Abstract
Inappropriate activation of MET, the receptor tyrosine kinase for hepatocyte growth factor (HGF), has been implicated in tumorigenesis. Although we have previously shown that HGF/MET signaling controls survival and proliferation of multiple myeloma (MM), its role in the pathogenesis of other B-cell malignancies has remained largely unexplored. Here, we have examined a panel of 110 B-cell malignancies for MET expression, which, apart from MM (48%), was found to be largely confined to diffuse large B-cell lymphomas (DLBCLs) (30%). No amplification of the MET gene was found; however, mutational analysis revealed 2 germ-line missense mutations: R1166Q in the tyrosine kinase domain in 1 patient, and R988C in the juxtamembrane domain in 4 patients. The R988C mutation has recently been shown to enhance tumorigenesis. In MET-positive DLBCL cells, HGF induces MEK-dependent activation of ERK and PI3K-dependent phosphorylation of PKB, GSK3, and FOXO3a. Furthermore, HGF induces PI3K-dependent α4β1 integrin-mediated adhesion to VCAM-1 and fibronectin. Within the tumor microenvironment of DLBCL, HGF is provided by macrophages, whereas DLBCL cells themselves produce the serine protease HGF activator (HGFA), which autocatalyzes HGF activation. Taken together, these data indicate that HGF/MET signaling, and secretion of HGFA by DLBCL cells, contributes to lymphomagenesis in DLBCL. (Blood. 2006;107:760-768)
Introduction
B-cell lymphomas represent the malignant counterparts of normal B cells, arrested at specific maturational stages. They are classified into distinct disease categories based on their stage-specific morphologic features, molecular profile, and B-cell receptor (BCR) configuration.1 The initial step in lymphomagenesis is the acquisition of a genetic aberration, most often a chromosomal translocation involving a proto-oncogene, causing an increased life span and/or enhanced proliferation.2 In general, this event per se is not tumorigenic, but further (multiple) genetic alterations are required for the development of a fully malignant phenotype. In addition to these oncogenic events, B-cell malignancies require signals from the microenvironment for their growth, survival, and progression. These signals, which include B-cell receptor (BCR) stimulation by antigen,3 physical contact of (malignant) B cells with stromal cells via integrin adhesion receptors,4-7 as well as a number of cytokines/growth factors,8 activate intracellular signaling cascades and present potential targets for therapeutic intervention. One of the candidate growth factors in B-cell malignancies is hepatocyte growth factor (HGF).9-11
HGF induces complex biologic responses in target cells, including adhesion, motility, growth, survival, and morphogenesis, by activating the tyrosine kinase receptor MET. HGF/MET signaling is indispensable for mammalian development, while uncontrolled activation of MET is oncogenic and has been implicated in the growth, invasion, and metastasis of a variety of tumors.12,13 Several distinct mechanisms may underlie uncontrolled MET activation. These include translocation, amplification, or mutation of the MET gene,12,14-19 and autocrine or paracrine HGF production.14,20,21
In B cells, the HGF/MET pathway has been implicated in differentiation, specifically in the regulation of adhesion and migration.13,22 We have previously demonstrated that the MET protein is expressed on GC B cells,22 whereas follicular dendritic cells (FDCs) and stromal cells express HGF.22,23 Furthermore, we and others have identified the HGF/MET pathway as a potentially important signaling route in lymphomagenesis.9,13,24,25 In several B-cell malignancies, including multiple myeloma (MM),24,26,27 primary effusion lymphoma (PEL),28 and Hodgkin lymphoma (HL),9 coexpression of HGF and MET has been observed, suggesting autocrine activation of HGF/MET signaling. Furthermore, in MM, HL, and diffuse large B-cell lymphoma (DLBCL), elevated serum HGF levels correlate with unfavorable prognosis.9-11 Moreover, we have recently shown that HGF induces a potent proliferative and antiapoptotic response in MM cell lines and primary MMs.24,29 Together with our observation that MM cells themselves produce the serine protease HGF activator (HGFA), and thereby are able to autocatalyze HGF activation,30 this suggests an important role for the HGF/MET pathway in the pathogenesis of MM. To examine the possible role of HGF/MET in the pathogenesis of other B-cell malignancies, we have studied the expression of MET and HGF in a large panel of B-cell malignancies, including all major B-cell non-Hodgkin lymphoma (B-NHL) subtypes, and we analyzed the MET gene in MET-positive lymphomas for the presence of amplification and mutations. Furthermore, we have defined the role of the HGF/MET pathway, including HGFA, in DLBCL the most common type of B-NHL.
Materials and methods
Antibodies and reagents
Mouse monoclonal antibodies used were as follows: anti-CD68 (IgG1), anti-CD21L (DRC-1, IgM), and FITC-conjugated anti-IgD (all DAKO, Glostrup, Denmark); anti-MET, DO24 (IgG2a) (Upstate Biotechnology, Lake Placid, NY), and IgG1; APC-conjugated anti-CD38 (IgG1; BD Biosciences, Erembodegem, Belgium); anti-HGFA A-1 (IgG1) and P1-4 (IgG1)31 ; anti-factor XIIa and OT-2 (IgG1; Sanquin, Amsterdam, the Netherlands); anti-CD20 (L26; DAKO, Glostrup, Denmark); fluorescein isothiocyanate (FITC)-conjugated anti-human IgD (DAKO); allophycocyanin (APC)-conjugated anti-human CD38 (IgG1; BD Biosciences); phycoerythrin (PE)-conjugated anti-human CD20 (DAKO); antibodies against the integrin subunit α4 (CD49d) (HP2/1, IgG1; Immunotech, Marseilles, France); and α4β7 (Act-1, IgG132 ; a gift from A. Lazarovits, University of Western Ontario, London, ON). Polyclonal antibodies used were as follows: goat anti-human HGF (R&D Systems, Abingdon, United Kingdom); rabbit anti-MET (C12), rabbit anti-PKB, and rabbit anti-ERK1 and anti-ERK2 (all from Santa Cruz Biotechnology, Santa Cruz, CA); AP-conjugated goat antimouse and biotin-conjugated rabbit antimouse (both from DAKO); RPE-conjugated rabbit anti-mouse IgG2a (BD Biosciences); AP-conjugated antidigoxygenin (Roche, Almere, the Netherlands); HRP-conjugated swine antigoat (Biosource, Camarillo, CA); and HRP-conjugated rabbit antimouse (DAKO). Antibodies against phosphorylated MET pY1230/1234/1235 (Biosource); phospho-FOXO3a (FKHRL1, Thr32; Upstate, Charlottesville, VA); and rabbit anti-phospho-GSK3 α/β (Ser21 and Ser9), rabbit anti-phospho PKB/AKT (Ser 473), and rabbit anti-phospho p44/42 MAP kinase (Thr202/Tyr204; all from Cell Signaling, Beverly, MA). Reagents used were as follows: recombinant HGF (Relia-Tech, Braunschweig, Germany); recombinant single-chain HGF (R&D Systems); the PI3K inhibitors LY294002 and wortmannin; and the MEK inhibitor PD98059 (Biomol, Plymouth Meeting, PA).
B-cell tumors and DLBCL cell lines
Tissue samples of 89 cases of B-NHL and 21 cases of MM were obtained were obtained during standard diagnostic procedure at the Academic Medical Center Amsterdam, The Netherlands, and the University Medical Center Utrecht (UMCU, Utrecht, The Netherlands), and frozen at -80°C until further use. Mononuclear cells from BM-derived MM samples were obtained by standard Ficoll-Paque density gradient centrifugation (Amersham Pharmacia, Uppsala, Sweden). Approval was obtained from the Academic Medical Center institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. All B-cell malignancies were classified according to the WHO classification.1 DLBCLs cell lines OCI-LY-1, OCI-LY-3, OCI-LY-7, and OCI-LY-18 were cultured in Iscoves medium (Life Technologies, Breda, the Netherlands) supplemented with 10% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT), penicillin (50 U/mL), and streptomycin (50 μg/mL) (both from Life Technologies). DLBCL cell line OCI-LY-10 was cultured in the presence of 20% FCS, penicillin, streptomycin, and β-mercaptoethanol (55 μM). OCI-LY-1, OCI-LY-7, and OCI-LY-18 were kindly provided by Dr U. Klein (Institute for Cancer Genetics, Colombia University, NY); OCI-LY-3 and OCI-LY-10 were kindly provided by Dr R. Küppers (Institute for Genetics and Department of Internal Medicine, University of Cologne, Germany). B cells were purified from human tonsils obtained from children undergoing routine tonsillectomy as described.7 Briefly, mononuclear cells were isolated by Ficoll-Isopaque density gradient centrifugation (Amersham Pharmacia). Monocytes and T cells were depleted by plastic adherence and sheep red blood cell rosetting, respectively. The B-cell fraction was more than 97% pure and contained approximately 60% naive B cells and approximately 35% GC B cells as determined by fluorescence-activated cell sorter (FACS) analysis using FITC-conjugated anti-human IgD, PE-conjugated anti-human CD20, and APC-conjugated anti-CD38. To obtain GC B cells, total B cells were stained with FITC-conjugated anti-human IgD, PE-conjugated anti-human CD20, and APC-conjugated anti-CD38 and sorted using a FACS aria (BD Biosciences).
Single-stranded conformation polymorphism (SSCP)
High-molecular-weight genomic DNA was obtained using standard methods by lysis in SDS, proteinase K digestion, phenol-chloroform extraction, and ethanol precipitation (all from Sigma, Bornem, Belgium). Polymerase chain reaction (PCR) was performed by amplifying exon 14, and exons 16 to 19 of MET, using intron-specific primers as described.16 Integrity of the DNA was confirmed through amplification of the β-globin gene using primers pair GH20/PCO4. The radioactive PCR amplification was carried out in a 30-μL reaction mixture containing 200 ng genomic DNA, 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 10 mM dATP, dTTP, and dGTP, 15 pmol primer, 5.5 × 107 Bq [α32P] dCTP (Amersham Pharmacia), and 0.3 U Taq DNA polymerase (Life Technologies). Following PCR, samples were diluted 1:7 in loading buffer (10 mM EDTA, pH 8.0, 0.05% SDS, 95% deionized formamid, 0.25% bromophenolblue, and 0.25% xylene cyanole FF [all from Sigma]), denatured for 3 minutes at 95°C, and slowly cooled to 4°C (1°C/s). Samples were loaded onto a 8% nondenaturing, 1× TBE acrylamide-bisacrylamide gel (50:1) (Life Technologies), containing 10% glycerol (Sigma), and run in for 16 hours at 8 W. Aberrant migrating amplicons were excised from the gel, reamplified using Pfu DNA polymerase (Stratagene, La Jolla, CA), cloned into EcoRV-digested pZeRO (Life Technologies), and sequenced using M13 primers and big-dye terminators (Amersham Pharmacia). Nucleotide and amino-acid numbering of MET was done according to the MET sequence as described by Schmidt et al.16
Immunohistochemistry and immunocytochemistry
Immunohistochemical stainings were performed as described previously33 on acetone-fixed cryostat sections (MET and DRC-1) or formalin-fixed paraffin-embedded sections (CD68). Substrate was developed with either 3,3-diaminobenzidine (Sigma) (anti-MET and CD68 staining) or fast blue BB (DAKO) (anti-CD21L, DRC-1) staining. Immunocytochemical stainings were performed on acetone-fixed cytospins. The cytospins were preincubated with 1% bovine serum albumin (BSA; Sigma) in PBS for 15 minutes. After incubating with the primary antibody (overnight at 4°C), endogenous peroxidase was blocked with 0.1% NaN3 and 0.3% H2O2 in PBS for 10 minutes. Subsequently, the cytospins were stained with postantibody of Powervision (Immunovision Technologies, Duiven, the Netherlands) for 15 minutes, followed by poly-HRP-conjugated goat anti-mouse/rabbit IgG for 30 minutes. Substrate was developed with 3,3-amino-9-ethylcarbazole. The immunohistochemical stainings were examined by use of an Olympus BX51 microscope (Olympus Optical, Hamburg, Germany) with a 40×/0.85 objective. Images were acquired by an Olympus DP11 camera and processed with Adobe Photoshop 7 (Adobe Systems, San Jose, CA).
Assay for HGF activation
HGF activation was assayed as described previously.34 Conditioned medium was obtained as described previously.35 In brief, 20 μL DLBCL conditioned medium was pretreated with 1 U thrombin (Sigma) and added to 0.1 μg single-chain HGF. Inhibitor studies were done in the presence of leupeptin (500 μg/mL; Sigma) or neutralizing antibody against HGFA (P1-4, 40 μg/mL).
ELISA for HGF
Conditioned medium of DLBCL cell lines or, as positive controls, of MM cell line UM-3 and follicular dendritic cells was used to detect the production of HGF.22 The enzyme-linked immunosorbent assay (ELISA) kit for HGF was used according to the manufacturer's instructions (R&D Systems). The lower detection limit was 125 pg/mL.
Immunoblot analysis
Immunoblotting was performed as described.33 For signaling experiments, cells were serum-starved for 2 hours and lysed after the indicated treatments. The samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The phosphorylation of MET, PKB, FOXO3a, GSK3, and ERK1 and ERK2 was examined by phosphorylation state-specific antibodies. After stripping, the same blot was restained with antibodies against MET, PKB, and ERK. The samples of the HGF conversion assay or DLBCL cell lines were also analyzed by SDS-PAGE. The immunoblots were stained with anti-HGF or anti-HGFA (A-1) and detected with HRP-conjugated swine antigoat and HRP-conjugated rabbit antimouse, respectively.
Cell-adhesion assay
Adhesion assays were done essentially as described.36 Briefly, 96-well plates were coated with 1 μg/mL soluble vascular cell-adhesion molecule-1 (VCAM-1; R&D Systems) or 10 μg/mL foreskin fibronectin (FN; Sigma). Cells (1.5 × 105) were preincubated for 30 minutes at 4°C in the presence or absence of either 1 μg/mL anti-α4β1 or 3 μg/mL anti-α4β7 integrin antibodies, or at 37°C with inhibitors. Next, the cells were plated in the absence or presence of recombinant HGF (ReliaTech) or phorbol-12-myristate-13-acetate (PMA; Sigma) in 100 μL/well, and incubated at 37°C for 30 minutes. The adherent cells were stained with crystal violet and washed; the dye was eluted; and absorbance was measured on a spectrophotometer (Microplate Reader 450; Biorad, Hercules, CA). Background absorbance (no cells added) was subtracted. Maximal adhesion (=100%) was determined by measuring nonspecific adhesion to poly-l-lysin-coated wells.
RNA isolation, cDNA synthesis, and reverse-transcriptase (RT)-PCR
RNA isolation and cDNA synthesis was done as described previously.22 Primers used were as follows: HGFA forward (5′-AGGACACAAGTGCCAGATTG-3); HGFA reverse (5′-GTTGATCCAGTCCACACATAGT-3′); MET forward (5′-GAGACTCATAATCCAACTG-3′); MET reverse (5′-AGCATACAGTTTCTTGCAG-3′); HGF forward (5′-CAGCATGTCCTCCTGCATCTCC-3′); and HGF reverse (5′-TCGTGTGGTATCATGGAACTCC-3′).
HGF mRNA in situ hybridization
Snap-frozen tissues were collected and frozen at -80°C until further use. Sections (10 μm thick) were cut, recovered on silianized slides, fixed for 15 minutes in 4% paraformaldehyde in PBS, dehydrated using ethanol, dried overnight, and stored at -20°C. Before use, tissue sections were rehydrated, washed in PBS, and incubated for 10 minutes in PBS containing 0.1 M glycine (Sigma). Sections were permeabilized for 15 minutes in 10 μg/mL proteinase K (Roche), after which the sections were washed 3 times with PBS, treated with 4% PFA/PBS for 10 minutes, and washed with 4× SSC. Acetylation was done using acetic acid anhydride and triethanolamide-hydrochloride for 15 minutes and prehybridized in hybridization mixture (50% formamid, 5× SSC, 5× Denhardt, 25 μg/mL baker's yeast tRNA, 500 μg/mL herring sperm DNA) for 1 hour. cRNA probes were synthesized as run-off transcripts using either T3 or T7 RNA polymerase and a digoxygenin RNA labeling kit (Roche). Probes were made from an 847-bp human HGF EcoR1 cDNA fragment (nt's 186-1033, kindly provided by Dr W. Birchmeier, MDC, Berlin, Germany) in pBSSK+. Overnight hybridization was done at 58°C. After hybridization, the slides were rinsed with 4× SSC/50% formamid, and subsequently washed in 4× SSC and 1× SSC, successively. Sections were treated with 1% PFA/PBS for 30 minutes, washed in 0.1M glycine/PBS, and equilibrated in TRIC buffer (100 mM Tris, 150 mM NaCl, pH 7.5). Blocking of nonspecific binding was performed in TRICB buffer (TRIC buffer containing 1% blocking reagent [Roche]). Slides were incubated with AP-conjugated antidigoxygenin antibody in TRICB buffer. Color development was done using nitro blue tetrazolium-5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP; Roche) in color buffer (100 mM Tris, 100 mM NaCl, 50 mM MgCl2, pH 9.5) containing 2.4 mg/mL levamisole (Sigma).
Results
MET expression in B-cell malignancies
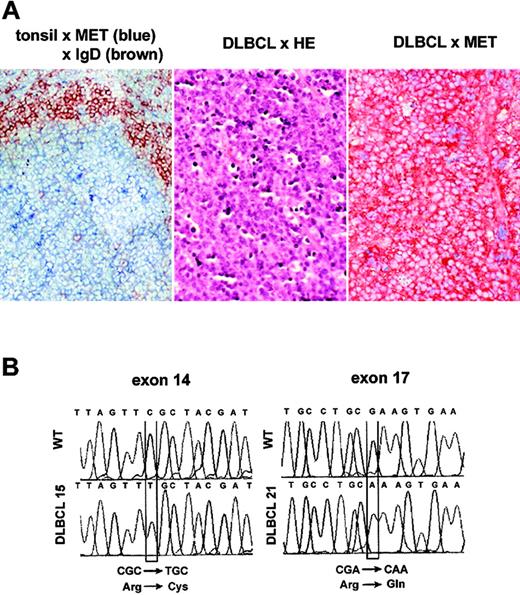
To investigate the expression of MET in B-cell malignancies, a panel of 110 B-cell tumors of different subtypes, representing a broad spectrum of differentiation stages ranging from precursor B-cell to plasma cell, was analyzed by immunohistochemistry and/or immunoblotting. A few cases of follicular lymphoma (FL), Burkitt lymphoma (BL), and chronic lymphocytic lymphoma (CLL) showed MET expression (Table 1), whereas MET was not detected on precursor-B-cell lymphomas, mantle-cell lymphomas, and marginal-zone lymphomas (Table 1). MET expression was largely confined to MMs (48%) and DLBCLs (30%) (Table 1). In several cases of DLBCL, which is the most common type of B-cell non-Hodgkin lymphoma (B-NHL) (30%-40% of cases), staining of MET was very strong (Figure 1A, right panel), indicating overexpression relative to the expression levels in normal GCs (Figure 1A, left panel).
Expression and mutational analysis of MET in B-cell tumors
WHO classification . | n . | % positive (n) . | Intensity* . | No. mutated/no. examined . |
|---|---|---|---|---|
| Precursor B lymphoblastic | 3 | 0 (0) | NA | 0/3 |
| Mantle cell | 5 | 0 (0) | NA | 0/3 |
| Follicular | 15 | 7 (1) | 2 | 1/15 |
| Burkitt | 12 | 8 (1) | 3 | 1/12 |
| DLBCL | 43 | 30 (13) | 2-3 | 2/39 |
| Marginal zone | 3 | 0 (0) | NA | ND |
| B-CLL | 8 | 25 (2) | 1 | 1/8 |
| Multiple myeloma | 21 | 48 (10) | NA (ϕ) | 0/21 |
WHO classification . | n . | % positive (n) . | Intensity* . | No. mutated/no. examined . |
|---|---|---|---|---|
| Precursor B lymphoblastic | 3 | 0 (0) | NA | 0/3 |
| Mantle cell | 5 | 0 (0) | NA | 0/3 |
| Follicular | 15 | 7 (1) | 2 | 1/15 |
| Burkitt | 12 | 8 (1) | 3 | 1/12 |
| DLBCL | 43 | 30 (13) | 2-3 | 2/39 |
| Marginal zone | 3 | 0 (0) | NA | ND |
| B-CLL | 8 | 25 (2) | 1 | 1/8 |
| Multiple myeloma | 21 | 48 (10) | NA (ϕ) | 0/21 |
n indicates number of samples; DLBCL, diffuse large B-cell lymphoma; B-CLL, B-cell chronic lymphocytic leukemia; ND, not done; NA, not applicable; and (φ), expression of MET by multiple myeloma was determined by Western blot analysis using a polyclonal anti-MET antibody.
1 indicates weak; 2, moderate; and 3, strong staining with a monoclonal anti-MET antibody.
MET expression and missense germ-line MET mutations in DLBCL. (A) MET expression in normal lymphoid tissue and primary DLBCL. Immunohistochemical double staining of tonsillar sections for MET (blue) and IgD (brown), showing MET expression on germinal center B cells (left panel). Frozen tissue sections of DLBCL were stained with hematoxylin-eosin (HE) (middle panel) or with hematoxylin and anti-MET (brown, right panel). Image magnification: × 200. (B) Sequence analysis showing mutational transitions. PCR products were excised, reamplified, cloned, and sequenced. Shown are wild-type (WT) and mutant sequences. Mutational transitions are boxed.
MET expression and missense germ-line MET mutations in DLBCL. (A) MET expression in normal lymphoid tissue and primary DLBCL. Immunohistochemical double staining of tonsillar sections for MET (blue) and IgD (brown), showing MET expression on germinal center B cells (left panel). Frozen tissue sections of DLBCL were stained with hematoxylin-eosin (HE) (middle panel) or with hematoxylin and anti-MET (brown, right panel). Image magnification: × 200. (B) Sequence analysis showing mutational transitions. PCR products were excised, reamplified, cloned, and sequenced. Shown are wild-type (WT) and mutant sequences. Mutational transitions are boxed.
MET mutations in B-cell lymphomas
Amplification of the MET gene resulting in MET overexpression has been described in several types of cancer.14,20,37 However, Southern blot analysis using a MET-specific cDNA probe did not reveal MET gene amplification in the MET-positive lymphomas (data not shown). Furthermore, missense germ-line or somatic MET mutations have been found in hereditary papillary renal carcinoma (HPRC),16,17 and in several other types of cancer.18,19,38 The affected regions of MET are the catalytic or the juxtamembrane (JM) domain, resulting in deregulated activation or degradation of MET, respectively.19,39,40 Upon HGF stimulation, cells with MET mutations in these regions display enhanced or prolonged kinase activity, resulting in transformation, invasive growth, and enhanced tumor-cell survival.39-41
To investigate whether MET mutations may also contribute to lymphomagenesis, we screened the tumor samples listed in Table 1 for mutations in exon 14, encoding the JM region, and exons 16 to 19, encoding the tyrosine kinase and docking site regions of MET, by means of SSCP analysis. Using this approach, 2 distinct missense mutations were detected in MET (Figure 1B). The first was at position 2961 (C to T) in exon 14, resulting in a nonconservative transition from arginine to cysteine at position 988 (R988C), and was found in 4 individual cases of CLL, FL, BL, and DLBCL. The second mutation (3496 G to A) in exon 17, resulting in a transition from arginine to glutamine at position 1166 (R1166Q), was detected in one case of DLBCL. Analysis of normal tissues from the affected individuals revealed that these mutations were germ-line mutations. Of note, these mutations were found in none of the 5 DLBCL cell lines (data not shown). During the course of our study, the R988C mutation was also reported in several (non-) small-cell lung cancer ([N]SCLC) cell lines,42,43 and in 2 lung cancer patients.42,44 Of interest, these studies revealed that R988C conveys enhanced in vitro tumorigenicity as well as lung tumor susceptibility in mice,43,44 indicating that R988C is a gain-of-function mutation.
HGF/MET signaling in DLBCL cells
Apart from MM, MET expression was largely restricted to DLBCLs (Table 1). Of interest, in DLBCL high serum HGF levels were previously found, which were shown to be correlated with unfavorable prognosis.10,45 In addition, a gene-profiling study showed significantly enhanced expression of MET upon transformation of low-grade FLs into DLBCLs,46 suggesting a pathogenic role for HGF/MET signaling in tumor progression. Therefore, we decided to explore the functionality of MET signaling in DLBCL cells and to examine which signaling pathways become activated upon HGF stimulation. For this purpose, we analyzed a panel of DLBCL cell lines for MET expression. In a subset of DLBCL cell lines (3/5), expression of MET was observed at mRNA (Figure 2A) and protein (Figure 2B) level. HGF stimulation resulted in enhanced phosphorylation of MET in the strongly MET-positive DLBCL cell lines OCI-LY-3 and OCI-LY-10, whereas the weakly MET-positive cell line OCI-LY-1 showed a weak response (Figure 2C). Moreover, HGF stimulation of the MET-positive cells leads to phosphorylation of the mitogen-activated protein kinases ERK1 and ERK2, as well as to phosphorylation of PKB and of the PKB substrates GSK3 and the forkhead transcription factor FOXO3a (FKHRL1) (Figure 2C). The unrelated PI3K inhibitors wortmannin (WM) or LY294002 (LY) both completely abrogated the HGF-induced phosphorylation of PKB, GSK3, and FOXO3a, but hardly affected the phosphorylation of ERK1/2 (Figure 2D). Vice versa, the HGF-stimulated activation of ERK1/2 was specifically blocked by the MEK inhibitor PD98059 (PD), which did not affect phosphorylation of PKB, GSK3, or FOXO3a (Figure 2D). The RAS downstream effector components MEK and ERK1/2 have been directly linked to the regulation of cell proliferation,47,48 whereas PKB, GSK3, and FOXOs, targets of PI3K-derived signals, have been implicated in both the proliferation and survival.49,50
HGF induces phosphorylation of MET in DLBCL cells and activates the RAS/MAPK and PI3K/PKB pathway. (A) mRNA expression of MET in DLBCL cell lines. After RNA isolation and cDNA synthesis, RT-PCR for MET was performed. β2-Microglobulin was used as housekeeping gene control. (B) MET protein expression in DLBCL cell lines. DLBCL cell lines were analyzed by immunoblotting for the expression of MET. The (weak) expression of MET by OCI-LY-1 cells is clearly demonstrated by means of a 3-times longer exposure (right). Staining with anti-β-actin represents the loading control. (C) HGF induces tyrosine phosphorylation of MET, PKB, FOXO3a, GSK3, and ERK. The DLBCL cells OCI-LY-1, OCI-LY-3, and OCI-LY-10, and MET-transfected Namalwa cells (V3M) were stimulated with HGF for the indicated time periods. Cell lysates were immunoblotted with phosphorylation-specific antibodies against MET, FOXO3a, GSK3, PKB, and ERK. The blots were stripped and restained with antibodies against MET, PKB, and ERK. (D) HGF-induced phosphorylation of FOXO3a and GSK3 requires PI3K activity, whereas phosphorylation of ERK1 and ERK2 is MEK dependent. OCI-LY-3 and OCI-LY-10 cells were pretreated with the PI3K inhibitors wortmannin (WM, 50 μM) or LY294002 (LY, 20 μM), the MEK inhibitor PD98059 (PD, 50 μM), or DMSO (C) for 30 minutes, prior to incubation with HGF (200 ng/mL). Phosphorylation of ERK1 and ERK2, PKB, GSK3, and FOXO3a was determined by immunoblotting with phosphorylation-specific antibodies. The blots were stripped and restained for ERK1 and ERK2, and PKB.
HGF induces phosphorylation of MET in DLBCL cells and activates the RAS/MAPK and PI3K/PKB pathway. (A) mRNA expression of MET in DLBCL cell lines. After RNA isolation and cDNA synthesis, RT-PCR for MET was performed. β2-Microglobulin was used as housekeeping gene control. (B) MET protein expression in DLBCL cell lines. DLBCL cell lines were analyzed by immunoblotting for the expression of MET. The (weak) expression of MET by OCI-LY-1 cells is clearly demonstrated by means of a 3-times longer exposure (right). Staining with anti-β-actin represents the loading control. (C) HGF induces tyrosine phosphorylation of MET, PKB, FOXO3a, GSK3, and ERK. The DLBCL cells OCI-LY-1, OCI-LY-3, and OCI-LY-10, and MET-transfected Namalwa cells (V3M) were stimulated with HGF for the indicated time periods. Cell lysates were immunoblotted with phosphorylation-specific antibodies against MET, FOXO3a, GSK3, PKB, and ERK. The blots were stripped and restained with antibodies against MET, PKB, and ERK. (D) HGF-induced phosphorylation of FOXO3a and GSK3 requires PI3K activity, whereas phosphorylation of ERK1 and ERK2 is MEK dependent. OCI-LY-3 and OCI-LY-10 cells were pretreated with the PI3K inhibitors wortmannin (WM, 50 μM) or LY294002 (LY, 20 μM), the MEK inhibitor PD98059 (PD, 50 μM), or DMSO (C) for 30 minutes, prior to incubation with HGF (200 ng/mL). Phosphorylation of ERK1 and ERK2, PKB, GSK3, and FOXO3a was determined by immunoblotting with phosphorylation-specific antibodies. The blots were stripped and restained for ERK1 and ERK2, and PKB.
HGF induces integrin-mediated adhesion of DLBCL cells in a PI3K-dependent fashion
Previous studies, including from our laboratory, revealed that HGF/MET signaling induces survival and proliferation of MM cells24,29 and can control integrin-mediated adhesion of Burkitt lymphoma cells6,22 and MM cells.51 Despite the activation of several survival- and proliferation-regulatory signaling proteins (Figure 2C), we did not observe an effect of HGF on the survival or proliferation of DLBCL cells (data not shown). To further define the role of HGF/MET signaling in DLBCLs, we examined whether HGF may be able to control integrin-mediated adhesion. HGF stimulation of both OCI-LY-1 and OCI-LY-10 cells strongly augmented adhesion to both VCAM-1 (Figure 3A) and FN (data not shown). In contrast, as expected, the MET-negative DLBCL cell lines OCI-LY-7 and OCI-LY-18 did not exhibit any HGF-induced adhesion (Figure 3A). Of note, the OCI-LY-3 cells displayed extensive (constitutive) cell aggregation. As a consequence, neither HGF nor PMA could enhance adhesion of OCILY-3, thus rendering this cell line useless for adhesion analysis (Figure 3A).
HGF induces α4β1-mediated adhesion of DLBCL cells in a PI3K-dependent fashion. (A) HGF induces adhesion of DLBCL cell lines OCI-LY-1 and OCI-LY-10 to VCAM-1. Cells were stimulated with 200 ng/mL HGF or 50 ng/mL PMA followed by adhesion to VCAM-1. The OCI-LY-3 cells displayed extensive (constitutive) cell aggregation. Neither HGF nor PMA could enhance adhesion of OCI-LY-3 (three left panels). MET-negative OCI-LY-7 and OCI-LY-18 cells were used as negative controls (two right panels). The results are expressed as a percentage of maximal adhesion. The bars represent the means ± the standard deviation of a triplicate experiment representative of at least 3 independent experiments. (B) HGF-induced adhesion involves α4β1 integrin. The effect of preincubation with anti-α4β1 (HP2/1) and anti-α4β7 (Act-1) integrin antibodies on the HGF-induced binding of DLBCL cell lines OCI-LY-10 to VCAM-1 was established. Cells were preincubated for 30 minutes at 4°C in the presence or absence of anti-integrin monoclonal antibodies or isotype control antibody, as indicated. Next, adhesion to VCAM-1 in the presence of 200 ng/mL HGF was measured. Error bars represent the means ± standard deviation of a triplicate experiment representative of 2 independent experiments. (C) HGF-induced adhesion requires PI3K activity. HGF-induced adhesion of OCI-LY-10 was determined after pretreatment with the PI3K inhibitors wortmannin (WM, 100 nM) and LY294002 (LY, 20 μM), the MEK inhibitor PD98059 (PD, 50 μM), or DMSO (C) for 30 minutes at 37°C, followed by adhesion to VCAM-1 in the presence of 200 ng/mL HGF. The bars represent the means ± standard deviation of a triplicate experiment representative of 2 independent experiments.
HGF induces α4β1-mediated adhesion of DLBCL cells in a PI3K-dependent fashion. (A) HGF induces adhesion of DLBCL cell lines OCI-LY-1 and OCI-LY-10 to VCAM-1. Cells were stimulated with 200 ng/mL HGF or 50 ng/mL PMA followed by adhesion to VCAM-1. The OCI-LY-3 cells displayed extensive (constitutive) cell aggregation. Neither HGF nor PMA could enhance adhesion of OCI-LY-3 (three left panels). MET-negative OCI-LY-7 and OCI-LY-18 cells were used as negative controls (two right panels). The results are expressed as a percentage of maximal adhesion. The bars represent the means ± the standard deviation of a triplicate experiment representative of at least 3 independent experiments. (B) HGF-induced adhesion involves α4β1 integrin. The effect of preincubation with anti-α4β1 (HP2/1) and anti-α4β7 (Act-1) integrin antibodies on the HGF-induced binding of DLBCL cell lines OCI-LY-10 to VCAM-1 was established. Cells were preincubated for 30 minutes at 4°C in the presence or absence of anti-integrin monoclonal antibodies or isotype control antibody, as indicated. Next, adhesion to VCAM-1 in the presence of 200 ng/mL HGF was measured. Error bars represent the means ± standard deviation of a triplicate experiment representative of 2 independent experiments. (C) HGF-induced adhesion requires PI3K activity. HGF-induced adhesion of OCI-LY-10 was determined after pretreatment with the PI3K inhibitors wortmannin (WM, 100 nM) and LY294002 (LY, 20 μM), the MEK inhibitor PD98059 (PD, 50 μM), or DMSO (C) for 30 minutes at 37°C, followed by adhesion to VCAM-1 in the presence of 200 ng/mL HGF. The bars represent the means ± standard deviation of a triplicate experiment representative of 2 independent experiments.
To identify the adhesion receptors on the DLBCL cell lines responsible for the enhanced VCAM-1 binding, integrin expression analysis and antibody blocking experiments were performed. OCI-LY-1, OCI-LY-3, and OCI-LY-10 showed high expression of integrin α4β1 but do not express α4β7, as established by FACS analysis (data not shown). Notably, HGF treatment did not enhance α4β1 or α4β7 expression during the course of the adhesion assay (data not shown). The adhesion to VCAM-1 was completely blocked by the α4β1-blocking antibody HP2/1, whereas the α4β7-blocking antibody Act-1 or an isotype control antibody had no effect (Figure 3B).
To investigate the functional importance of PI3K and MEK/MAPK in the HGF-induced adhesion of DLBCL cells, we analyzed the effect of the PI3K inhibitors WM or LY, and the MEK inhibitor PD. The specificity and effectivity of these inhibitors in DLBCL cells is shown in Figure 2D. Both WM and LY completely abolished the HGF-induced adhesion of OCI-LY-10 cells, whereas hardly any effect by PD was observed (Figure 3C). Hence, HGF-induced adhesion of DLBCL cells is dependent on PI3K, but not on MEK or MAPK activity. Taken together, these results show that the HGF/MET pathway controls α4β1 integrin-mediated adhesion of DLBCL cells in a PI3K-dependent manner.
HGF expression in the DLBCL microenvironment
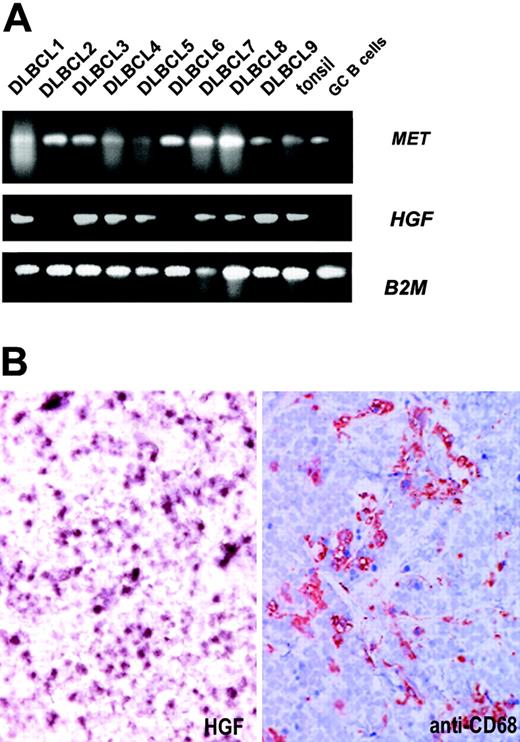
In several epithelial tumors as well as in MM, autocrine HGF secretion by tumor cells as well as paracrine HGF production by fibroblasts and macrophages in the tumor stroma is crucial for the growth and invasion of MET-positive tumor cells.13 To investigate whether autocrine or paracrine stimulation of MET by HGF takes place in DLBCLs and is responsible for the signaling and adhesion effects observed in the DLBCL cell lines, we first studied the expression of HGF mRNA in a number of primary MET-positive DLBCL samples. RT-PCR showed HGF mRNA expression in most of the MET-positive primary DLBCL tumors (7/9) (Figure 4A). By mRNA in situ hybridization, we found that HGF was localized in single cells and small cell clusters within the DLBCLs (n = 8). Staining of serial sections with anti-CD68 showed a similar staining pattern, suggesting that these cells were (activated) macrophages (Figure 4B). Furthermore, analysis of the conditioned medium of the DLBCL cell lines by means of HGF ELISA did not reveal any autocrine production of HGF (data not shown). Our findings suggest that DLBCL cells are stimulated via a paracrine rather than an autocrine mechanism.
HGF expression in DLBCL. (A) MET-positive primary DLBCLs were analyzed for HGF mRNA expression. After RNA isolation and cDNA synthesis, RT-PCR for MET and HGF was performed. β2-Microglobulin was used as housekeeping gene control. (B) Frozen sections of DLBCL cases were analyzed for the presence of expression of HGF by mRNA in situ hybridization, using DIG-labeled antisense cRNA run-off transcripts. Serial sections were stained with anti-CD68 to identify macrophages. The section was counterstained with hematoxylin. The result shown is a representative of 8 tested DLBCL cases. Image magnification: × 200.
HGF expression in DLBCL. (A) MET-positive primary DLBCLs were analyzed for HGF mRNA expression. After RNA isolation and cDNA synthesis, RT-PCR for MET and HGF was performed. β2-Microglobulin was used as housekeeping gene control. (B) Frozen sections of DLBCL cases were analyzed for the presence of expression of HGF by mRNA in situ hybridization, using DIG-labeled antisense cRNA run-off transcripts. Serial sections were stained with anti-CD68 to identify macrophages. The section was counterstained with hematoxylin. The result shown is a representative of 8 tested DLBCL cases. Image magnification: × 200.
DLBCL cells activate HGF by secretion of HGFA
The serine protease HGFA has been shown to mediate proteolytic conversion of single-chain HGF (scHGF, HGF precursor) to its active heterodimeric form,31 which is essential for the activation and biologic function of HGF.52 HGFA, a factor XIIa-related serine protease, has been identified as the most potent activator of HGF.31 We have previously demonstrated that MM plasma cells produce HGFA, and in this way may activate HGF in the bone marrow microenvironment.30 To assess whether HGFA is expressed and mediates HGF conversion in DLBCLs, we evaluated the expression of HGFA in MET-positive DLBCLs and cell lines. Of interest, all primary DLBCLs and cell lines expressed HGFA mRNA (Figure 5A-B). Moreover, contrary to normal tonsillar naive, GC, or memory B cells, which do not or very weakly express HGFA53 (Figure 5C), the DLBCL cells express HGFA protein (Figure 5C-D).
HGFA expression in DLBCL cells. (A) Expression of HGFA in GC B cells and primary DLBCLs at mRNA level. After RNA isolation and cDNA synthesis, RT-PCR for MET was performed. β2-Microglobulin was used as housekeeping gene control. (B) mRNA expression of HGFA in DLBCL cell lines. (C) HGFA protein is expressed in DLBCL cell lines, but not in GC B cells. Cell lysates were immunoblotted using a monoclonal anti-HGFA antibody (A-1). MM cell line LME-1 was used as positive control. β-Actin was used as loading control. (D) Expression of HGFA protein in DLBCL lines and tonsillar B cells by immunocytochemical staining. DLBCL and tonsillar B cells were immunocytochemically stained with mAb A-1 against HGFA (A-1), CD20 (L26), or isotype control, as indicated. Image magnification: × 400.
HGFA expression in DLBCL cells. (A) Expression of HGFA in GC B cells and primary DLBCLs at mRNA level. After RNA isolation and cDNA synthesis, RT-PCR for MET was performed. β2-Microglobulin was used as housekeeping gene control. (B) mRNA expression of HGFA in DLBCL cell lines. (C) HGFA protein is expressed in DLBCL cell lines, but not in GC B cells. Cell lysates were immunoblotted using a monoclonal anti-HGFA antibody (A-1). MM cell line LME-1 was used as positive control. β-Actin was used as loading control. (D) Expression of HGFA protein in DLBCL lines and tonsillar B cells by immunocytochemical staining. DLBCL and tonsillar B cells were immunocytochemically stained with mAb A-1 against HGFA (A-1), CD20 (L26), or isotype control, as indicated. Image magnification: × 400.
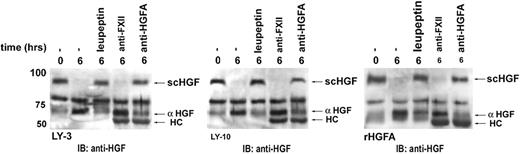
Subsequently, we examined whether DLBCL cells are able to process scHGF (precursor of HGF) to its active form. Indeed, we observed processing of scHGF to its α-chain by conditioned media from the DLBCL cell lines. This conversion was completely inhibited by addition of the serine protease inhibitor leupeptin (Figure 6). Since proteases other than HGFA are, although with low efficiency, capable of activating scHGF in vitro, we explored whether the conversion of scHGF by DLBCLs could be specifically inhibited by interfering with HGFA activity. Indeed, we observed that the anti-HGFA monoclonal P1-4, which blocks HGFA function, effectively inhibits scHGF conversion by DLB-CLs (Figure 6). These findings demonstrate that HGFA is the serine protease responsible for the conversion of scHGF in DLBCLs and identifies the DLBCL cells themselves as an important source of HGFA, thereby regulating HGF activity within the tumor microenvironment.
DLBCL cells autocatalyze HGF activation by producing HGFA. Conditioned medium of DLBCL cell lines OCI-LY-3 (left panel) and OCI-LY-10 (middle panel) was incubated with scHGF for 6 hours in the presence of thrombin, combined with either the serine protease inhibitor leupeptin, neutralizing antibody against HGFA (PI-4), or factor XIIa (OT-2), as indicated. As positive control, HGF conversion by recombinant HGFA is shown (right panel). HGF conversion was determined by immunoblotting with anti-HGF. αHGF indicates active α heavy chain of HGF; scHGF, inactive single chain of HGF; and HC, heavy chain of immunoglobulin.
DLBCL cells autocatalyze HGF activation by producing HGFA. Conditioned medium of DLBCL cell lines OCI-LY-3 (left panel) and OCI-LY-10 (middle panel) was incubated with scHGF for 6 hours in the presence of thrombin, combined with either the serine protease inhibitor leupeptin, neutralizing antibody against HGFA (PI-4), or factor XIIa (OT-2), as indicated. As positive control, HGF conversion by recombinant HGFA is shown (right panel). HGF conversion was determined by immunoblotting with anti-HGF. αHGF indicates active α heavy chain of HGF; scHGF, inactive single chain of HGF; and HC, heavy chain of immunoglobulin.
Discussion
Uncontrolled activation of the HGF/MET signaling pathway has been implicated in tumor growth, invasion, and metastasis in both mice and humans.13 Here, we have investigated the expression of MET protein on a large panel of B-cell malignancies. We have found that MET is frequently expressed in MM (48%) and DLBCL (30%) (Table 1), and that MET is occasionally mutated in B-cell malignancies, including DLBCL (Figure 1). Furthermore, we have demonstrated that HGF is produced within the DLBCL microenvironment (Figure 4), that DLBCL cells themselves produce HGFA thereby activating HGF (Figures 5-6), and that HGF/MET signaling in DLBCL cells is functional and controls integrin-mediated adhesion (Figures 2-3). Previously, overexpression of either HGF or MET in DLBCL tumor sections, as well as high levels of HGF in the serum of DLBCL patients, has been found to associate with poor prognosis.10,45,54 Notably, the treatment response was associated with changes in serum HGF levels.10 More recently, a gene-profiling study showed significantly enhanced expression of MET upon transformation of low-grade FLs into DLBCLs within the same patients.46 Combined, these data strongly suggest a pathogenic role for HGF/MET signaling in DLBCL.
Overexpression of MET in tumor cells may be due to MET amplification, defective transcriptional regulation of the MET gene, or mutations affecting MET protein stability.14 Although amplification of MET has been reported in several types of human cancer, this was not found in any of the MET-expressing lymphomas studied (data not shown). Of importance, however, the high MET-expressing cell lines OCI-LY-3 and OCI-LY-10 are so-called activated B-cell (ABC)-like DLBCLs. These ABC-like DLBCLs are characterized by a nuclear factor κB (NFκB) expression profile, and indeed OCI-LY-3 and OCI-LY-10 exhibit constitutive NFκB activation.55 This is particularly interesting since the MET promoter contains several putative NFκB responsive elements.
Thus far, no MET mutations have been described for B-cell malignancies. However, here we found 2 distinct germ-line missense mutations in MET: R1166Q in the kinase domain in 1 DLBCL patient, and R988C in the JM domain in 4 patients with either DLBCL, CLL, FL, or BL (Figure 2). Notably, no mutations in MET were found in the DLBCL cell lines (data not shown). In hereditary and sporadic papillary renal-cell cancer, most missense mutations are located in the tyrosine kinase domain of MET, causing constitutive activity and/or a lower threshold for HGF-induced activation of the tyrosine kinase.39,41,56,57 Thus, the R1166Q mutant may have a similar effect. Since the JM region of MET harbors important negative regulatory sites involved in receptor ubiquitination, degradation, and inhibition of kinase activity,58-61 the R988C MET mutation may affect these processes, leading to aberrant MET signaling. Recently, the R988C mutation has also been reported in 2 SCLC cell lines,43 and, during the preparation of this paper, in an NSCLC cell line and in 2 lung cancer patients.42,44 Of interest, upon transfection the R988C mutant promoted proliferation, motility, and overall tyrosine phosphorylation of the pre-B-cell line BaF3.43 Moreover, expression of the R988C mutant in a SCLC cell line resulted in enhanced focus formation and soft-agar colony formation.43 These observations, combined with the recent demonstration that a mouse MET mutation homologous to R988C plays an important role in lung tumor susceptibility,44 strongly suggest that the R988C mutation is a true gain-of-function mutation. Since a recent study has shown that mice expressing oncogenic MET mutants develop lymphomas,62 it is conceivable that the R988C MET mutation can convey B-cell lymphoma susceptibility in humans.
Within the DLBCL microenvironment, we found that HGF was localized in single cells and small cell clusters, most likely representing (activated) macrophages (Figure 4B). This, combined with the lack of HGF expression by the DLBCL cell lines as measured in conditioned medium (data not shown), indicates a paracrine rather than an autocrine mechanism of MET activation in DLBCL. Of note, since HGF itself has angiogenic properties,63 and has been demonstrated to induce expression of vascular endothelial growth factor (VEGF) as well,64,65 HGF might also stimulate angiogenesis in DLBCL, thereby promoting tumor growth. Proteolytic activation of HGF in the extracellular milieu is a critical limiting step in HGF/MET signaling. Here we have demonstrated that DLBCLs and cell lines express HGFA and are able to process scHGF to its active form (Figure 6). This is in sharp contrast to tonsillar naive, GC, or memory B cells, which do not or very weakly express HGFA53 (Figure 5C). Hence, autocrine production of HGFA by DLBCL cells may support tumorigenesis via autocatalyzation of HGF conversion, consequently providing a constant source of active HGF in the tumor microenvironment.
HGF-induced activation of MET in DLBCL cells resulted in MEK-dependent phosphorylation of the MAP kinases ERK1 and ERK2 (Figure 2C). The consecutive activation of MEK-1 and ERK1/2, the phosphorylation of the transcription factors ELK1 and ETS2, and the expression of immediate early genes such as FOS have been directly linked to regulation of cell proliferation.47,48 Furthermore, upon HGF stimulation of DLBCL cells, we observed PI3K-dependent phosphorylation of PKB/Akt and its substrates GSK3 and FOXO3a (Figure 2D). By direct phosphorylation, PKB can inhibit BAD and caspase-9, activate IKKα resulting in activation of NFκB, and inhibit GSK3 and forkhead transcription factors of the FOXO subfamily, including FOXO3a (FKHRL1), all of which contributes to its antiapoptotic function.66 Inhibition of GSK3 and FOXOs by PKB can also induce cell proliferation through enhanced cyclin D1 stabilization and transcription, respectively.66,67 Of note, overexpression of cyclin D1, often as a consequence of chromosomal translocations, is frequently observed in lymphomas.68 Phosphorylation of FOXOs by PKB prevents their nuclear translocation and thereby the expression of FOXO target genes, which include the proapoptotic genes FasL and Bim, and the antiproliferative genes p27KIP and Rb2.69 Indirectly, FOXOs can suppress expression of the antiapoptotic gene FLIP70 and the proproliferative genes cyclin D1 and D2.66 Recently, PI3K/PKB-mediated inactivation of FOXO3a was shown to be important for B-cell proliferation.71 The observed low expression of p27kip1 in GC B-type DLBCL, as well as the constitutive activation of NFκB and high expression of FLIP and cyclin D2 in ABC-type DLBCL,1,55 illustrates the relevance of these HGF/MET-controlled signaling cascades for DLBCL.
Similar to our previous studies with the GC B-cell-like Burkitt lymphoma cell line Namalwa,22 we have shown that HGF induces integrin-mediated adhesion of DLBCL cells to VCAM-1 and FN (Figure 3A and data not shown). Furthermore, this HGF-induced adhesion involved α4β1 integrin (Figure 3B) and required activation of PI3K (Figure 3C). The HGF-induced integrin activation can control the interaction of the DLBCL cells with extracellular matrix, stromal cells, and FDCs within the tumor microenvironment. By analogy to B-cell antigen receptor-controlled adhesion of GC B cells,7,36,72 this may provide important integrin-mediated outside-in, as well as paracrine, growth, and survival, signals.
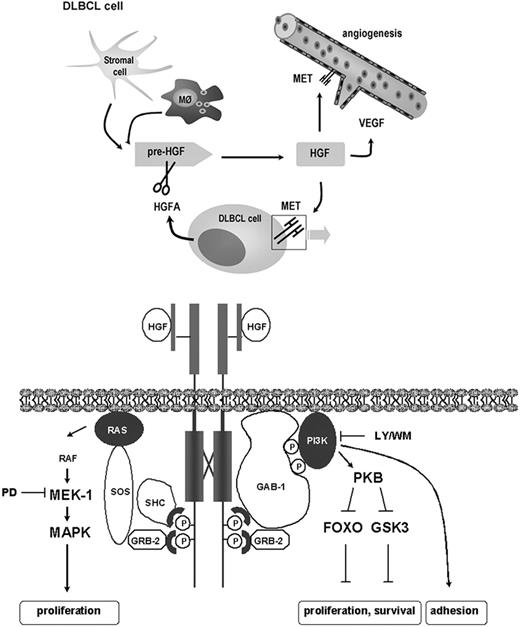
A better understanding of the biology of B-cell malignancies is needed in the development of potential therapeutic agents that target specific intracellular pathways and the cross talk that occurs between malignant B cells and the microenvironment. Our data indicate that aberrant HGF/MET signaling and conversion of HGF by DLBCL cell-secreted HGFA can play an important role in the control of adhesion, survival, and proliferation of DLBCL cells, and thereby in the maintenance of DLBCL in the tumor microenvironment (Figure 7). Thus, our data provide new insights into the pathogenesis of DLBCL and identify the HGF/MET pathway as a potential novel therapeutic target for the treatment of DLBCL.
Activation and biologic actions of HGF in the DLBCL microenvironment. HGF is produced by macrophages (M∅) and/or stromal cells. Expression and secretion of HGFA by DLBCL cells regulates the bioavailability of active HGF in the DLBCL microenvironment. Catalyzation of HGF activation by DLBCL cells can directly stimulate HGF/MET signaling (bottom panel), promoting DLBCL adhesion, growth, and survival. In addition, HGF can directly or indirectly stimulate angiogenesis. Bottom panel: schematic representation of the HGF-induced signaling events in DLBCL cells. HGF-induced activation of MAPK and PKB is mediated by Grb2/SOS coupling to RAS and GAB-1 coupling to PI3K, respectively.73 Activation of RAS/MAPK may lead to proliferation of DLBCL cells. Activation of PI3K/PKB leads to phosphorylation of FOXO3a and GSK3, which may control proliferation and survival (see “Discussion” for further details). Furthermore, PI3K mediates HGF-induced adhesion of DLBCL cells. Pre-HGF indicates inactive precursor of HGF.
Activation and biologic actions of HGF in the DLBCL microenvironment. HGF is produced by macrophages (M∅) and/or stromal cells. Expression and secretion of HGFA by DLBCL cells regulates the bioavailability of active HGF in the DLBCL microenvironment. Catalyzation of HGF activation by DLBCL cells can directly stimulate HGF/MET signaling (bottom panel), promoting DLBCL adhesion, growth, and survival. In addition, HGF can directly or indirectly stimulate angiogenesis. Bottom panel: schematic representation of the HGF-induced signaling events in DLBCL cells. HGF-induced activation of MAPK and PKB is mediated by Grb2/SOS coupling to RAS and GAB-1 coupling to PI3K, respectively.73 Activation of RAS/MAPK may lead to proliferation of DLBCL cells. Activation of PI3K/PKB leads to phosphorylation of FOXO3a and GSK3, which may control proliferation and survival (see “Discussion” for further details). Furthermore, PI3K mediates HGF-induced adhesion of DLBCL cells. Pre-HGF indicates inactive precursor of HGF.
Prepublished online as Blood First Edition Paper, September 27, 2005; DOI 10.1182/blood-2005-05-1929.
Supported by grants from the Netherlands Organisation for Health Research and Development (ZonMW) and the Dutch Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Prof Dr H. Kataoka for the antibodies against HGFA and recombinant active HGFA.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal