Abstract
Cytomegalovirus (CMV) infection is a major complication after allogeneic stem cell transplantation (SCT). Valganciclovir (V-GCV) is an oral prodrug hydrolyzed to the anti-CMV drug ganciclovir (GCV). A randomized, multicenter, crossover, open-label clinical trial compared exposure to GCV after V-GCV and intravenous GCV (IV-GCV) as preemptive therapy for CMV disease in SCT. The primary objective was to compare exposure to GCV in patients with CMV infection stratified for intestinal graft-versus-host disease (I-GVHD). Secondary objectives were the assessment of safety and efficacy. Patients without I-GVHD had a higher exposure to GCV after V-GCV when compared with IV-GCV (area under the concentration-time curve from drug administration to last observed concentration after 12 hours [AUC0-12] 53.8 ± 17.97 μg/mL · h [mean ± SD] vs 39.5 ± 13.91; P < .001; ratio of V-GCV/IV-GCV was 1.4; 90% confidence interval [CI], 1.2-1.5). This was also true in patients with I-GVHD grades I-II (AUC0-12 52.9 ± 21.75 vs 33.1 ± 12.97 μg/mL · h; P = .018; ratio 1.6; 90% CI, 1.3-2.0). Absolute bioavailability of GCV after V-GCV was approximately 75% in individuals with or without I-GVHD grades I-II. No severe GCV-related toxicity was observed and efficacy and safety was comparable (84-day follow-up). This supports the use of V-GCV in SCT, even in patients with I-GVHD grades I-II. Due to higher exposure after V-GCV compared with IV-GCV, patients should be monitored carefully for safety reasons.
Introduction
Cytomegalovirus (CMV) infection is a major cause of morbidity and mortality after allogeneic stem cell transplantation (SCT).1,2 Currently, preemptive therapy for CMV infection has to be performed intravenously (ie, using intravenous ganciclovir [IV-GCV; ganciclovir sodium] and/or foscarnet [Foscavir, trisodium phosphonoformate]) and is associated with hospitalization, high costs, and increased morbidity due to catheter-related complications. In addition, new transplantation modalities (eg, reduced-intensity conditioning protocols and haploidentical SCT) are associated with increased risks for late-onset CMV reactivation and disease. This underlines the need for an oral anti-CMV drug with sufficient bioavailability that can be used for outpatient care.
Prophylactic drug treatment is used to prevent CMV reactivation in every patient at risk,3-5 whereas preemptive therapy means initiating antiviral treatment only in patients with documented CMV infection in order to prevent the development of CMV disease.6-9 GCV preemptive therapy minimizes drug exposure to patients but relies on the availability of sensitive and specific CMV monitoring procedures.6-11
Valganciclovir (V-GCV; valganciclovir hydrochloride) is a monovalyl ester prodrug that, when administered orally, is rapidly hydrolyzed to GCV.12,13 It is indicated for the treatment of CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS) and for the prevention of CMV disease in high-risk kidney, heart, and kidney-pancreas transplantation patients. A previous study in liver transplant recipients has demonstrated that a dose of 1 × 900 mg V-GCV results in a GCV AUC0-24 (area under the concentration-time curve from drug administration to last observed concentration after 24 hours) similar to those obtained with 1 × 5 mg IV-GCV/kg body weight (BW).14 The safety and efficacy of its prophylactic use in solid organ transplantation (SOT) was comparable to3gof oral GCV.15 In addition, orally administered 2 × 900 mg V-GCV was as effective as 2 × 5 mg IV-GCV/kg BW for the induction and maintenance therapy of acute CMV retinitis in HIV-infected patients.16 The exposure to GCV (AUC0-24) after V-GCV treatment was less in the HIV population compared with patients after liver transplantation.
After SCT, graft-versus-host disease (GVHD) grades II-IV develops in approximately 30% to 50% of patients,17 and intestinal manifestations (I-GVHD) can alter the absorption of drugs from the gut.18 Allogeneic SCT-related myeloablative chemotherapy and total body irradiation are additional risk factors for malabsorption in the early phase after SCT. V-GCV has already been advocated in the medical literature for CMV preemptive therapy after allogeneic SCT,17 but because of safety reasons pharmacokinetics (pk) data need to be generated for the exposure to GCV as well as initial safety and efficacy data.19 We addressed this need using a randomized clinical trial.
Patients, materials, and methods
Study design
This was a prospective, randomized, multicenter, crossover, open-label clinical trial with V-GCV and IV-GCV. The primary objective was to compare the GCV AUC0-12 after administration of IV-GCV and V-GCV. Secondary objectives were to assess safety and efficacy aspects for the 2 treatment groups at crossover and after completing the treatment. Approval was obtained from the ethics committee at the University of Tübingen (272/2001). Informed consent was provided according to the Declaration of Helsinki.
The study patients were adults who underwent an allogeneic SCT and were at risk for CMV disease (Table 1). Patients were recruited and stratified according to the presence of I-GVHD. The patients who received transplants were screened for CMV infection using Cobas Amplicor CMV MONITOR (CMV-M; Roche Diagnostics, Mannheim, Germany), a commercially available quantitative polymerase chain reaction (PCR) test for plasma specimens with a limit of detection of 400 copies of CMV DNA/mL.11 Patients who tested positive up to day 100 after SCT were eligible for randomization. Other eligibility criteria were ability to take oral medication, an absolute neutrophil count (ANC) greater than 0.75 × 109/L (750 cells/μL) on 2 consecutive visits within 10 days prior to randomization, and a negative serum pregnancy test at randomization for female patients.
Baseline characteristics of the patients
. | Group A, val/gan; n = 26 . | Group B, gan/val; n = 22 . |
|---|---|---|
| Age, y* | 44.7 ± 12.91 | 44.9 ± 12.60 |
| Male sex, no. (%) | 15 (57.7) | 15 (68.2) |
| Ethnic origin, no. (%) | ||
| White | 26 (100) | 21 (95.5) |
| Other | 0 (0.0) | 1 (4.5) |
| Body mass index, kg/m2* | 23.3 ± 3.96 | 23.2 ± 3.39 |
| Intestinal GVHD, no. (%) | 3 (11.5) | 3 (13.6) |
| Day of randomization after transplantation, d | 51.5 ± 36.98 | 41.7 ± 13.91 |
| CMV status donor/recipient, no. (%) | ||
| Positive/positive | 17 (65.4) | 12 (54.5) |
| Positive/negative | 1 (3.8) | 3 (13.6) |
| Negative/positive | 8 (30.8) | 7 (31.8) |
| Donor/recipient relationship, no. (%)† | ||
| HLA-identical sibling donor | 14 (53.8) | 8 (36.4) |
| Matched unrelated donor | 9 (34.6) | 9 (40.9) |
| Mismatched related donor | 0 (0.0) | 2 (9.1) |
| Mismatched unrelated donor | 3 (11.5) | 2 (9.1) |
| Nonmyeloablative low intensity SCT, no. (%) | 6 (23.1) | 6 (27.3) |
| CMV-M positive, no.(%) | 24 (92.3) | 21 (95.5) |
. | Group A, val/gan; n = 26 . | Group B, gan/val; n = 22 . |
|---|---|---|
| Age, y* | 44.7 ± 12.91 | 44.9 ± 12.60 |
| Male sex, no. (%) | 15 (57.7) | 15 (68.2) |
| Ethnic origin, no. (%) | ||
| White | 26 (100) | 21 (95.5) |
| Other | 0 (0.0) | 1 (4.5) |
| Body mass index, kg/m2* | 23.3 ± 3.96 | 23.2 ± 3.39 |
| Intestinal GVHD, no. (%) | 3 (11.5) | 3 (13.6) |
| Day of randomization after transplantation, d | 51.5 ± 36.98 | 41.7 ± 13.91 |
| CMV status donor/recipient, no. (%) | ||
| Positive/positive | 17 (65.4) | 12 (54.5) |
| Positive/negative | 1 (3.8) | 3 (13.6) |
| Negative/positive | 8 (30.8) | 7 (31.8) |
| Donor/recipient relationship, no. (%)† | ||
| HLA-identical sibling donor | 14 (53.8) | 8 (36.4) |
| Matched unrelated donor | 9 (34.6) | 9 (40.9) |
| Mismatched related donor | 0 (0.0) | 2 (9.1) |
| Mismatched unrelated donor | 3 (11.5) | 2 (9.1) |
| Nonmyeloablative low intensity SCT, no. (%) | 6 (23.1) | 6 (27.3) |
| CMV-M positive, no.(%) | 24 (92.3) | 21 (95.5) |
Positive CMV infection was determined as CMV DNA ≥ 400 copies/mL. All plus/minus values are mean ± SD.
val indicates valganciclovir; gan, ganciclovir.
Based on n = 24 (group A) and n = 21 (group B)
Based on n = 26 (group A) and n = 21 (group B)
Patients were ineligible if they had established CMV disease or had received any systemic anti-CMV therapy within 30 days prior to randomization. Patients unable to take oral medication and those with impaired renal function (creatinine clearance < 0.41 mL/s [25 mL/min]) were also ineligible. Patients showing less than 30% or more than twice the average weight for their sex, age, and height were excluded (determined by the Metropolitan Life Insurance Company Weight Tables, National Center for Health Statistics, Hyattsville, MD).
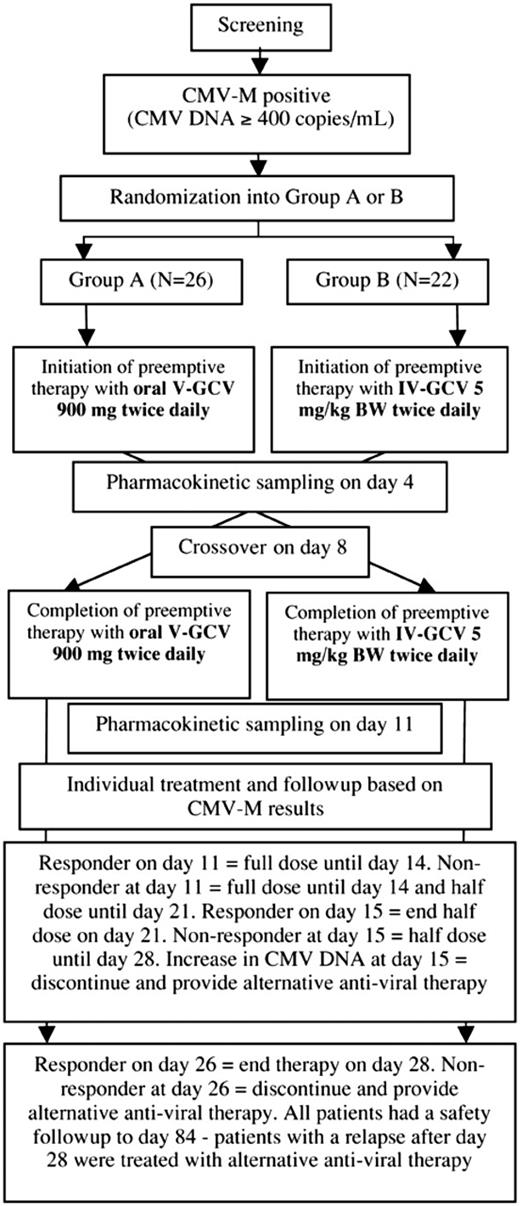
Eligible patients were randomized to 1 of 2 treatment groups (Figure 1): group A received 900 mg oral V-GCV (2 × 900 mg/d) for 7 days, crossing over on day 8 to IV-GCV given twice daily (2 × 5 mg/kg BW/d) until day 14; group B was treated vice versa. Compliance with the treatment regimen was assessed by maintaining drug dispensing records, and treatments were administered by clinical staff. For the purposes of the study, the administration of 90% of the planned study medication throughout the treatment period (until day 14) was considered adequate compliance. Patients experiencing impaired renal function (creatinine clearance < 1.00 mL/s [60 mL/min]) received a reduced dose of IV-GCV or V-GCV according to the manufacturer's recommendation. Individual decisions about the duration of therapy were made based on the results of CMV-M (Figure 1). All patients were monitored for 84 days (safety follow-up).
For safety reasons, the screening of vital signs, laboratory parameters for renal impairment, and analysis of the ANC were performed daily. Hematopoietic growth factors could be given in case of moderate neutropenia (ANC < 0.75 × 109/L [750 cells/μL]), or in the case of severe neutropenia (ANC < 0.50 × 109/L [500 cells/μL] for 2 or more consecutive days). Any dose adjustments due to changing renal function during the initial 14 days of treatment led to the exclusion of the patient from the pk analysis.
Study design. After completion of pharmacokinetic sampling on day 11, all treatment decisions were made based on the results of the Cobas Amplicor CMV MONITOR (CMV-M) assay. Using this assay, responders were defined as patients with CMV DNA less than 400 copies/mL. Nonresponders were defined as patients with CMV DNA at least 400 copies/mL. V-GCV indicates valganciclovir; and IV-GCV, intravenous ganciclovir.
Study design. After completion of pharmacokinetic sampling on day 11, all treatment decisions were made based on the results of the Cobas Amplicor CMV MONITOR (CMV-M) assay. Using this assay, responders were defined as patients with CMV DNA less than 400 copies/mL. Nonresponders were defined as patients with CMV DNA at least 400 copies/mL. V-GCV indicates valganciclovir; and IV-GCV, intravenous ganciclovir.
Pharmacokinetics
During the initial 14-day treatment period pk profiles of GCV were obtained after administration of V-GCV and IV-GCV in each of both study arms (crossover design). Pk assessments were scheduled at days 4 and 11 (Figure 1). Plasma samples were drawn 30 minutes prior to the dosing interval (minimal concentration [Cmin]); at administration of study drug (T0); 30 minutes thereafter; and subsequently 1, 2, 3, 4, 6, 8, and 12 hours after dosing. GCV plasma levels were used to generate individual pk profiles and to calculate the exposure to GCV (AUC0-12) following V-GCV and IV-GCV administration. Crossover to the second study medication was performed on day 8. A washout period was not applicable under clinical conditions for safety reasons (ongoing CMV infection), but pk analysis confirmed that 10 GCV half-lives were sufficient to prevent any overlapping effect resulting from the former study drug. No fasting period was required but a standardized breakfast was provided for patients receiving V-GCV (study drug was swallowed within 10 minutes of breakfast [T0]). For this study a standard breakfast was comparable to the following: bowl of cereal (cornflakes) with 100 mL whole milk; 2 slices toast with butter, marmalade, and cheese/sausage; 100 mL fresh orange juice; 150 mL decaffeinated tea or coffee. The dose of IV-GCV was given over a period of 1 hour at the rate of 50 mL/h using an intravenous infusion pump. For IV-GCV, T0 was the start of the infusion. Blood samples were collected using a catheter according to a standardized procedure in order to prevent any contamination. Samples were analyzed by Analytico Research B.V. (Bergschot, Breda, The Netherlands) using high-pressure liquid chromatography analysis and the following parameters were calculated for GCV: area under the concentration-time curve from drug administration to last observed concentration after 12 hours (AUC0-12), maximal concentration (Cmax), minimal concentration (Cmin), time to Cmax (tmax), and half-life (t1/2). It was accepted that up to one pk sample was not evaluable per pk profile. Absolute bioavailability of V-GCV was calculated as follows: [AUC0-12(GCV after V-GCV)/dose(GCV after V-GCV)]/[AUC0-12(IV-GCV)/dose(IV-GCV)], where GCV after V-GCV values are expressed in GCV equivalents, calculated as follows: doseV-GCV × molecular weight of GCV (free base)/molecular weight of V-GCV (free base) = doseV-GCV × 255/354.4.
CMV monitoring
Outcome parameters
The primary end point was the GCV AUC0-12 after V-GCV compared with IV-GCV based on the V-GCV/IV-GCV ratio. Efficacy was monitored regarding time to first response by treatment group determined as plasma CMV DNA clearance proven by CMV-M-negative testing (CMV DNA < 400 copies/mL) and the incidence of CMV disease until day 84 according to previously reported criteria.20 Safety analysis was based primarily on the incidence of serious adverse events (SAEs), CMV-related mortality, 100-day mortality, and overall mortality by treatment group. The numbers of patients with ANC values less than 1.0 × 109/L (1000 cells/μL) and less than 0.5 × 109/L (500 cells/μL) at days 8, 15, and 22 were also compared between treatment groups.
Statistical analysis
Patients were randomized using a dynamic treatment allocation procedure and stratified by the presence of I-GVHD.21 The results were summarized and presented using summary statistics. A Kaplan-Meier analysis22 was used to estimate time to first response between groups A and B. All pk parameters were analyzed separately in patients with and without I-GVHD. Geometric means were calculated for concentration-related parameters. For this exploratory study, no sample size calculation was performed but it was planned to have a minimum of 20 patients without I-GVHD with an evaluable pk profile.
For the primary end point AUC0-12 of GCV after V-GCV compared with IV-GCV, the ratio of AUC0-12 means (V-GCV/IV-GCV) and the 2-sided 90% confidence interval for the ratio of AUC0-12 means were calculated. AUC0-12 was calculated using the linear trapezoidal rule. An analysis of variance with the factors patient, sequence, period, and treatment was applied to the logarithmically transformed variable in AUC0-12 using the main effects model to estimate the difference between treatment sequences and within patient variance (σ2) and using the 2-sided 90% confidence interval for the difference. To obtain the estimates of the ratio of AUC0-12 means and the 2-sided 90% confidence interval for the ratio of AUC0-12 means, the derived least squares means difference and the respective confidence limits were back transformed using the exponential function. In addition, pk parameters were compared using a paired t test. Safety and efficacy parameters were analyzed descriptively for the 2 groups.
Results
Characteristics of the patients
Between February 2002 and July 2003, 48 allograft recipients were randomized to treatment: 26 to group A (receiving V-GCV first) and 22 to group B (receiving IV-GCV first). In each group there were 3 patients with I-GVHD (Table 1) as defined clinically (n = 1) or by histopathology (n = 5; Table 2). Twenty patients were excluded from the pk analysis due to 23 protocol violations (n = 9 in group A and n = 11 in group B; patients could have more than one protocol violation leading to exclusion from the pk analysis); these were patients who received the incorrect dose of IV-GCV and/or V-GCV (eg, due to dose adjustment for changes in creatinine clearance or the administered dose was too high or too low by error; this occurred in 8 patients), discontinued the study drug without completing both pk profiles (this occurred in 8 patients), had violations in sampling (this occurred in 6 patients), or were entered more than 200 days after SCT (this occurred for 1 patient). No patients were excluded from the pk analysis specifically for a lack of compliance with study medication (< 90% of the planned study medication until day 14), and no patients with I-GVHD were excluded from the pk analysis due to protocol violations. Three patients who were randomized after testing positive with an in-house PCR assay but without being tested positive by CMV-M during the screening period were excluded from the efficacy analysis. Therefore pk parameters were determined for 28 patients (17/26 groupAand 11/22 group B; n = 22 for patients without I-GVHD and n = 6 for patients with I-GVHD); 45 patients (24/26 group A and 21/22 group B) were included in the efficacy analysis and all 48 randomized patients in the safety analysis. One I-GVHD patient had severe I-GVHD grade III (Table 2) and, because of proven severe malabsorption, this patient is presented separately (n = 5 for patients with I-GVHD grades I-II).
Clinical characteristics of intestinal GVHD (I-GVHD) patients
. | GVHD grading . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Patient . | Overall . | I-GVHD . | Biopsy-proven I-GVHD . | GCV pk after V-GCV administration . | Remark . | |||
| Group A, val/gan | ||||||||
| 1 | I | I | Yes | Adequate | Diarrhea > 500 mL/day | |||
| 2 | III | II | Yes | Adequate | Diarrhea > 1000 mL/day | |||
| 3 | II | III | Yes | Severe malabsorption | Severe I-GVHD (diarrhea > 1000 mL/day) for weeks, decreasing prior to randomization; GVHD of the liver (grade II) | |||
| Group B, gan/val | ||||||||
| 1 | II | I | No* | Adequate | Diarrhea (≤ 10 × in total 600 mL/day); severe GVHD of the skin (grade III) | |||
| 2 | II | II | Yes | Adequate | Outpatient care | |||
| 3 | III | II | Yes | Adequate | Outpatient care | |||
. | GVHD grading . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Patient . | Overall . | I-GVHD . | Biopsy-proven I-GVHD . | GCV pk after V-GCV administration . | Remark . | |||
| Group A, val/gan | ||||||||
| 1 | I | I | Yes | Adequate | Diarrhea > 500 mL/day | |||
| 2 | III | II | Yes | Adequate | Diarrhea > 1000 mL/day | |||
| 3 | II | III | Yes | Severe malabsorption | Severe I-GVHD (diarrhea > 1000 mL/day) for weeks, decreasing prior to randomization; GVHD of the liver (grade II) | |||
| Group B, gan/val | ||||||||
| 1 | II | I | No* | Adequate | Diarrhea (≤ 10 × in total 600 mL/day); severe GVHD of the skin (grade III) | |||
| 2 | II | II | Yes | Adequate | Outpatient care | |||
| 3 | III | II | Yes | Adequate | Outpatient care | |||
val indicates valganciclovir; and gan, ganciclovir.
This patient had diarrhea between 500 and 1000 mL/day and a coexisting cutaneous aGVHD grade III, indicating the diarrhea to be due to intestinal GVHD
Dose adjustments due to changes in creatinine clearance occurred 7 times in 4 patients. During the study 9 of 48 patients discontinued treatment with V-GCV and 6 of 48 treatment with IV-GCV. Discontinuation from treatment before both pk profiles were complete also led to exclusion from the pk analysis (see above). The main reason for discontinuation was treatment failure (2 and 3 patients for V-GCV and IV-GCV, respectively), with all these patients showing an increase in CMV DNA during treatment. Only 4 patients discontinued due to dose adjustments (2 patients each for V-GCV and IV-GCV). Other reasons for discontinuation were adverse events (2 patients from V-GCV for a central line infection and neutropenia, respectively, and 1 patient from IV-GCV due to neutropenia) and protocol violations (3 patients for V-GCV).
Pharmacokinetics
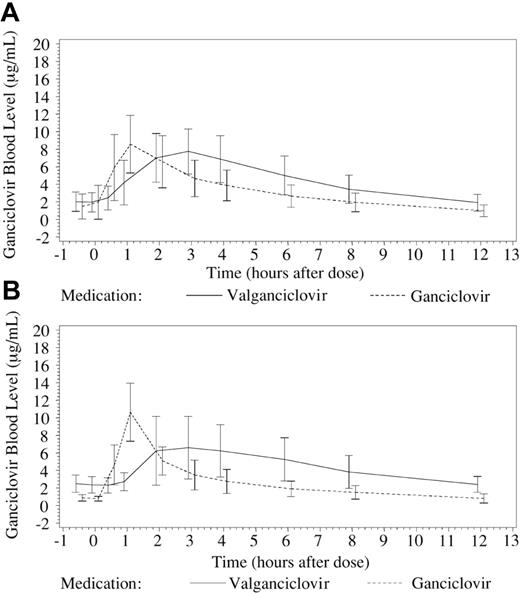
In patients without I-GVHD (n = 22), using a fixed oral dose of V-GCV 2 × 900 mg/d led to a significantly higher exposure to GCV (AUC0-12 53.8 ± 17.97 μg/mL · h [mean ± SD]) compared with a BW-adjusted dose of IV-GCV 2 × 5 mg/kg/d (AUC0-12 39.5 ± 13.91 μg/mL · h; P < .001, paired t test for difference). The V-GCV/IV-GCV ratio was 1.4 (Table 3). For 3 patients without I-GVHD, creatinine clearance was slightly below 1.00 mL/s (60 mL/min) at the day of pk analysis (0.98, 0.95, 0.93 mL/s [59, 57, 56 mL/min]). Excluding these patients from the pk analysis (n = 19) does not alter the overall result (AUC0-12 52.1 ± 15.26 μg/mL · h vs AUC0-12 38.5 ± 13.99 μg/mL · h for V-GCV vs IV-GCV, respectively; P < .001, paired t test for difference; ratio 1.4). A significant difference was also observed in exposure to GCV for 5 patients with I-GVHD grades I-II between treatment with V-GCV (AUC0-12 52.9 ± 21.75 μg/mL · h) and IV-GCV (AUC0-12 33.1 ± 12.97 μg/mL · h; P = .018, paired t test for difference; Table 3). The V-GCV/IV-GCV ratio within this subpopulation was 1.6. Profiles for blood levels of GCV are shown in Figure 2 for both populations. For one patient with biopsy-proven severe I-GVHD grade III, oral absorption following V-GCV was inadequate, and a low trough level (Cmin; 0.4 μg/mL) and an inadequate AUC0-12 (14.8 μg/mL · h) following dosing were documented.
Pharmacokinetic parameters for ganciclovir after treatment with intravenous ganciclovir and oral valganciclovir
. | No. of patients . | Body weight, kg . | AUC0-12, μg/mL · h . | Cmax, μg/mL . | Cmin, μg/mL . | Tmax, h . | t1/2, h . |
|---|---|---|---|---|---|---|---|
| Patients without intestinal GVHD | |||||||
| Oral valganciclovir | 22 | 72.26 ± 11.49 | 53.8 ± 17.97 | 8.8 ± 2.38 | 1.7 ± 0.94 | 2.7 ± 0.75 | 4.2 ± 1.08 |
| Intravenous ganciclovir | 22 | 72.26 ± 11.49 | 39.5 ± 13.91 | 10.3 ± 2.12 | 1.0 ± 0.65 | 1.1 ± 0.64 | 3.4 ± 0.81 |
| Ratio, 90% CI (range) | 22 | 72.26 ± 11.49 | 1.4 (1.2-1.5) | 0.8 (0.8-0.9) | ND | ND | ND |
| P | 22 | 72.26 ± 11.49 | < .001 | .013 | < .001 | < .001 | < .001 |
| Patients with intestinal GVHD I-II | |||||||
| Oral valganciclovir | 5 | 60.22 ± 5.06 | 52.9 ± 21.75 | 8.0 ± 3.25 | 1.9 ± 0.77 | 2.9 ± 0.82 | 6.1 ± 1.92 |
| Intravenous ganciclovir | 5 | 60.22 ± 5.06 | 33.1 ± 12.97 | 10.6 ± 3.30 | 0.7 ± 0.36 | 1.0 ± 0.07 | 3.4 ± 0.74 |
| Ratio, 90% CI (range) | 5 | 60.22 ± 5.06 | 1.6 (1.3-2.0) | 0.7 (0.5-1.0) | ND | ND | ND |
| P | 5 | 60.22 ± 5.06 | .018 | .084 | .016 | .007 | .041 |
| Patient with intestinal GVHD III and severe malabsorption | |||||||
| Oral valganciclovir | 1 | 49.7 | 14.8 | 2.6 | 0.4 | 2.0 | 3.4 |
| Intravenous ganciclovir | 1 | 49.7 | 46.6 | 13.2 | 1.1 | 2.0 | 3.0 |
. | No. of patients . | Body weight, kg . | AUC0-12, μg/mL · h . | Cmax, μg/mL . | Cmin, μg/mL . | Tmax, h . | t1/2, h . |
|---|---|---|---|---|---|---|---|
| Patients without intestinal GVHD | |||||||
| Oral valganciclovir | 22 | 72.26 ± 11.49 | 53.8 ± 17.97 | 8.8 ± 2.38 | 1.7 ± 0.94 | 2.7 ± 0.75 | 4.2 ± 1.08 |
| Intravenous ganciclovir | 22 | 72.26 ± 11.49 | 39.5 ± 13.91 | 10.3 ± 2.12 | 1.0 ± 0.65 | 1.1 ± 0.64 | 3.4 ± 0.81 |
| Ratio, 90% CI (range) | 22 | 72.26 ± 11.49 | 1.4 (1.2-1.5) | 0.8 (0.8-0.9) | ND | ND | ND |
| P | 22 | 72.26 ± 11.49 | < .001 | .013 | < .001 | < .001 | < .001 |
| Patients with intestinal GVHD I-II | |||||||
| Oral valganciclovir | 5 | 60.22 ± 5.06 | 52.9 ± 21.75 | 8.0 ± 3.25 | 1.9 ± 0.77 | 2.9 ± 0.82 | 6.1 ± 1.92 |
| Intravenous ganciclovir | 5 | 60.22 ± 5.06 | 33.1 ± 12.97 | 10.6 ± 3.30 | 0.7 ± 0.36 | 1.0 ± 0.07 | 3.4 ± 0.74 |
| Ratio, 90% CI (range) | 5 | 60.22 ± 5.06 | 1.6 (1.3-2.0) | 0.7 (0.5-1.0) | ND | ND | ND |
| P | 5 | 60.22 ± 5.06 | .018 | .084 | .016 | .007 | .041 |
| Patient with intestinal GVHD III and severe malabsorption | |||||||
| Oral valganciclovir | 1 | 49.7 | 14.8 | 2.6 | 0.4 | 2.0 | 3.4 |
| Intravenous ganciclovir | 1 | 49.7 | 46.6 | 13.2 | 1.1 | 2.0 | 3.0 |
P values are for paired t tests for difference. All plus/minus values are mean ± SD.
AUC indicates the area under the concentration-time curve; Cmax, the maximal concentration; Cmin, the minimum concentration; Tmax, the time to Cmax; t1/2, the half-life of ganciclovir; and ND, not done.
The absolute bioavailability of GCV after V-GCV in patients without I-GVHD was 76.4% ± 18.27% (mean ± SD; n = 22) and 74.5% ± 14.39% in SCT patients suffering from I-GVHD grades I-II (n = 5).
Efficacy
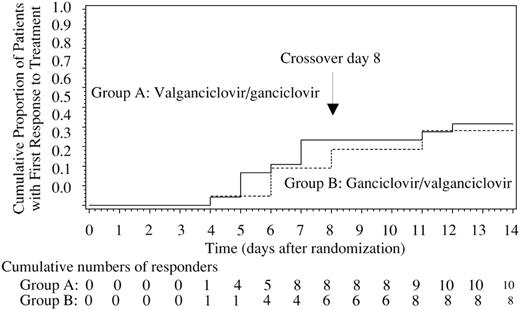
All 45 patients analyzed for efficacy recorded a positive CMV-M test at randomization. The Kaplan-Meier estimates of time to first response and cumulative responders within the initial 14-day treatment period are provided in Figure 3.
Overall, at the end of the safety follow-up (day 84), the total number of patients with response to therapy (CMV DNA < 400 copies/mL) was 37 of 45 (82.2%): 21 of 24 in group A and 16 of 21 in group B (87.5% vs 76.2%). For 9 of 45 (20.0%) patients, CMV infection recurred after first response to treatment (7/21 [33.3%] in group A and 2/16 [12.5%] in group B).
Blood levels for ganciclovir over 12-hour dosing period (mean ± SD). Plasma samples were drawn 30 minutes prior to the dosing interval; at administration of study drug (T0); 30 minutes thereafter; and subsequently 1, 2, 3, 4, 6, 8, and 12 hours after dosing. GCV plasma levels (mean ± SD) were determined using high-pressure liquid chromatography and were used to generate pk profiles following administration of valganciclovir (V-GCV) and intravenous ganciclovir (IV-GCV). (A) Pk profiles for patients without intestinal graft-versus-host disease (I-GVHD; n = 22). (B) Pk profiles for patients with I-GVHD grades I-II (n = 5). One patient with I-GVHD grade III is not included. Time points are offset for clarity.
Blood levels for ganciclovir over 12-hour dosing period (mean ± SD). Plasma samples were drawn 30 minutes prior to the dosing interval; at administration of study drug (T0); 30 minutes thereafter; and subsequently 1, 2, 3, 4, 6, 8, and 12 hours after dosing. GCV plasma levels (mean ± SD) were determined using high-pressure liquid chromatography and were used to generate pk profiles following administration of valganciclovir (V-GCV) and intravenous ganciclovir (IV-GCV). (A) Pk profiles for patients without intestinal graft-versus-host disease (I-GVHD; n = 22). (B) Pk profiles for patients with I-GVHD grades I-II (n = 5). One patient with I-GVHD grade III is not included. Time points are offset for clarity.
Only 2 patients developed CMV disease, both cases occurred during the follow-up period on days 40 and 55 in group B. Both patients developed CMV interstitial pneumonia (CMV-IP) and responded to IV-GCV plus CMV hyperimmunoglobulin.
Safety
The primary safety analysis did not show major differences between group A and group B (Table 4). There was no study medication-related mortality and the overall safety was excellent with regard to SCT patients. The overall incidence, severity, and type of adverse events (AEs) were similar between treatments (25/26 [96.2%] experienced 264 AEs and 22/22 [100%] experienced 229 AEs in group A and group B, respectively).
Serious adverse events and overall mortality
. | Group A, val/gan; n = 26 . | Group B, gan/val; n = 22 . |
|---|---|---|
| Serious adverse events* | ||
| Total no. of patients with a serious adverse event (%) | 15 (57.7) | 16 (72.7) |
| Myelotoxicity (%) | 3 (11.5) | 3 (13.6) |
| Anemia | 2 | 0 |
| Febrile neutropenia | 0 | 1 |
| Severe neutropenia†/pancytopenia | 1 | 1 |
| Thrombocytopenic purpura | 0 | 1 |
| Gastrointestinal disorders (%) | 2 (7.7) | 1 (4.5) |
| Diarrhea | 0 | 1 |
| Gastrointestinal hemorrhage | 2 | 0 |
| Secondary infections (%) | 8 (30.8) | 8 (36.4) |
| Fungal infection | 1 | 0 |
| Recurrent CMV infections | 3 | 4 |
| CMV interstitial pneumonia (CMV-IP) | 0 | 2§ |
| Total no. of patients w/serious adverse event leading to withdrawal (%) | 0 (0.0) | 1 (4.5) |
| Severe neutropenia | 0 (0.0) | 1 (4.5) |
| Mortality | ||
| Overall mortality (%)‡ | 2 (7.7) | 4 (18.2) |
| 100-day mortality | 1∥ | 3¶ |
| Fungal pneumonia | 1; day 32 | 0 |
| Non-CMV respiratory failure | 0 | 1; day 23 |
| Progressive disease | 0 | 2; days 62 and 76 |
. | Group A, val/gan; n = 26 . | Group B, gan/val; n = 22 . |
|---|---|---|
| Serious adverse events* | ||
| Total no. of patients with a serious adverse event (%) | 15 (57.7) | 16 (72.7) |
| Myelotoxicity (%) | 3 (11.5) | 3 (13.6) |
| Anemia | 2 | 0 |
| Febrile neutropenia | 0 | 1 |
| Severe neutropenia†/pancytopenia | 1 | 1 |
| Thrombocytopenic purpura | 0 | 1 |
| Gastrointestinal disorders (%) | 2 (7.7) | 1 (4.5) |
| Diarrhea | 0 | 1 |
| Gastrointestinal hemorrhage | 2 | 0 |
| Secondary infections (%) | 8 (30.8) | 8 (36.4) |
| Fungal infection | 1 | 0 |
| Recurrent CMV infections | 3 | 4 |
| CMV interstitial pneumonia (CMV-IP) | 0 | 2§ |
| Total no. of patients w/serious adverse event leading to withdrawal (%) | 0 (0.0) | 1 (4.5) |
| Severe neutropenia | 0 (0.0) | 1 (4.5) |
| Mortality | ||
| Overall mortality (%)‡ | 2 (7.7) | 4 (18.2) |
| 100-day mortality | 1∥ | 3¶ |
| Fungal pneumonia | 1; day 32 | 0 |
| Non-CMV respiratory failure | 0 | 1; day 23 |
| Progressive disease | 0 | 2; days 62 and 76 |
val indicate valganciclovir; gan, ganciclovir.
Details are provided for selected serious adverse events
ANC < 500 cells/μL for 2 or more consecutive days
No deaths occurred in patients with intestinal GVHD
Both patients who developed CMV-IP had completed study drug administration since 11 days (n = 1) and 40 days (n = 1), respectively, and recovered completely
One additional patient died after safety follow-up from relapse of acute myeloid leukemia
One additional patient died from RSV pneumonia at day 110 after randomization
Kaplan-Meier curves showing time to first response within the first 14 days of treatment. Responders were identified using the Cobas Amplicor CMV MONITOR (CMV-M) assay. The cumulative proportion of patients with a first response to treatment (CMV DNA < 400 copies/mL) and the cumulative number of responders are provided.
Kaplan-Meier curves showing time to first response within the first 14 days of treatment. Responders were identified using the Cobas Amplicor CMV MONITOR (CMV-M) assay. The cumulative proportion of patients with a first response to treatment (CMV DNA < 400 copies/mL) and the cumulative number of responders are provided.
Pyrexia was the most common AE reported for 12 (46.2%) of 26 patients in group A and 11 (50.0%) of 22 patients in group B. Neutropenia was reported as an AE for 5 (19.2%) of 26 patients in group A and 8 (36.4%) of 22 patients in group B. For patients with ANC counts at days 8, 15, and 22, the numbers of patients with ANC values less than 1.0 × 109/L (1000 cells/μL) in group A and group B were 3 (14.3%) of 21 and 4 (20.0%) of 20 at day 8, 2 (10.5%) of 19 and 3 (17.6%) of 17 at day 15, and 5 (31.3%) of 16 and 2 (15.4%) of 13 at day 22, respectively. Patients with ANC values less than 0.5 × 109/L (500 cells/μL) in group A and group B were 0 and 1 (5.0%) of 20 at day 8, none at day 15, and 2 (12.5%) of 16 and 1 (7.7%) of 13 at day 22, respectively. Hematopoietic growth factors were administered to a total of 17 patients during the study (11/26 [42.3%] in group A and 6/22 [27.3%] in group B).
Discussion
This is the first clinical trial investigating an oral V-GCV induction dose for preemptive therapy in patients with CMV infection following allogeneic SCT. A twice-daily dose of 900 mg V-GCV led to a significantly higher exposure to GCV (AUC0-12) than 2 × 5 mg/kg/d IV-GCV, even in patients with I-GVHD grades I-II. The absolute bioavailability of GCV in SCT patients was comparable for patients with I-GVHD grades I-II and without I-GVHD (approximately 75%). This excellent bioavailability in the SCT population was linked with an increased GCV AUC0-12 during V-GCV induction therapy when compared with HIV patients treated for acute CMV retinitis (53.8 ± 17.97 vs 32.8 ± 10.1 μg/mL · h).16 A potential damage to the intestinal tract following myeloablative conditioning therapy may contribute to the increase in bioavailability of GCV after V-GCV in SC transplant recipients when compared with HIV and SOT patients. For both HIV and SOT patients the bioavailability of GCV after V-GCV treatment was demonstrated to be approximately 60%, although overall exposure was different between HIV and SOT patients (AUC0-24 34.9 vs 41.7 μg/mL · h).14,16 As reasons to explain the differences in exposure variations in glomerular filtration and tubular secretion of GCV, underlying disease and comedication between these patient populations are possible.
The concentration time profile (AUC0-12) of GCV following V-GCV treatment demonstrated a slower rate of absorption, with a lower maximal blood concentration (Cmax) but a longer time to elimination (t1/2), leading to higher dosing interval trough levels (Cmin) compared with IV-GCV treatment. This was observed both for patients with and without I-GVHD grades I-II, indicating that these patients can be offered treatment with oral V-GCV. Due to the observed malabsorption of GCV after V-GCV treatment in one patient suffering from severe I-GVHD grade III, the authors recommend IV-GCV treatment for patients with severe I-GVHD associated with significant malabsorption.
The lower GCV Cmax observed with oral V-GCV treatment than IV-GCV treatment is a known phenomenon for pk comparisons of V-GCV and IV-GCV16 and was statistically significant in the current trial. However, previous authors have indicated that the clinical efficacy of GCV is driven by total drug exposure (AUC) and not by Cmax,13 indicating that a lower Cmax should not compromise any potential efficacy. In the current paper, the AUC0-12 of GCV was significantly greater after V-GCV treatment when compared with a standard, effective dose of IV-GCV and is higher when compared with HIV patients treated for acute CMV retinitis.
Because this pk trial was performed under clinical conditions, a washout period was not feasible for safety reasons. However, for the pk analysis, no contamination issues were expected after crossover, as the steady-state of GCV has been described to be completed within 24 hours and in our protocol the patient was treated for at least 72 hours before the pk profile was generated. The crossover design also allowed determination of intersubject/patient variability in the GCV pk profile following administration of IV-GCV and V-GCV, respectively. In addition, the bioavailability of GCV can be estimated in this patient population. A disadvantage of this crossover design was that safety and efficacy aspects were not attachable to the individual study drug intervals. Patients received both treatments in a short time frame and there is a known delay in the onset of key safety issues (ie, neutropenia) and readout parameters for efficacy (ie, CMV viral load clearance). To confirm the safety and efficacy of V-GCV treatment in SCT, another prospective clinical trial is necessary.
Renal toxicity was minimal, and dose adjustments due to decreased creatinine clearance were performed in only 4 of 48 patients. However physicians should be aware that renal function can change rapidly in SCT patients and that immediate dose adaptation of GCV is required. Alteration of renal function within the study period was a major reason that randomized patients were not eligible for pk analysis. However it must be noted that the exclusion of 3 patients without I-GVHD with a known reduced creatinine clearance from the pk analysis did not alter the overall result and the exposure to GCV after V-GCV treatment was still higher compared with IV-GCV treatment.
Clearance of viral load in patients who received V-GCV as their initial therapy was similar to patients who received IV-GCV first (response rates). Only 2 patients developed CMV disease (CMV-IP) during follow-up when receiving no antiviral chemotherapy and recovered completely. No patient died due to CMV disease or from events related to study drug. The rate of serious neutropenia was low and favorable when compared with earlier reports on prophylactic or preemptive treatment with GCV after SCT.3-6,23,24
In conclusion, the exposure to GCV after the administration of 1800 mg/d V-GCV for preemptive therapy is significantly higher compared with standard therapy using 10 mg/kg/d IV-GCV. This is also true for patients suffering from I-GVHD grades I-II. Taking into account more than one decade of experience in using IV-GCV in SCT, the authors do not consider that a change in the standard dosing of this medication should be considered (10 mg/kg/d IV-GCV). Moreover there should be careful consideration before transferring these patients to oral preemptive therapy using V-GCV, as there will be a significantly higher exposure to GCV and currently V-GCV is not approved for use in SCT (any use would be “off-label”). Daily ANC and creatinine clearance monitoring would be essential tools to improve the safety of V-GCV in SCT patients, and in the case of reduced renal function it is essential to adjust the dose of V-GCV appropriately. Therapeutic drug monitoring of GCV concentrations is also possible and may be an option if dose finding for V-GCV is a problem in a single patient. Overall, the results support the use of short-term preemptive therapy with V-GCV in combination with adequate CMV monitoring,25,26 but the crossover design means that clear statements regarding efficacy and safety cannot be made and there is still a need for a larger, confirmative study evaluating the safety and efficacy of V-GCV preemptive therapy in patients after allogeneic SCT.
Appendix
The study group was as follows: Einsele H (University of Würzburg, Würzburg, Germany); Hebart H, Schmalzing M (University of Tübingen, Tübingen, Germany); Reusser P, Gratwohl A (Division of Hematology, University Hospital Basel, and Hospital of Jura, Porrentruy, Switzerland); Bornhäuser M, Ehninger G (University of Dresden, Dresden, Germany); Kröger N, Kratochwille A, Zander AR (University of Hamburg, Hamburg, Germany); Hertenstein B, Mischak-Weissinger EM, Buchholz S, Ganser A (Hannover Medical School, Hannover, Germany); Kalhs P, Rabitsch W (Medical University of Vienna, Vienna, Austria); Chalandon Y, Chapuis B (University of Geneva, Geneva, Switzerland); Schüler P, Raab C, Dawes K, Bruns R, Reister M, Leskopf W, Esken W, Straub C, Bruckmann K, Strunck E, Wiecker B (PRA International, Mannheim, Germany); Rohde F, Birras C, Rose MT (Hoffmann-La Roche AG, Grenzach-Wyhlen, Germany).
Prepublished online as Blood First Edition Paper, December 13, 2005; DOI 10.1182/blood-2005-09-3786.
A complete list of the members of the European Group for Blood and Marrow Transplantation (EBMT) Working Group for Infectious Disease appears in the “Appendix.”
This study (MX16451) was sponsored by Hoffmann-La Roche AG (Grenzach-Wyhlen, Germany) on behalf of F. Hoffmann-La Roche Ltd (Basel, Switzerland) in cooperation with H.E., P.R., M.B., P.K., G.E., H.H., Y.C., N.K., and B.H.
One of the authors (F.R.) has declared a financial interest in Hoffmann-La Roche, whose products were studied in the present work. One of the authors (F.R.) is employed by Hoffmann-La Roche, whose products were studied in the present work.
H.E. and P.R. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank all members of the study group for their participation in this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal