic GVHD was correlated to the percentage of donor-type T cells in the recipients. While about 100% of recipients with more than 85% donor-type T cells developed chronic GVHD, none of the recipients with about 20% of donor T cells developed the disease (Figures 1A and 2). These results indicate that host T cells may play a role in preventing GVHD. It was reported that residual host CD4+ T cells prevented chronic GVHD in an MHC-matched model,28 although it was also reported that host CD4+ T cells play an essential role in the induction of chronic GVHD in an MHC-mismatched model.29,30
Sclerodermatous histopathology in GVHD BALB/c recipients given DBA/2 donor cells
Since B10D2 spleen cells were reported to induce chronic GVHD in lethally irradiated BALB/c recipients, which resembles scleroderma features in human chronic GVHD,17-19 we compared the clinical features of GVHD in sublethally irradiated BALB/c recipients induced by 50 × 106 spleen cells from DBA/2 or B10D2 donors. While the BALB/c recipients given DBA/2 spleen cells developed severe proteinuria and hair loss, the BALB/c recipients given B10D2 spleen cells developed severe diarrhea and hair loss (data not shown). Furthermore, we compared the histopathology of the recipients given DBA/2 or B10D2 spleen cells 30 days after cell injection. As shown in Figure 1B, both skin tissues of the GVHD recipients showed hyperplasia compared with control recipients given irradiation only, but the epidermis of the GVHD recipients given DBA/2 spleen cells was much thicker than that of GVHD recipients given B10D2 spleen cells. In addition, it appeared that there was less mononuclear-cell infiltration in the skin dermis of GVHD recipients given DBA/2 donor cells compared with the recipients given B10D2 donor cells.
Donor T-cell chimerism. Blood mononuclear cells of BALB/c recipients given graded numbers of DBA/2 donor spleen cells were stained with anti-TCRαβ versus anti-CD5.1. The TCRαβ+ T cells among blood mononuclear cells are gated and the percentage of T cells is shown above the gating boxes. Gated T cells are shown in TCRαβ versus CD5.1 in the right panels. The donor T cells (CD5.1+) are gated and the percentage of donor T cells in total T cells is shown above the gating boxes. The donor-cell dose for each group was shown on the right side of the panels. A representative of 10 recipients in each group is shown.
Donor T-cell chimerism. Blood mononuclear cells of BALB/c recipients given graded numbers of DBA/2 donor spleen cells were stained with anti-TCRαβ versus anti-CD5.1. The TCRαβ+ T cells among blood mononuclear cells are gated and the percentage of T cells is shown above the gating boxes. Gated T cells are shown in TCRαβ versus CD5.1 in the right panels. The donor T cells (CD5.1+) are gated and the percentage of donor T cells in total T cells is shown above the gating boxes. The donor-cell dose for each group was shown on the right side of the panels. A representative of 10 recipients in each group is shown.
Excess collagen deposition in the dermis is one of the characteristic features of human chronic GVHD.31 Thus, we also stained the skin tissues with anti-collagen I as described previously.19 As shown in Figure 1B (second row), there was excess collagen deposition in the dermis of both GVHD recipients compared with control recipients, but it appeared that there was more collagen deposition in the dermis of recipients given DBA/2 donor cells compared with the recipients given B10D2 donor cells.
Human chronic GVHD was reported to have IgG immune complex deposition in dermis,32 and human and murine systemic lupus was reported to have IgG immune complex deposition in glomerular capillary loops.33,34 Thus, skin and kidney tissues of GVHD recipients given DBA/2 or B10D2 donor spleen cells were checked for IgG immune complex deposition 30 days after donor-cell injection. As shown in Figure 1B (bottom 2 rows), IgG immune complex deposition was found in the dermis of both GVHD recipients. It was also found in glomerular capillary loops and mesangium of GVHD recipients given DBA/2 donor cells but only in the glomerular mesangium of GVHD recipients given B10D2 donor cells. The control recipients showed a little IgG deposition in either skin dermis or glomerular mesangium. Taken together, similar to BALB/c recipients given B10D2 donor cells, BALB/c recipients given DBA/2 donor cells developed a sclerodermatous syndrome. In addition, the latter recipients also developed a lupuslike syndrome.
High levels of serum anti-dsDNA antibodies of DBA/2 donor origin in the BALB/c recipients with chronic GVHD
Since BALB/c recipients given DBA/2 donor cells showed immune complex glomerulonephritis (Figure 1B), serum levels of total IgG1 and IgG2a and anti-dsDNA IgG1 and IgG2a of BALB/c recipients given 50 × 106 DBA/2 spleen cells were measured with ELISA 15 days after cell injection. As shown in Table 1, compared with recipients given irradiation only, the recipients given additional DBA/2 spleen cells had 10-fold higher levels of serum IgG1, 100-fold higher levels of IgG2a, and more than 100-fold higher levels of anti-dsDNA IgG1 and IgG2a (P < .001). BALB/c or DBA/2 recipients given syngeneic BALB/c or DBA/2 spleen cells did not show significant increase of total or anti-dsDNA IgG (data not shown).
Serum levels of autoantibodies of BALB/c recipients on day 15 after donor-cell injection
Donor cells, 50 × 106 . | Total IgG1, μg/mL . | Total IgG2a, μg/mL . | Anti-dsDNA IgG1, U/mL . | Anti-dsDNA IgG2a, U/mL . | Anti-dsDNA IgG2aa, U/mL . |
|---|---|---|---|---|---|
| DBA/2 whole SPL | 1157 ± 58 | 4033 ± 279 | 3162 ± 603 | 1459 ± 163 | <10 |
| DBA/2 CD4--SPL | 484 ± 41 | 731 ± 60 | 407 ± 43 | 335 ± 41 | <10 |
| DBA/2 CD8--SPL | 1953 ± 204 | 5051 ± 729 | 2630 ± 276 | 1073 ± 481 | <10 |
| DBA/2 B220--SPL | 346 ± 43 | 455 ± 42 | 225 ± 46 | 140 ± 16 | <10 |
| No injection | 97 ± 5 | 43 ± 4.2 | <10 | <10 | <10 |
| NZB SPL* | NA | NA | NA | 366 ± 79 | 293 ± 32 |
Donor cells, 50 × 106 . | Total IgG1, μg/mL . | Total IgG2a, μg/mL . | Anti-dsDNA IgG1, U/mL . | Anti-dsDNA IgG2a, U/mL . | Anti-dsDNA IgG2aa, U/mL . |
|---|---|---|---|---|---|
| DBA/2 whole SPL | 1157 ± 58 | 4033 ± 279 | 3162 ± 603 | 1459 ± 163 | <10 |
| DBA/2 CD4--SPL | 484 ± 41 | 731 ± 60 | 407 ± 43 | 335 ± 41 | <10 |
| DBA/2 CD8--SPL | 1953 ± 204 | 5051 ± 729 | 2630 ± 276 | 1073 ± 481 | <10 |
| DBA/2 B220--SPL | 346 ± 43 | 455 ± 42 | 225 ± 46 | 140 ± 16 | <10 |
| No injection | 97 ± 5 | 43 ± 4.2 | <10 | <10 | <10 |
| NZB SPL* | NA | NA | NA | 366 ± 79 | 293 ± 32 |
Data are presented as means ± SE; n = 10.
CD4--SPL indicates CD4+ T cell–depleted spleen cells; CD8--SPL, CD8+ T cell–depleted spleen cells; B220--SPL, B220+ B cell–depleted spleen cells; and NA, not applicable.
IgG2a antibodies secreted by B cells from NZB mice are allotype a, and serum of BALB/c recipients given donor NZB spleen cells was used as positive control for anti-dsDNA IgG2aa
Next, we determined which B cells (donor or host) were the source of autoantibodies in the irradiated BALB/c recipients given DBA/2 spleen cells. IgG2a antibodies secreted by DBA/2 B cells express allotype c, but those secreted by BALB/c B cells express allotype a.35 Since there is anti-allotype a, but no anti-allotype c, mAb available, we used anti-allotype a mAb to identify the origin of anti-dsDNA IgG2a in the serum of BALB/c recipients. In addition, because IgG2a secreted by New Zealand Black (NZB) B cells is allotype a, we use serum from sublethally irradiated BALB/c recipients given NZB donor spleen cells as the positive control of anti-allotype a. As shown in Table 1, almost all of serum anti-dsDNA IgG2a (366 ± 79 U/mL) of BALB/c recipients given NZB donor cells was allotype a (293 ± 32 U/mL; P > .1). In contrast, no allotype a anti-dsDNA IgG2a was detectable in the serum of recipients given DBA/2 donor cells with high levels of anti-dsDNA IgG2a (1459 ± 163 U/mL), indicating that those anti-dsDNA IgG2a antibodies are not secreted by the B cells of irradiated BALB/c recipients with allotype a but are instead secreted by the B cells of DBA/2 donors. B cells are very sensitive to irradiation.36-38 Thus, it is not surprising that autoantibodies in the sublethally irradiated BALB/c recipients given DBA/2 donor cells are from donor B cells, although autoantibodies in the nonirradiated (C57BL/6 x DBA/2) F1 recipients given parental DBA/2 donor cells are from host B cells.26
Transplanted donor CD4+ but not CD8+ T cells were required for the induction of chronic GVHD with autoimmune manifestations
As shown in Figure 3A, while all the recipients given 50 × 106 whole donor spleen (whole-SPL) cells developed severe proteinuria (> 4+) and hair loss and about 25% died by day 50 after donor-cell injection, the recipients given 50 × 106 CD4+ T-cell-depleted donor spleen (CD4--SPL) cells showed no signs of proteinuria or hair loss for more than 50 days after cell injection (P < .001). Depletion of donor CD4+ T cells also reduced serum levels of IgG1 and IgG2a and anti-dsDNA IgG1 and IgG2a antibodies by about 5 fold (Table 1) compared with the recipients given whole-SPL cells (P < .001). In contrast, the recipients given donor CD8+ T-cell-depleted spleen (CD8--SPL) cells showed similar tempo of hair loss and proteinuria development and survival and similar levels of serum autoantibodies compared with recipients given whole-SPL cells (P > .1; Figure 3A; Table 1). Depletion of donor CD4+ but not CD8+ T cells in transplants also prevented sclerodermatous skin damage (Figure 3B).
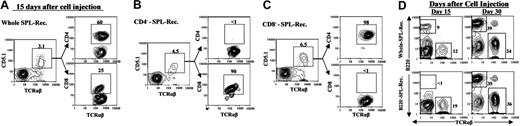
Furthermore, we analyzed the percentage of donor CD4+ and CD8+ T cells in the peripheral blood of recipients given different donor cells 15 and 30 days after cell injection. As shown in Figure 4, 15 days after cell injection, the recipients given whole donor spleen cells had both CD4+ and CD8+ donor (CD5.1+) T cells in blood mononuclear cells (Figure 4A). In contrast, the recipients given donor CD4--SPL cells had only CD8+ donor T cells (Figure 4B), and the recipients given donor CD8--SPL cells had only CD4+ donor T cells (Figure 4C). However, all 3 kinds of recipients had CD4+ and CD8+ donor T cells by day 30 after cell transfer (data not shown). The presence of de novo-developed donor CD4+ T cells was not associated with the disease development, since no new disease onset was observed in recipients given CD4--SPL cells 30 days after cell injection (Figure 3A). These results indicate that donor CD4+ but not CD8+ T cells in transplants are required for the induction of chronic GVHD and that de novo-developed donor CD4+ T cells do not induce the disease.
Donor CD4+ T and B220+ B cells in transplants were required for chronic GVHD induction. (A) Sublethally irradiated (6.5 Gy [650 rad]) BALB/c recipients were injected with 50 × 106 whole spleen cells (whole-SPL), CD4+ T-cell-depleted spleen cells (CD4--SPL), CD8+ T-cell-depleted spleen cells (CD8--SPL), or B220+ B-cell-depleted spleen cells (B220--SPL). The recipients were checked twice a week for proteinuria, hair loss, and survival. The percentage of mice without proteinuria is shown in the left panel, and the percentage of survival is shown in the right panel. There were 8 mice in each group combined from 2 replicate experiments. (B) HE staining of skin tissue sections of BALB/c recipients given CD4--SPL cells, CD8--SPL cells, or B220--SPL cells. One representative is shown of 4 examined recipients in each group.
Donor CD4+ T and B220+ B cells in transplants were required for chronic GVHD induction. (A) Sublethally irradiated (6.5 Gy [650 rad]) BALB/c recipients were injected with 50 × 106 whole spleen cells (whole-SPL), CD4+ T-cell-depleted spleen cells (CD4--SPL), CD8+ T-cell-depleted spleen cells (CD8--SPL), or B220+ B-cell-depleted spleen cells (B220--SPL). The recipients were checked twice a week for proteinuria, hair loss, and survival. The percentage of mice without proteinuria is shown in the left panel, and the percentage of survival is shown in the right panel. There were 8 mice in each group combined from 2 replicate experiments. (B) HE staining of skin tissue sections of BALB/c recipients given CD4--SPL cells, CD8--SPL cells, or B220--SPL cells. One representative is shown of 4 examined recipients in each group.
Transplanted donor B cells were required for the induction of chronic GVHD with autoimmune manifestations
As shown in Figure 3A, the recipients given 50 × 106 B-cell-depleted DBA/2 donor spleen (B220--SPL) cells did not develop proteinuria or hair loss over an observation period of more than 50 days, although those recipients showed signs of acute GVHD (ie, weight loss and swollen face) during the first 2 weeks after donor-cell injection (data not shown) and showed significant reduction of thymocytes compared with that of recipients given total body irradiation (TBI) conditioning only or recipients given CD4--SPL donor cells (P < .001; Table 2). Depletion of donor B cells in transplants reduced serum autoantibody levels by greater than 10 fold compared with whole-spleen transplants (P < .001; Table 1) and prevented the development of sclerodermatous skin damage (Figure 3B). Add-back of 10 × 106 B220+ cells to B220--SPL cells (50 × 106) induced overt disease again (data not shown). In addition, FACS analysis of blood mononuclear cells from the recipients showed that there was less than 1% of B cells in the recipients given donor B220--SPL cells but 9% B cells in the recipients given whole-SPL cells on day 15 after donor-cell injection (Figure 4D). However, a high percentage of B cells was seen in both recipients by day 30 (Figure 4D). The presence of de novo-developed B cells was not associated with the disease development, since there was no new disease onset in recipients given B220--SPL cells 30 days after donor-cell injection (Figure 3A). These results indicate that donor but not host B cells are required for the induction of chronic GVHD and that donor B220+ B cells in transplants but not de novo-developed donor B cells are required for the induction of chronic GVHD.
Thymocyte yield in TBI-conditioned recipients given different DBA/2 donor spleen cells 17 days after donor-cell injection
Donor cells . | Recipient thymocyte yield, × 106 . |
|---|---|
| TBI conditioning only | 24 ± 4 |
| Whole SPL* | 6 ± 1.4 |
| CD4–-SPL | 59 ± 10 |
| B220–-SPL | 11 ± 4 |
| CD4–-SPL + CD25–CD4+ T† | 0.2 ± 0.06 |
| CD4–-SPL + CD25–CD4+ T + CD25+CD4+ T‡ | 35 ± 6 |
Donor cells . | Recipient thymocyte yield, × 106 . |
|---|---|
| TBI conditioning only | 24 ± 4 |
| Whole SPL* | 6 ± 1.4 |
| CD4–-SPL | 59 ± 10 |
| B220–-SPL | 11 ± 4 |
| CD4–-SPL + CD25–CD4+ T† | 0.2 ± 0.06 |
| CD4–-SPL + CD25–CD4+ T + CD25+CD4+ T‡ | 35 ± 6 |
Data are mean ± SE; n = 8.
50 × 106 SPL cells/recipient
5 × 106 CD25–CD4+ T cells/recipient
2.5 × 106 CD25+CD4+ T cells/recipient
It is noteworthy that although depletion of CD4+ T or B cells in transplants markedly reduced serum levels of autoantibodies of the recipients, there were still significantly higher levels of serum autoantibodies in those recipients compared with recipients given irradiation only (Table 1). These may be due to the residual CD4+ T or B220+ cells in the transplants, since we found that there was about 1% to 2% of CD4+ T or B220+ cells among the spleen mononuclear cells of recipients given CD4--SPL or B220--SPL cells 15 days after donor-cell injection (data not shown).
Donor T-cell subsets and B cells in recipients given different donor cells. (A-C) The blood mononuclear cells from recipients given whole DBA/2 donor spleen cells (whole SPL-Rec), CD4+ T-cell-depleted spleen cells (CD4--SPL-Rec), or CD8+ T-cell-depleted spleen cells (CD8--SPL-Rec) were stained with anti-TCRαβ, CD4, CD8, and CD5.1 (donor T-cell marker) on day 15 after cell injection. The gated TCRαβ+CD5.1+ donor T cells are shown as TCRαβ versus CD4 or CD8. The percentage of CD5.1+ T cells among blood mononuclear cells and the percentage of CD4+ and CD8+ T cells among CD5.1+ donor T cells are shown above the gating boxes. One representative is shown of 8 recipients in each group. (D) The blood mononuclear cells from recipients given whole DBA/2 donor spleen or B220+ B-cell-depleted spleen (B220--SPL-Rec) were stained with anti-TCRαβ versus anti-B220 15 and 30 days after donor-cell injection. The TCRαβ+ T cells and B220+ B cells are gated, and the percentage of those cells among mononuclear cells is shown beside the gating boxes. One representative is shown of 8 recipients in each group.
Donor T-cell subsets and B cells in recipients given different donor cells. (A-C) The blood mononuclear cells from recipients given whole DBA/2 donor spleen cells (whole SPL-Rec), CD4+ T-cell-depleted spleen cells (CD4--SPL-Rec), or CD8+ T-cell-depleted spleen cells (CD8--SPL-Rec) were stained with anti-TCRαβ, CD4, CD8, and CD5.1 (donor T-cell marker) on day 15 after cell injection. The gated TCRαβ+CD5.1+ donor T cells are shown as TCRαβ versus CD4 or CD8. The percentage of CD5.1+ T cells among blood mononuclear cells and the percentage of CD4+ and CD8+ T cells among CD5.1+ donor T cells are shown above the gating boxes. One representative is shown of 8 recipients in each group. (D) The blood mononuclear cells from recipients given whole DBA/2 donor spleen or B220+ B-cell-depleted spleen (B220--SPL-Rec) were stained with anti-TCRαβ versus anti-B220 15 and 30 days after donor-cell injection. The TCRαβ+ T cells and B220+ B cells are gated, and the percentage of those cells among mononuclear cells is shown beside the gating boxes. One representative is shown of 8 recipients in each group.
DBA/2 spleen cells induced chronic GVHD in BALB/cnu/nu and adult-thymectomized BALB/c recipients
It was proposed that chronic GVHD resulted from generation of autoreactive T cells in the GVHD recipient's thymus.31,39,40 We also observed a marked reduction of thymocytes in the chronic GVHD recipients given donor whole-SPL cells compared with GVHD-free recipients given CD4--SPL cells (P < .01; Table 2). To test whether thymus is required for induction of chronic GVHD, 50 × 106 DBA/2 spleen cells were injected into sublethally irradiated athymic BALB/cnu/nu as well as adult-thymectomized and sham-thymectomized BALB/c recipients. As shown in Table 3, all of the recipients given DBA/2 spleen cells developed severe proteinuria (4+) and high levels of serum anti-dsDNA IgG autoantibodies by day 20 after donor-cell injection. The recipients also showed hyperplasia in epidermis and proliferative glomerulonephritis (data not shown). There was no significant difference between thymectomized and sham-thymectomized recipients in clinical features and serum autoantibody levels (Table 3; P > .1). These results indicate that induction of autoimmune-like chronic GVHD dose not require the host thymus or de novo-developed donor T cells from the recipient thymus.
Chronic GVHD with autoimmune manifestations in sublethally irradiated athymic BALB/cnu/nu and adult-thymectomized BALB/c recipients given DBA/2 donor cells
Host* . | Hair loss† . | Proteinuria, ++++ . | Anti-dsDNA IgG1, U/mL . | Anti-dsDNA IgG2a, U/mL . |
|---|---|---|---|---|
| BALB/cnu/nu | NA | 12/12 | 2583 ± 360 | 1650 ± 274 |
| Adult-thymectomized BALB/c | 8/8 | 8/8 | 2708 ± 412 | 1119 ± 172 |
| Sham-thymectomized BALB/c | 8/8 | 8/8 | 2099 ± 482 | 1229 ± 286 |
Host* . | Hair loss† . | Proteinuria, ++++ . | Anti-dsDNA IgG1, U/mL . | Anti-dsDNA IgG2a, U/mL . |
|---|---|---|---|---|
| BALB/cnu/nu | NA | 12/12 | 2583 ± 360 | 1650 ± 274 |
| Adult-thymectomized BALB/c | 8/8 | 8/8 | 2708 ± 412 | 1119 ± 172 |
| Sham-thymectomized BALB/c | 8/8 | 8/8 | 2099 ± 482 | 1229 ± 286 |
Recipients received 50 × 106 donor cells. Data in the last 2 columns are presented as mean ± SE.
All of the recipients developed severe proteinuria (4+) by 15 to 20 days after donor-cell injection, and serum levels of anti-dsDNA antibodies were measured on day 15 after donor-cell injection
Greater than 2 cm2 of hair loss in the neck and back by day 30 after donor-cell injection
DBA/2 donor splenic CD25+CD4+ T cells prevent chronic GVHD induced by CD25-CD4+ T cells
Donor CD25+CD4+ T cells prevent acute GVHD in a variety of animal models.28,41-44 We tested whether CD25+CD4+ T cells in donor DBA/2 spleen prevented chronic GVHD. DBA/2 spleen CD4+ T cells were sorted into CD25+ and CD25- subsets (Figure 5A). We first found that CD25+CD4+ T cells suppressed the proliferation of CD25-CD4+ T cells in vitro in a cell-dose-dependent manner (Figure 5B). We then observed that 12 of 12 of BALB/c recipients given 100 × 106 CD4+ T-cell-depleted (CD4--SPL) cells and 5 × 106 CD25-CD4+ T cells developed proteinuria and hair loss by day 30 after cell injection and 3 of 12 of them died by day 40; the clinical signs of hair loss and proteinuria in the survival recipients lasted for more than 100 days after cell injection (data not shown). In contrast, 0 of 12 of the BALB/C recipients given the same numbers of donor CD4--SPL and CD25-CD4+ T cells and additional 2.5 × 106 CD25+CD4+ T cells developed the disease during an observation period of more than 50 days (Figure 5C). Compared with recipients given donor CD4--SPL and CD25-CD4+ T cells, the recipients given the same numbers of donor CD4--SPL and CD25-CD4+ T cells and additional 2.5 × 106 CD25+CD4+ T cells showed a more than 100-fold increase in thymocyte yield (P < .001; Table 2), a 2-fold reduction in serum levels of anti-dsDNA IgG2a (P < .01; Table 4), and a marked improvement in sclerodermatous skin damage (Figure 5D).
CD25+CD4+ T cells suppress autoantibody production in chronic GVHD BALB/c recipients on day 15 after donor-cell injection
Donor cells . | Total IgG1, μg/mL . | Total IgG2a, μg/mL . | Anti-dsDNA IgG1, U/mL . | Anti-dsDNA IgG2a, U/mL . | Anti-dsDNA IgG2aa, U/mL . |
|---|---|---|---|---|---|
| CD4–-SPL* + CD25–CD4+ T† | 1678 ± 207 | 2119 ± 218 | 1464 ± 239 | 700 ± 79 | <10 |
| CD4–SPL + CD25–CD4+ T + CD25+CD4+ T‡ | 1279 ± 149 | 1994 ± 166 | 1097 ± 167 | 308 ± 38 | <10 |
Donor cells . | Total IgG1, μg/mL . | Total IgG2a, μg/mL . | Anti-dsDNA IgG1, U/mL . | Anti-dsDNA IgG2a, U/mL . | Anti-dsDNA IgG2aa, U/mL . |
|---|---|---|---|---|---|
| CD4–-SPL* + CD25–CD4+ T† | 1678 ± 207 | 2119 ± 218 | 1464 ± 239 | 700 ± 79 | <10 |
| CD4–SPL + CD25–CD4+ T + CD25+CD4+ T‡ | 1279 ± 149 | 1994 ± 166 | 1097 ± 167 | 308 ± 38 | <10 |
Data are mean ± SE, n = 12.
100 × 106 CD4+ T cell–depleted spleen cells/recipient
5 × 106 CD25–CD4+ T cells/recipient
2.5 × 106 CD25+CD4+ T cells/recipient
In additional experiments, we attempted to study the mechanisms whereby donor CD25+CD4+ T cells prevent chronic GVHD. Seven days after donor-cell injection, the yield of donor CD4+ T cells in spleens of recipients and in vitro spontaneous proliferation and IFN-γ secretion of donor CD4+ T cells were measured. We found that coinjection of donor CD25+CD4+ T cells reduced the yield of donor CD4+ T cells by 2 fold (Figure 5E; P < .01), suppressed the in vitro spontaneous proliferation of donor CD4+ T cells by 5 fold (Figure 5F; P < .01), reduced the percentage of IFN-γ-secreting donor CD4+ T cells in spleen from 56% ± 7% to 28% ± 3% (mean ± SE, n = 8; P < .01; Figure 5G), and reduced serum levels of IFN-γ from 818 ± 69 to 377 ± 38 pg/mL (mean ± SE, n = 8; P < .01). These results indicate that donor CD25+CD4+ T cells may suppress the expansion and IFN-γ secretion of donor CD25-CD4+ T cells to reduce the production of autoantibodies and prevent the development of chronic GVHD with autoimmune manifestations. IFN-γ was reported to play an important role in autoantibody isotype switch to IgG2a45,46 and augmentation of collagen deposition in chronic GVHD.31
Discussion
We have demonstrated here that CD4+ T and B cells in transplants from nonautoimmune DBA/2 donors induced chronic GVHD with high levels of serum autoantibodies, sclerodermatous skin damage, and glomerulonephritis in sublethally irradiated MHC-matched but minor antigen-mismatched euthymic and athymic BALB/c recipients. The disease induction did not require host thymus but did require both donor CD4+ T and B cells. In contrast, donor CD25+CD4+ T cells suppressed the disease induction.
The scleroderma and lupus manifestations with high levels of serum autoantibodies in the BALB/c recipients given DBA/2 donor cells reflect important clinical features of human chronic GVHD.1,31,47 However, the relevance of this model to human chronic GVHD is unclear because the temporal time course of chronic GVHD in those BALB/c recipients does not mirror that in human chronic GVHD, which can have a long latency period of at least several months. In addition, it is still unknown whether or not the features of chronic GVHD in those BALB/c recipients were caused by autoimmunity and/or alloimmunity, although those recipients showed high levels of serum anti-dsDNA.
Natural CD25+CD4+ regulatory T cells prevented chronic GVHD. (A) DBA/2 spleen cells were stained with anti-TCRαβ, CD4, and CD25 and then CD4+TCRαβ+ cells were sorted into CD25+ and CD25-CD4+ T cells. (B) CD25-CD4+T cells (0.1 × 106) were stimulated with anti-CD3 mAb and cocultured with titrated numbers of CD25+CD4+ T cells for 72 hours; the proliferation of CD25-CD4+ T cells was measured by 3H-TdR incorporation. Mean ± SE of triplicate culture, and one representative of 3 replicate experiments are shown. (C) Sublethally irradiated BALB/c mice were injected with CD4+ T-cell-depleted DBA/2 spleen cells (CD4-dep alone; 100 × 106), CD4-dep plus CD25-CD4+ T cells (CD25-; 5 × 106), and CD4-dep plus CD25- and CD25+CD4+ T cells (CD25+; 2.5 × 106). The development of proteinuria and hair loss in the 3 groups were compared. There were 12 mice in each group. (D) HE staining of skin tissue sections of the recipients given CD4-dep cells, CD4-dep plus CD25-CD4+ T cells, or CD4-dep plus CD25- and CD25+ CD4+ T cells 30 days after cell injection. One representative is shown of 4 examined recipients in each group. (E) Seven days after cell injection, yield of donor CD4+ T cells from the spleens of recipients given donor CD25-CD4+ T cells and recipients given both CD25- and CD25+ CD4+ T cells was measured. Mean ± SE of 4 recipients in each group is shown. (F) Sorted donor CD4+ T cells (0.3 × 106/well) from recipient in panel E were cultured in vitro for 5 days without additional stimulation. T-cell proliferation was measured with 3H-TdR incorporation. Mean ± SE of 4 examined recipients in each group is shown. (G) Intracellular staining of IFN-γ in CD4+ T cells from the spleens of recipients injected with CD25-CD4+ T cells (bold solid line) or from the recipients injected with both CD25- and CD25+ CD4+ T cells (thin solid line) 7 days after cell injection. The isotype control staining was shown as thin dashed line. One representative is shown of 4 examined recipients in each group.
Natural CD25+CD4+ regulatory T cells prevented chronic GVHD. (A) DBA/2 spleen cells were stained with anti-TCRαβ, CD4, and CD25 and then CD4+TCRαβ+ cells were sorted into CD25+ and CD25-CD4+ T cells. (B) CD25-CD4+T cells (0.1 × 106) were stimulated with anti-CD3 mAb and cocultured with titrated numbers of CD25+CD4+ T cells for 72 hours; the proliferation of CD25-CD4+ T cells was measured by 3H-TdR incorporation. Mean ± SE of triplicate culture, and one representative of 3 replicate experiments are shown. (C) Sublethally irradiated BALB/c mice were injected with CD4+ T-cell-depleted DBA/2 spleen cells (CD4-dep alone; 100 × 106), CD4-dep plus CD25-CD4+ T cells (CD25-; 5 × 106), and CD4-dep plus CD25- and CD25+CD4+ T cells (CD25+; 2.5 × 106). The development of proteinuria and hair loss in the 3 groups were compared. There were 12 mice in each group. (D) HE staining of skin tissue sections of the recipients given CD4-dep cells, CD4-dep plus CD25-CD4+ T cells, or CD4-dep plus CD25- and CD25+ CD4+ T cells 30 days after cell injection. One representative is shown of 4 examined recipients in each group. (E) Seven days after cell injection, yield of donor CD4+ T cells from the spleens of recipients given donor CD25-CD4+ T cells and recipients given both CD25- and CD25+ CD4+ T cells was measured. Mean ± SE of 4 recipients in each group is shown. (F) Sorted donor CD4+ T cells (0.3 × 106/well) from recipient in panel E were cultured in vitro for 5 days without additional stimulation. T-cell proliferation was measured with 3H-TdR incorporation. Mean ± SE of 4 examined recipients in each group is shown. (G) Intracellular staining of IFN-γ in CD4+ T cells from the spleens of recipients injected with CD25-CD4+ T cells (bold solid line) or from the recipients injected with both CD25- and CD25+ CD4+ T cells (thin solid line) 7 days after cell injection. The isotype control staining was shown as thin dashed line. One representative is shown of 4 examined recipients in each group.
Donor-type autoreactive CD4+ T cells were reported to play a critical role in induction of chronic GVHD.16,48 However, it is still unclear how the autoreactive T cells are generated in chronic GVHD recipients. It was previously proposed that those donor-type autoreactive T cells were de novo developed from recipients' thymuses that were deficient in negative selection of autoreactive T cells and/or deficient in production of autoregulatory T cells due to thymic damage caused by acute GVHD.31,39,40,48-50 However, it was also reported that chronic GVHD recipients did not have a defect in thymic negative selection.51 In our report, induction of chronic GVHD did not require host thymus or de novo-developed donor CD4+ T cells but required mature CD4+ T cells in transplants from nonautoimmune donors. It is not yet clear how donor alloreactive CD4+ T cells in transplants lead to the development of chronic GVHD with autoimmune manifestations. We hypothesize that alloimmune responses lead to the activation and expansion of quiescent autoreactive CD4+ T cells in transplants from nonautoimmune donors. Quiescent autoreactive T cells are found in the periphery of healthy individuals.52
This is the first report that B cells in the transplants from nonautoimmune donors play a critical role in the pathogenesis of chronic GVHD, although it was reported that B cells augmented acute GVHD53 and that depletion of B cells using anti-CD20 mAb ameliorated refractory chronic GVHD in some patients.7,8 In the current report, mature donor B cells in transplants but not de novo-developed donor B cells are required for autoantibody production and induction of chronic GVHD, since depletion of mature donor B cells in transplants prevented the autoantibody production and disease induction. The mechanisms whereby donor B cells lead to the development of autoimmune-like chronic GVHD are unclear. We hypothesize that quiescent autoreactive donor B cells in transplants from nonautoimmune donors are activated and expanded by alloreactive donor CD4+ T cells via recognition of host minor alloantigens presented by donor B cells. Autoreactive B cells are commonly found in the peripheral lymphoid tissues of healthy individuals.54,55
We observed that both donor CD4+ T and B cells in transplants were required for induction of chronic GVHD and that depletion of either one prevented the disease induction. The mechanisms whereby donor CD4+ T and B cells interact to induce chronic GVHD are not yet clear. We recently found that CD4+ T cells from chronic GVHD recipients given whole DBA/2 spleen cells augmented the in vitro and in vivo autoantibody production by B cells from the chronic GVHD recipients, however, CD4+ T cells from the nonchronic GVHD recipients given B-cell-depleted DBA/2 spleen cells did not augment the autoantibody production by the B cells from the chronic GVHD recipients (Supplemental Figure S1, available on the Blood website; click on the Supplemental Figure link at the top of the online article). Although further investigation is needed, these results may indicate that there are autoreactive CD4+ T cells in the chronic GVHD recipients given donor DBA/2 spleen cells and the activation and expansion of donor autoreactive CD4+ T cells in the recipients required donor B cells. It was previously reported that activation of autoreactive B cells led to activation of autoreactive T cells.56 It has also been proposed that autoreactive B cells are important antigen-presenting cells that play a central role in amplifying autoimmune responses and epitope spreading of autoreactive T and B cells due to the fact that B-cell surface Ig receptors are very efficient at capturing soluble antigens and one B cell can present multiple antigens that activate multiple CD4+ T-cell clones.57-60
We observed that the recipients given B-cell-depleted DBA/2 donor spleen cells showed clinical signs of acute GVHD and marked reduction of thymocytes early after donor-cell injection but did not go on to develop chronic GVHD. In contrast, recipients given whole donor spleen cells developed signs of acute GVHD early after donor-cell injection and went on to develop chronic GVHD with autoimmune manifestations. We also found that transplantation of DBA/2 spleen cells into sublethally irradiated syngenic DBA/2 recipients did not induce chronic GVHD or autoantibody production. These results indicate that alloreactive CD4+ T cells are required but not sufficient for induction of chronic GVHD. Our observation is consistent with clinical observations that chronic GVHD is not simply a consequence of tissue damage caused by acute GVHD.
CD25+CD4+ Treg cells have been reported to prevent GVHD and autoimmune disease in a variety of models.28,41-44,61,62 Consistent with previous reports, we observed that donor CD25+CD4+ Treg cells suppressed donor CD25-CD4+ T-cell proliferation and expansion and IFN-γ secretion when coinjected into allogeneic recipients. The CD25+CD4+ Treg cells also suppressed autoantibody production and prevented induction of chronic GVHD. These results indicate that the breakdown of peripheral tolerance of donor lymphocytes triggered by HCT procedures and alloimmune responses can be restored by infusion of large numbers of donor CD25+CD4+ T cells. It is noteworthy that our data showed that add-back of donor CD25+CD4+ Treg cells to CD25-CD4+ T cells markedly reduced the incidence of proteinuria and chronic GVHD tissue damage, but the reduction of anti-dsDNA was relatively limited. These results may indicate that CD25+CD4+ T cells do not completely suppress the in vivo function of donor CD25-CD4+ T cells so that some of the latter CD4+ T cells can still activate donor B cells to secrete autoantibodies. These results may also indicate that autoantibody alone may not play a critical role in the tissue damage of chronic GVHD. Shlomchik and colleagues (Chan et al59 ) had demonstrated that secreted autoantibodies are not required for tissue damage in autoimmune lupus mice.
Taken together, the current experimental results can be explained by the following hypotheses. After injection of DBA/2 donor spleen cells into sublethally irradiated BALB/c recipients, host/donor dendritic cells activate the alloreactive CD4+ T cells, which subsequently activate donor B cells by recognizing the host alloantigens presented by donor B cells, including autoreactive B cells that present both alloantigens and nuclear autoantigens. The activated autoreactive B cells then activate the autoreactive CD4+ T cells; in turn, the activated autoreactive CD4+ T cells augment the antibody secretion and isotype switch of autoreactive B cells via costimulatory molecules and their secretion of cytokines (ie, IFN-γ). The autoreactive T- and B-cell interaction also leads to the epitope spreading of T and B cells and the amplification of autoimmune response. Finally, autoreactive CD4+ T infiltration and/or autoantibody-immune complex deposition in the tissues cause chronic GVHD. In contrast, Treg cells suppress the autoimmune response at the above different steps.
In conclusion, our studies indicate that alloreactive CD4+ T cells and quiescent autoreactive donor CD4+ T and B cells in transplants from nonautoimmune donors induce chronic GVHD with autoimmune manifestations and that donor CD25+CD4+ Treg cells can prevent the disease induction. Therefore, depletion of CD25-CD4+ T and B cells in transplants and infusion of donor Treg cells may be an effective approach in preventing chronic GVHD in patients.
Prepublished online as Blood First Edition Paper, December 13, 2005; DOI 10.1182/blood-2005-09-3623.
Supported by the Leslie and Susan Gonda (Goldschmied) Foundation, an Arthritis Foundation Investigator Award, and National Institutes of Health grant R01-AI66008 (D.Z.).
C.Z. designed research, performed experiments, analyzed data, and prepared the manuscript; I.T. performed experiments and analyzed data in immunohistology; Z.Z. performed experiments in cell transfer and flow cytometry analysis; Y.L. performed experiments in autoantibody production; F.K. advised on experimental design and reviewing of manuscript; S.S. advised on experimental design and manuscript preparation; S.F. advised on experimental design and manuscript preparation; and D.Z. designed research, analyzed data, and prepared the manuscript.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Tammy Huang, Stephen Scott, and Noe Gonzales in our laboratory; Lucy Brown and Claudia Spalla at City of Hope (COH) Flow Cytometry Facility; and Sofia Loera at COH Anatomic Pathology Laboratory for their excellent technical assistance. We thank Dr Richard Ermel and his staff at COH Research Animal Facility for providing excellent animal care and Dr Robertson Parkman for critical review of our manuscript.

![Figure 1. DBA/2 spleen cells induced sclerodoma-like chronic GVHD in a dose-dependent manner. (A) Sublethally irradiated (6.5 Gy [650 rad]) BALB/c recipients were injected with graded numbers (3.125 × 106 to 100 × 106) of DBA/2 spleen cells. The left panel shows percentage of mice without proteinuria and the right panel shows the percentage of survival. There were 10 mice in each group combined from 2 replicate experiments. (B) Tissue sections of skin and kidney from GVHD BALB/c recipients injected 30 days earlier with DBA/2 or B10D2 spleen cells (50 × 106) and control recipients without injection. The top row shows hematoxylin and eosin (HE) staining of skin tissues. The second row shows anti-collagen I staining of skin tissue. The third row shows immunofluorescent staining of IgG immune complex deposition in frozen skin tissues. The bottom row shows immunofluorescent staining of IgG immune complex deposition in frozen kidney tissues. Arrows show IgG immune complex deposition in glomerular capillary loops. One representative is shown of 4 examined recipients in each group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/7/10.1182_blood-2005-09-3623/5/m_zh80070693470001.jpeg?Expires=1763504797&Signature=efBU-TYlMdCSN0xGw~dRoX3S67~g1b46aOs9WWURJQqPkIDxrHK3xmQhQBt5-td6qTPlzQN~2zN-5CD1STcDb82UCwEhmUEaUMxsPyblQSXbZGv2A3lDeVR5~A4oTnjn4VvWyd~p4TTXvlKp00znUz~tHra38Idb-rqDPNTQXtwK4-3~W56RAc14vj-qxMRo2CoNU3e6ll8FKNGokbyQLQS1iO4ib4~HR2iHCdY9foWzIhwGruMT-sLT8Pi-0N5RBhqWcElsD8z30U1A6j0CLUWw0j9yLKO64rLAiHdhqQBNdPaZCDYgiK3MIyIK76ocekuyuhiruhABP-rlBdzMfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Donor CD4+ T and B220+ B cells in transplants were required for chronic GVHD induction. (A) Sublethally irradiated (6.5 Gy [650 rad]) BALB/c recipients were injected with 50 × 106 whole spleen cells (whole-SPL), CD4+ T-cell-depleted spleen cells (CD4--SPL), CD8+ T-cell-depleted spleen cells (CD8--SPL), or B220+ B-cell-depleted spleen cells (B220--SPL). The recipients were checked twice a week for proteinuria, hair loss, and survival. The percentage of mice without proteinuria is shown in the left panel, and the percentage of survival is shown in the right panel. There were 8 mice in each group combined from 2 replicate experiments. (B) HE staining of skin tissue sections of BALB/c recipients given CD4--SPL cells, CD8--SPL cells, or B220--SPL cells. One representative is shown of 4 examined recipients in each group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/7/10.1182_blood-2005-09-3623/5/m_zh80070693470003.jpeg?Expires=1763504797&Signature=vjF55ECYKbl9xPOlt3q9N959QD3SJ6KrckguCbxDDu7fB-lV7xJRhaZ3JBdfZ1ZuXqgTZKtr6Vs~sFGx5XhvNzJSkoDXuQAwZjEUSYDpPuZKKbjlSwZJKBJFYgTiHw9mOiyYFOT5Vk-6rZn9kraEB9nOt9wdu5UbU4Y2899VqqYNuaXbujeKhZkzI~j~dxetz4uy1cmMCo-WNHa7vfvMT813uyDXj8hWsTQaCo-iGCORQDsHE0uUmBeMd1RFbKQYkiXDjd~izf2KMTi-2E9~VIokGPj4ST5t7chkCMaE6p2MtFelc~apl26MQRAC2fPhxJh071U6ARbX5exoPwALYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal