Abstract
In most patients with systemic mastocytosis (SM), including aggressive SM and mast cell leukemia (MCL), neoplastic cells express the oncogenic KIT mutation D816V. KIT D816V is associated with constitutive tyrosine kinase (TK) activity and thus represents an attractive drug target. However, imatinib and most other TK inhibitors fail to block the TK activity of KIT D816V. We show that the novel TK-targeting drugs PKC412 and AMN107 counteract TK activity of D816V KIT and inhibit the growth of Ba/F3 cells with doxycycline-inducible expression of KIT D816V as well as the growth of primary neoplastic mast cells and HMC-1 cells harboring this KIT mutation. PKC412 was a superior agent with median inhibitory concentration (IC50) values of 50 to 250 nM without differences seen between HMC-1 cells exhibiting or lacking KIT D816V. By contrast, AMN107 exhibited more potent effects in KIT D816V- HMC-1 cells. Corresponding results were obtained with Ba/F3 cells exhibiting wild-type or D816V-mutated KIT. The growth-inhibitory effects of PKC412 and AMN107 on HMC-1 cells were associated with induction of apoptosis and down-regulation of CD2 and CD63. PKC412 was found to cooperate with AMN107, imatinib, and cladribine (2CdA) in producing growth inhibition in HMC-1, but synergistic drug interactions were observed only in cells lacking KIT D816V. Together, PKC412 and AMN107 represent promising novel agents for targeted therapy of SM. (Blood. 2006;107: 752-759)
Introduction
Several emerging modalities for the treatment of myeloid neoplasms are based on novel drugs targeting critical tyrosine kinases (TKs) or downstream signaling molecules.1-5 Systemic mastocytosis (SM) is a hematopoietic neoplasm that behaves as an indolent myeloproliferative disease in most patients, but it can also present as an aggressive disease (aggressive SM [ASM]) or even as a leukemia (mast cell leukemia [MCL]).6-11 In patients with ASM and MCL, the response to conventional therapy is poor, and the prognosis is grave.6-12 Therefore, a number of attempts have been made to identify novel targets for therapy in neoplastic mast cells (MCs) and to define new treatment strategies for these patients.9-12
In the majority of all patients with SM, including those who have ASM or MCL, the somatic KIT point mutation D816V (Asp816Val) is detectable in neoplastic (mast) cells.13-17 This mutation is associated with ligand-independent phosphorylation and activation of KIT and autonomous differentiation and growth of affected cells.17,18 Based on this concept, the D816V-mutated variant of KIT is an attractive target of therapy.9-12,19 Thus, a number of efforts have been made in recent years to identify suitable drugs that would inhibit the TK activity of KIT D816V.9-12,19-24 The TK inhibitor imatinib (STI571), which is widely used in clinical hematology, has recently been found to counteract growth of neoplastic MCs exhibiting wild-type (wt) KIT or the rarely occurring F522C-mutated variant of KIT.20-23 In addition, this drug was found to block the growth of neoplastic cells in patients who have SM associated with clonal eosinophilia and a FIP1L1/PDGFRA fusion gene.24-26 However, imatinib failed to inhibit the growth of neoplastic MCs harboring the KIT mutation D816V,20-22 which points to a clear need for the identification and development of novel TK inhibitors that block KIT D816V and thus inhibit growth of neoplastic MCs in SM.
In the current study, we show that the novel TK inhibitors PKC4125 and AMN10727 counteract the growth of neoplastic human MCs and Ba/F3 cells expressing KIT D816V. PKC412 appears to be the more potent compound in this regard. We also show that PKC412 and AMN107 cooperate in producing growth inhibition in neoplastic MCs. These data suggest that PKC412 and AMN107 may be novel promising targeted drugs for the treatment of mastocytosis.
Patient, materials, and methods
Reagents
The TK inhibitors imatinib, AMN107,27 and PKC4125 (all from Novartis Pharma, Basel, Switzerland) were used in this study. Stock solutions of AMN107 and PKC412 were prepared by dissolving in dimethyl sulfoxide (DMSO; Merck, Darmstadt, Germany). Recombinant human (rh) stem cell factor (SCF) was purchased from Strathmann Biotech (Hannover, Germany), RPMI 1640 medium and fetal calf serum (FCS) from PAA Laboratories (Pasching, Austria), L-glutamine and Iscove modified Dulbecco medium (IMDM) from Gibco Life Technologies (Gaithersburg, MD),3 H-thymidine from Amersham (Buckinghamshire, United Kingdom), and propidium iodide from Sigma (St Louis, MO). Interferon α (IFN-α) was from Roche (Basel, Switzerland), 2-chlorodeoxyadenosine (cladribine [2CdA]) from Janssen Cilag (Titusville, NJ), and rh interleukin-4 (IL-4) from PeproTech (Rocky Hill, NJ). The phycoerythrin (PE)-labeled monoclonal antibodies (mAbs) RPA-2.10 (CD2), WM15 (CD13), YB5.B8 (CD117), and N6B6.2 (CD164) were purchased from Becton Dickinson (San Jose, CA), and the PE-conjugated mAbs CLB-gran12 (CD63) and 97A6 (CD203c) from Immunotech (Marseille, France).
HMC-1 cells expressing or lacking KIT-D816V
The human mast cell line HMC-1,28 generated from a patient with MCL, was kindly provided by Dr J. H. Butterfield (Mayo Clinic, Rochester, MN). Two subclones of HMC-1 were used, namely, HMC-1.1 harboring the KIT mutation V560G but not the KIT mutation D816V,20 and a second subclone, HMC-1.2, harboring both KIT mutations, that is, V560G and D816V.20 HMC-1 cells were grown in IMDM supplemented with 10% FCS, L-glutamine, and antibiotics at 37°C and 5% CO2. HMC-1 cells were rethawed from an original stock every 4 to 8 weeks and were passaged weekly. As control of “phenotypic stability,” HMC-1 cells were periodically checked for (1) the presence of metachromatic granules, (2) expression of KIT, and (3) the down-modulating effect of IL-4 (100 U/mL, 48 hours) on KIT expression.29
Ba/F3 cells with inducible expression of wt KIT or KIT D816V
The generation of Ba/F3 cells with doxycycline-inducible expression of wt KIT (Ton.Kit.wt) or KIT D816V has recently been described.30 In brief, Ba/F3 cells expressing the reverse tet-transactivator31,32 were cotransfected with pTRE2 vector (Clontech, Palo Alto, CA) containing KIT D816V cDNA (or wt KIT cDNA, both kindly supplied by Dr J. B. Longley, Columbia University, New York, NY) and pTK-Hyg (Clontech) by electroporation. Stably transfected cells were selected by growing in hygromycin and cloned by limiting dilution. In this study, the subclone Ton.Kit.D816V.2730 was used in all experiments. As assessed by Western blotting, immunocytochemistry, polymerase chain reaction (PCR), and restriction fragment length polymorphism (RFLP) analysis,16 expression of KIT-D816V can be induced in Ton.Kit.D816V.27 cells within 12 hours by exposure to doxycycline (1 μg/mL).30
Isolation of primary neoplastic MCs
Primary bone marrow (BM) MCs were obtained from a female patient (aged 54 years) with smouldering systemic mastocytosis (SSM), a distinct subvariant of SM characterized by involvement of multiple hematopoietic lineages and detection of KIT D816V in MC- and non-MC-lineage myeloid cells.33-36 For control purpose, BM obtained from a patient suffering from malignant lymphoma (without BM involvement) was analyzed. Both patients gave informed consent before BM puncture, in accordance with the Declaration of Helsinki. The BM aspirate was collected in syringes containing preservative-free heparin. Cells were layered over Ficoll to isolate mononuclear cells (MNCs). The MNC fractions were found to contain 5% MCs in the patient with SSM and fewer than 1% MCs in the control sample (normal BM). Cell viability was more than 90%. The presence of the KIT mutation D816V in BM MNCs in the patient with SSM was confirmed by reverse transcription-PCR (RT-PCR) and RFLP analysis.16
Analysis of KIT phosphorylation by Western blotting
HMC-1 cells (106/mL) and Ba/F3 cells (106/mL) containing either wt KIT (Ton.Kit.wt) or KIT D816V (Ton.Kit.D816V.27) were incubated with PKC412 (1 μM), AMN107 (1 μM), imatinib (1 μM), or control medium at 37°C for 4 hours. Prior to exposure to inhibitory drugs, Ton.Kit.wt cells and Ton.Kit.D816V.27 cells were incubated with doxycycline (1 μg/mL) at 37°C for 24 hours to induce expression of KIT. In the case of Ton.Kit.wt cells, KIT phosphorylation was induced by adding rhSCF (100 ng/mL). Immunoprecipitation (IP) and Western blotting were performed as described.32 In brief, cells were washed at 4°C and resuspended in RIPA buffer (1 mL buffer/108 cells) containing 50 mM Tris, 150 mM NaCl, 1% Nonidet P40 (NP-40), 0.25% deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS), 1 mM EDTA, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and 1 mM Na3VO4. After incubation in RIPA buffer supplemented with proteinase inhibitor cocktail (Roche) for 30 minutes at 4°C, lysates were centrifuged. For IP, lysates from 107 cells were incubated with anti-KIT antibody SR1 (kindly provided by Dr V. Broudy, University of Washington, Seattle)37 or with anti-KIT antibody 1C1 (kindly provided by Dr H.-J. Bühring, University of Tübingen, Tübingen, Germany)38 and protein G-Sepharose beads (Amersham) in IP buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 100 mM NaF, and 1% NP-40) at 4°C overnight. Beads were then washed 3 times in IP buffer. Lysates and immunoprecipitates were separated under reducing conditions by 7.5% SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Protran, Schleicher & Schuell, Keene, NH) in buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol at 4°C. Membranes were blocked for 1 hour in 5% blocking reagent (Roche) and were then incubated with anti-KIT antibody 1C1 or with antiphospho protein mAb 4G10 (Upstate Biotechnology, Lake Placid, NY) at 4°C overnight. Antibody reactivity was made visible by sheep anti-mouse IgG antibody and Lumingen PS-3 detection reagent (both from Amersham), with CL-Xposure film (Pierce Biotechnology, Rockford, IL).
Measurement of 3H-thymidine uptake
To determine growth-inhibitory drug effects, HMC-1 cells and Ba/F3 cells containing either SCF-activated wt KIT (Ton.Kit.wt) or KIT D816V (Ton.Kit.D816V.27) were incubated with various concentrations of PKC412 (100 pM to10 μM), AMN107 (1 nM to 100 μM), or imatinib (3 nM to 300 μM) in 96-well culture plates (TPP, Trasadingen, Switzerland) at 37°C for 48 hours. In time-course experiments, HMC-1 cells were exposed to PKC412 (300 nM) for 1, 12, 24, 36, or 48 hours. In select experiments, HMC-1 cells (both subclones) were incubated with various concentrations of IFN-α (0.1-500 000 U/mL) or 2CdA (0.005-10 μg/mL). Primary cells (BM cells from a patient with SSM and control BM) were cultured in control medium, PKC412 (50-500 nM), AMN107 (100 nM to 30 μM), or imatinib (1 μM) for 48 hours. After incubation, 1 μCi (0.037 MBq) 3H-thymidine was added (37°C, 12 hours). Cells were then harvested on filter membranes (Packard Bioscience, Meriden, CT) in a Filtermate 196 harvester (Packard Bioscience). Filters were air-dried, and the bound radioactivity was counted in a β counter (Top-Count NXT, Packard Bioscience).
To determine potential additive or synergistic drug effects on growth of neoplastic MCs, HMC-1 cells (both subclones) were exposed to various combinations of drugs (PKC412, AMN107, imatinib, IFN-α, 2CdA) at a fixed ratio of drug concentrations. Drug interactions (additive, synergistic) were determined by calculating combination index values using a commercially available software (Calcusyn; Biosoft, Ferguson, MO).39 All experiments were performed in triplicate.
Evaluation of apoptosis by conventional morphology and electron microscopy
The apoptosis-inducing effects of TK inhibitors were analyzed by morphologic examination, flow cytometry, and electron microscopy. In typical experiments, HMC-1 cells were incubated with various concentrations of PKC412 (500 nM to 1 μM), AMN107 (50 nM to 10 μM), imatinib (50 nM to 10 μM) or control medium in 6-well culture plates (TPP) in IMDM containing 10% FCS at 37°C for 24 hours. The percentage of apoptotic cells was quantified on cytospin preparations stained with Wright-Giemsa stain. Apoptosis was defined according to conventional cytomorphologic criteria (cell shrinkage, condensation of chromatin structure).40
To confirm apoptosis in HMC-1 cells, electron microscopy was performed as described,41,42 using HMC-1 cells (both subclones) exposed to PKC412 (500 nM, 900 nM, or 1 μM), AMN107 (1 μM), imatinib (1 μM), or control medium for 24 hours. After incubation, cells were washed and fixed in 2% paraformaldehyde, 2.5% glutaraldehyde, and 0.025% CaCl2 buffered in 0.1 M sodium cacodylate buffer (pH 7.4) for 1 hour. Cells were then washed, suspended in 2% agar, and centrifuged. Pellets were postfixed with 1.3% OsO4 (buffered in 0.66 M collidine) and stained en bloc in 2% uranyl acetate and sodium maleate buffer (pH 4.4) for 2 hours. Pellets were then rinsed, dehydrated in alcohol series, and embedded in EPON 812. Ultrathin sections were cut and placed on gold grids. Sections were contrasted in uranyl acetate and lead citrate and viewed in a JEOL 1200 EX II transmission electron microscope (JEOL, Tokyo, Japan).
Evaluation of apoptosis by TUNEL assay and flow cytometry
To confirm that apoptosis occurred in HMC-1 cells following a 24-hour exposure to either PKC412 (1 μM), AMN107 (1 μM), or imatinib (1 μM), an in situ terminal transferase-mediated dUTP-fluorescence nick end-labeling (TUNEL) assay was used as reported.43,44 In brief, cells were fixed in 1% formaldehyde at pH 7.4 at 0°C for 15 minutes. Cells were then treated with 70% ethanol (ice-cold) for 1 hour, washed, and incubated in terminal-transferase reaction solution containing CoCl2, DNA deoxynucleotidyl-exotransferase, and biotin-16-2′-deoxy-uridin-5′-triphosphate (Boehringer Mannheim, Mannheim, Germany) at 37°C for 10 minutes. After incubation, cells were washed and incubated with streptavidin fluorescein (10 μg/mL; Boehringer Mannheim) at 37°C for 20 minutes. Cells were then washed and analyzed with a Nikon Eclipse E 800 fluorescence microscope (Tokyo, Japan).
For flow cytometric determination of apoptosis and cell viability, combined annexin V/propidium iodide staining was performed. HMC-1 cells were exposed to PKC412 (0.5, 1, and 2.5 μM), AMN107 (0.5, 1, and 2.5 μM), imatinib (0.5, 1, and 2.5 μM), or control medium at 37°C for 24 hours. Thereafter, cells were washed and incubated with annexin V-fluorescein isothiocyanate (FITC; Alexis Biochemicals, San Diego, CA) in binding buffer containing HEPES (10 mM, pH 7.4), NaCl (140 mM), and CaCl2 (2.5 mM). Thereafter, propidium iodide (1 μg/mL) was added. Cells were then washed and analyzed by flow cytometry on a FACScan (Becton Dickinson).
Evaluation of expression of activation-linked surface antigens on HMC-1 cells
Expression of cell-surface antigens on HMC-1.2 cells was determined by flow cytometry after culture in control medium or medium supplemented with TK inhibitors (PKC412, 1 μM; AMN107, 1 μM; imatinib, 1 μM) for 24 hours. In select experiments, various concentrations of PKC412 (50, 100, 250, 500, and 1000 nM) were applied. After incubation with drugs, cells were washed and subjected to single-color flow cytometry using PE-conjugated antibodies against MC antigens known to be overexpressed on neoplastic MCs (compared with normal MCs) or are expressed at an early stage of mastopoiesis (CD2, CD13, CD63, CD117, CD164, CD203c).45-47 Flow cytometry was performed on a FACScan (Becton Dickinson) as described.29
Approval was obtained from the Institutional Review Board (Department of Internal Medicine I, Division of Hematology and Hemostaseology, Medical University of Vienna) for all series of experiments of this study.
Statistical analysis
To determine the significance in differences between proliferation rates, apoptosis, and surface expression levels after exposure of HMC-1 cells to inhibitors, the Student t test for dependent samples was applied. Results were considered statistically significant at P less than .05.
Results
Effects of PKC412 and AMN107 on TK activity of D816V-mutated KIT
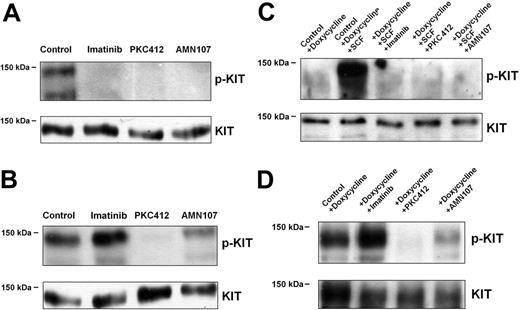
As assessed by IP and Western blotting, PKC412 (1 μM) decreased phosphorylation of KIT in HMC-1.1 cells (expressing KIT V560G but not KIT D816V) as well as in HMC-1.2 cells harboring both mutations (Figure 1A-B).AMN107 (1 μM) strongly reduced KIT phosphorylation in HMC-1.1 cells but showed only weak effects on KIT phosphorylation in HMC-1.2 cells at 1 μM. Similarly, imatinib (1 μM) reduced KIT phosphorylation in HMC-1.1 cells but did not inhibit KIT phosphorylation in HMC-1.2 cells (Figure 1A-B). We next examined effects of the TK inhibitors on Ba/F3 cells expressing either wt KIT (Ton.Kit.wt) or KIT D816V (Ton.Kit.D816V.27) after exposure to doxycycline. In Ton.Kit.wt cells, KIT appeared to be phosphorylated in the presence of SCF, whereas KIT was found to be constitutively phosphorylated in Ton.Kit.D816V.27 cells. As visible in Figure 1C, all 3 TK inhibitors (PKC412, AMN107, imatinib, each at 1 μM) decreased the SCF-induced phosphorylation of KIT in Ton.Kit.wt cells. By contrast, only PKC412, and to a lesser degree AMN107, decreased KIT phosphorylation in Ton.Kit.D816V-27 cells. Imatinib (1 μM) showed no effect on KIT phosphorylation in these cells (Figure 1D).
Effects of TK inhibitors on KIT phosphorylation in neoplastic cells. (A-B) KIT phosphorylation in HMC-1.1 cells (A) and HMC-1.2 cells (exhibiting KIT D816V) (B) after incubation in control medium, imatinib (1 μM), PKC412 (1 μM), or AMN107 (1 μM) for 4 hours. (C-D) KIT phosphorylation in Ton.Kit.wt cells (C) and Ton.Kit.D816V.27 cells (D) after incubation in control medium (control), imatinib (1 μM), PKC412 (1 μM), or AMN107 (1 μM) for 4 hours. Prior to drug exposure, Ton.Kit.wt cells and Ton.Kit.D816V.27 cells were kept in doxycycline for 24 hours to induce expression of KIT. In case of the Ton.Kit.wt clone, cells were also exposed to SCF (100 ng/mL, 4 hours) to induce KIT phosphorylation (p-KIT). In all cells, immunoprecipitation was conducted using the anti-KIT mAb SR-1. Western blotting was performed using the antiphospho mAb 4G10 for p-KIT detection and the anti-KIT mAb 1C1 for detection of total KIT protein.
Effects of TK inhibitors on KIT phosphorylation in neoplastic cells. (A-B) KIT phosphorylation in HMC-1.1 cells (A) and HMC-1.2 cells (exhibiting KIT D816V) (B) after incubation in control medium, imatinib (1 μM), PKC412 (1 μM), or AMN107 (1 μM) for 4 hours. (C-D) KIT phosphorylation in Ton.Kit.wt cells (C) and Ton.Kit.D816V.27 cells (D) after incubation in control medium (control), imatinib (1 μM), PKC412 (1 μM), or AMN107 (1 μM) for 4 hours. Prior to drug exposure, Ton.Kit.wt cells and Ton.Kit.D816V.27 cells were kept in doxycycline for 24 hours to induce expression of KIT. In case of the Ton.Kit.wt clone, cells were also exposed to SCF (100 ng/mL, 4 hours) to induce KIT phosphorylation (p-KIT). In all cells, immunoprecipitation was conducted using the anti-KIT mAb SR-1. Western blotting was performed using the antiphospho mAb 4G10 for p-KIT detection and the anti-KIT mAb 1C1 for detection of total KIT protein.
Effects of TK inhibitors on 3H-thymidine uptake in HMC-1 cells
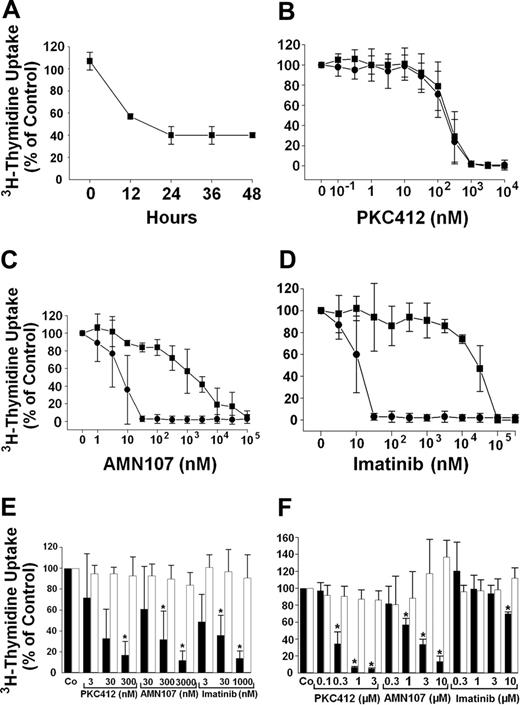
In time-course experiments, maximum inhibitory effects of PKC412 on growth of HMC-1.1 and HMC-1.2 cells were seen after 36 to 48 hours. Figure 2A shows the time-dependent effect of PKC412 (300 nM) on growth of HMC-1.2 cells. As shown in Figure 2B-C, PKC412 and AMN107 counteracted 3H-thymidine uptake in HMC-1.1 and HMC-1.2 cells in a dose-dependent manner. Interestingly, the median inhibitory concentration (IC50) for the effects of PKC412 in these 2 subclones appeared to be in the same range (50-250 nM; Figure 2B). In contrast, the IC50 values for the effects of AMN107 on proliferation were significantly higher in HMC-1.2 cells (1-5 μM) compared to those found in HMC-1.1 cells (3-10 nM; Figure 2C). As expected, imatinib was effective only in HMC-1.1 cells (IC50, 10-30 nM), whereas no significant effects of imatinib on HMC-1.2 cells were seen at pharmacologically relevant concentrations (Figure 2D), confirming previous data.20-22 An interesting observation was that AMN107 was the most potent compound when comparing growth-inhibitory effects of the 3 drugs on HMC-1.1 cells exhibiting KIT V560G (Figure 2B-D).
Effects of PKC412 and AMN107 on proliferation of HMC-1 cells and BaF/3 cells. (A) Time-dependent effects of PKC412 on 3H-thymidine uptake in HMC-1.2 cells. HMC-1.2 cells were incubated with control medium or PKC412 (300 nM) at 37°C and 5% CO2 for various time periods as indicated. After incubation,3H-thymidine uptake was measured. Results are expressed as percent of control (3H-thymidine uptake in control medium at each time point) and represent the mean ± SD of triplicates. (B-D) Dose-dependent effects of TK inhibitors on 3H-thymidine uptake in HMC-1 cells. HMC-1.1 cells (•) and HMC-1.2 cells (▪) were incubated in control medium in the absence or presence of various concentrations of either PKC412 (B), AMN107 (C), or imatinib (D) at 37°C for 48 hours. After incubation,3H-thymidine uptake was measured. Results are expressed as percent of control (100%) and represent the mean ± SD from at least 3 independent experiments. (E-F) Effects of PKC412, AMN107, and imatinib on 3H-thymidine uptake in Ton.Kit cells. (E) Ton.Kit.wt cells were kept in IL-3-containing control medium (□) or were induced to express activated wt KIT by adding doxycycline (1 μg/mL) and SCF (in the absence of IL-3; ▪). In both conditions, cells were exposed to either control medium (Co) or various concentrations of PKC412, AMN107, or imatinib, as indicated, for 48 hours (37°C, 5% CO2). (F) Ton.Kit.D816V.27 cells were kept in control medium (□) or were induced to express KIT D816V by adding doxycycline (1 μg/mL; ▪), and then were exposed to either control medium (Co) or various concentrations of PKC412, AMN107, or imatinib, as indicated, for 48 hours (37°C, 5% CO2). Thereafter,3H-thymidine uptake was determined. Results are expressed as percent of control (Co) and represent the mean ± SD from 3 independent experiments. *P < .05 compared with control.
Effects of PKC412 and AMN107 on proliferation of HMC-1 cells and BaF/3 cells. (A) Time-dependent effects of PKC412 on 3H-thymidine uptake in HMC-1.2 cells. HMC-1.2 cells were incubated with control medium or PKC412 (300 nM) at 37°C and 5% CO2 for various time periods as indicated. After incubation,3H-thymidine uptake was measured. Results are expressed as percent of control (3H-thymidine uptake in control medium at each time point) and represent the mean ± SD of triplicates. (B-D) Dose-dependent effects of TK inhibitors on 3H-thymidine uptake in HMC-1 cells. HMC-1.1 cells (•) and HMC-1.2 cells (▪) were incubated in control medium in the absence or presence of various concentrations of either PKC412 (B), AMN107 (C), or imatinib (D) at 37°C for 48 hours. After incubation,3H-thymidine uptake was measured. Results are expressed as percent of control (100%) and represent the mean ± SD from at least 3 independent experiments. (E-F) Effects of PKC412, AMN107, and imatinib on 3H-thymidine uptake in Ton.Kit cells. (E) Ton.Kit.wt cells were kept in IL-3-containing control medium (□) or were induced to express activated wt KIT by adding doxycycline (1 μg/mL) and SCF (in the absence of IL-3; ▪). In both conditions, cells were exposed to either control medium (Co) or various concentrations of PKC412, AMN107, or imatinib, as indicated, for 48 hours (37°C, 5% CO2). (F) Ton.Kit.D816V.27 cells were kept in control medium (□) or were induced to express KIT D816V by adding doxycycline (1 μg/mL; ▪), and then were exposed to either control medium (Co) or various concentrations of PKC412, AMN107, or imatinib, as indicated, for 48 hours (37°C, 5% CO2). Thereafter,3H-thymidine uptake was determined. Results are expressed as percent of control (Co) and represent the mean ± SD from 3 independent experiments. *P < .05 compared with control.
Effects of TK inhibitors on growth of Ba/F3 cells expressing wt KIT or KIT D816V
As shown in Figure 2E, all 3 TK inhibitors counteracted SCF-dependent growth of doxycycline-exposed (KIT-expressing) Ton. Kit.wt cells with IC50 values of 3 to 30 nM for PKC412, 30 to 300 nM for AMN107, and 3 to 30 nM for imatinib. By contrast, in Ton.Kit.D816V cells, only PKC412 (IC50, 100-300 nM), and to a lesser degree AMN107 (IC50, 1-3 μM), inhibited 3H-thymidine incorporation, whereas no significant effect was obtained with imatinib at pharmacologic concentrations (Figure 2F). None of the inhibitors counteracted growth of Ton.Kit.wt or Ton.Kit.D816V.27 cells in the absence of doxycycline, that is, in the absence of KIT (Figure 2E-F). In further control experiments, neither doxycycline (1 μg/mL) nor the TK inhibitors showed growth-inhibitory effects on non-transfected Ba/F3 cells (not shown).
PKC412 and AMN107 counteract growth of primary neoplastic (mast) cells expressing KIT D816V
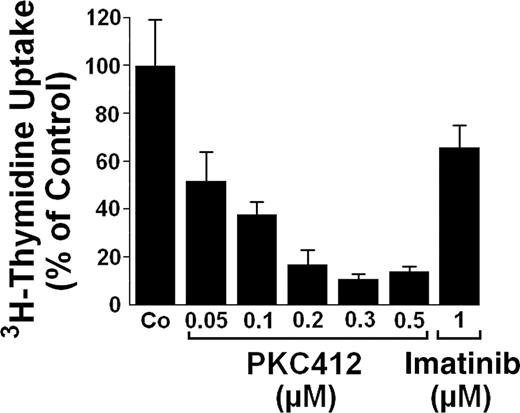
To confirm antiproliferative drug effects in SM, we examined primary neoplastic BM-derived MCs in a patient with SSM, a special subvariant of SM in which most myeloid cells (MCs as well as non-MC-lineage cells) exhibit KIT D816V. Although the purity of MCs was only 5%, most of the myeloid cells in this patient exhibited KIT D816V. In these neoplastic cells, PKC412 (and to a lesser degree AMN107, not shown) inhibited the spontaneous uptake of 3H-thymidine in a dose-dependent manner, whereas no significant effect was seen with imatinib at 1 μM (Figure 3). In the control sample (normal BM), PKC412 showed no effect on 3H-thymidine uptake (not shown).
PKC412 down-regulates growth of primary neoplastic (mast) cells exhibiting D816V. Primary neoplastic BM cells exhibiting KIT D816V were isolated from a patient with SSM. Isolated cells were incubated in control medium (Co) or with various concentrations of PKC412 or imatinib (1 μM) as indicated. Cell growth was quantified by measuring 3H-thymidine uptake. Results are expressed as percent of control (Co, 100%) and represent the mean ± SD of triplicates. In normal BM cells, no effects of PKC412 were seen (not shown).
PKC412 down-regulates growth of primary neoplastic (mast) cells exhibiting D816V. Primary neoplastic BM cells exhibiting KIT D816V were isolated from a patient with SSM. Isolated cells were incubated in control medium (Co) or with various concentrations of PKC412 or imatinib (1 μM) as indicated. Cell growth was quantified by measuring 3H-thymidine uptake. Results are expressed as percent of control (Co, 100%) and represent the mean ± SD of triplicates. In normal BM cells, no effects of PKC412 were seen (not shown).
PKC412 and AMN107 induce apoptosis in HMC-1 cells
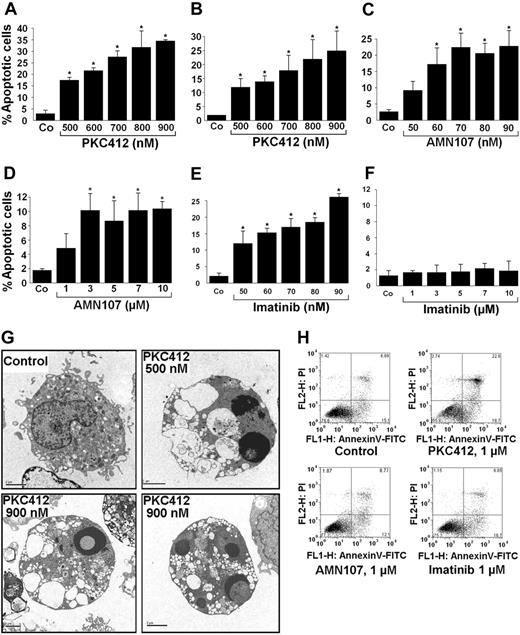
To explore the mechanisms underlying the growth-inhibitory effects of PKC412 and AMN107, we analyzed morphologic and biochemical signs of apoptosis in HMC-1 cells after drug exposure. In these experiments, PKC412 induced apoptosis in both HMC-1 subclones (Figure 4A-B). AMN107 also induced apoptosis in both subclones, but the effect of this compound was much more pronounced in HMC-1.1 cells (Figure 4C) compared with HMC-1.2 cells (Figure 4D). Similarly, imatinib produced apoptosis in HMC-1.1 cells (Figure 4E), but showed no effect on HMC-1.2 cells (Figure 4F). The apoptosis-inducing effects of the drugs on HMC-1 cells were confirmed by electron microscopy. Again, all 3 drugs (each at 1 μM) induced apoptosis in HMC-1.1 cells, whereas in HMC-1.2, only PKC412 and, to a lesser degree AMN107, were found to produce apoptosis. Figure 4G shows the apoptosis-inducing effect of PKC412 (24 hours) on HMC-1.2 cells. Finally, we were able to demonstrate the apoptosis-inducing effect of PKC412 in HMC-1 cells by combined annexin V/propidium iodide staining and flow cytometry (Figure 4H) as well as in a TUNEL assay (Figure 5). In both assays, PKC412 (1 μM), and to a lesser degree AMN107 (1 μM), induced apoptosis in HMC-1.2 cells, whereas imatinib showed no effects. In contrast, in HMC-1.1 cells, all 3 compounds induced apoptosis as assessed by the TUNEL assay (Figure 5).
Effects of TK inhibitors on apoptosis of HMC-1 cells. (A-F) HMC-1.1 cells (A,C,E) and HMC-1.2 cells (B,D,F) were cultured in the absence (Co) or presence of various concentrations of PKC412 (A-B), AMN107 (C-D), or imatinib (E-F), as indicated, for 24 hours. Thereafter, the percentages of apoptotic cells were quantified by light microscopy. Results represent the mean ± SD of 3 independent experiments. *P < .05 compared with control. (G) Electron microscopic examination of PKC412-induced apoptosis in HMC-1 cells. HMC-1.2 cells were incubated with control medium or PKC412 (500 nM or 900 nM as indicated) at 37°C for 24 hours. Then, cells were harvested and analyzed for ultrastructural signs of apoptosis. Whereas apoptotic cells were rarely seen in cultures kept with control medium, HMC-1.2 cells cultured in PKC412 frequently displayed signs of apoptosis including cell shrinkage, membrane ruffling, vacuolization, and condensation of the nuclear chromatin. Original magnification, 5000 ×. Images were captured using a Gatan Bioscan Camera model 792 and Digital Micrograph acquisition software (Gatan, Pleasanton, CA). (H) HMC-1.2 cells were exposed to control medium (Control), PKC412 (1 μM), AMN107 (1 μM), or imatinib (1 μM) at 37°C for 24 hours. Then, cells were examined for viability and apoptosis by combined propidium iodide (PI)/annexin V-FITC staining.
Effects of TK inhibitors on apoptosis of HMC-1 cells. (A-F) HMC-1.1 cells (A,C,E) and HMC-1.2 cells (B,D,F) were cultured in the absence (Co) or presence of various concentrations of PKC412 (A-B), AMN107 (C-D), or imatinib (E-F), as indicated, for 24 hours. Thereafter, the percentages of apoptotic cells were quantified by light microscopy. Results represent the mean ± SD of 3 independent experiments. *P < .05 compared with control. (G) Electron microscopic examination of PKC412-induced apoptosis in HMC-1 cells. HMC-1.2 cells were incubated with control medium or PKC412 (500 nM or 900 nM as indicated) at 37°C for 24 hours. Then, cells were harvested and analyzed for ultrastructural signs of apoptosis. Whereas apoptotic cells were rarely seen in cultures kept with control medium, HMC-1.2 cells cultured in PKC412 frequently displayed signs of apoptosis including cell shrinkage, membrane ruffling, vacuolization, and condensation of the nuclear chromatin. Original magnification, 5000 ×. Images were captured using a Gatan Bioscan Camera model 792 and Digital Micrograph acquisition software (Gatan, Pleasanton, CA). (H) HMC-1.2 cells were exposed to control medium (Control), PKC412 (1 μM), AMN107 (1 μM), or imatinib (1 μM) at 37°C for 24 hours. Then, cells were examined for viability and apoptosis by combined propidium iodide (PI)/annexin V-FITC staining.
Apoptosis in HMC-1 cells assessed by TUNEL assay. HMC-1.1 cells (A) and HMC-1.2 cells (B) were incubated in control medium (i,v), PKC412, 1 μM (ii,vi), AMN107, 1 μM (iii,vii), or imatinib, 1 μM (iv,viii) at 37°C for 24 hours. Thereafter, cells were harvested and subjected to TUNEL assay. As visible, PKC412 produced apoptosis in most HMC-1.1 and HMC-1.2 cells, whereas AMN107 and imatinib showed potent apoptosis-inducing effects in HMC-1.1 cells (iii-iv), but not in HMC-1.2 cells exhibiting KIT D816V (vii-viii). Images were obtained using a Nikon Plan Apo 40 ×/1.0 numeric aperture oil objective. Images were acquired from FITC-labeled cells using a Hamamatsu high-resolution digital camera (model C4242-95; Hamamatsu, Japan) and HPD-CPX32 Microsoft Windows 95 software (Microsoft, Redmond, WA). Citifluor (Agar Science, Stansted, United Kingdom) was used as imaging solution.
Apoptosis in HMC-1 cells assessed by TUNEL assay. HMC-1.1 cells (A) and HMC-1.2 cells (B) were incubated in control medium (i,v), PKC412, 1 μM (ii,vi), AMN107, 1 μM (iii,vii), or imatinib, 1 μM (iv,viii) at 37°C for 24 hours. Thereafter, cells were harvested and subjected to TUNEL assay. As visible, PKC412 produced apoptosis in most HMC-1.1 and HMC-1.2 cells, whereas AMN107 and imatinib showed potent apoptosis-inducing effects in HMC-1.1 cells (iii-iv), but not in HMC-1.2 cells exhibiting KIT D816V (vii-viii). Images were obtained using a Nikon Plan Apo 40 ×/1.0 numeric aperture oil objective. Images were acquired from FITC-labeled cells using a Hamamatsu high-resolution digital camera (model C4242-95; Hamamatsu, Japan) and HPD-CPX32 Microsoft Windows 95 software (Microsoft, Redmond, WA). Citifluor (Agar Science, Stansted, United Kingdom) was used as imaging solution.
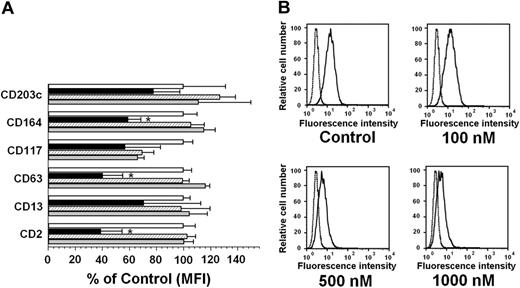
PKC412 down-regulates expression of activation-linked cell-surface antigens on HMC-1 cells
Several cell-surface antigens are typically (over)expressed on neoplastic MCs in SM.45,46 Some of these molecules may be up-regulated directly by KIT D816V.30 We therefore investigated whether PKC412, AMN107, or imatinib would influence expression of surface antigens on HMC-1.2 cells. Unstimulated cells expressed CD2, CD13, CD63, CD117, CD164, and CD203c, confirming previous data.45-47 Incubation of HMC-1.2 cells with PKC412 resulted in a significant decrease in expression of CD2, CD63, and CD164 (P < .05; Figure 6A). In contrast, no significant effects of PKC412 on expression of CD13 or CD203c were seen (Figure 6A). In the case of CD117/KIT, a slight decrease of expression on HMC-1.2 cells was found on exposure to PKC412 (as well as on exposure to either AMN107 or imatinib), but the effects were not significant (P > .05; Figure 6A). The effects of PKC412 on expression of CD2 and CD63 were dose dependent. Figure 6B shows the dose-dependent effect of PKC412 on expression of CD63 on HMC-1.2 cells. In contrast to PKC412, no significant effects of either AMN107 or imatinib on expression of CD antigens on HMC-1.2 cells were seen (Figure 6A).
Effects of TK inhibitors on expression of cell-surface antigens on HMC-1.2 cells. (A) HMC-1.2 cells were exposed to control medium (Co, □), PKC412, 1 μM (▪), AMN107, 1 μM (▨), or imatinib, 1 μM (▦) at 37°C for 24 hours. After incubation, cells were examined for expression of various CD antigens by flow cytometry using CD-specific mAbs. The figure shows the mean fluorescence intensity (MFI) levels as percent of control (Co, □). Results represent the mean ± SD of 3 independent experiments. *P < .05 compared with control. (B) Dose-dependent effect of PKC412 on expression of CD63 in HMC-1.2 cells. Cells were incubated with various concentrations of PKC412 as indicated at 37°C for 24 hours. Cells were then examined for expression of CD63 by flow cytometry. As visible, PKC412 dose dependently decreased expression of CD63.
Effects of TK inhibitors on expression of cell-surface antigens on HMC-1.2 cells. (A) HMC-1.2 cells were exposed to control medium (Co, □), PKC412, 1 μM (▪), AMN107, 1 μM (▨), or imatinib, 1 μM (▦) at 37°C for 24 hours. After incubation, cells were examined for expression of various CD antigens by flow cytometry using CD-specific mAbs. The figure shows the mean fluorescence intensity (MFI) levels as percent of control (Co, □). Results represent the mean ± SD of 3 independent experiments. *P < .05 compared with control. (B) Dose-dependent effect of PKC412 on expression of CD63 in HMC-1.2 cells. Cells were incubated with various concentrations of PKC412 as indicated at 37°C for 24 hours. Cells were then examined for expression of CD63 by flow cytometry. As visible, PKC412 dose dependently decreased expression of CD63.
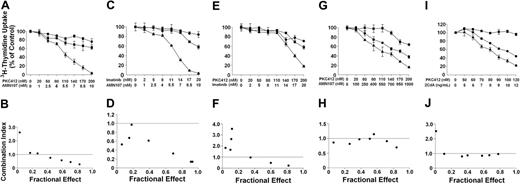
PKC412 cooperates with other drugs in producing growth inhibition in HMC-1 cells
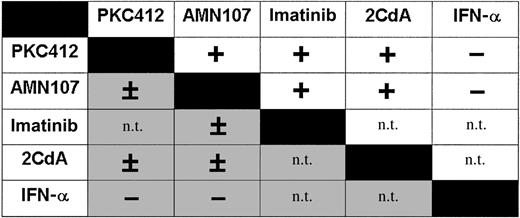
As assessed by 3H-thymidine incorporation, PKC412 was found to cooperate with AMN107 in causing growth inhibition in HMC-1.1 and HMC-1.2 cells (Figures 7-8). In HMC-1.1 cells, the drug interaction was synergistic, whereas in HMC-1.2 cells, interactions were additive rather than synergistic (Figures 7-8). In addition, PKC412 and 2CdA, a drug used to treat ASM/MCL, inhibited growth of HMC-1.1 cells in a synergistic manner (Figure 8). By contrast, no synergistic effect of PKC412 and 2CdA on HMC-1.2 cells was seen. Similarly, AMN107 and imatinib produced synergistic inhibitory effects in HMC-1.1 cells (Figure 7), but not in HMC-1.2 cells carrying KIT D816V (Figure 8). No synergistic or additive effects on growth of HMC-1 cells were seen when combining PKC412 and IFN-α or AMN107 and IFN-α (Figure 8). Single-drug effects (IC50) on growth of HMC-1.2 cells were: 2CdA, 10-20 ng/mL, and IFN-α, more than 100 U/mL. IC50 values for growth-inhibitory effects on HMC-1.1 cells were: 2CdA, 100-300 ng/mL, and IFN-α, more than 100 U/mL. A summary of cooperative drug effects is shown in Figure 8.
Synergistic drug effects on growth of HMC-1 cells. HMC-1.1 cells lacking KIT D816V (A-F) and HMC-1.2 cells exhibiting KIT D816V (G-J) were incubated with control medium or various combinations of drugs (at a fixed ratio) as indicated, at 37°C for 48 hours to determine cooperative antiproliferative effects. (A,C,E,G,I) After incubation with single drugs (A) PKC412 (▪) and AMN107 (•); (C) imatinib (▪) and AMN107 (•); (E) imatinib (▪) and PKC412 (•); (G) PKC412 (▪) and AMN107 (•); (I) PKC412 (▪) and 2CdA (•) or drug combinations (▴), cells were analyzed for 3H-thymidine uptake. Results show 3H-thymidine uptake as percentage of control (medium control, 100%) and represent the mean ± SD of triplicates from one typical experiment (corresponding results were obtained in at least 2 other experiments for each drug combination). Images in the bottom row (B,D,F,H,J) show combination index values determined for each fraction affected according to the method described by Chou and Talalay39 using Calcusyn software. A combination index (CI) value of 1.0 indicates an additive effect, a CI greater than 1.0 indicates antagonism, and a CI of less than 1.0 indicates synergism.
Synergistic drug effects on growth of HMC-1 cells. HMC-1.1 cells lacking KIT D816V (A-F) and HMC-1.2 cells exhibiting KIT D816V (G-J) were incubated with control medium or various combinations of drugs (at a fixed ratio) as indicated, at 37°C for 48 hours to determine cooperative antiproliferative effects. (A,C,E,G,I) After incubation with single drugs (A) PKC412 (▪) and AMN107 (•); (C) imatinib (▪) and AMN107 (•); (E) imatinib (▪) and PKC412 (•); (G) PKC412 (▪) and AMN107 (•); (I) PKC412 (▪) and 2CdA (•) or drug combinations (▴), cells were analyzed for 3H-thymidine uptake. Results show 3H-thymidine uptake as percentage of control (medium control, 100%) and represent the mean ± SD of triplicates from one typical experiment (corresponding results were obtained in at least 2 other experiments for each drug combination). Images in the bottom row (B,D,F,H,J) show combination index values determined for each fraction affected according to the method described by Chou and Talalay39 using Calcusyn software. A combination index (CI) value of 1.0 indicates an additive effect, a CI greater than 1.0 indicates antagonism, and a CI of less than 1.0 indicates synergism.
Drug interactions on HMC-1.1 cells and HMC-1.2 cells. The effects of various drug combinations on growth of HMC-1.1 cells (upper right, □) and HMC-1.2 cells (lower left, ▦) were determined by 3H-thymidine incorporation assay. Each drug combination was tested in at least 3 independent experiments. Drugs were applied at fixed ratio and resulting effects (and the type of drug interaction) were determined by Calcusyn software. Score: +, synergistic growth-inhibitory effect; ±, additive effect; -, less than additive (antagonistic) effect; n.t., not tested.
Drug interactions on HMC-1.1 cells and HMC-1.2 cells. The effects of various drug combinations on growth of HMC-1.1 cells (upper right, □) and HMC-1.2 cells (lower left, ▦) were determined by 3H-thymidine incorporation assay. Each drug combination was tested in at least 3 independent experiments. Drugs were applied at fixed ratio and resulting effects (and the type of drug interaction) were determined by Calcusyn software. Score: +, synergistic growth-inhibitory effect; ±, additive effect; -, less than additive (antagonistic) effect; n.t., not tested.
Discussion
The somatic KIT mutation D816V is a gene defect that leads to constitutive activation of the KIT receptor, which is critically involved in growth of neoplastic MCs and thus in the pathogenesis of SM.13-17 Therefore, recent attempts have focused on the identification and development of pharmacologic compounds that inhibit the TK activity of KIT D816V and thereby can inhibit growth of neoplastic MCs in patients with SM.9-12 We here describe that the TK inhibitors PKC412, and to a lesser degree AMN107, inhibit TK activity of KIT D816V as well as growth of neoplastic human MCs carrying this particular KIT mutation. In addition, we show that both drugs cooperate with each other as well as with other targeted and conventional drugs in producing growth inhibition.
PKC412 is a staurosporine-related inhibitor of PKC and of several TKs including PDGFRA, FLT3, and KIT.5 In this study, we show that PKC412 counteracts the growth of neoplastic human MCs and Ba/F3 cells expressing the D816V-mutated variant of KIT. With regard to Ba/F3 cells, our data are in line with the results of Growney et al.48 Interestingly, the effective dose range for Ba/F3 cells was found to be the same as that found in HMC-1 cells. Another interesting observation was that the IC50 for the effects of PKC412 on the 2 subclones of HMC-1 (expressing or lacking KIT D816V) appeared to be in the same range. Finally, we were able to confirm growth-inhibitory effects of PKC412 for primary neoplastic (mast) cells expressing KIT D816V. Because the KIT mutation D816V is detectable in a majority of all patients with SM,13-17 these data are of considerable importance. In fact, PKC412 seems to be the first TK inhibitor that counteracts growth of KIT D816V-bearing human MCs in the same way as MCs expressing wt KIT. It is also noteworthy in this regard that the inhibitory effects of PKC412 on KIT D816V+ cells clearly exceeded the effects of AMN107. Thus, PKC412 seems to be a novel attractive targeted drug worthy to be considered for use in clinical trials in ASM or MCL.
Recent data suggest that AMN107 is a highly potent inhibitor of BCR/ABL TK activity.27 It has also been reported that AMN107 inhibits TK activity of wt KIT.27 We found that AMN107 exerts potent effects on HMC-1 cells carrying the KIT mutation V560G, but exhibits relatively weak effects on HMC-1 cells harboring both KIT V560G and KIT D816V. Similarly, AMN107 showed only weak effects on growth of Ba/F3 cells expressing the D816V-mutated variant of KIT. These data suggest that the KIT mutation D816V, but not the KIT mutation V560G, confers relative resistance against AMN107, although AMN107 still retains inhibitory effects on KIT D816V+ cells compared with imatinib. The impressive antiproliferative effects of AMN107 on V560G+ cells also suggest that this compound may be an attractive “drug candidate” for gastrointestinal stroma cell tumors, in which mutations at codon 560 of KIT have been reported.49
Several pharmacologic inhibitors targeting TK activity of pro-oncogenic molecules have recently been developed in clinical hematology.5,12,19,27 The growth-inhibitory effects of these TK inhibitors on neoplastic cells (expressing the appropriate target) are usually associated with loss of TK activity and consecutive apoptosis. In the present study, we were also able to demonstrate that the growth-inhibitory effects of PKC412 on neoplastic MCs are associated with TK inhibition of (mutated) KIT as well as with apoptosis. The apoptosis-inducing effect of PKC412 was demonstrable by light and electron microscopy as well as by flow cytometry and in a TUNEL assay. As expected, both AMN107 and imatinib showed significant apoptosis-inducing effects on HMC-1.1 cells, but did not exhibit significant effects on HMC-1.2 cells.
A number of cell-surface antigens are typically (over)expressed on neoplastic MCs. Likewise, in contrast to normal MCs, neoplastic MCs in SM express CD2 and CD25.45,46 We show that PKC412 down-regulates expression of CD2, CD63, and CD164 in HMC-1.2 cells exhibiting KIT D816V. A slight albeit insignificant effect of PKC412 on KIT expression was also seen. An interesting observation was that AMN107 failed to suppress expression of CD2 and CD63 on HMC-1.2 cells, which is probably due to its weaker effect on TK activity of KIT D816V compared with the effect of PKC412.
Recent data suggest that treatment of myeloid neoplasms with TK inhibitors as single agents may be insufficient to control the disease for prolonged time periods. This has been documented for the use of imatinib in advanced chronic myelogenous leukemia (CML)50,51 and may also apply for patients with SM or MCL treated with imatinib or PKC412.52,53 In these patients, drug resistance may occur. To overcome resistance, a number of pharmacologic strategies may be envisaged. One possibility is to apply drug combinations. We were therefore interested to learn whether PKC412 and AMN107 would exhibit additive or synergistic antiproliferative effects on HMC-1 cells. Indeed, our data show that PKC412 cooperates with imatinib and AMN107 in producing growth inhibition in both HMC-1 clones. Furthermore, PKC412 and 2CdA, a drug that has been described to counteract growth of neoplastic MCs in vivo in (aggressive) SM,54 showed cooperative growth-inhibitory effects on HMC-1.1 and HMC-1.2 cells. However, drug interactions were synergistic on HMC-1.1 cells but not in HMC-1.2 cells. This may be explained by the relatively weak (AMN107) or absent (imatinib) effects of coapplied drugs on KIT TK activity. Whether drug combinations consisting of PKC412 and other (targeted) drugs will be of clinical value in patients with ASM or MCL remains to be elucidated. Thus, so far, only a few agents with documented antiproliferative effects on neoplastic MCs in vivo in SM have been presented, and none of these drugs produce long-lasting complete remissions in patients with ASM or MCL.9-12,52-54
In summary, we show that PKC412 and AMN107 are novel promising drugs targeting KIT in SM. Whereas each of the 2 drugs exhibits a distinct pharmacologic profile with unique effects on mutated variants of KIT, a promising approach may be to combine both drugs with each other or with 2CdA to treat ASM or MCL.
Prepublished online as Blood First Edition Paper, September 27, 2005; DOI 10.1182/blood-2005-07-3022.
Supported by Fonds zur Förderung der Wissenschaftlichen Forschung (FWF) in Österreich, grant no. P-17205-B14, and Austrian Federal Ministry for Education, Science, and Culture grant GZ 200.062/2-VI/1/2002. S.D. and W.F.P. were supported by grant no. 20030 from CeMM, the Center of Molecular Medicine, Austrian Academy of Sciences.
P.W.M. and D.F. are employed by Novartis Pharma AG (Basel, Switzerland) whose potential products were studied in the present work.
K.V.G. performed research experiments on KIT expression and phosphorylation as well as cell growth and drug interactions, analyzed the data, and contributed by drafting the article; M.M. and C.S. contributed by establishing vital new analytic tools (Ba/F3 cells with inducible expression of KIT), by analyzing data, and by drafting and critically reviewing the manuscript; K.J.A., S.D., A.G., and W.F.P. contributed by performing key laboratory experiments on cell growth and proliferation and by analyzing respective data; K.S. performed flow cytometry experiments; A.B. conducted experiments with primary bone marrow-derived cells; P.S. performed electron microscopy experiments as well as TUNEL assay experiments; P.W.M. and D.F. contributed essential new reagents (PKC412, AMN107, imatinib); and P.V. contributed by designing the study, establishing the research plan, providing logistic and budget support, and approving the data and the final version of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal