Abstract
Adult T-cell leukemia (ATL), an aggressive malignancy of CD4+ T cells associated with human T-cell leukemia virus type I (HTLV-I) infection, carries a very poor prognosis because of the resistance of leukemic cells to any conventional regimen, including chemotherapy. We examined the effect of ritonavir, an HIV protease inhibitor, on HTLV-I-infected T-cell lines and primary ATL cells and found that it induced apoptosis and inhibited transcriptional activation of NF-κB in these cells. Furthermore, ritonavir inhibited expression of Bcl-xL, survivin, c-Myc, and cyclin D2, the targets of NF-κB. In nonobese diabetic/severe combined immunodeficient (NOD/SCID)/γcnull (NOG) mice, ritonavir very efficiently prevented tumor growth and leukemic infiltration in various organs of NOG mice at the same dose used for treatment of patients with AIDS. Our data indicate that ritonavir has potent anti-NF-κB and antitumor effects and might be clinically applicable for treatment of ATL. These results would provide a new concept and novel platform for new drug development of leukemia and solid cancer as well. (Blood. 2006;107:716-724)
Introduction
Human T-cell leukemia virus type I (HTLV-I) is the causative agent of an aggressive form of CD4+ T-cell leukemia designated adult T-cell leukemia (ATL).1-3 ATL was first identified in Japan in 1977.4,5 Common findings for patients with ATL include enlargement of peripheral lymph nodes, hepatomegaly, splenomegaly, hypercalcemia, and skin lesions. At present, there is no accepted curative therapy for ATL, and patients progress to death with a median survival duration of 13 months in aggressive ATL.6 ATL has a poor prognosis mainly because of its resistance to conventional as well as high-dose chemotherapy.
ATL develops after a long period of latent infection. This long latency suggests that multiple genetic events, which accumulate in HTLV-I-infected cells, are involved in the development of ATL. However, the precise molecular mechanism of leukemogenesis and the development of ATL after HTLV-I infection are not fully elucidated. A unique viral gene Tax is considered to play a central role in HTLV-I-induced transformation, which is responsible for transactivation of the HTLV-I long terminal repeat,7,8 as well as numerous cellular genes involved in T-cell activation and growth, such as those encoding IL-2,9 and the α-chain of IL-2 receptor (IL-2Rα) (CD25, Tac).10,11 Induction of many cellular genes by Tax is mediated through the transcription factor NF-κB. The malignant cells associated with all phases of ATL express very high levels of IL-2Rα12-14 without expressing a significant amount of Tax.
HTLV-I-infected cell lines derived from a leukemic cell clone and primary ATL cells failed to express significant amounts of Tax and other viral proteins, suggesting that the expression of viral proteins is not always necessary for leukemic proliferation at the late stage of the disease. However, HTLV-I-infected cell lines and leukemic cells from patients with ATL display constitutive NF-κB binding activity and increased degradation of a specific inhibitor, IκBα.15 In resting cells, NF-κB is sequestered as an inactive precursor by association with inhibitory IκBs in the cytoplasm. On stimulation, IκBs are rapidly phosphorylated, ubiquitinated, and degraded by a proteasome-dependent pathway, allowing active NF-κB to translocate into the nucleus where it can activate the expression of a number of genes.16 NF-κB activation has been connected with multiple processes of oncogenesis, including control of apoptosis, cell cycle, differentiation, and cell migration16 ; therefore, inhibition of NF-κB was suggested to be a useful strategy for cancer therapy.17-20 Despite the diversity in clinical manifestations of ATL, strong and constitutive NF-κB activation was reported to be a unique and common characteristic of ATL cells.15 Thus, the indispensability of NF-κB for the maintenance of the malignant phenotype of HTLV-I provides a possible molecular target for ATL therapy.21-24
Ritonavir, a human immunodeficiency virus type 1 (HIV-1) protease inhibitor (PI), has been successfully used in clinical treatments of HIV infection, with patients exhibiting a marked decrease in HIV viral load and a subsequent increase in CD4+ T-cell counts.25-28 Evidence of other effects by ritonavir on cellular proteases, such as the cysteine proteases cathepsin D and E, was presented in the drug's original description, albeit at concentrations greater than 500-fold above the concentration required for inhibition of HIV protease.29 PIs have also been shown to directly affect cell metabolism, interfere with host or fungal proteases, and block T-cell activation and dendritic cell function.30,31 Recently, ritonavir has been shown to inhibit the chymotrypsin-like activity of the 20S proteasome, and it activates the chymotrypsin-like activity of the 26S proteasome conversely.30,32,33 Ritonavir also has been reported to inhibit the transactivation of NF-κB induced by activators such as TNFα, HIV-1 Tat protein, and the human herpesvirus 8 protein ORF74. It is possible that inhibition of NF-κB activation by ritonavir is linked to additional pathways other than inhibition of proteasome.34 PIs also have been shown to have direct antiangiogenic and antitumor activity34,35
In this study, we investigated the antitumor effects of ritonavir on HTLV-I-infected cell lines and primary ATL cells. We found that ritonavir decreases NF-κB activity linked to the inhibition of IκBα phosphorylation and induces apoptosis of these cells. In addition, we established preclinical models to evaluate the efficacy of anti-ATL and anti-NF-κB therapies. In the ATL model, ritonavir potently inhibited the growth and infiltration of leukemic cells from patients at concentrations used for treatment of patients with AIDS.
Materials and methods
Cell lines
The T-cell leukemia cell line Jurkat, HTLV-I-infected T-cell lines MT-2,36 MT-4,37 C5/MJ,38 SLB-1,39 HUT-102,2 MT-1,40 and ED-40515(-),41 and bcr-abl+ leukemic cell line K562 were cultured in RPMI 1640 medium supplemented with 2% heat-inactivated fetal bovine serum (JRH Biosciences, Lenexa, KS), 100 U/mL penicillin, and 10 μg/mL streptomycin. MT-2, MT-4, C5/MJ, and SLB-1 are HTLV-I-transformed T-cell lines. MT-1 and ED-40515(-) are T-cell lines of leukemic cell origin established from patients with ATL. The clonal origin of HUT-102 is unclear.
Human specimens
Leukemic cells from 38 patients (8 patient samples for in vitro studies, 20 for establishment of ATL model, 10 for in vivo ritonavir studies) diagnosed as either acute type or chronic type were used in this study. The diagnosis of ATL was based on clinical features, hematologic findings, and the presence of anti-HTLV-I antibodies in the sera. Baseline characteristics for the patients who entered the study are shown in Table 1. Monoclonal HTLV-I provirus integration into the DNA of leukemia cells was confirmed by Southern blot hybridization in all cases (data not shown). All samples were collected after obtaining informed consent from patients. Peripheral blood mononuclear cells (PBMCs) from healthy volunteers and patients with ATL were purified by Ficoll-Hypaque gradient centrifugation (Amersham Biosciences, Uppsala, Sweden) and washed with RPMI 1640.
Clinical characteristics of patients
Patient . | Age, y/sex . | Diagnosis . | WBC count, cells × 109/L . | Lymphocytes, % . | Atypical cells, % . | Treatment status . |
|---|---|---|---|---|---|---|
| 1 | 54/M | Acute | 192.80 | 65.0 | 91.0 | Untreated |
| 2 | 77/F | Acute | 186.0 | 41.0 | 70.0 | Untreated |
| 3 | 67/F | Chronic | 10.40 | 38.0 | 89.0 | Treated |
| 4 | 58/M | Acute | 67.30 | 71.0 | 80.0 | Treated |
| 5 | 71/M | Acute | 19.70 | 51.0 | 61.0 | Treated |
| 6 | 66/F | Chronic | 29.40 | 49.0 | 75.0 | Untreated |
| 7 | 48/F | Chronic | 9.40 | 29.0 | 55.0 | Untreated |
| 8 | 69/F | Acute | 53.80 | 44.0 | 95.0 | Treated |
| 9 | 58/M | Chronic | 11.30 | 59.0 | 70.0 | Treated |
| 10 | 60/M | Chronic | 9.12 | 61.0 | 80.0 | Treated |
| 11 | 65/F | Acute | 29.40 | 25.0 | 60.0 | Untreated |
| 12 | 49/F | Chronic | 15.10 | 48.0 | 75.0 | Treated |
| 13 | 57/M | Chronic | 10.00 | 50.0 | 57.0 | Treated |
| 14 | 72/F | Acute | 39.00 | 70.0 | 80.0 | Treated |
| 15 | 79/F | Chronic | 10.10 | 47.0 | 27.0 | Untreated |
| 16 | 68/F | Acute | 7.00 | 86.0 | 86.0 | Treated |
| 17 | 59/F | Chronic | 8.99 | 74.5 | 40.0 | Treated |
| 18 | 70/M | Acute | 31.60 | 71.7 | 68.0 | Treated |
| 19 | 49/M | Acute | 5.00 | 19.5 | 78.0 | Treated |
| 20 | 44/M | Chronic | 36.40 | 33.0 | 43.0 | Untreated |
| 21 | 65/F | Chronic | 14.70 | 76.0 | 22.0 | Untreated |
| 22 | 63/F | Acute | 12.40 | 71.0 | 82.0 | Untreated |
| 23 | 56/F | Chronic | 7.20 | 46.0 | 15.0 | Treated |
| 24 | 78/F | Acute | 94.50 | 63.3 | 49.0 | Treated |
| 25 | 62/F | Acute | 10.10 | 53.0 | 27.0 | Untreated |
| 26 | 66/M | Chronic | 38.10 | 63.0 | 39.0 | Treated |
| 26 | 39/F | Chronic | 18.50 | 16.0 | 57.0 | Untreated |
| 28 | 48/F | Acute | 53.50 | 39.0 | 64.0 | Untreated |
| 29 | 75/F | Acute | 15.00 | 72.0 | 65.0 | Untreated |
| 30 | 84/F | Acute | 14.40 | 69.0 | 61.0 | Treated |
| 31 | 73/M | Chronic | 7.80 | 59.0 | 47.0 | Untreated |
| 32 | 43/F | Chronic | 18.60 | 63.0 | 43.0 | Untreated |
| 33 | 54/F | Acute | 69.00 | 49.0 | 50.0 | Untreated |
| 34 | 66/F | Acute | 10.20 | 38.0 | 51.0 | Untreated |
| 35 | 73/F | Chronic | 15.70 | 58.0 | 39.0 | Untreated |
| 36 | 63/F | Acute | 32.90 | 71.0 | 95.0 | Treated |
| 37 | 44/F | Chronic | 22.60 | 51.0 | 45.0 | Untreated |
| 38 | 68/M | Acute | 30.00 | 79.0 | 81.0 | Untreated |
Patient . | Age, y/sex . | Diagnosis . | WBC count, cells × 109/L . | Lymphocytes, % . | Atypical cells, % . | Treatment status . |
|---|---|---|---|---|---|---|
| 1 | 54/M | Acute | 192.80 | 65.0 | 91.0 | Untreated |
| 2 | 77/F | Acute | 186.0 | 41.0 | 70.0 | Untreated |
| 3 | 67/F | Chronic | 10.40 | 38.0 | 89.0 | Treated |
| 4 | 58/M | Acute | 67.30 | 71.0 | 80.0 | Treated |
| 5 | 71/M | Acute | 19.70 | 51.0 | 61.0 | Treated |
| 6 | 66/F | Chronic | 29.40 | 49.0 | 75.0 | Untreated |
| 7 | 48/F | Chronic | 9.40 | 29.0 | 55.0 | Untreated |
| 8 | 69/F | Acute | 53.80 | 44.0 | 95.0 | Treated |
| 9 | 58/M | Chronic | 11.30 | 59.0 | 70.0 | Treated |
| 10 | 60/M | Chronic | 9.12 | 61.0 | 80.0 | Treated |
| 11 | 65/F | Acute | 29.40 | 25.0 | 60.0 | Untreated |
| 12 | 49/F | Chronic | 15.10 | 48.0 | 75.0 | Treated |
| 13 | 57/M | Chronic | 10.00 | 50.0 | 57.0 | Treated |
| 14 | 72/F | Acute | 39.00 | 70.0 | 80.0 | Treated |
| 15 | 79/F | Chronic | 10.10 | 47.0 | 27.0 | Untreated |
| 16 | 68/F | Acute | 7.00 | 86.0 | 86.0 | Treated |
| 17 | 59/F | Chronic | 8.99 | 74.5 | 40.0 | Treated |
| 18 | 70/M | Acute | 31.60 | 71.7 | 68.0 | Treated |
| 19 | 49/M | Acute | 5.00 | 19.5 | 78.0 | Treated |
| 20 | 44/M | Chronic | 36.40 | 33.0 | 43.0 | Untreated |
| 21 | 65/F | Chronic | 14.70 | 76.0 | 22.0 | Untreated |
| 22 | 63/F | Acute | 12.40 | 71.0 | 82.0 | Untreated |
| 23 | 56/F | Chronic | 7.20 | 46.0 | 15.0 | Treated |
| 24 | 78/F | Acute | 94.50 | 63.3 | 49.0 | Treated |
| 25 | 62/F | Acute | 10.10 | 53.0 | 27.0 | Untreated |
| 26 | 66/M | Chronic | 38.10 | 63.0 | 39.0 | Treated |
| 26 | 39/F | Chronic | 18.50 | 16.0 | 57.0 | Untreated |
| 28 | 48/F | Acute | 53.50 | 39.0 | 64.0 | Untreated |
| 29 | 75/F | Acute | 15.00 | 72.0 | 65.0 | Untreated |
| 30 | 84/F | Acute | 14.40 | 69.0 | 61.0 | Treated |
| 31 | 73/M | Chronic | 7.80 | 59.0 | 47.0 | Untreated |
| 32 | 43/F | Chronic | 18.60 | 63.0 | 43.0 | Untreated |
| 33 | 54/F | Acute | 69.00 | 49.0 | 50.0 | Untreated |
| 34 | 66/F | Acute | 10.20 | 38.0 | 51.0 | Untreated |
| 35 | 73/F | Chronic | 15.70 | 58.0 | 39.0 | Untreated |
| 36 | 63/F | Acute | 32.90 | 71.0 | 95.0 | Treated |
| 37 | 44/F | Chronic | 22.60 | 51.0 | 45.0 | Untreated |
| 38 | 68/M | Acute | 30.00 | 79.0 | 81.0 | Untreated |
WBC indicates white blood cells.
Growth inhibition assay
The effect of ritonavir on cell growth was assayed by the WST-8 method as described previously.42 The WST-8 Cell Counting Kit was obtained from Wako Chemicals (Osaka, Japan). Briefly, 2 × 105 cells were incubated in a 96-well microculture plate in the absence or presence of various concentrations of ritonavir. After 72 hours of culture, 10 μL WST-8 solution was added, and the cells were incubated for another 2 hours. The number of surviving cells was measured with a microplate reader at a reference wavelength of 655 nm and test wavelength of 450 nm. Cell viability was determined as the percentage of the control (ie, absence of ritonavir).
Assay for apoptosis
Quantification of apoptosis was performed by immunostaining cells with Apo2.7, which specifically detects the 38-kDa mitochondrial membrane antigen 7A6 present only on the mitochondrial membrane of apoptotic cells, and so can be used as an early apoptotic marker in cells.43,44 Cells cultured for 72 hours in the absence or presence of various concentrations of ritonavir were labeled with the Apo2.7-phycoerythrin-conjugated monoclonal antibody (Beckman-Coulter/Immunotech, Miami, Florida) or mouse IgG1 isotype control (Beckman-Coulter/Immunotech) and subsequently analyzed by flow cytometry.
EMSA
Cells were placed in culture at 1 × 106 cells/mL (cell line) or 5 × 106 cells/mL (PBMCs) and examined for inhibition of NF-κB after exposure to ritonavir. Nuclear proteins were extracted, and NF-κB binding activities to κB element were examined by electrophoretic mobility shift assay (EMSA) as described previously.15 In brief, 5 μg nuclear extracts were preincubated in a binding buffer containing 1 μg poly(dI:dC) (Amersham Biosciences), followed by addition of 32P-labeled oligonucleotide probe containing NF-κB element (5 × 104 cpm). These mixtures were incubated for 15 minutes at room temperature. The DNA-protein complexes were separated on a 4% polyacrylamide gel and visualized by autoradiography. To examine the specificity of the NF-κB element probe, unlabeled competitor oligonucleotides were preincubated with nuclear extracts for 15 minutes before incubation with probes. The probe or competitors used were prepared by annealing the sense and antisense synthetic oligonucleotides as follows: a typical NF-κB element from the IL2RA gene, 5′-gatcCGGCAGGGGAATCTCCCTCTC-3′; and AP-1 element of the IL8 gene, 5′-gatcGTGATGACTCAGGTT-3′. Underlined sequences represent the NF-κB or AP-1 binding site. To identify NF-κB proteins in the DNA protein complex revealed by EMSA, we used antibodies specific for various NF-κB proteins, including p65, p50, c-Rel, and p52 (Santa Cruz Biotechnology, Santa Cruz, CA), to elicit a supershift DNA protein complex formation. These antibodies were incubated with the nuclear extracts for 45 minutes at room temperature before incubation with radiolabeled probes.
Western blot analysis
Treated cells were solubilized at 4°C in lysis buffer containing 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 6% 2-mercaptoethanol, and 0.01% bromophenol blue. Samples were subjected to electrophoresis on SDS-polyacrylamide gels followed by transfer to a polyvinylidene difluoride membrane and probing with the following specific antibodies: polyclonal antibodies against IκBα, cIAP2, survivin, cyclin D2 (Santa Cruz Biotechnology), Bcl-XL (Transduction Laboratories, San Jose, CA), and c-Myc (NeoMarkers, Fremont, CA) and monoclonal antibodies against phospho-IκBα, hyperphosphorylated form of pRb (Cell Signaling Technology, Beverly, MA), Bcl-2, and actin (NeoMarkers). The protein bands recognized by the antibodies were visualized using the enhanced chemiluminescence system (Amersham, Piscataway, NJ).
Plasmids and transfection
Reporter plasmid κB-LUC (kindly provided by J. Fujisawa, Kansai Medical University, Osaka, Japan) is a luciferase expression plasmid controlled by 5 tandem repeats of a NF-κB binding site from the IL2RA gene. Reporter plasmid AP-1-LUC (kindly provided by N. Mukaida, Kanazawa University, Kanazawa, Japan) is a luciferase expression plasmid controlled by 2 copies of the AP-1 binding site from the IL-8 promoter. The expression plasmid for HTLV-I Tax has been described previously.45 Transient transfections were performed in Jurkat and HUT-102 cells by electroporation using 5 × 106 cells and reporter and effecter plasmids. To normalize transfection efficiencies, a thymidine kinase (TK) promoter-driven Renilla luciferase plasmid (phRL-TK; Promega, Madison, WI) was cotransfected as an internal control plasmid. Then, 16 hours after transfection, ritonavir was added to the cultures at various concentrations, and cells were further cultured for 24 hours for assay of luciferase activity. Transfected cells were collected by centrifugation, washed with phosphate-buffer saline (PBS), and lysed in reporter lysis buffer (Promega). Lysates were assayed for reporter gene activity with the dual-luciferase reporter assay system (Promega).
Inoculation of ATL cells and collection of samples
NOG mice were obtained from the Central Institute for Experimental Animals (Kawasaki, Japan). All mice were maintained under specific pathogen-free conditions in the Animal Center of National Institute of Infectious Diseases (Tokyo, Japan). The Ethical Review Committee of the Institute approved the experimental protocol. Mice were anesthetized with ether, and cells were inoculated either intraperitoneally in abdominal region or subcutaneously in the postauricular region of NOG mice without injection of human recombinant IL-2 at a dose of 1 to 2 × 107 cells per mouse. All mice were killed 30 or 60 days after inoculation with primary ATL cells. Blood was collected from the tail to make a smear, as well as from the heart with heparinized syringes. PBMCs and splenocytes were isolated by density gradient concentration with Ficoll-Hypaque. Blood smear slides were fixed in methyl alcohol for May-Grunwald and Giemsa staining. PBMCs and splenocytes were stored at -80°C for further experiments. Tissues and various organs of mice were collected and fixed with Streck Tissue Fixative, then processed to paraffin wax-embedded sections for staining with hematoxylin and eosin (HE) and immunostaining.
Treatment of ATL mice with ritonavir
Ritonavir was obtained from Abbott Labs, North Chicago, IL. Primary ATL cells (2 × 107) from 10 patients were inoculated subcutaneously in the postauricular region of NOG mice. One day after inoculation of ATL cells, mice were treated with either RPMI 1640 (control mice) or drug (ritonavir 30 mg/kg/d) intraperitoneally daily for 30 days followed by observation for another 30 days without treatment. ATL cell growth and progression were monitored by observation of physical condition of mice during a 2-month follow-up period.
Immunohistochemistry
Paraffinized cryosections of various organs were deparaffinized and hydrated in xylenes or clearing agents and graded alcohol series, then rinsed for 5 minutes in water. Deparaffinized samples were incubated with 0.025% trypsin/PBS for 30 minutes followed by washing, and then incubated with 0.3% methanol for 30 minutes at room temperature and washed 2 times with PBS. Immunostaining was done as described previously46 using Vector MOM immunodetection kit (Vector Labs, Burlingame CA) for ATL cells with a 1:500 dilution of primary mouse monoclonal antibody specific for human CD4 and CD25 (Dako, Caterpillar, CA). This was followed by washing in PBS and incubation with a secondary antibody MOM biotinylated anti-mouse IgG, after which cells were again washed in PBS and incubated with VECTASTAIN Elite ABC for 20 minutes at room temperature. Positive staining was visualized after incubation of these samples with a mixture of 0.05% 3,3′-diaminobenzidine tetrahydrochloride in 50 mM Tris-HCl buffer and 0.01% hydrogen peroxide for 5 minutes. The samples were counterstained with hematoxylin for 2 minutes, hydrated completely, cleaned in xylene, and then mounted. Photographs were taken by light microscopy (BX41, Olympus, Tokyo, Japan) using UplanF1 lenses (DP70, Olympus; magnification ×40).
Results
Ritonavir reduces cell growth and induces apoptosis of HTLV-I-infected cell lines and primary ATL cells
Ritonavir was examined for its effect on proliferation of HTLV-I-infected cell lines (Figure 1A). Ritonavir effectively inhibited the proliferation of HTLV-I-infected cell lines as measured by WST-8 on the third day of culture in a dose-dependent manner, but not that of K562 cells. Further experiments using Apo2.7 showed that ritonavir caused apoptosis of HTLV-I-infected cell lines in a dose-dependent manner, but not that of K562 cells (Figure 1B-C). In addition, we explored the anti-ATL effect of ritonavir on freshly isolated ATL cells from patients. In all ATL cases, ritonavir reduced the survival of ATL cells in a dose-dependent manner (Figure 1D). Ritonavir also caused apoptosis of ATL cells (Figure 1E). In contrast, ritonavir hardly affected the survival of peripheral blood mononuclear cells (PBMCs) from 3 healthy volunteers as measured by WST-8 (Figure 1D).
Effect of ritonavir on the growth and induction of apoptosis of HTLV-I-infected cell lines and freshly isolated ATL cells. (A) Dose-response effect of ritonavir on the growth of HTLV-I-infected cell lines. Cells (105/mL) were cultured for 72 hours in the presence of various concentrations (2.5-40 μM) of ritonavir. Cell growth was assessed by the water-soluble tetrazolium (WST)-8 method and is expressed as a percentage of control (untreated cells) and represents the mean of triplicate cultures. (B) Effect of ritonavir on induction of apoptosis of HTLV-I-infected cell lines. Cells were cultured for 72 hours with ritonavir (40 μM), and apoptosis was measured by Apo2.7 immunostaining. Data represent the mean percentages of apoptotic cells from both untreated (□) and ritonavir-treated (▪) cells. (C) Dose-response effect of ritonavir on induction of apoptosis of MT-4 and HUT-102 cells. (D) Dose-response effect of ritonavir on the cell viability of freshly isolated ATL cells. Cells (106/mL) were cultured for 72 hours in the presence of various concentrations (2.5-40 μM) of ritonavir. (E) Dose-response effect of ritonavir on induction of apoptosis of ATL cells.
Effect of ritonavir on the growth and induction of apoptosis of HTLV-I-infected cell lines and freshly isolated ATL cells. (A) Dose-response effect of ritonavir on the growth of HTLV-I-infected cell lines. Cells (105/mL) were cultured for 72 hours in the presence of various concentrations (2.5-40 μM) of ritonavir. Cell growth was assessed by the water-soluble tetrazolium (WST)-8 method and is expressed as a percentage of control (untreated cells) and represents the mean of triplicate cultures. (B) Effect of ritonavir on induction of apoptosis of HTLV-I-infected cell lines. Cells were cultured for 72 hours with ritonavir (40 μM), and apoptosis was measured by Apo2.7 immunostaining. Data represent the mean percentages of apoptotic cells from both untreated (□) and ritonavir-treated (▪) cells. (C) Dose-response effect of ritonavir on induction of apoptosis of MT-4 and HUT-102 cells. (D) Dose-response effect of ritonavir on the cell viability of freshly isolated ATL cells. Cells (106/mL) were cultured for 72 hours in the presence of various concentrations (2.5-40 μM) of ritonavir. (E) Dose-response effect of ritonavir on induction of apoptosis of ATL cells.
Ritonavir suppresses constitutive NF-κB expressed by HTLV-I-infected cell lines and primary ATL cells
To examine the effect of ritonavir on NF-κB DNA binding, electrophoretic mobility shift assay (EMSA) was performed. We first examined the minimum duration of exposure to ritonavir required for suppression of NF-κB. For this, HUT-102 cells were incubated with 40 μM ritonavir for different periods of time, and nuclear extracts were prepared and examined for NF-κB by EMSA. Down-regulation of NF-κB occurred at 24 hours in HUT-102 cells. However, no change in binding activity of AP-1 was observed (Figure 2A). The observed protein/DNA binding was specific for NF-κB, because the binding was effectively competed and abrogated by excess unlabeled NF-κB oligonucleotide but not by AP-1 (Figure 2C). The NF-κB complex contained p50, p65, and c-Rel (Figure 2C). Forty micromolar concentration of ritonavir was sufficient to suppress constitutive NF-κB activation in residual HTLV-I-infected T-cell lines (data not shown). It should be noted that K562 cells did not show constitutive NF-κB activation (data not shown). We also found a concentration-dependent inhibitory effect of ritonavir on the constitutive increase of NF-κB DNA binding activity (Figure 2B). Twenty micromolar concentration of ritonavir caused only a partial inhibition of NF-κB/DNA binding, whereas strong inhibition was observed at 30 and 40 μM in HUT-102 cells. However, AP-1 binding was not inhibited. This inhibition coincided with an accumulation of unphosphorylated IκBα and a decrease of the slower-migrating form of phosphorylated IκBα, a prerequisite for its subsequent degradation (Figure 2D, top). Thirty micromolar concentration of ritonavir caused only a partial decrease of the slower-migrating form of phosphorylated IκBα, whereas significant decrease of the slower-migrating form of phosphorylated IκBα and an accumulation of unphosphorylated IκBα were observed at 40 μM. We determined the alteration of phosphorylation of IκBα using antibody against phosphospecific IκBα. Results in Figure 2D (middle) show that 40 μM ritonavir decreased the phosphorylated IκBα content. We also determined whether the same results were obtained in primary ex vivo ATL specimens. As shown in Figure 2E, the amount of NF-κB that translocates to the nucleus is also decreased, as determined by EMSA. Twenty micromolar concentration of ritonavir caused only a partial inhibition of NF-κB/DNA binding, whereas strong inhibition was observed at 30 μM in primary ATL cells from acute type. However, AP-1 binding was not inhibited. Ritonavir abolished proximal signaling events leading to IκBα phosphorylation (Figure 2F). Twenty micromolar concentration of ritonavir caused only a partial decrease of the slower-migrating form of phosphorylated IκBα, whereas significant decrease of the slower-migrating form of phosphorylated IκBα and an accumulation of unphosphorylated IκBα were observed at 30 μM (top). Thirty micromolar concentration of ritonavir abolished the phosphorylated IκBα content (middle). We obtained similar results using another acute-type ATL cells (data not shown).
Ritonavir inhibits constitutive NF-κB activation. (A) HUT-102 cells treated with ritonavir (40 μM) for the indicated time were evaluated for NF-κB and AP-1 activation. (B) HUT-102 cells treated with the indicated concentration of ritonavir for 24 hours were evaluated for NF-κB and AP-1 activation. (C) Cold competition using 100-fold molar excess of unlabeled NF-κB and AP-1 oligonucleotides (lanes 2 and 3) demonstrated the specificity of the protein/DNA binding complexes. Specificity of NF-κB binding was also determined by using antibodies to the NF-κB components p50, p65, c-Rel, and p52, resulting in supershift (lanes 4-7). (D) HUT-102 cells were treated with the indicated concentration of ritonavir for 24 hours, and cell lysates were immunoblotted for IκBα (top) and phospho-IκBα (middle). Actin immunoblots confirm that similar amounts of cell extracts were analyzed (bottom). (E) Primary acute-type ATL cells treated with concentrations of ritonavir as indicated for 24 hours were evaluated for NF-κB and AP-1 activation. Where indicated, 100-fold excess amounts of competitor oligonucleotides were added to the reaction mixture (lanes 4 and 5). (F) The bands of phosphorylated IκΒα were down-regulated by ritonavir treatment. Detection of actin expression was used as an internal control.
Ritonavir inhibits constitutive NF-κB activation. (A) HUT-102 cells treated with ritonavir (40 μM) for the indicated time were evaluated for NF-κB and AP-1 activation. (B) HUT-102 cells treated with the indicated concentration of ritonavir for 24 hours were evaluated for NF-κB and AP-1 activation. (C) Cold competition using 100-fold molar excess of unlabeled NF-κB and AP-1 oligonucleotides (lanes 2 and 3) demonstrated the specificity of the protein/DNA binding complexes. Specificity of NF-κB binding was also determined by using antibodies to the NF-κB components p50, p65, c-Rel, and p52, resulting in supershift (lanes 4-7). (D) HUT-102 cells were treated with the indicated concentration of ritonavir for 24 hours, and cell lysates were immunoblotted for IκBα (top) and phospho-IκBα (middle). Actin immunoblots confirm that similar amounts of cell extracts were analyzed (bottom). (E) Primary acute-type ATL cells treated with concentrations of ritonavir as indicated for 24 hours were evaluated for NF-κB and AP-1 activation. Where indicated, 100-fold excess amounts of competitor oligonucleotides were added to the reaction mixture (lanes 4 and 5). (F) The bands of phosphorylated IκΒα were down-regulated by ritonavir treatment. Detection of actin expression was used as an internal control.
Ritonavir represses Tax-induced and constitutive transcriptional activity of NF-κB
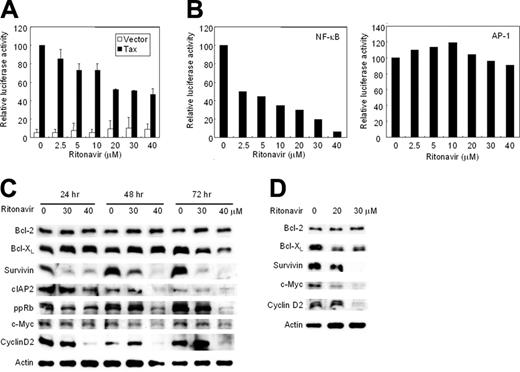
We examined whether ritonavir inhibits the transcriptional activity of NF-κB. First, we tested the effect of ritonavir on Tax-induced NF-κB transcriptional activity in Jurkat cells (HTLV-I-uninfected cell line) transfected with Tax expression plasmid. Ritonavir caused only a partial inhibition of proliferation in Jurkat cells cultured for 24 hours even at the highest dose. Tax-induced NF-κB transcriptional activation was suppressed by ritonavir in a dose-dependent manner (Figure 3A). Next, we determined the effect of ritonavir on constitutively activated NF-κB and AP-1 transcriptional activity in an HTLV-I-infected cell line HUT-102. Ritonavir inhibited the constitutively activated transcriptional activity of NF-κB in a dose-dependent manner, but not that of AP-1 (Figure 3B). Twenty micromolar concentration of ritonavir caused only a partial inhibition of NF-κB/DNA binding (Figure 2B), whereas strong inhibition of NF-κB transcriptional activity was observed at the concentration less than 20 μM. This discrepancy might derive from differences in sensitivity between these 2 assays. These results indicate that ritonavir inhibits both Tax-induced and constitutive NF-κB transcriptional activity.
Ritonavir inhibits NF-κB transcriptional activation and expression of apoptosis- and cell-cycle-associated proteins. (A) Ritonavir inhibits Tax-induced NF-κB transcriptional activation. κB-LUC was transfected into Jurkat cells with Tax-expressing plasmid (▪) or empty vector (□). After transfection, cells were treated with increasing concentrations of ritonavir. Luciferase activity is expressed relative to the basal level measured in cells transfected with the reporter plasmid and Tax-expressing plasmid without further treatment, which was defined as 100. Data represent the mean + SD from 3 independent experiments. (B) Ritonavir inhibits constitutive active NF-κB transcriptional activity in HUT-102 cells. κB-LUC and AP-1-LUC were transfected into HUT-102 cells. After transfection, cells were treated as in panel A. Luciferase activity was normalized, based on the Renilla luciferase activity from phRL-TK. Relative luciferase activity is expressed relative to the basal level measured in cells transfected with the reporter plasmid without further treatment, which was defined as 100. (C-D) Western blot analyses. HUT-102 cells (C) and primary acute-type ATL cells (D) were cultured with the indicated concentration of ritonavir for 24 to 72 hours (HUT-102 cells) and 24 hours (ATL cells). Cells were harvested and subjected to Western blot analysis. The polyvinylidene fluoride membrane was sequentially probed with indicated antibodies.
Ritonavir inhibits NF-κB transcriptional activation and expression of apoptosis- and cell-cycle-associated proteins. (A) Ritonavir inhibits Tax-induced NF-κB transcriptional activation. κB-LUC was transfected into Jurkat cells with Tax-expressing plasmid (▪) or empty vector (□). After transfection, cells were treated with increasing concentrations of ritonavir. Luciferase activity is expressed relative to the basal level measured in cells transfected with the reporter plasmid and Tax-expressing plasmid without further treatment, which was defined as 100. Data represent the mean + SD from 3 independent experiments. (B) Ritonavir inhibits constitutive active NF-κB transcriptional activity in HUT-102 cells. κB-LUC and AP-1-LUC were transfected into HUT-102 cells. After transfection, cells were treated as in panel A. Luciferase activity was normalized, based on the Renilla luciferase activity from phRL-TK. Relative luciferase activity is expressed relative to the basal level measured in cells transfected with the reporter plasmid without further treatment, which was defined as 100. (C-D) Western blot analyses. HUT-102 cells (C) and primary acute-type ATL cells (D) were cultured with the indicated concentration of ritonavir for 24 to 72 hours (HUT-102 cells) and 24 hours (ATL cells). Cells were harvested and subjected to Western blot analysis. The polyvinylidene fluoride membrane was sequentially probed with indicated antibodies.
Ritonavir down-regulates the expression of NF-κB-regulated gene products
The antiproliferative and proapoptotic effects of ritonavir were explored by examining the level of intracellular regulators of cell cycle and apoptosis after exposure to ritonavir (Figure 3C). Ritonavir down-regulated levels of Bcl-XL, survivin, cIAP2, c-Myc, cyclin D2, regulated by NF-κB,47-51 and the phosphorylated form of pRb in HUT-102 cells cultured with 40 μM ritonavir for 72 hours. Thirty micromolar concentration of ritonavir caused only a partial down-regulation of Bcl-XL and the phosphorylated form of pRb, whereas significant down-regulation of survivin was observed in HUT-102 cells cultured for 72 hours, suggesting that NF-κB-regulated genes have the differential sensitivity to ritonavir. However, ritonavir did not modulate levels of Bcl-2 and Tax proteins in these cells (Figure 3C; data not shown). We also explored the effect of ritonavir on NF-κB-regulated gene products in ATL cells freshly isolated from an acute-type patient. As shown in Figure 3D, Bcl-XL, survivin, cyclin D2, and c-Myc, but not Bcl-2, showed a decline. Thus, expression of NF-κB regulated genes, the induction of which are involved in antiapoptosis (Bcl-XL, survivin, and cIAP2) and cell cycle (cyclin D2 and c-Myc), apparently had been down-regulated in the presence of ritonavir. We obtained a similar result using other acute-type ATL cells (data not shown). Taken together these data suggest that constitutively high NF-κB activity in ATL cells is indispensable for their survival, and that specific inhibition of this activity by a clinically available drug results in the induction of apoptosis.
Successful engraftment and massive infiltration of primary ATL cells into various organs of NOG mice inoculated with PBMCs from patients with ATL. (A) Photographs of whole organs of normal and ATL cell-challenged mice with enlarged spleen, liver, and lungs (left 2 panels). Right 2 panels show May-Grunwald and Giemsa staining of PBMCs collected from normal and ATL cell-challenged mice, 2 months after inoculation of ATL cells, respectively. (B) HE (top) and immunohistochemical staining of various organs of NOG mice inoculated with ATL cells. Immunohistochemical staining using anti-CD4 (middle) and anti-CD25 (bottom) was conducted on various organs of mice tissues 2 months after inoculation of ATL cells. Magnification ×40.
Successful engraftment and massive infiltration of primary ATL cells into various organs of NOG mice inoculated with PBMCs from patients with ATL. (A) Photographs of whole organs of normal and ATL cell-challenged mice with enlarged spleen, liver, and lungs (left 2 panels). Right 2 panels show May-Grunwald and Giemsa staining of PBMCs collected from normal and ATL cell-challenged mice, 2 months after inoculation of ATL cells, respectively. (B) HE (top) and immunohistochemical staining of various organs of NOG mice inoculated with ATL cells. Immunohistochemical staining using anti-CD4 (middle) and anti-CD25 (bottom) was conducted on various organs of mice tissues 2 months after inoculation of ATL cells. Magnification ×40.
Establishment of a novel ATL model
PBMCs from patients with ATL were inoculated either intraperitoneally into the abdominal region or subcutaneously in the postauricular region of unconditional NOD/SCID/γcnull (NOG) mice. All mice developed clinical sign of near-death, such as piloerection, weight loss, and cachexia 6 to 8 weeks after inoculation of ATL cells in addition to the enlargement of lymph nodes, spleen, lungs, and liver, whereas no tumors were found in the postauricular region or abdominal cavity where primary ATL cells were inoculated (Figure 4A). There was no difference in respect to the successful engraftment of ATL cells inoculated either intraperitoneally or subcutaneously in NOG mice. Histologic analysis of ATL-bearing mice showed massive infiltration of leukemic cells in various organs of NOG mice that were efficiently expressing human CD4 and CD25 molecules (Figure 4B). A higher level of IL-2Rα (CD25) expression was observed on the surface of malignant cells associated with all stages of ATL12-14 as well as ATL cells infiltrated into various organs of patients.52,53 Thus, results from this model indicated successful engraftment and massive infiltration of primary ATL cells in various organs of NOG mice, like leukemia but without producing tumors at the sites of inoculation.
Effect of ritonavir on ATL cell growth and infiltration. (A) Mice were injected with ATL cells (2 × 107 cells) subcutaneously in the postauricular region. One day after inoculation, the mice were administered either RPMI 1640 or ritonavir (30 mg/kg/d) intraperitoneally every day for 30 days followed by observation for another month without therapy. Photographs of whole organs of RPMI 1640-treated ATL mice (left) and ritonavir-treated ATL mice (right) with enlarged spleen, liver, and lungs. (B-D) HE staining (B) and immunohistochemical staining using anti-CD4 (C) or anti-CD25 (D) in various organs of NOG mice 2 months after inoculation of ATL cells. Upper panels show various organs of mice treated with RPMI 1640, and lower panels represent various organs of mice treated with ritonavir. Magnification ×40.
Effect of ritonavir on ATL cell growth and infiltration. (A) Mice were injected with ATL cells (2 × 107 cells) subcutaneously in the postauricular region. One day after inoculation, the mice were administered either RPMI 1640 or ritonavir (30 mg/kg/d) intraperitoneally every day for 30 days followed by observation for another month without therapy. Photographs of whole organs of RPMI 1640-treated ATL mice (left) and ritonavir-treated ATL mice (right) with enlarged spleen, liver, and lungs. (B-D) HE staining (B) and immunohistochemical staining using anti-CD4 (C) or anti-CD25 (D) in various organs of NOG mice 2 months after inoculation of ATL cells. Upper panels show various organs of mice treated with RPMI 1640, and lower panels represent various organs of mice treated with ritonavir. Magnification ×40.
Ritonavir inhibits ATL cell growth and infiltration in NOG mice
To study the effect of ritonavir on ATL, we injected primary ATL cells (2 × 107) from 10 patients subcutaneously into the postauricular region of NOG mice. One day after inoculation, mice were treated with either RPMI-1640 (as control) or ritonavir (30 mg/kg/d) intraperitoneally daily for 30 days followed by observation for another 30 days without any treatment. ATL cell inoculation promoted the development of piloerection, weight loss, and cachexia, all of which are signs of near-death, in addition to the enlargement of lymph nodes, spleen, lungs, and liver in all control mice 2 months after inoculation (Figure 5A). In contrast, ritonavir-treated mice appeared to be healthy and had almost no enlargement of these organs (Figure 5A). Clinical evaluation of organ invasion 2 months after injection of primary ATL cells showed that ritonavir treatment inhibited their infiltration into lymph nodes, spleen, lungs, and liver (Figure 5B-D). Samples from 7 patients of 10 injected in mice treated with ritonavir presented substantial inhibition of organ invasion, and 2 (patients 5 and 7) showed partial inhibition, whereas one sample (patient 6) failed to do so (Table 2). In contrast, all control mice showed formation of new lymph nodes and infiltration with ATL cells into various organs (Figure 5B-D; Table 2). Organ infiltration of primary ATL cells was analyzed and evaluated by HE staining and immunostaining of CD4 and CD25. Together, these data indicate that ritonavir significantly inhibits ATL cell growth and infiltration in various organs of NOG mice (Figure 5).
Effect of ritonavir on infiltration of ATL cells in various organs of SCID mice
Patient no. and treatment . | Liver . | Spleen . | Lung . | Lymph node . |
|---|---|---|---|---|
| 1 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | ++ | + | + | NF |
| 2 | ||||
| Control | +++ | +++ | ++ | +++ |
| Ritonavir | + | + | − | NF |
| 3 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | ± | ± | ± | NF |
| 4 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | + | + | + | NF |
| 5 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | ++ | ++ | ++ | NF |
| 6 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | +++ | ++ | +++ | ++ |
| 7 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | ++ | + | ++ | NF |
| 8 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | ± | ± | ± | NF |
| 9 | ||||
| Control | +++ | +++ | +++ | ++ |
| Ritonavir | ++ | + | + | NF |
| 10 | ||||
| Control | +++ | +++ | ++ | +++ |
| Ritonavir | + | + | + | NF |
Patient no. and treatment . | Liver . | Spleen . | Lung . | Lymph node . |
|---|---|---|---|---|
| 1 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | ++ | + | + | NF |
| 2 | ||||
| Control | +++ | +++ | ++ | +++ |
| Ritonavir | + | + | − | NF |
| 3 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | ± | ± | ± | NF |
| 4 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | + | + | + | NF |
| 5 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | ++ | ++ | ++ | NF |
| 6 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | +++ | ++ | +++ | ++ |
| 7 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | ++ | + | ++ | NF |
| 8 | ||||
| Control | +++ | +++ | +++ | +++ |
| Ritonavir | ± | ± | ± | NF |
| 9 | ||||
| Control | +++ | +++ | +++ | ++ |
| Ritonavir | ++ | + | + | NF |
| 10 | ||||
| Control | +++ | +++ | ++ | +++ |
| Ritonavir | + | + | + | NF |
PBMCs from patients with ATL patients were inoculated subcutaneously into postauricular region of NOG mice, which were treated with ritonavir or RPMI-1640 as a control 1 day after inoculation for 1 month. Various organ infiltration was evaluated 2 months after inoculation.
NF indicates no formation of new lymph node; +++, massive; ++, marked; +, slight; ±, focally present or no infiltration; −, no infiltration.
Discussion
ATL is a malignancy of CD4+ T-lymphocytes etiologically linked to a retrovirus, HTLV-I.1-3 The malignant cells associated with all phases of ATL express very high levels of IL-2Rα (CD25).12-14 The median survival duration of all patients with aggressive ATL was 13 months, and overall survival at 2 years was estimated to be 31.3%.6 The various chemotherapies so far developed have not increased significantly the survival of patients with ATL. Given disappointing results using conventional chemotherapy, new approaches for the treatment of ATL are required. Previous reports have shown that primary ATL cells proliferate and infiltrate into various organs of SCID mice.54-56 Our ATL model was consistent with others, but it represented more aggressive features about cell growth and infiltration in SCID mice. The tumor cells massively infiltrated into various organs in a manner similar to a leukemia-expressing T-cell marker CD4 and an activating marker CD25, especially into the spleen, lymph nodes, liver, and lungs of NOG mice. Our NOG ATL model presents many features 6 to 8 weeks after inoculation of ATL cells such as the clinical signs observed in patients with ATL. Two clinical types, acute and chronic, carry very different prognosis. However, no difference of cell growth, surface phenotype, and NF-κB activity is observed in primary leukemic cells from patients with acute- and chronic-type ATL. Therefore, the same characteristics of freshly isolated ATL cells with acute- and chronic-type were observed in NOG mice. Thus, it represents a novel model to evaluate tissue toxicity and the efficacy of therapeutic agents directed toward the treatment of ATL.
Constitutive NF-κB activation was demonstrated not only in HTLV-I-infected cell lines but also on fresh ATL cells regardless of Tax expression,15 which could contribute to the drug-resistance of ATL cells through overexpression of Bcl-XL.51 We and others have previously reported that suppression of high NF-κB activity inhibited cell growth and induced apoptosis of HTLV-I-infected cell lines and primary ATL cells both in vitro and in vivo.21-24 Recently, ritonavir has been shown to inhibit NF-κB activity induced by activators such as TNFα, Tat, and ORF74.34 This led us to investigate whether this drug exhibits anti-ATL effects in vitro and in our preclinical murine ATL model. In the present study, we revealed that ritonavir treatment of HTLV-I-infected cell lines and ATL cells inhibited phosphorylation of IκBα, allowing suppression of NF-κB activity. Our results also suggest that inhibition of NF-κB activity by ritonavir reduced cell growth and induced apoptosis of these cells. This is consistent with down-modulation of NF-κB-regulated genes such as antiapoptotic (Bcl-XL,50,51 cIAP2,57 and survivin49 ) and cell-cycle-related (cyclin D248 and c-Myc47 ) genes. We also examined the effect of ritonavir on the proliferation of an HTLV-I-negative T-cell line, Jurkat, in vitro. Exposure of ritonavir for 72 hours reduced the rate of proliferation (data not shown). Because ritonavir is suggested to affect proteasomal proteolysis,33 it may effect a stabilization of p21, p27, and p53 proteins. Like proteasome inhibitors, ritonavir may also affect multiple pathways critical for survival of HTLV-I-positive and -negative malignant T cells. Additional experiments will be necessary to elucidate the mechanisms of anti-ATL activity.
In the therapy of HIV infection, the blood plasma ritonavir concentrations are between 5 and 15 μM,58 but much higher maximal concentrations (up to 46 μM) have been determined in individual patients.59 In the present study, we used the concentration of ritonavir for doing in vitro experiments from 0 to 40 μM and in vivo 30 mg/kg/d used for treatment of patients with AIDS. Our murine ATL model clearly indicates that 30 mg/kg/d of ritonavir significantly inhibits ATL cell growth and infiltration into various organs of NOG mice. The plasma exposure produced by this dose in mice is only approximately one half of the plasma exposure observed with the licensed dose of ritonavir in humans (600 mg twice daily). In our NOG ATL model, ritonavir at this treatment dosage is well tolerated without severe adverse effects observed in the mice during the treatment period. These data strongly suggest that the HIV PI, ritonavir, is a promising antitumor agent against ATL and could be used clinically for ATL regimens. Ritonavir exhibited anti-ATL activity against leukemic cells from patients with acute- and chronic-type ATL in vitro and in vivo. The expression of CD25 and NF-κB activity do not differ between acute and chronic ATL cells.12-15 These results suggest that anti-ATL activity of ritonavir correlates with suppression of NF-κB activity. It is also of interest to note that HTLV-I uses an aspartic protease analogous to HIV protease in its replication. To our knowledge, the activity of ritonavir against HTLV-I protease has not been assessed. Although Tax expression is very low or undetectable in ATL malignancy, a direct antiviral effect of ritonavir cannot be ruled out at this time.
In summary, using a large number of patient samples we have established a novel NOG ATL model that presents features similar to patients with ATL. These results also indicate that the HIV PI, ritonavir, showed antitumor and anti-NF-κB activity against primary ATL cells. Finally, our results strongly suggest that NF-κB serves as a potential molecular target to treat ATL, and that ritonavir might be used clinically as a single compound or in combination with the reducing dose of chemotherapeutic agents for treatment of patients with ATL.
Prepublished online as Blood First Edition Paper, September 20, 2005; DOI 10.1182/blood-2005-02-0735.
Supported by grants from the Ministry of Education, Science, and Culture; the Ministry of Health, Labor, and Welfare; and Human Health Science of Japan.
M.Z.D. and J.U. contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank D. Kempf and T. Yamada of Abbott Laboratories, N. Yamamoto and S. Takeda of Molecular Virology, S. Ichinose of the Instrumental Analysis Research Center, S. Endo of the Animal Research Center, Tokyo Medical and Dental University, and P.J. Richard of Cardiff University for their advice and assistance with the experiments, and Y. Sato of the National Institute of Infectious Diseases for her excellent technical assistance. We thank J. Fujisawa for providing a luciferase reporter construct, κB-LUC, K. Matsumoto for providing the Tax expression plasmid, M. Maeda for providing ED-40 515(-), and the Fujisaki Cell Center, Hayashibara Biomedical Laboratories, for providing the MT-1, HUT-102, and C5/MJ cell lines.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal