Abstract
NF-κB is a transcription factor that controls the expression of a number of genes important for mediating immune and inflammatory responses. In this study, we examined whether bortezomib and PS-1145, each of which inhibits NF-κB, could protect mice from lethal graft-versus-host disease (GVHD), which is characterized by immune activation and proinflammatory cytokine production. When administered within the first 2 days after transplantation, bortezomib and PS-1145 both protected mice from fatal GVHD, did not compromise donor engraftment, and effected marked reduction in the levels of serum cytokines that are normally increased during GVHD. Extending the course of bortezomib administration or delaying the initiation of this agent for as few as 3 days after bone marrow transplantation (BMT), however, significantly exacerbated GVHD-dependent mortality because of severe pathological damage in the colon. In contrast, prolonged administration of PS-1145, which, unlike bortezomib, is a selective inhibitor of NF-κB, caused no early toxicity and resulted in more complete protection than that observed with an abbreviated PS-1145 treatment schedule. These results confirm a critical role for NF-κB in the pathophysiology of GVHD and indicate that targeted inhibition of NF-κB may have a superior therapeutic index and may constitute a viable therapeutic approach to reduce GVHD severity. (Blood. 2006;107:827-834)
Introduction
Graft-versus-host disease (GVHD) is the major complication after allogeneic stem cell transplantation. It is a complex pathophysiologic process best conceptualized as a series of events that begins with the conditioning regimen and is amplified by cooperative interactions of multiple effector cell populations resident in the donor graft and persistent in the host.1 The initial event in GVHD pathophysiology derives from the conditioning regimen that results in host tissue damage, proinflammatory cytokine release, MHC and adhesion molecule up-regulation, and chemokine production.2-4 Donor T-cell recognition of host alloantigens subsequently results in T-cell activation,5,6 further cytokine secretion,7 and acquisition of effector cell function, resulting in damage to host tissues through perforin/granzyme, fas/fas ligand, and other cytotoxic pathways.8-11 Moreover, the release of cytokines leads to the recruitment of secondary effector cell populations (eg, macrophages, NK cells) that cause additional proinflammatory cytokine production.12 Damage to host tissue, particularly the gastrointestinal tract, results in the translocation of endotoxin across damaged mucosal barriers, further exacerbating the cytokine cascade.13 The sum effect of these events is a proinflammatory environment that results in significant tissue destruction and, in some cases, death.
NF-κB is a transcription factor that is found in virtually all cell types and that controls the expression of a number of genes important for mediating immune and inflammatory responses. NF-κB is a heterodimer composed of different combinations of members of the NF-κB/Rel family, of which the p50/p65 complex appears to be the most abundant.14 NF-κB is retained in an inactive form in the cytoplasm by the IκB family of inhibitors.15,16 Various stimuli, including proinflammatory cytokines such as IL-1 and TNF-α, which are produced during the course of GVHD, activate IκB kinase (IKK), which then phosphorylates IκBα.17 Phosphorylation of IκBα on 2 serine residues results in the ubiquitination and degradation of IKβ by the 26S proteasome, allowing the translocation of NF-κB into the nucleus, where it is able to induce gene transcription,18 resulting in the production of proteins necessary for immune and inflammatory responses. The critical role of this molecule has, therefore, made it a viable target for the treatment of patients with a number of inflammatory disorders.19,20
Bortezomib (PS-341; Velcade, Millenium Pharmaceuticals, Cambridge, MA) is a dipeptidyl boronic acid analog and reversible proteasome inhibitor that has been shown to be a potent inhibitor of NF-κB.21 Bortezomib inhibits chymotryptic activity within the 26S proteasome where multi-ubiquitinated proteins are targeted for degradation, thereby blocking the nuclear translocation of NF-κB.22 This has been exploited in the therapy of malignancies in which tumor cells have been shown to have preferential sensitivity to the proapoptotic effects of proteasome inhibition.23-27 Studies in murine tumor models28,29 and small clinical studies30,31 have shown activity against a spectrum of malignancies. In addition, bortezomib has recently been approved for the treatment of patients with relapsed multiple myeloma in whom response rates of 40% have been observed after the failure of high-dose therapy followed by stem cell transplantation.32 The clinical availability of bortezomib and its potential to modulate inflammatory responses by inhibiting NF-κB make this agent a potentially attractive option for the prevention of GVHD. However, though the inhibition of NF-κB is a major pharmacologic action of bortezomib, recent studies have demonstrated that this agent has other mechanisms of action that contribute to mediating antitumor and anti-inflammatory effects.33,34 These other actions potentially confound the ability to determine whether the inhibition of NF-κB is a relevant strategy for the mitigation of GVHD severity. To address the potential clinical usefulness of bortezomib and to define the precise role of NF-κB inhibition on GVHD prevention, we comparatively analyzed the effect of bortezomib with that of PS-1145, an IKK inhibitor that selectively inhibits NF-κB. Results of these studies demonstrate that though bortezomib is able to protect mice from the development of GVHD, extended administration of this agent is associated with substantial GVHD-associated mortality. In contrast, PS-1145 is equally protective for GVHD, does not compromise engraftment, and has less GVHD-related toxicity when administered on an extended schedule, strongly suggesting that selective inhibition of NF-κB may be a superior therapeutic strategy for the prevention of GVHD.
Materials and methods
Mice
C57BL/6 (B6) (H-2b, CD45.2), B10.BR (H-2k), and B6.SJL (H-2b, CD45.1) mice were bred in the Animal Resource Center at the Medical College of Wisconsin (MCW) or were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were housed in the American Association for Laboratory Animal Care (AALAC)-accredited Animal Resource Center of the Medical College of Wisconsin. Mice received regular mouse chow and acidified tap water ad libitum.
Reagents
Bortezomib (PS-341; Velcade) and PS-1145 were kindly provided by Millennium Pharmaceuticals. Bortezomib was dissolved in phosphatebuffered saline (PBS) and was stored at -20°C before use in transplantation studies. PS-1145 was resuspended in 10% dimethylsulfoxide (DMSO) and was stored at 4°C for up to 3 days before administration to mice. Bortezomib was administered intravenously to animals at a dose of 1 mg/kg, whereas PS-1145 was given intraperitoneally at a dose of 50 mg/kg. The first dose of each agent was administered before conditioning with total body irradiation (TBI).
Bone marrow transplantation
Bone marrow (BM) was flushed from donor femurs and tibias with Dulbecco modified Eagle medium (DMEM) and passed through sterile mesh filters to obtain single-cell suspensions. BM was T-cell depleted in vitro with anti-Thy1.2 monoclonal antibody plus low toxicity rabbit complement (C-6 Diagnostics, Mequon, WI). The hybridoma for 30-H12 (anti-Thy1.2, rat IgG2b) antibody was purchased from the American Type Culture Collection (Rockville, MD). BM cells were washed and resuspended in DMEM before injection. Red blood cells were removed from spleen cell suspensions by hypotonic lysis using distilled water. Host mice were conditioned with TBI administered as a single exposure at a dose rate of 67 cGy using a Shepherd Mark I Cesium Irradiator (J.L. Shepherd and Associates, San Fernando, CA). Irradiated recipients received a single intravenous injection each of TCD BM (107 cells) with or without added spleen cells.
Flow cytometric analysis and assessment of chimerism
Monoclonal antibodies (mAbs) conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE) were used to assess chimerism in recipients of marrow transplants. PE-anti-TCR αβ (clone H57-597, hamster IgG), FITC-anti-H-2Kb (clone AF6-88.5, mouse IgG2a), PE-CD45R (clone RA3-6B2, rat IgG2a), annexin V-FITC, and propidium iodide were all purchased from BD Biosciences PharMingen (San Diego, CA). Spleen cells were obtained from chimeras at defined intervals after transplantation, processed into single-cell suspensions, and stained for 2-color analysis. Cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). The absolute number of splenic B and T cells was determined by analyzing cells within a gate that included the entire spleen cell population after exclusion of debris and residual red cells. Donor T-cell chimerism was determined by analyzing cells within a lymphocyte gate. At least 10 000 cells were analyzed for each determination whenever possible.
Mixed lymphocyte culture
Responder B10.BR T cells (1 × 105 cells/well) were cocultured with 5 × 104 B6 dendritic cell-enriched stimulator cells in U-bottomed microwell plates (Becton Dickinson, Lincoln Park, NJ) at 37°C. Responder T cells were obtained by positive selection of Thy 1.2+ T cells using the MACS magnetic cell separation system (Miltenyi Biotech, Auburn, CA). Stimulators were obtained by digestion with collagenase D (1 mg/mL) (Roche, Indianapolis, IN) of spleens, followed by positive selection of CD11c+ “dendritic cells” using the MACS system. Nonirradiated stimulator cells were then seeded into microwell plates. One microcurie (0.037 MBq) of 3H-thymidine was added to triplicate wells for the final 12 to 18 hours before harvest. Proliferation was assessed using a liquid scintillation counter (Micromedics Systems, Huntsville, AL). Control wells consisted of responders only without stimulators.
Cytokine analysis
B10.BR T cells were purified using Thy 1.2 magnetic beads and cocultured with B6 dendritic cell-enriched stimulator cells for 5 days in a mixed lymphocyte culture (MLC), as described in “Mixed lymphocyte culture.” Culture supernatant from triplicate wells was obtained and assayed for γ-interferon in standard ELISA assays (BD Biosciences PharMingen). In other experiments, serum was collected from mice by retroorbital bleeds and assayed on a Bio-Plex (BioRad Laboratories, Hercules, CA) using a mouse TH1/TH2 cytokine panel, according to the manufacturer's instructions.
Histologic analysis
Representative samples of liver, colon, small intestines, kidney, pancreas, and lung were obtained from recipients after transplantation and were fixed in 10% neutral-buffered formalin. Samples were then embedded in paraffin, cut into 5-μm micron-thick sections, and stained with hematoxylin and eosin. A semiquantitative scoring system was used to account for histologic changes in the colon, which was the primary target organ for bortezomib-induced toxicity in mice undergoing GVHD. Changes compatible with GVHD were deemed to be goblet cell depletion, apoptotic enterocytes, crypt cell destruction, and sloughing of the colonic mucosa. Histologic tissue damage was graded as 0 for normal, 1 for mild, 2 for moderate, and 3 for severe (maximal score, 3/mouse). All slides were coded and read in a blinded fashion. Images were taken with a Nikon Eclipse E400 microscope and a Nikon Plan APO 10 ×/0.45 numeric aperture lens (Nikon, Tokyo, Japan). They were then acquired with a Zeiss Axiom camera and Axiovision 3.0.6 SPZ software (Carl Zeiss, Oberkochen, Germany).
Statistical analysis
Group comparisons of donor T-cell chimerism, splenic and thymic cellularity, thymidine incorporation, cytokine levels, and pathology scores were performed using the Mann-Whitney U test. The percentage of apoptotic cells among groups was assessed using the Student t test. Survival curves were constructed using the Kaplan-Meier product limit estimator and compared using the log rank rest. P ≤ .05 was deemed to be significant in all experiments.
Administration of bortezomib immediately before transplantation protects mice from lethal GVHD. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone or together with B10.BR spleen cells adjusted to yield a dose of either 2 × 106 (A) or 4 × 106 T cells (B). Mice that received transplanted TCD BM only either were left untreated (□, n = 8) or were administered bortezomib (1 mg/kg, day 0) (○, n = 6) immediately before transplantation. Animals that received transplanted TCD BM plus B10.BR T cells also either were left untreated (▪, n = 8) or were administered bortezomib (•, n = 10) on day 0 immediately before TBI. Data are cumulative results from 2 experiments at each T-cell dose. Percentages of donor T-cell chimerism in the spleen (C) and overall spleen (D) and thymic (E) cellularity of mice described in panel A that survived 60 days after transplantation are shown (□, TCD BM; ▪, TCD BM/bortezomib; ▨, TCD BM/spleen cells/bortezomib). Data in bar graphs are presented as the mean ± SEM. Spleen cellularity: TCD versus TCD/bortezomib, P = .53; TCD/bortezomib versus TCD/spleen/bortezomib, P = .002. Thymus cellularity: TCD versus TCD/bortezomib, P = 0.94; TCD/bortezomib versus TCD/spleen/bortezomib, P = .02.
Administration of bortezomib immediately before transplantation protects mice from lethal GVHD. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone or together with B10.BR spleen cells adjusted to yield a dose of either 2 × 106 (A) or 4 × 106 T cells (B). Mice that received transplanted TCD BM only either were left untreated (□, n = 8) or were administered bortezomib (1 mg/kg, day 0) (○, n = 6) immediately before transplantation. Animals that received transplanted TCD BM plus B10.BR T cells also either were left untreated (▪, n = 8) or were administered bortezomib (•, n = 10) on day 0 immediately before TBI. Data are cumulative results from 2 experiments at each T-cell dose. Percentages of donor T-cell chimerism in the spleen (C) and overall spleen (D) and thymic (E) cellularity of mice described in panel A that survived 60 days after transplantation are shown (□, TCD BM; ▪, TCD BM/bortezomib; ▨, TCD BM/spleen cells/bortezomib). Data in bar graphs are presented as the mean ± SEM. Spleen cellularity: TCD versus TCD/bortezomib, P = .53; TCD/bortezomib versus TCD/spleen/bortezomib, P = .002. Thymus cellularity: TCD versus TCD/bortezomib, P = 0.94; TCD/bortezomib versus TCD/spleen/bortezomib, P = .02.
Results
Bortezomib administration protects mice from lethal GVHD
Initial studies were performed to determine whether bortezomib could prevent GVHD in an MHC-incompatible murine bone marrow transplantation (BMT) model. Lethally irradiated B6 mice underwent transplantation with TCD B10.BR BM alone (control animals) or with spleen cells adjusted to yield a T-cell dose of 2 × 106 or 4 × 106 B10.BR T cells. Cohorts of control or GVHD mice were then left untreated or were administered a single dose each of bortezomib (1 mg/kg) before transplantation. Animals that underwent transplantation with TCD BM alone and then were treated with bortezomib had survival times similar to those of untreated control mice because mice in both groups survived for the duration of the experiment (Figure 1A-B). Transplantation of 2 × 106 T cells along with TCD BM resulted in lethal GVHD in nearly all animals (Figure 1A). In contrast, the administration of bortezomib to a similar group of mice with GVHD significantly prolonged survival (P = .04). When the dose of T cells in the spleen cell inoculum was increased to 4 × 106, all untreated mice died within 55 days of BMT (Figure 1B). Bortezomib administration again significantly prolonged survival (P = .02), with 50% of mice becoming long-term survivors. To assess the effect of bortezomib on engraftment and immune reconstitution, surviving mice from experiments depicted in Figure 1A were examined to determine the extent of donor T-cell engraftment and overall splenic and thymic cellularity. Animals undergoing GVHD and treated with bortezomib had complete donor T-cell chimerism (Figure 1C), indicating that protection from GVHD did not compromise alloengraftment. Bortezomib-treated mice, however, were not completely protected from GVHD, as evidenced by significantly decreased splenic (Figure 1D) and thymic (Figure 1E) cellularity when compared with animals that underwent transplantation with TCD BM alone and then were administered bortezomib (key statistical comparisons are given in the legend to Figure 1).
Delayed administration of bortezomib significantly exacerbates GVHD-associated mortality
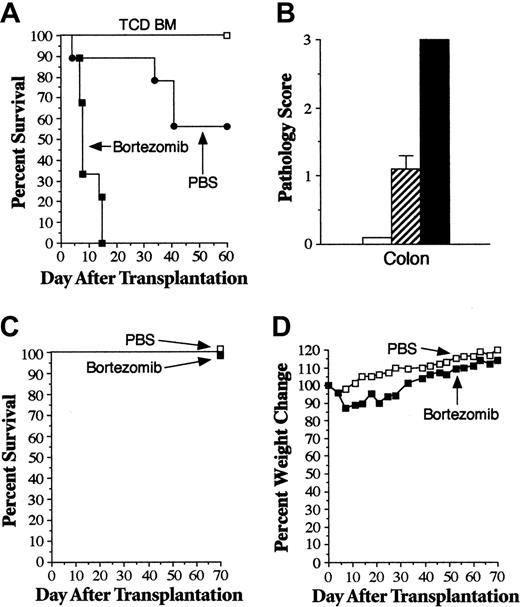
Donor T cells traffic to regional lymph nodes within the first 24 hours of transplantation, where they become activated and then emigrate to GVHD target organs.35 Therefore, we sought to examine whether bortezomib administration was capable of attenuating GVHD severity after T-cell activation had already occurred in secondary lymphoid organs. In addition, because current clinical practice is to administer bortezomib on a twice-weekly schedule for the treatment of patients with hematologic malignancies,32,36 we examined whether bortezomib could be given to murine recipients on an extended administration schedule. Lethally irradiated B6 mice underwent transplantation with TCD B10.BR BM alone (control animals) or with spleen cells adjusted to yield a T-cell dose of 2 × 106 B10.BR T cells. Mice undergoing GVHD were then treated with bortezomib twice weekly beginning on day 3 or 4 after transplantation, after donor T cells had trafficked to nodal sites.35 In contrast to what was observed with the administration of a single dose of bortezomib on the day of BMT, delaying the administration of bortezomib resulted in significantly greater mortality than did PBS treatment of GVHD control animals (Figure 2A). Notably, mice treated with bortezomib were able to receive a maximum of only 3 doses because all animals died abruptly within 10 days of transplantation. To determine the cause of death in bortezomib-treated animals, replicate experiments were performed and histopathologic analysis was conducted on lung, liver, colon, kidney, pancreas, and small intestine from representative mice 1 week after BMT, immediately before animals died of bortezomib administration. No abnormalities in the kidney, lung, small intestines, pancreas, or liver of bortezomib-treated mice were observed compared with control or PBS-treated GVHD animals. Histologic analysis, however, did show marked damage to the colons of all bortezomib-treated mice compared with PBS-treated GVHD control animals (mean pathologic score, 3.0 vs 1.1; P = .003) (Figure 2B). Microscopic examination revealed the depletion of goblet cells, crypt destruction, and sloughing of the colonic mucosa in bortezomib-treated mice, whereas animals administered PBS had only modest colon injury (Figure 3). In additional experiments, we examined whether the GVHD protective effect conferred by day 0 bortezomib administration persisted when treatment was extended after BMT using a similar twice-weekly schedule. Animals under-going GVHD (n = 10) and treated with twice-weekly bortezomib (beginning on day 0) all died within 8 days of transplantation (data not shown), indicating that there was a narrow therapeutic window in which bortezomib could be administered without increased mortality.
To determine whether the extended administration of bortezomib induced gut toxicity in the absence of GVHD, similar experiments were performed in recipients of syngeneic marrow transplants. Lethally irradiated B6 mice underwent transplantation with B6.SJL BM cells (10 × 106) and then were treated with twice-weekly PBS or bortezomib (1 mg/kg) for a total of 4 weeks. Treatment with bortezomib had no adverse effect on overall survival because all mice in both groups survived 60 days after transplantation (Figure 2C). Bortezomib-treated animals, however, did have greater weight loss during the period of drug administration, but this was transient and improved once treatment was completed (Figure 2D). Given that weight loss did occur in bortezomib-treated mice, we performed similar experiments and killed mice in both cohorts 13 to 14 days after BMT, when weight loss was maximal. Histologic analysis of representative tissues (ie, colon, small intestines, liver, lung, kidney, and pancreas) from mice in each group (n = 10/group) demonstrated no pathologic differences between PBS- and bortezomib-treated mice (data not shown), indicating that bortezomib did not cause gut toxicity in the absence of underlying GVHD.
Extended posttransplantation administration of bortezomib exacerbates GVHD-dependent mortality. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone (□, n = 6) or with spleen cells adjusted to yield a dose of 2 × 106 B10.BR T cells. Mice that received transplanted B10.BR T cells were treated with PBS (•, n = 9) or bortezomib (▪, n = 9) (1 mg/kg twice weekly beginning on days 3-4). Survival is shown in panel A. In 2 additional experiments, cohorts of mice that underwent transplantation as in panel A were killed 6 to 7 days after BMT, and colon tissue was obtained for histologic analysis. Panel B shows pathology scores in the colons of mice that received TCD BM alone (□, n = 6) or with B10.BR T cells, followed by treatment with PBS (▨, n = 8) or bortezomib (▪, n = 7). In panels C and D, lethally irradiated B6 mice underwent transplantation with 10 × 106 B6.SJL BM cells and then were treated with twice-weekly PBS (□, n = 10) or bortezomib (▪, n = 10) (1 mg/kg/dose) beginning 3 to 4 days after BMT. Survival is shown in panel C, and panel D shows mean percentages of pretransplantation weight loss/gain. Data are cumulative results from 2 independent experiments for each strain combination (allogeneic and syngeneic).
Extended posttransplantation administration of bortezomib exacerbates GVHD-dependent mortality. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone (□, n = 6) or with spleen cells adjusted to yield a dose of 2 × 106 B10.BR T cells. Mice that received transplanted B10.BR T cells were treated with PBS (•, n = 9) or bortezomib (▪, n = 9) (1 mg/kg twice weekly beginning on days 3-4). Survival is shown in panel A. In 2 additional experiments, cohorts of mice that underwent transplantation as in panel A were killed 6 to 7 days after BMT, and colon tissue was obtained for histologic analysis. Panel B shows pathology scores in the colons of mice that received TCD BM alone (□, n = 6) or with B10.BR T cells, followed by treatment with PBS (▨, n = 8) or bortezomib (▪, n = 7). In panels C and D, lethally irradiated B6 mice underwent transplantation with 10 × 106 B6.SJL BM cells and then were treated with twice-weekly PBS (□, n = 10) or bortezomib (▪, n = 10) (1 mg/kg/dose) beginning 3 to 4 days after BMT. Survival is shown in panel C, and panel D shows mean percentages of pretransplantation weight loss/gain. Data are cumulative results from 2 independent experiments for each strain combination (allogeneic and syngeneic).
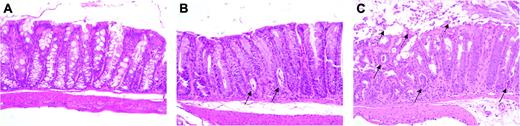
Lethality caused by extended administration of bortezomib is attributable to gut toxicity. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone or with spleen cells adjusted to yield a dose of 2 × 106 B10.BR T cells. Mice that received transplanted B10.BR T cells were treated with PBS or bortezomib (1 mg/kg twice weekly beginning on day 3). Eight days after transplantation, prior to demise, mice from each of the 3 cohorts were killed, and samples of colon tissue were analyzed for evidence of pathologic damage. Hematoxylin and eosin stains of colon tissue obtained from these mice are depicted (original magnification, 200 ×). (A) Colon tissue from a representative mouse that underwent transplantation with TCD BM only showing normal-appearing colon tissue with intact crypts lined by mucin-filled enterocytes. (B) PBS-treated mouse undergoing GVHD showing increased mitotic activity at the base of crypts associated with a few crypt abscesses (solid arrows). (C) Bortezomib-treated animal demonstrating extensive crypt destruction with sloughing of the colonic mucosa (dashed arrows), goblet cell depletion, and numerous crypt abscesses (solid arrows).
Lethality caused by extended administration of bortezomib is attributable to gut toxicity. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone or with spleen cells adjusted to yield a dose of 2 × 106 B10.BR T cells. Mice that received transplanted B10.BR T cells were treated with PBS or bortezomib (1 mg/kg twice weekly beginning on day 3). Eight days after transplantation, prior to demise, mice from each of the 3 cohorts were killed, and samples of colon tissue were analyzed for evidence of pathologic damage. Hematoxylin and eosin stains of colon tissue obtained from these mice are depicted (original magnification, 200 ×). (A) Colon tissue from a representative mouse that underwent transplantation with TCD BM only showing normal-appearing colon tissue with intact crypts lined by mucin-filled enterocytes. (B) PBS-treated mouse undergoing GVHD showing increased mitotic activity at the base of crypts associated with a few crypt abscesses (solid arrows). (C) Bortezomib-treated animal demonstrating extensive crypt destruction with sloughing of the colonic mucosa (dashed arrows), goblet cell depletion, and numerous crypt abscesses (solid arrows).
PS-1145 inhibits T-cell alloreactivity in vitro but is less potent than bortezomib
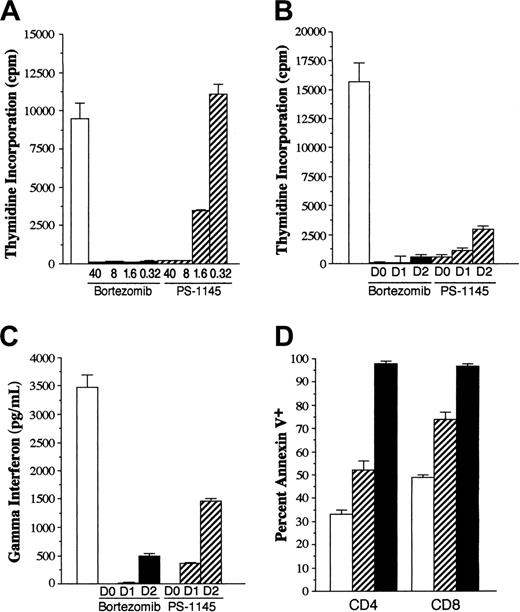
Given that the therapeutic window for bortezomib administration after transplantation was narrow, we examined whether more selective inhibition of NF-κB could prevent GVHD without inducing the toxicity observed with bortezomib. To address this question, we used PS-1145, which is an IKK inhibitor and has been shown to specifically inhibit NF-κB in myeloma cell lines.23 Studies were first performed to determine whether PS-1145 was capable of inhibiting T-cell alloreactivity in vitro and to compare this agent directly with bortezomib. In standard mixed lymphocyte cultures (MLCs), bortezomib potently inhibited T-cell proliferation with negligible thymidine incorporation observed at concentrations as low as 0.32 μM (Figure 4A). Adding PS-1145 to MLCs also effected a dose-dependent reduction in the proliferative response; however, inhibition was less complete than it was for bortezomib when tested at equivalent concentrations. Specifically, at a concentration of 1.6 μM, thymidine incorporation was approximately 33% that of control, whereas at 0.32 μM, PS-1145 had no inhibitory effect on T-cell proliferation. To determine whether either agent could inhibit the proliferative response once T-cell activation had occurred, bortezomib or PS-1145 was added to established MLCs at varying time points after culture initiation. Concentrations of bortezomib (0.32 μM) and PS-1145 (8 μM) were used that completely suppressed proliferation when added on day 0 of culture. The delayed addition of bortezomib for up to 2 days significantly reduced thymidine incorporation compared with control cultures (P < .01) (Figure 4B). Measurement of IFN-γ levels also revealed complete inhibition when bortezomib was delayed for 1 day and 85% inhibition when it was delayed for 2 days (Figure 4C). A similar trend was observed with PS-1145; however, inhibition of T-cell proliferation and IFN-γ levels was less than that observed with bortezomib. Bortezomib and PS-1145 both significantly increased apoptosis of alloreactive CD4+ and CD8+ T cells in MLCs (P < .01 vs control), though the percentage of annexin V-positive T cells was greater in bortezomib than in PS-1145-treated cultures (P < .008) (Figure 4D). These data demonstrated that PS-1145 was also capable of suppressing T-cell alloreactivity and inducing apoptosis in vitro but that it was not as potent as bortezomib on a molar basis.
PS-1145 inhibits alloreactive T-cell responses in vitro but is less potent than bortezomib. B10.BR T cells (1 × 105/well) were cultured with B6 CD11c+ dendritic cells (5 × 104) in a standard mixed lymphocyte culture for 5 days. (A) Replicate wells were cultured alone (□) or in the presence of 5-fold μM dilutions of bortezomib (▪) or PS-1145 (▨) for 5 days. Cells were pulsed with 3H-Tdr for the last 18 hours of culture, and the percentages of incorporated radioactivity were determined. (B-C) B10.BR T cells (1 × 105/well) were cultured with B6 CD11c+ dendritic cells (5 × 104) alone or with bortezomib (0.32 μM) or PS-1145 (8 μM) for 5 days. Bortezomib and PS-1145 were added to microwells on days 0, 1, or 2 after culture initiation. Cells were pulsed with 3H-Tdr for the last 18 hours of culture and the percentages of incorporated radioactivity were determined (B); in separate experiments, supernatants were collected and tested for the presence of IFN-γ (C). Data are presented as the mean ± SEM from triplicate control wells. Data shown are from 1 of 3 experiments that produced similar results. (D) B10.BR T cells were cultured with B6 CD11c+ dendritic cells alone or with bortezomib (0.32 μM) or PS-1145 (8 μM). Cells were harvested and pooled from triplicate microwells after 3 or 4 days and were surface stained for CD4 or CD8 and annexin V. Data are presented as the mean ± SEM. Data shown are cumulative results of 4 experiments.
PS-1145 inhibits alloreactive T-cell responses in vitro but is less potent than bortezomib. B10.BR T cells (1 × 105/well) were cultured with B6 CD11c+ dendritic cells (5 × 104) in a standard mixed lymphocyte culture for 5 days. (A) Replicate wells were cultured alone (□) or in the presence of 5-fold μM dilutions of bortezomib (▪) or PS-1145 (▨) for 5 days. Cells were pulsed with 3H-Tdr for the last 18 hours of culture, and the percentages of incorporated radioactivity were determined. (B-C) B10.BR T cells (1 × 105/well) were cultured with B6 CD11c+ dendritic cells (5 × 104) alone or with bortezomib (0.32 μM) or PS-1145 (8 μM) for 5 days. Bortezomib and PS-1145 were added to microwells on days 0, 1, or 2 after culture initiation. Cells were pulsed with 3H-Tdr for the last 18 hours of culture and the percentages of incorporated radioactivity were determined (B); in separate experiments, supernatants were collected and tested for the presence of IFN-γ (C). Data are presented as the mean ± SEM from triplicate control wells. Data shown are from 1 of 3 experiments that produced similar results. (D) B10.BR T cells were cultured with B6 CD11c+ dendritic cells alone or with bortezomib (0.32 μM) or PS-1145 (8 μM). Cells were harvested and pooled from triplicate microwells after 3 or 4 days and were surface stained for CD4 or CD8 and annexin V. Data are presented as the mean ± SEM. Data shown are cumulative results of 4 experiments.
Administration of PS-1145 early after transplantation protects mice from lethal GVHD. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone or with B10.BR spleen cells adjusted to yield a dose of 4 × 106 T cells. Mice that received transplanted TCD BM only were untreated (□, n = 8) or were administered PS-1145 (50 mg/kg, days 0-2) (○, n = 5). Animals that received transplanted TCD BM plus B10.BR T cells were also left untreated (▪, n = 8) or were administered PS-1145 (•, n = 10) on days 0-2. (A) Survival percentages. (B) Weight loss percentages. Data are cumulative results from 2 experiments.
Administration of PS-1145 early after transplantation protects mice from lethal GVHD. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone or with B10.BR spleen cells adjusted to yield a dose of 4 × 106 T cells. Mice that received transplanted TCD BM only were untreated (□, n = 8) or were administered PS-1145 (50 mg/kg, days 0-2) (○, n = 5). Animals that received transplanted TCD BM plus B10.BR T cells were also left untreated (▪, n = 8) or were administered PS-1145 (•, n = 10) on days 0-2. (A) Survival percentages. (B) Weight loss percentages. Data are cumulative results from 2 experiments.
PS-1145 administration early after BMT protects mice from lethal GVHD
After intraperitoneal administration of PS-1145 at a dose of 50 mg/kg, the half-life is approximately 16 hours and results in a mean concentration of approximately 4 μM after 24 hours (personal communication, L. Dang, Millennium Pharmaceuticals, October 2003). We observed that this concentration was sufficient to suppress T-cell proliferation in MLC assays (Figure 4) and, therefore, used this dose in BMT studies. Furthermore, given that maximum proteasome inhibition after bortezomib administration occurs within 1 hour but does not recover to the baseline level for 72 to 96 hours, we administered PS-1145 to mice for 3 days beginning on day 0 after transplantation to approximate the degree of suppression occurring after bortezomib administration. Lethally irradiated B6 mice underwent transplantation with TCD B10.BR BM alone (control animals) or together with spleen cells adjusted to yield a T-cell dose of 4 × 106 B10.BR T cells. Cohorts of control or GVHD mice were then left untreated or were administered PS-1145 (50 mg/kg) on days 0 to 2 after transplantation. The first dose was given before TBI. Administration of PS-1145 had no deleterious effect on the survival of mice undergoing reconstitution with TCD BM alone (Figure 5A). Moreover, PS-1145 administration significantly decreased the risk for death in mice that underwent transplantation with adjunctive spleen cells compared with untreated controls, though surviving PS-1145-treated animals had evidence of weight loss, indicating that they were not completely protected from GVHD (Figure 5B). PS-1145 did not compromise engraftment as all treated mice undergoing GVHD had complete donor T-cell chimerism (data not shown). Notably, survival in PS-1145-treated, GVHD-protected mice was comparable to that observed in animals treated with bortezomib after similar transplantation procedures (Figure 1B), suggesting that the respective doses and schedules of these 2 agents gave equivalent GVHD protection.
Bortezomib and PS-1145 reduce serum cytokine levels in mice undergoing GVHD
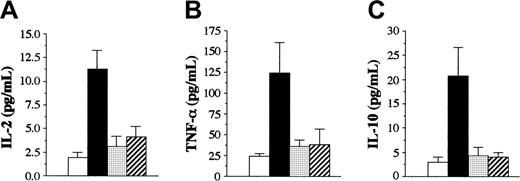
We examined serum cytokine levels to determine whether bortezomib and PS-1145 affected cytokine levels that were increased during the course of GVHD. Lethally irradiated B6 mice underwent transplantation with TCD B10.BR BM plus B10.BR spleen cells adjusted to yield a T-cell dose of 2 × 106 T cells. Cohorts of mice then were left untreated or were administered bortezomib on day 0 or PS-1145 on days 0 to 2 after BMT. Animals were bled 7 days after BMT and were analyzed for serum levels of TNF-α, IL-2, IL-10, IFN-γ, IL-4, and IL-12. Untreated mice with GVHD had increased levels of TNF-α, IL-10, and IL-2 compared with TCD BM control animals (P < .01 for all 3 cytokines) (Figure 6). Administration of either bortezomib or PS-1145 resulted in a significant decrease in serum levels of all 3 cytokines (Figure 6) that was nonsignificantly different from those in mice that underwent transplantation with TCD BM alone. Serum levels of IFN-γ, IL-12, and IL-4 were also examined in untreated, bortezomib-treated, or PS-1145-treated animals. No differences were observed among these 3 groups with respect to each of these cytokines (data not shown).
Extended administration of PS-1145 protects mice from lethal GVHD and does not cause gut toxicity
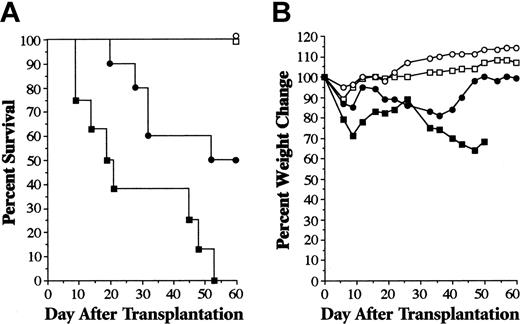
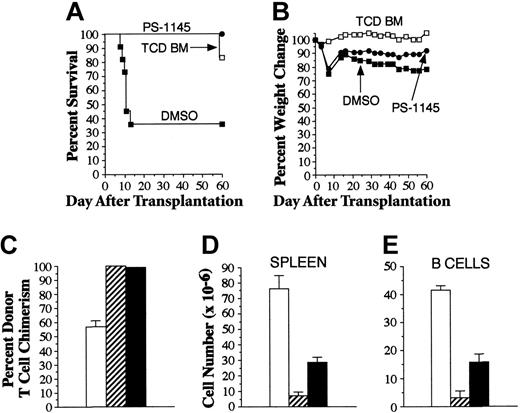
Studies were performed to determine whether more prolonged treatment with PS-1145 abrogated GVHD protection and resulted in gastrointestinal toxicity, as had been observed with bortezomib. In vitro experiments demonstrated that PS-1145 was less effective in preventing T-cell proliferation when addition was delayed for 2 days after the initiation of culture (Figure 4C), leading us to conclude that an extended treatment course of PS-1145 would likely be most effective if begun at the time of BMT. Mice underwent transplantation as described in the legend to Figure 1A and then were administered PS-1145 beginning on day 0 for a total of 10 days. In contrast to what was observed with bortezomib, PS-1145-treated mice were completely protected from GVHD death and experienced significantly superior survival when compared with vehicle-treated (DMSO) GVHD control mice (P = .002) (Figure 7A), even though serial weights indicated that mice were not free of GVHD (Figure 7B). The absence of early death was indicative that PS-1145 did not induce fatal gastrointestinal toxicity when administered over 10 days. Moreover, histopathologic analysis of all mice 60 days after BMT revealed that only 2 of 11 animals had any evidence of GVHD of the colon (moderate in one mouse, severe in the other). GVHD in affected animals was morphologically indistinguishable from that observed in mice that uniformly developed the disease after extended bortezomib administration (Figures 2, 3). To assess whether PS-1145 compromised alloengraftment, all surviving mice were analyzed for the extent of donor T-cell engraftment. Mice that underwent transplantation with TCD BM only had mixed T-cell chimerism, indicative of incomplete donor engraftment (Figure 7C). PS-1145-treated animals, however, had complete donor T-cell engraftment that was not significantly different from that observed in surviving GVHD control mice (key statistical comparisons are given in the legend to Figure 7). Overall splenic cellularity (Figure 7D) and absolute numbers of B cells (Figure 7E) were significantly greater in recipients administered PS-1145, though these cell numbers were lower than those observed in mice receiving transplanted TCD BM alone. PS-1145 also significantly reduced donor T-cell expansion in the spleen when assessed 7 days after BMT (mean, 3.6 × 106 vs 7.1 × 106; n = 4/group; P = .03). Collectively, these data indicated that PS-1145 protected mice from lethal GVHD and that, in contrast to the effect of bortezomib, extending the treatment course did not induce toxicity.
Bortezomib and PS-1145 both effectively reduce serum cytokine levels in mice with GVHD. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone (□) or with B10.BR spleen cells adjusted to yield a dose of 2 × 106 T cells. Mice that received transplanted T cells were left untreated (▪) or were administered bortezomib (1 mg/kg, day 0) (▦) or PS-1145 (50 mg/kg, days 0-2) (▨). Mice in all groups were bled on day 7 after BMT and were analyzed for the specified cytokines. Data are cumulative results from 2 independent experiments with 5 to 7 mice per group. Data are presented as the mean ± SEM. IL-2: bortezomib or PS-1145 versus GVHD control, P < .005. TNF-α: bortezomib or PS-1145 versus GVHD control, P = .02. IL-10: bortezomib or PS-1145 versus GVHD control, P < .005.
Bortezomib and PS-1145 both effectively reduce serum cytokine levels in mice with GVHD. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone (□) or with B10.BR spleen cells adjusted to yield a dose of 2 × 106 T cells. Mice that received transplanted T cells were left untreated (▪) or were administered bortezomib (1 mg/kg, day 0) (▦) or PS-1145 (50 mg/kg, days 0-2) (▨). Mice in all groups were bled on day 7 after BMT and were analyzed for the specified cytokines. Data are cumulative results from 2 independent experiments with 5 to 7 mice per group. Data are presented as the mean ± SEM. IL-2: bortezomib or PS-1145 versus GVHD control, P < .005. TNF-α: bortezomib or PS-1145 versus GVHD control, P = .02. IL-10: bortezomib or PS-1145 versus GVHD control, P < .005.
Extended posttransplantation administration of PS-1145 protects animals from lethal GVHD and does not induce fatal gut toxicity. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone (□, n = 6) or together with spleen cells adjusted to yield a dose of 2 × 106 B10.BR T cells. (A) Animals that received transplanted B10.BR T cells were administered DMSO (▪, n = 11) or PS-1145 (•, n = 11) (50 mg/kg for 10 days beginning the day of transplantation). Survival is shown in panel A, and weight loss, in panel B. Overall cellularity (C), absolute number of B cells (D), and donor T-cell chimerism (E) in the spleens of mice that survived 60 days after transplantation are shown (□, TCD BM; ▨, DMSO; ▪, PS-1145). Data in bar graphs are presented as the mean ± SEM. Spleen cellularity: DMSO versus PS-1145, P = .006; TCD versus PS-1145, P < .001. Total splenic B cells: DMSO versus PS-1145, P = .04; TCD versus PS-1145, P < .001. Percentage donor T cells: DMSO versus PS-1145, P = .66; TCD versus PS-1145, P < .001
Extended posttransplantation administration of PS-1145 protects animals from lethal GVHD and does not induce fatal gut toxicity. Lethally irradiated (1000 cGy) B6 mice underwent transplantation with TCD B10.BR BM alone (□, n = 6) or together with spleen cells adjusted to yield a dose of 2 × 106 B10.BR T cells. (A) Animals that received transplanted B10.BR T cells were administered DMSO (▪, n = 11) or PS-1145 (•, n = 11) (50 mg/kg for 10 days beginning the day of transplantation). Survival is shown in panel A, and weight loss, in panel B. Overall cellularity (C), absolute number of B cells (D), and donor T-cell chimerism (E) in the spleens of mice that survived 60 days after transplantation are shown (□, TCD BM; ▨, DMSO; ▪, PS-1145). Data in bar graphs are presented as the mean ± SEM. Spleen cellularity: DMSO versus PS-1145, P = .006; TCD versus PS-1145, P < .001. Total splenic B cells: DMSO versus PS-1145, P = .04; TCD versus PS-1145, P < .001. Percentage donor T cells: DMSO versus PS-1145, P = .66; TCD versus PS-1145, P < .001
Discussion
One of the major findings of this study was that lethal GVHD could be prevented by the administration of bortezomib and PS-1145, whose mechanisms of action involve, in part or exclusively, the inhibition of NF-κB. Protection from GVHD required only a brief administration of either agent in the immediate peritransplantation period and did not compromise donor engraftment. With respect to bortezomib, these results confirmed those of Sun et al,37 who demonstrated that administration of this agent within the first several days of BMT protected mice from fatal GVHD. In their report, mice received a cumulative dose of 40 μg over a 4-day period. In our study mice were treated only once before transplantation, but the dose of 1 mg/kg was similar to the cumulative dose administered in this earlier report. In contrast, PS-1145—which binds directly to the 20S component, the catalytic complex of the 26S proteasome38 —is a specific IKK inhibitor and has been shown to inhibit the phosphorylation and degradation of IKBα by TNF-α.23 Complete inhibition of phosphorylation of IKBα has been observed to occur at levels of 5 μM or higher. This correlated with our in vitro studies in which T-cell proliferation and IFN-gamma production were not completely suppressed until a concentration of 8 μM PS-1145 was achieved. This was, again, in contrast to bortezomib, for which inhibition of T-cell alloreactivity in vitro was achieved at much lower concentrations. The reduced potency of PS-1145, as opposed to that of bortezomib, has also been supported by studies showing that bortezomib is able to induce more significant tumor cell inhibition at equimolar concentrations compared with PS-1145.23 Despite the reduced potency on a molar basis, we were able to achieve equivalent protection from lethal GVHD by administering higher doses of PS-1145 in vivo for a more extended period of time (ie, days 0-2). Each agent directly affected T cells, as evidenced by the inhibition of T-cell proliferation in vitro (Figure 4) and by reduced levels of cytokines, such as IL-2 (Figure 6), which peaks within the first week of BMT39 and is secreted in an autocrine fashion to enhance further T-cell proliferation in vivo. Additionally, bortezomib and PS-1145 reduced serum levels of TNF-α, which is a major cytokine mediator of GVHD-induced tissue damage.40-42 Thus, both agents inhibited cytokines capable of enhancing donor T-cell proliferation and causing direct target organ damage.
The other major finding of this study was that proteasome inhibition with bortezomib and IKK inhibition with PS-1145 were not therapeutically equivalent strategies with respect to GVHD prevention. Although pretransplantation administration of bortezomib protected mice from lethal GVHD, we observed that delayed or extended treatment exacerbated the risk for death (Figure 2). Deaths occurred early after BMT, typically after 2 doses of bortezomib, and were attributable to severe colonic damage. Conversely, with PS-1145, extended treatment not only resulted in complete protection from lethal GVHD (Figure 7) but also caused no apparent toxicity. Bortezomib-induced toxicity was GVHD dependent because mice that underwent transplantation with syngeneic marrow grafts and were treated on the same schedule had transient weight loss but did not die or have evidence of tissue damage. Thus, TBI conditioning alone was an insufficient stimulus to potentiate the deleterious effect of bortezomib on colonic tissue. Notably, the gut was the only target organ affected adversely by bortezomib in recipients with GVHD as no other tissues had evidence of increased damage.
There are several possible explanations for the striking toxicity observed in animals treated with extended courses of bortezomib. One is that toxicity was attributable to the inhibition of NF-κB. NF-κB has been shown to play an important role in radioprotection in the intestines of mice subsequently exposed to TBI. Studies using p50-deficient mice have demonstrated increased apoptosis in intestinal epithelial cells after TBI but no evidence of tissue injury in BM, spleen, liver, heart, lung, or kidney, indicating a critical role for NF-κB in the radioprotection of the gut compared with other organs.43 Moreover, mice deficient in IKKβ, which is an NF-κB activation kinase, have increased radiation-induced epithelial cell apoptosis.44 The finding that extended treatment with PS-1145, which only inhibits NF-κB, did not cause fatal gut toxicity, however, argues against this premise, unless pharmacokinetic differences between these 2 agents affected the extent and duration of NF-κB inhibition. For example, bortezomib is a transition-state analog with extremely tight binding and a slow off rate. This is in contrast to PS-1145, which is a less potent inhibitor that has an off rate against IKK on the order of seconds (personal communication, L. Dang, Millennium Pharmaceuticals, October 2003). Consequently, the duration of inhibition by PS-1145 is contingent on the maintenance of a drug concentration greater than 4 to 5 μM. Thus, it is possible that a lack of sustained NF-κB inhibition by PS-1145 over the course of treatment, caused by a decay in serum levels, could have reduced the susceptibility of intestine cells to the combination of TBI and alloactivated donor T cells.
A more likely explanation, which we favor, derives from that fact that proteasome inhibition has effects in addition to those of NF-κB blockade. Specifically, bortezomib has been shown to affect a number of other proteins, including cyclin-dependent kinases such as p21 and p27, along with bax and p53.38 Using human cancer cell lines to investigate the mechanism by which bortezomib induces apoptosis, Ling et al33 showed that bortezomib also increased the generation of reactive oxygen species (ROS) and mitochondrial membrane permeability. In subsequent studies, Fribley et al34 demonstrated that the inhibition of NF-κB was not sufficient to induce apoptosis in squamous cell cancer lines. Rather, ROS was found to play a critical role in bortezomib-induced apoptosis and resulted in increased expression of endoplasmic reticulum stress proteins. Conversely, the inhibition of ROS significantly suppressed bortezomib-induced apoptosis in these same cell lines. Moreover, bortezomib is able to induce fas and fas ligand expression,45 reduce levels of c-FLIP,46 and activate the c-Jun terminal kinase, leading to caspase activation.45,47,48 The up-regulation of death effector molecules, such as fas ligand, is of particular significance in that increased oxidative stress, as occurs in GVHD, induces the expression of fas on intestinal epithelial cells.49,50 Thus, bortezomib may induce gut toxicity by increasing the susceptibility of intestine cells to immune-mediated death by fas/fas ligand interactions with alloactivated T cells.
Relevant to this premise are studies by Panoskaltsis-Mortari et al,35 who, using eGFP transgenic T cells to track alloreactive donor T cells in BMT recipients, have shown that T cells home to lymph nodes within 24 hours of transplantation. Expansion of allogeneic T cells occurs within 48 to 72 hours, and T cells have already localized to GVHD target organs by 72 hours after BMT. Therefore, when bortezomib is administered early after BMT, T-cell proliferation is prevented by the inhibition of proliferation and the induction of apoptosis.37 Thus, there is a reduction in the number of effector T cells that are available to mediate tissue damage in the gut that has been exposed to radiation damage. When bortezomib is delayed, however, T-cell activation has already occurred, as has the up-regulation of death effector molecules. These T cells are now resident in target organs such as the intestinal tract, where they are capable of mediating tissue damage. The fact that continued administration of bortezomib, even when begun on day 0, fails to protect mice is likely due to the fact that GVHD is not completely eliminated in these animals (Figures 1, 5); and residual GVHD T cell effectors are sufficient to induce GI toxicity in the presence of prolonged exposure to bortezomib.
In summary, our studies demonstrate that bortezomib and PS-1145 are capable of protecting mice from lethal GVHD. Protection was associated with significant reductions in cytokine production and did not compromise donor engraftment. The therapeutic index for bortezomib, however, was narrow because extended or delayed administration uniformly resulted in fatal GI toxicity, indicating that caution should be applied when considering the clinical administration of this agent to patients with established GVHD. In contrast, treatment with PS-1145 was equally protective and had a broader therapeutic index. This translated into more complete GVHD protection than was observed with bortezomib because of the ability for sustained administration. These results confirm a critical role for NF-κB in GVHD pathogenesis and suggest that targeted inhibition of NF-κB may be a viable therapeutic approach to reduce GVHD severity.
Prepublished online as Blood First Edition Paper, September 20, 2005; DOI 10.1182/blood-2005-05-1820.
Supported by grants from the National Institutes of Health (HL55388) and the Midwest Athletes Against Childhood Cancer Fund (Milwaukee, WI).
S.V.-J. performed research; P.H. performed research and analyzed the data;
R.K. analyzed pathology specimens; P.J. performed research; and W.R.D. designed research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal