Infection with HHV-8 in transplant recipients has been associated with severe HHV-8 primary infection and is responsible for the death of 3 of the 5 reported cases.1-4 Here we report a case of severe HHV-8 primary infection that was transmitted through a renal allograft and successfully treated with rituximab.
A 47-year-old man, seronegative for HHV-8, received a first renal transplant from an HHV-8 seropositive cadaveric donor to treat end-stage renal failure due to nephroangiosclerosis. Eight months later, the patient was hospitalized due to fever, night sweats, pharyngeal pain, and weakness. Upon physical examination, weight loss, hepatosplenomegaly, and pharyngeal erythema were found, and laboratory tests showed pancytopenia. Bone marrow aspiration showed normal cellularity, with a slight excess of myeloblasts, dysplastic maturing granulocyte precursors, moderate plasmacytosis, and no evidence of hemophagocytosis. Routine blood and urines cultures for common bacterial and fungal pathogens were negative. No evidence of type 1 or type 2 herpesviruses, varicella-zoster virus, Epstein-Barr virus, or parvovirus B19 DNA was detected in the patient's serum. Severe HHV-8 primary infection transmitted through the renal allograft was suspected and was confirmed by the detection of HHV-8 DNA by quantitative polymerase chain reaction (PCR) assay in peripheral blood mononuclear cells (PBMCs), bone marrow, and pharyngeal samples.
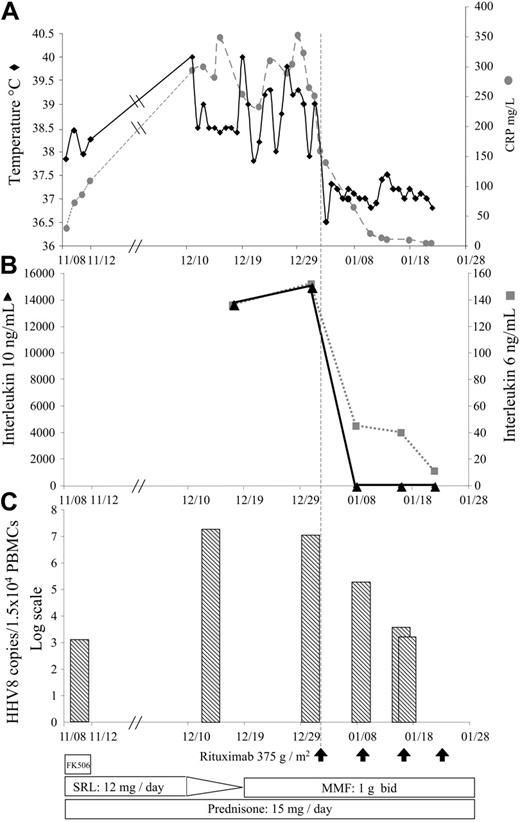
Despite a drastic reduction of the immunosuppression (Figure 1), the patient's clinical condition worsened, and rituximab was started with the written informed consent of the patient. Its initiation was followed by a rapid reduction of circulating B cells and was associated with a dramatic improvement in the patient's general condition (Figure 1A) and performance status. Hepatosplenomegaly and pharyngitis were no longer found on physical examination 2 weeks after the beginning of treatment. At one month, both routine laboratory tests and bone marrow aspiration were normal. The HHV-8 load in PBMCs started to fall after the first infusion of rituximab and became negative after the third infusion (Figure 1C), as did the levels of serum interleukin 6 (IL-6) and IL-10 (Figure 1B). The patient was finally discharged the day after the fourth injection of rituximab.
After a follow-up of 3 years, the patient is clinically well, and neither Kaposi sarcoma nor lymphoproliferative disease has been diagnosed. Serologic tests remain negative for HHV-8 LANA-1, and HHV-8 DNA in PBMCs remains undetectable by PCR assay.
We resorted to rituximab as rescue therapy because we postulated that the rapid eradication of HHV-8-infected B cells5 might reduce viral burden and prevent the proliferation and activation of reactive T cells and macrophages. Our decision was further supported by previous demonstrations that CD20 antigen was expressed by HHV-8-infected cells6,7 and later by the results reported by Milone et al8 for 2 patients with primary Epstein-Barr virus infection and X-linked lymphoproliferative disease.
Although further studies are required to confirm this efficacy, we believe that rituximab therapy should be considered as the initial management of severe HHV-8 primary infection.
Summary of the patient's clinical history from admission to discharge. (A) Changes in body temperature and serum C reactive protein. (B) Changes in the serum IL-6 and IL-10 levels. (C) Changes in the human herpesvirus-8 viral load evaluated in peripheral blood mononuclear cells by quantitative PCR assay (log scale). Reduction of the immunosuppressive regimen are indicated at the bottom of the figure. Rituximab infusions (375 g/m2) are indicated by arrows. FK506 indicates tacrolimus; SRL, sirolimus; and MMF, mycophenolate mofetil.
Summary of the patient's clinical history from admission to discharge. (A) Changes in body temperature and serum C reactive protein. (B) Changes in the serum IL-6 and IL-10 levels. (C) Changes in the human herpesvirus-8 viral load evaluated in peripheral blood mononuclear cells by quantitative PCR assay (log scale). Reduction of the immunosuppressive regimen are indicated at the bottom of the figure. Rituximab infusions (375 g/m2) are indicated by arrows. FK506 indicates tacrolimus; SRL, sirolimus; and MMF, mycophenolate mofetil.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal