Abstract
The most common type of renal injury in multiple myeloma is chronic tubulointerstitial nephropathy associated with casts in tubule lumens, an entity referred to as “myeloma kidney” that often progresses to end-stage kidney diseases. Myeloma kidney is associated with a significant increase in all-cause mortality, yet no effective intervention, except a limited use of steroid, is available. Here, we report that pituitary adenylate cyclase-activating polypeptide with 38 residues (PACAP38) dramatically prevents injury of cultured renal proximal tubule cells caused by myeloma light chains through suppression of proinflammatory cytokines production, by inhibiting p38 MAPK and translocation of NFκB via both PAC1 and VPAC1 receptors. The suppressive effects of PACAP was as effective as dexamethasone in all of their cytokine assays and demonstrated both in vitro and in vivo. Furthermore, PACAP38 inhibits myeloma cell growth directly and may also indirectly by suppressing production of the growth factor, IL-6, from bone marrow stromal cells, that is stimulated by adhesion of myeloma cells. These findings render PACAP38 worth evaluation as a promising candidate for an effective and safe renoprotectant in myeloma kidney, and possibly other nephropathy, and also as a new antitumor agent in multiple myeloma.
Introduction
Multiple myeloma is the sixth most common cancer in the United States. About 13 200 new cases of multiple myeloma were diagnosed in 2000. According to the US Renal Data System, renal morbidity from multiple myeloma is substantial.1 Myeloma kidney disease was associated with 250% increase in all-cause mortality.1 The kidney is vulnerable to pathogenic effects of monoclonal light chains in multiple myeloma and other disease associated with overproduction of immunoglobulin light chains. The most common type of renal injury in multiple myeloma is chronic tubulointerstitial nephropathy associated with casts in tubule lumens, an entity referred to as “myeloma kidney” that often progresses to end-stage kidney disease.2,3 Although the mechanisms of cast formation have been clarified in studies that showed that certain types of light chains behave as ligand binding to defined sites on Tamm-Horsfall proteins,4 the mechanisms responsible for chronic tubulointerstitial injury have only recently been explored.5,6 These studies showed that increased endocytosis and overloading by myeloma light chains in proximal tubule cells produce inflammatory cytokines. These cytokine responses were mediated by activation of nuclear factor NFκB and signaled through MAPKs, ERK1/2, JNK, and p38 MAPK. Despite improved understanding of the pathophysiology, no effective treatment is known for myeloma kidney except for a limited use of steroids.
Pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) was isolated from ovine hypothalamic tissues based on the ability to stimulate adenylate cyclase in pituitary cell cultures.7 PACAP38 is a new member of the vasoactive intestinal peptide (VIP) family of peptides. It is a pleiotropic neuropeptide, exhibiting a variety of biologic actions, including activities as a neurotransmitter, neuromodulator, neurotrophic factor, as well as an immunomodulator, in immune cells through its effect on MAPK signaling and modulation of activation of NFκB.8,9 MAPK signaling and NFκB activation have also been involved in neurotrophic effects of PACAP38 in the brain.10 Although the kidney, particularly tubular epithelial cells, has never been considered a target site for PACAP, the apparent pivotal role of MAPKs and NFκB in light chain-mediated cytokine responses prompted us to evaluate PACAP38 as a potentially beneficial agent to counter the adverse effects of light chains in renal tubule cells.
The effects of PACAP38 on the production of proinflammatory cytokines stimulated by light chains in human proximal tubule cell culture were first examined. The effect was also examined in vivo using rats. Our results showed that PACAP38 was capable of inhibiting light chain-induced cytokine expression with a great potency and prevented the resulting cell damage. However, PACAP is also considered as an autoregulatory factor for certain tumors, stimulating their growth in an autocrine fashion.11 If PACAP38 stimulates myeloma cell growth directly or indirectly, its beneficial use as renoprotectant would be seriously challenged. This issue is thus addressed as well.
Materials and methods
Animals
Male Sprague-Dawley rats (3-4 weeks old) were obtained from Charles River Laboratories (Wilmington, MA). All procedures involving animals were reviewed and approved by the institutional animal care and use committee at Tulane University Health Sciences Center.
Antagonists
Potent VPAC1 receptor-specific antagonist, Ac-His, D-Phe2, K15, R16, L27 VIP(1-7)/GRF(8-27) (PG97-269), was provided by Drs P. Gourlet and P. Robberecht (Université Libre, Brussels, Belgium), and Dr A. Miyata (Kagoshima University, Kagoshima, Japan) provided the PAC1 receptor-specific antagonist, M65.
Isolation and purification of light chains (LCs)
The κ-light chain used in these experiments was isolated and purified from the urine of a patient with multiple myeloma and myeloma kidney as described previously.12,13 Briefly, the light chain was precipitated with a minimal amount of saturated ammonium sulfate (65%-90%), extensively dialyzed with distilled water, and lyophilized. The light chain protein was purified by dissolving the lyophilized desalted crude protein in buffer at pH 6.0 followed by chromatography on carboxymethyl-Sephadex (C-50; Pharmacia, Piscataway, NJ). Under these conditions, light chains are bound to the column, and the contaminants are not. Bound light chain was eluted with 0.6 M NaCl, redialyzed against distilled water, and lyophilized. The purity of the light chain was confirmed by sodium dodecyl sulfate (SDS)–gel electrophoresis, and the immunologic identity reported from the clinical laboratory was confirmed by Western blotting using goat anti–human κ and λ antibodies (Sigma, St Louis, MO). The light chain used in these studies was previously shown to undergo endocytosis by proximal tubule cells,13 bind to cubilin,12 and megalin14 and induced cytokines through phosphorylation of MAPKs and activation of NFκB.6,14 The light chain preparation used here was tested for endotoxin using the chromogenic Limulus amebocyte lysate test (Charles River Laboratories, Charleston, SC) and was found to be essentially endotoxin free (< 3.7 EU/mg light chain protein). Furthermore, the light chain preparation contained no measurable quantities of IL-1, IL-6, IL-8, MCP-1, or TNFα.
Intravenous infusion of myeloma light chain
Rats (180-200 g) were anesthetized with isoflurane in nitrous oxide/oxygen (7:3) to allow implantation of the intrajugular cannula. A PE50 polyethylene tubing filled with heparin (200 U/mL saline) was introduced through an incision into the right jugular vein toward the right atrium. The other end of the tubing was introduced through the subcutaneous tissue and pulled out through a small incision on the nuchal area. The tubing was then introduced through a flexible steel tubing which was fixed on the protecting vest and secured on the nuchal area. The tubing was connected to an infusion pump. Because 50 μM light chain markedly stimulated expression of TNFα and IL-6 and produced significant cell damage in vitro, the light chain protein dissolved in physiologic saline was first rapidly infused intravenously at 10 mL/h (150 mg light chain) over 1 hour, then by a slow infusion at 1 mL/h (15 mg light chain/h) over 3 days to maintain the level of the light chain in the blood to approximate 50 μM. Immediately after the termination of light chain infusion, the animal was killed, and the kidney was removed for determination of cytokine and histologic examination. The pilot experiment showed that the infusion of the light chain increased TNFα level in the kidney tissue several times as compared with the basal level. Control animals received saline only; some rats received light chain over 3 days, and other rats received the same amount of light chain and PACAP38. PACAP38 (2 nmol) dissolved in 10 mL saline containing the light chain was infused intravenously over 1 hour, and was then followed by 0.2 nmol PACAP38 per hour for 3 days with the light chain. Immediately after the termination of the treatment the animals were killed. The kidneys were removed and dissected into halves. Half of the kidney was extracted for cytokine and protein analyses, and the other half was fixed in Bouin solution.
Measurement of rat TNFα in kidney tissue
Fresh kidney tissues were weighted and immediately placed in 1-mL capacity chilled Kontes-Duall tissue grinders. Ice-cold extracting buffer, consisting of 0.1% Igepal CA-630 nonionic detergent (Sigma) in phosphate-buffered saline (PBS) with added protease inhibitor cocktail (Sigma) was then added at a rate of 50 μL/10 mg tissue. The mixture was ground by hand on ice until only fibrous white insoluble connective tissue remained. After incubating the mixture for at least 10 minutes on ice, it was transferred to a microcentrifuge tube and centrifuged for 10 minutes at 20 000g at 4°C. The resulting supernatant was divided into aliquots and stored at –80°C for later analysis. The total protein content in extracts diluted 1:75 in PBS was determined with DC protein assay reagents (Bio-Rad, Hercules, CA). Colorimetric enzyme-linked immunosorbent assay (ELISA) for rat TNFα (BioSource, Camarillo, CA) was used to detect the cytokine in kidney tissue extracts.
Cell cultures
Simian virus 40 (SV40) immortalized human renal proximal tubule cells were originally obtained from Dr L.C. Racusen, Johns Hopkins University Medical School (Baltimore, MD). These cells are the cell lines with extended in vitro growth potential from human renal proximal tubule. Their original characteristics and validation were described.5 They showed the marker brush-border enzymes and biochemical and morphologic characteristics similar to other widely used proximal tubule cell lines and human proximal tubule cells in stable culture. These cells were grown in DRM-23E medium supplemented with 0.5% (vol/vol) fetal bovine medium (FBS) at 37°C in a humidified atmosphere of 95% air–5% CO2 and nourished at intervals of 2 or 3 days. This transformed cell-line's response to inflammatory stimuli was compared with parental proximal tubule cells and found to be similar.
The NCI-H929 human multiple myeloma-derived cell line that produces κ light chain obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI 1640 medium containing 10% non-inactivated FBS and 0.05 mM 2-mercaptoethanol. Effects of PACAP38 and dexamethasone on multiple myeloma cell growth were assessed by determining BrdU incorporation (Roche, Indianapolis, IN).
The normal human bone marrow stem cells (BMSCs) were provided by the Center for Gene Therapy, Tulane University Health Sciences Center, and cultured in MEM containing 16.5% FBS. To evaluate cytokine secretion in BMSCs adherent to MM cells, the passage 3 to 5 normal human BMSCs were cultured (1 × 105 cells/mL) for 24 hours to obtain a confluent monolayer. After BMSCs became confluent, nonadherent cells were washed with Hanks buffered saline solution. The myelomonocytic leukemia (MM) cell suspension (4-5 × 105 cells/mL) was then added directly onto BMSCs. After incubation at 37°C for 1 to 72 hours, the supernatants were collected, and remaining cells were procured for RNA analysis. Human IL-6 levels in the supernatants of BMSCs cultured in media or with MM cells were determined using an ELISA (R&D Systems, Minneapolis, MN). All experiments were performed in quadruplicate.
RT-PCR analysis of PACAP/VIP receptor messenger RNAs
The oligonucleotides used for the reverse transcriptase–polymerase chain reaction (RT-PCR) were synthesized by Integrated DNA Technologies (Coralville, IA). Total RNA was isolated from the human proximal tubule cells (PTCs), MM cells, and BMSCs by extraction with the RNeasy Mini kit (Qiagen, Valencia, CA). Total RNA (0.5 μg) from each sample was used for RT-PCR, which was performed by SuperScript One-Step RT-PCR with Platinum Taq (Invitrogen, Carlsbad, CA) carried out on a GeneAmp PCR System 2400 (Applied Biosystems, Foster City, CA). The pairs of primers used for amplification of human PAC1-R (F4, 5′-ACA CGG TTG GCT ACA GCA CAT C-3′; B23, 5′-GCT TGA AGT CCA CAG CGA AGT AAC-3′), human VPAC1-R (F5, 5′-TGA CAA GGC AGC GAG TTT GG-3′; B5, 5′-GAA GCA GGA TTC GGA TGA TGC-3′), and human VPAC2-R (F1, 5′-TGT TCC TGT CCT TCA TCC TGA GAG-3′; B2, 5′-GGT CGT TTG TAT CCC AGC AAC C-3′) were designed with the MacVector program (Accelrys, San Diego, CA) based on the reported sequences of human PAC1-R (GenBank no. D17516), human VPAC1-R (GenBank no. NM 004624), and human VPAC2-R (GenBank no. NM 003382). These pairs can specifically discriminate among these 3 PACAP/VIP receptors. Twenty microliters of each PCR product were submitted to electrophoresis on a 2% NuSieve 3:1 agarose gel (BioWhittaker Molecular Applications, Walkersville, MD), stained with ethidium bromide, and visualized under ultraviolet light. The validity of the PCR products was confirmed by sequencing by SeqWright (Houston, TX).
Treatments of human renal proximal tubule cell line
Human renal proximal tubule cells plated onto 6-well tissue culture plates were grown at 37°C in DRM-23E medium supplemented with 0.5% (vol/vol) FBS in an incubator for 24 hours. After prewashing with serum-free medium, the cells were incubated with κ-LC (1.5 mg/ml, ∼ 50 μM) for 3 days in the presence and absence of various concentrations of PACAP38 or dexamethasone as well as kinase and transcription factor inhibitors. Cell viability was determined by trypan blue exclusion assays; in all experiments, at least 85% of cells remained viable. After exposure to test substances, culture supernatants were harvested and stored at –70°C for cytokine assays. After the medium was removed, the cells were washed with ice-cold PBS, and proteins were extracted by lysing cells with Sigma Mammalian Cell Lysis Reagents. Lysates were scraped and passed through a 21-gauge needle to shear DNA, and centrifuged at 12 000g for 10 minutes at 4°C. Finally, supernatants were harvested and used for kinase studies.
Measurements of human IL-6, MCP-1, and TNFα levels, and phosphorylated ERK1/2 and p38 MAPK by ELISA
Human IL-6, MCP-1, and TNFα were measured using a commercial human ELISA kit (Quantikine; R&D Systems). Experiments were conducted in triplicate using 96-well microplates. Cells were trypsinized and counted to express the amount of cytokine as picogram per 105 cells. The levels of phosphorylations of p44/42 ERK1/2 type MAPKs and p38 MAPK in cell lysates at both threonine and tyrosine were quantified (BioSource, Camarillo, CA).
Nuclear extracts and determination of NFκB activation
Confluent monolayers of tubule cells were incubated with 50 μmol LCs for 3 days in the presence or absence of PACAP38 or dexamethasone, and nuclear extracts were prepared by using Nuclear Extract Reagents (Active Motif, Carlsbad, CA). The activation of transcription factor NFκB, p50 and p65 subunits, were measured by a specific ELISA-based assay (TransAM; Active Motif).
Statistical analysis
Results were expressed as mean plus or minus SEM. Multiple comparisons were made by ANOVA and Tukey-Kramer or Bonferroni multiple-comparison tests (InStat; GraphPad Software, San Diego, CA). Statistical analyses, curve fitting, and calculations were done using GraphPad Prism 4 (GraphPad Software). Minimal level of significance was defined as P less than .05.
Results
Effect of PACAP38 on the light chain–induced cytokine production and cell damage in cultured human renal tubule cells
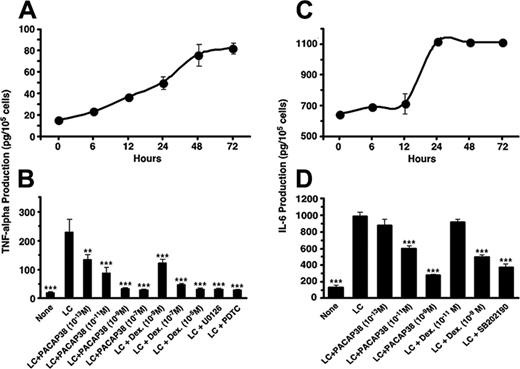
The light chain effect on human renal tubule cells was first examined in vitro using SV40 immortalized human tubule cell cultures. The κ light chain of immunoglobulin was purified from the urine of a patient with multiple myeloma and added to the tubule cell cultures in a concentration of 50 μM. Incubation of the cell cultures with the light chain stimulated release of proinflammatory cytokines, IL-6 and TNFα, in a time-dependent manner, reached maximum level at 24 to 72 hours (Figure 1).
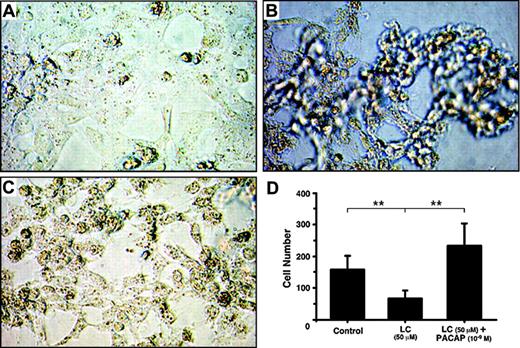
Effect of PACAP38 on the production of the cytokines was then examined at 3 days. Because dexamethasone is often used for controlling myeloma growth as well as protecting the kidney from injury by the myeloma light chain, we also compared its effects with the peptide. PACAP38 suppressed release of both IL-6 and TNFα dose dependently. Dexamethasone also suppressed cytokines production. The suppressive effect of PACAP was comparable to that of dexamethasone. (Figure 1B,D). Addition of the light chain induced considerable damage in cultured tubular cells as indicated by detachment of the cells from the cultured plate and aggregation and necrosis of the cells (Figure 2B). Addition of PACAP38 nearly completely prevented the cell injury resulted from the light chain (Figure 2C).
Production of inflammatory cytokines stimulated by the light chain in the cultured human renal proximal tubule cells and its suppression by PACAP38 or dexamethasone. (A,C) Time course of TNFα and IL-6 production, respectively. (B,D) Effect of PACAP38 or dexamethasone on LC-induced TNFα and IL-6 production, respectively. Effects of 20 μM U0126, a MEK1/2 inhibitor; 12.5 mM PDTC, a NFκB inhibitor; and 10 μM SB202190, a p38 MAPK inhibitor, are also shown. The vertical columns show the means of 4 replicate determinations with SE of means. ***P < .001 and **P < .01, as compared with respective LC-stimulated value.
Production of inflammatory cytokines stimulated by the light chain in the cultured human renal proximal tubule cells and its suppression by PACAP38 or dexamethasone. (A,C) Time course of TNFα and IL-6 production, respectively. (B,D) Effect of PACAP38 or dexamethasone on LC-induced TNFα and IL-6 production, respectively. Effects of 20 μM U0126, a MEK1/2 inhibitor; 12.5 mM PDTC, a NFκB inhibitor; and 10 μM SB202190, a p38 MAPK inhibitor, are also shown. The vertical columns show the means of 4 replicate determinations with SE of means. ***P < .001 and **P < .01, as compared with respective LC-stimulated value.
Effect of PACAP38 on light chain–induced activation of ERK and p38 MAPKs and NFκB in the renal tubule cell cultures
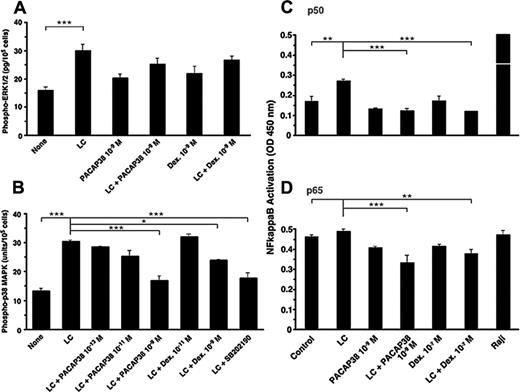
Proinflammatory cytokine production by the light chain in the tubule cells is mediated by activation of ERK1/2, JNK, and p38 MAPKs.5,6 Addition of the light chain significantly activated ERK1/2 MAPK, but neither PACAP38 nor dexamethasone suppressed the light chain–stimulated ERK1/2 activation (Figure 3A). However, light chain–induced activation of p38 MAPK was suppressed effectively by both PACAP38 and dexamethasone (Figure 3B). Both ERK1/2 inhibitor and p38 MAPK inhibitor in micromolar amounts also suppressed light chain–induced cytokine production (only the data with the p38 MAPK inhibitor shown in Figure 3B). H89, a cAMP-dependent protein kinase inhibitor, also suppressed light chain–induced p38 MAPK activation and cytokine production. This suggests that cAMP signaling may be the upstream event in p38 MAPK activation. It is unlikely that the cytokine inhibitory effect of PACAP is mediated through stimulation of adenylate cyclase. (Only the data with the p38 MAPK inhibitor shown in Figure 3B).
Light chain–stimulated cytokine production by tubule cells is mediated by the activation of NFκB.5 One nanomolar concentration of PACAP38 and 0.1 μM dexamethasone completely suppressed the light chain–induced activation of p50 subunit of NFκB (Figure 4A). The level of activated p65 subunit of NFκB was elevated in the basal state in cultured tubule cells before the light chain was added (Figure 3B). Nevertheless, both PACAP38 and dexamethasone reduced the activation of the p65 subunit of NFκB in the presence of the light chain.
Photomicrograph of cultured human renal proximal tubule cells. (A) Control; (B) proximal tubule cells cultured with 50 μM light chain for 3 days; (C) proximal tubule cells cultured with 50 μM light chain and 1 nM PACAP38 for 3 days. Images were obtained using a Nikon Diaphot inverted phase-contrast microscope (Nikon, Tokyo, Japan) with 10|×/0.30 numeric aperture objective. Images were acquired using a Nikon Coolpix 995 camera and processed using Adobe Photoshop 7 (Adobe Systems, San Jose, CA). (D) Number of healthy cells in each treatment group. Mean and SE of cells in 10 visual fields was used in each group.
Photomicrograph of cultured human renal proximal tubule cells. (A) Control; (B) proximal tubule cells cultured with 50 μM light chain for 3 days; (C) proximal tubule cells cultured with 50 μM light chain and 1 nM PACAP38 for 3 days. Images were obtained using a Nikon Diaphot inverted phase-contrast microscope (Nikon, Tokyo, Japan) with 10|×/0.30 numeric aperture objective. Images were acquired using a Nikon Coolpix 995 camera and processed using Adobe Photoshop 7 (Adobe Systems, San Jose, CA). (D) Number of healthy cells in each treatment group. Mean and SE of cells in 10 visual fields was used in each group.
Involvement of both PAC1 and VPAC1 receptors in the renoprotective effect of PACAP38
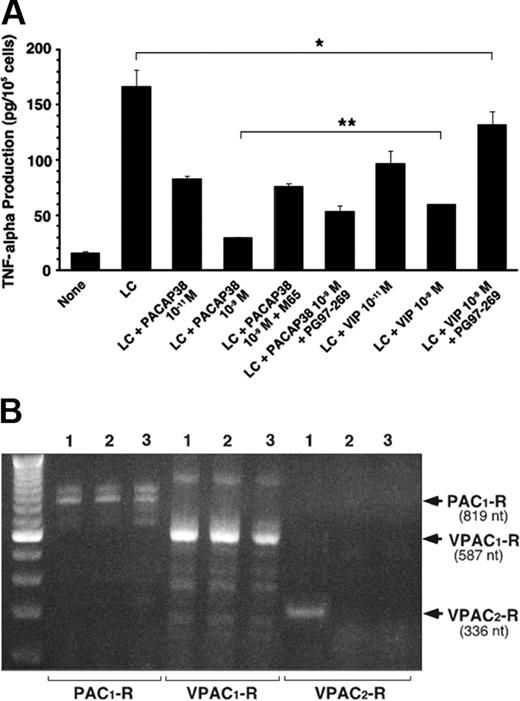
The peptide action is mediated by its interaction with its plasma membrane–associated receptors in the target cells. PACAP binds to 3 types of receptors, PAC1, VPAC1, and VPAC2 receptors, with similarly high affinity.10 Its paralog VIP also binds to VPAC1 and VPAC2 receptors with similarly high affinity, but not to PAC1 receptors. We found that the human renal tubule cell line expressed both PAC1 and VPAC1 receptors but not VPAC2 receptors (Figure 4B). Both PACAP38 and VIP induced dose-dependent suppression of TNFα production, but the suppressive effect of PACAP38 was greater than that induced by the same dose of VIP (Figure 4A). The suppressive effect of PACAP38 was attenuated by either M65, PAC1 receptor–specific antagonist, or PG97-269, VPAC1 receptor specific antagonist, indicating that both PAC1 and VPAC1 receptors are involved. As expected, the suppressive effect of VIP was considerably reduced by the VPAC1-receptor antagonist (Figure 4A).
Effect of PACAP38 and dexamethasone on LC-stimulated activation of MAPKs and NFκB. (A) The phosphorylation of ERK1/2 MAPK in the human renal proximal tubule cells cultured for 3 days with light chain (50 μM), PACAP38 (1 nM), or dexamethasone (1 nM) alone or light chain plus PACAP38 or dexamethasone. (B) The activation of p38 MAPK in the proximal tubule cells in a similar study. SB202190 (10 μM), a p38 MAPK inhibitor. (C-D) p50 and p65 subunits of activated NFκB, respectively. Raji cell extract, a positive control for NFκB. Each column represents mean of 3 replicates. Vertical bar is SE of mean. ***P < .001, **P < .01, and *P < .05.
Effect of PACAP38 and dexamethasone on LC-stimulated activation of MAPKs and NFκB. (A) The phosphorylation of ERK1/2 MAPK in the human renal proximal tubule cells cultured for 3 days with light chain (50 μM), PACAP38 (1 nM), or dexamethasone (1 nM) alone or light chain plus PACAP38 or dexamethasone. (B) The activation of p38 MAPK in the proximal tubule cells in a similar study. SB202190 (10 μM), a p38 MAPK inhibitor. (C-D) p50 and p65 subunits of activated NFκB, respectively. Raji cell extract, a positive control for NFκB. Each column represents mean of 3 replicates. Vertical bar is SE of mean. ***P < .001, **P < .01, and *P < .05.
PACAP receptors expressed in human renal tubule cell line, myeloma cells, and bone marrow stromal cells
The proximal tubule cells, multiple myeloma cells, and bone marrow stromal cells were examined for expression of the mRNA of 3 PACAP receptors by RT-PCR analysis using appropriate primers for each type of human PACAP receptors. Figure 4B shows PAC1 and VPAC1 receptors were constitutively expressed in cells of human bone marrow stroma, myeloma cells, and renal proximal tubule cell line. However, VPAC2 receptors were expressed only in the stromal cells. We found that addition of light chain did not induce the expression of VPAC2 receptor in human renal proximal tubule cell line (data not shown).
PACAP receptor involved in the suppressive effect of PACAP on LC-induced TNFα stimulation in tubule cells. (A) Effect of PACAP38, VIP, and their specific antagonists on light chain–induced TNFα secretion in cultured human renal proximal tubule cells. M65 and PG97-269 are specific antagonists for PAC1 and VPAC1 receptors, respectively. For each antagonist 10–7 M was added with 10–9 M PACAP38 or VIP, respectively. Each column represents the mean of 3 replicates. Vertical bar shows SE of mean. *P < .05, **P < .01, and ***P < .001. (B) RT-PCR analyses of PAC1,VPAC1, and VPAV2 receptors in human bone marrow stromal cells (lane 1), human multiple myeloma cells (lane 2), and human renal proximal tubule cell line (lane 3). PAC1- and VPAC1-Rs were constitutively expressed in human bone marrow stromal cells, multiple myeloma cells, and proximal tubule cell line. Otherwise, VPAC2-Rs were expressed only in the stromal cells. Stimulation of the tubule cell line by the LC did not induce VPAC2-Rs.
PACAP receptor involved in the suppressive effect of PACAP on LC-induced TNFα stimulation in tubule cells. (A) Effect of PACAP38, VIP, and their specific antagonists on light chain–induced TNFα secretion in cultured human renal proximal tubule cells. M65 and PG97-269 are specific antagonists for PAC1 and VPAC1 receptors, respectively. For each antagonist 10–7 M was added with 10–9 M PACAP38 or VIP, respectively. Each column represents the mean of 3 replicates. Vertical bar shows SE of mean. *P < .05, **P < .01, and ***P < .001. (B) RT-PCR analyses of PAC1,VPAC1, and VPAV2 receptors in human bone marrow stromal cells (lane 1), human multiple myeloma cells (lane 2), and human renal proximal tubule cell line (lane 3). PAC1- and VPAC1-Rs were constitutively expressed in human bone marrow stromal cells, multiple myeloma cells, and proximal tubule cell line. Otherwise, VPAC2-Rs were expressed only in the stromal cells. Stimulation of the tubule cell line by the LC did not induce VPAC2-Rs.
In vivo suppression of the light chain–induced TNFα production in the kidney
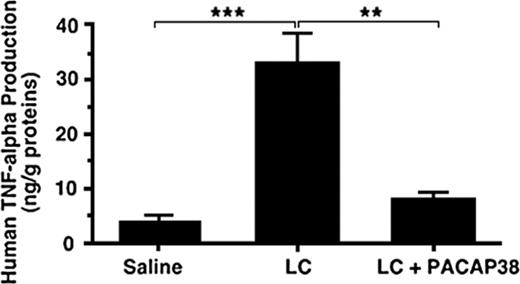
To examine whether administration of PACAP38 also suppresses the light chain–stimulated cytokine production in the kidney, the light chain protein dissolved in physiologic saline was administered intravenously into a freely moving rat through an implanted jugular cannula, allowing light chain concentration in the blood to approximate 50 μM to mimic the concentration used in the in vitro experiment. After administration of the light chain over 72 hours the rat was killed. TNFα level in the kidney increased more than 6-fold compared with the control level after the treatment with the light chain (Figure 5). When PACAP38 was intravenously administered with the light chain, the stimulation of TNFα production in the kidney was nearly completely suppressed (Figure 5).
Effect of PACAP38 on light chain–induced human TNFα production in rat kidney. κ-Light chain (50 μM) purified from the urine of a patient with multiple myeloma was administered intravenously into a freely moving rat through the chronically implanted jugular cannula. The control animals received the same amount of saline as that of light chain solution. On completion of administration of the light chain or saline over 72 hours the rat was killed to examine the expression of TNFα in the kidney. The level of TNFα in the kidney increased several times as compared with the control level after the treatment. When PACAP38 was intravenously administered with the light chains, the stimulation of TNFα production in the kidney was significantly suppressed. Each value represents the mean ± SE from 4 kidneys. ***P < .001 and **P < .01.
Effect of PACAP38 on light chain–induced human TNFα production in rat kidney. κ-Light chain (50 μM) purified from the urine of a patient with multiple myeloma was administered intravenously into a freely moving rat through the chronically implanted jugular cannula. The control animals received the same amount of saline as that of light chain solution. On completion of administration of the light chain or saline over 72 hours the rat was killed to examine the expression of TNFα in the kidney. The level of TNFα in the kidney increased several times as compared with the control level after the treatment. When PACAP38 was intravenously administered with the light chains, the stimulation of TNFα production in the kidney was significantly suppressed. Each value represents the mean ± SE from 4 kidneys. ***P < .001 and **P < .01.
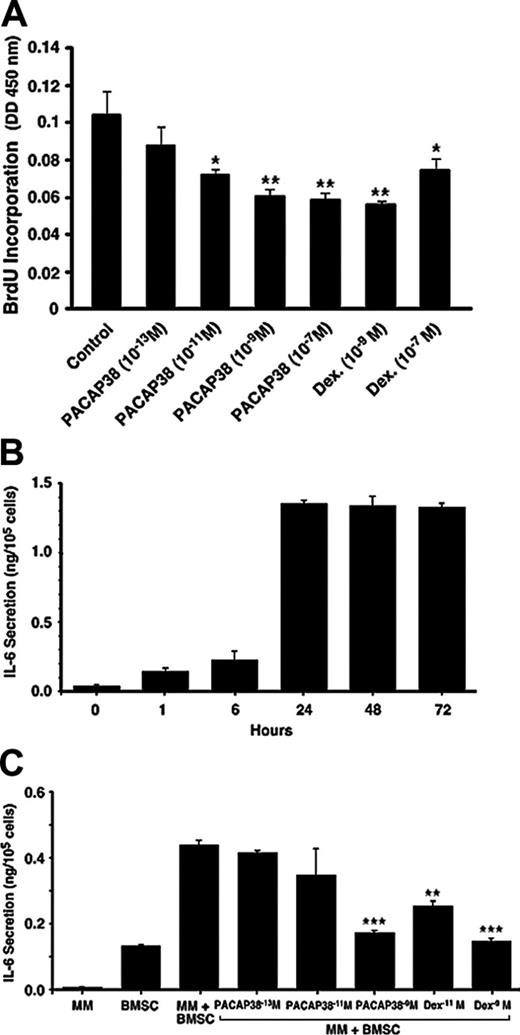
Suppressive effect of PACAP 38 on myeloma cell growth and IL-6 release by bone marrow stromal cells. (A) Effect of PACAP on cell proliferation in human myeloma cells. Human κ-light chain–producing multiple myeloma cell line (NCI-929) was cultured in RPMI 1640 medium supplemented with 10% FBS and 0.05 mM 2-mercaptoethanol. The cell growth was determined by incorporation of BrdU during DNA synthesis. Myeloma cells grew approximately twice during 24 hours. Coculture of the cells with PACAP38 suppressed cell growth dose dependently with the maximum suppression with 10–9 M. **P < .01 and *P < .05 compared with the control sample. (B) IL-6 secretion by human bone marrow stromal cells. The normal human bone marrow stromal cells produced low levels of IL-6. Addition of myeloma cells further increased expression of IL-6 by the BMSCs, reaching the maximum level during 24 hours of incubation. Myeloma cells alone do not secrete detectable levels of IL-6. (C) Addition of PACAP38 or dexamethasone suppressed myeloma cell–stimulated IL-6 secretion dose dependently, and maximum inhibition is seen at 10–9 M, which inhibited IL-6 production by 90% to 95%. ***P < .001 and **P < .01 compared with the multiple myeloma plus an untreated sample of bone marrow stromal cells.
Suppressive effect of PACAP 38 on myeloma cell growth and IL-6 release by bone marrow stromal cells. (A) Effect of PACAP on cell proliferation in human myeloma cells. Human κ-light chain–producing multiple myeloma cell line (NCI-929) was cultured in RPMI 1640 medium supplemented with 10% FBS and 0.05 mM 2-mercaptoethanol. The cell growth was determined by incorporation of BrdU during DNA synthesis. Myeloma cells grew approximately twice during 24 hours. Coculture of the cells with PACAP38 suppressed cell growth dose dependently with the maximum suppression with 10–9 M. **P < .01 and *P < .05 compared with the control sample. (B) IL-6 secretion by human bone marrow stromal cells. The normal human bone marrow stromal cells produced low levels of IL-6. Addition of myeloma cells further increased expression of IL-6 by the BMSCs, reaching the maximum level during 24 hours of incubation. Myeloma cells alone do not secrete detectable levels of IL-6. (C) Addition of PACAP38 or dexamethasone suppressed myeloma cell–stimulated IL-6 secretion dose dependently, and maximum inhibition is seen at 10–9 M, which inhibited IL-6 production by 90% to 95%. ***P < .001 and **P < .01 compared with the multiple myeloma plus an untreated sample of bone marrow stromal cells.
Suppression of myeloma cell growth by PACAP38
PACAP is also produced and secreted by certain tumors such as pheochromocytoma and prostate cancers, stimulating tumor growth by an autocrine and paracrine fashion.11 If PACAP38 stimulates myeloma cell growth, its beneficial effect of renoprotection would be compromised. To examine the effect of PACAP38 on myeloma cell growth, a human myeloma cell line was cultured in the media containing the non–inactivated bovine serum, which was required for myeloma cell growth. Cell growth was determined by incorporation of BrdU. Myeloma cells grew approximately 2-fold during 24 hours. Coculture of the cells with PACAP38 suppressed cell growth dose dependently with the maximum suppression with 10–9 M (Figure 6A). Dexamethasone induced similar amounts of suppression on the cell growth, but as the dose increased to 10–7 M cell growth was enhanced, whereas no such rebound reaction was observed with 10–7 M PACAP38.
Suppression of IL-6 production by bone marrow stromal cells
Myeloma cell growth in the bone marrow is stimulated by growth factors, especially IL-6, produced by bone marrow stromal cells.15 Adhesion of myeloma cells to the stromal cells stimulates expression and release of IL-6 from these cells. When myeloma cells were added to cultured human bone marrow stromal cells, IL-6 released into the culture media increased, reaching a maximum at 24 hours (Figure 6B). Both PACAP38 and dexamethasone suppressed the myeloma cell–stimulated production of IL-6 by the stromal cells dose dependently. PACAP38 and dexamethasone (10–9 M) nearly completely suppressed the IL-6 production to the basal level (Figure 6C). This finding suggests that PACAP38 not only suppresses myeloma cell growth by direct action but also inhibits by affecting the internal milieu in the bone marrow.
Discussion
This study shows that myeloma light chain–induced injury of a cultured human renal proximal tubule cell line was effectively prevented by PACAP38 in subnanomolar concentrations. The suppressive effect was mediated by inhibition of proinflammatory cytokine production stimulated by the light chain protein through suppression of p38 MAPK and p50 NFκB. The suppressive effect on light chain–stimulated production of cytokines in the kidney was also demonstrated in vivo, suggesting that PACAP38 would also protect the kidney from light chain–induced injury. Furthermore, the peptide suppressed myeloma cell growth directly and possibly indirectly via suppressing the release of its growth factor, IL-6, from bone marrow stromal cells. Considering the lack of an effective treatment of myeloma kidney, which eventually leads to the end stage of the renal failure, the present finding renders evaluation of PACAP38 as a promising therapeutic for myeloma kidney and myeloma.
Although dexamethasone is used to suppress myeloma and protect the kidney from the light chain–induced tubular injury, its long-term administration is limited because of its adverse side effects. However, PACAP38 is a natural and safe neuropeptide, which exhibited comparable potency to dexamethasone in the suppression of proinflammatory cytokine production by the tubule cells. No adverse effects have been reported when given to humans with doses producing significant biologic effects.16,17 In contrast to dexamethasone, PACAP may be safely given even in diabetic patients. PACAP might work in concert with other relevant neural and hormonal factors in feeding-induced insulin secretion.18 The potential of using PACAP in the treatment of diabetes also has been explored.19 Because circulating light chain may remain elevated for a long time in patients with myeloma kidney, a prolonged administration of PACAP may be necessary for protecting the kidney from injury. How long this therapy can be sustained without causing adverse effect remains to be studied.
Expression of PAC1 and VPAC1 receptors in the renal tubule cell line as well as myeloma cells suggests that PACAP's action is mediated by these receptors. Although VIP also suppressed the light chain–induced cytokine production from the tubule cells, the magnitude of the suppression was smaller than that of PACAP. Its effect may be mediated via VPAC1 receptor alone because VIP binds to PAC1 receptors with 1000 times lesser affinity than PACAP.10 Suppressive effects of PACAP were attenuated by either a PAC1 antagonist or VPAC1 antagonist, suggesting that both receptors are involved in the action of PACAP.
Although PACAP suppressed light chain–stimulated cytokine production in the kidney via its receptors, if the expression of these receptors on the renal tubule cells has any physiologic significance is unknown. The study of distribution of PACAP in the tissues showed that the kidney is the organ containing the least amount of PACAP as compared with other organs.20 In the peripheral tissues, PACAP immunoreactivity is mainly demonstrated in nerve fibers that occur around blood vessels, glands, and smooth muscle bundles.21 It also occurs within the sensory nerves.22 That the endogenous PACAP in the kidney plays a physiologic role as an endogenous renoprotective agent to protect the tubule cells from noxious stimuli apparently is remote. The current study showed that renoprotection generally required nanomolar levels of peptide. Where does this level of the physiologic ligand for the receptors in the tubule cells come from? PACAP is also expressed in lymphoid organs and released from these cells modulating the functions of other immune cells regulating expression of cytokines.8 The possibility that the physiologic ligand for the PACAP receptors in the renal tubule cells is conveyed from these immune cells cannot be excluded.
Myeloma kidney is characterized by tubular interstitial injury via toxic effect of an excess of light chains of immunoglobulin produced by cancerous plasma cells. Possibly prolonged overload of other proteins filtered through impaired glomeruli, which may happen in other proteinuric kidney diseases, could eventually damage tubule cells through the same mechanism.23 For 5 decades tubulointerstitial disease has been known to more closely correlate with progression to chronic renal failure than pure glomeruopathy.24 Also known is that tubulointerstitial injury exaggerates glomerulopathy by obstruction of the tubules, resulting in a vicious cycle.25,26 Until now, no effective treatment to suppress this vicious cycle is known. PACAP may be a strong candidate to evaluate for its beneficial effects on these tubulopathies.
Light chains stimulate MAPKs in the tubule cells.6 PACAP38, as well as dexamethasone, suppressed light chain–stimulated activation of p38 MAPK but not ERK1/2 MAPK. Both agents suppressed activation of NFκB which is recognized as the central transcription factor for driving inflammatory response to instigation. Use of a NFκB inhibitor as a possible agent for the treatment of myeloma kidney has been considered.27 However, NFκB is found in essentially all cell types and is involved in activation of an exceedingly large number of target genes. Because NFκB plays a pivotal role in the regulation of expression of numerous genes essential for normal function of the body, suppression of NFκB ubiquitously present in the body would eventually cause serious undesirable effects. Indeed, steroids are an inhibitor of NFκB, and many of its anti-inflammatory effects are mediated through NFκB inhibition, at the cost of its well-known side effects.
The present study shows that both PACAP38 and dexamethasone apparently elicit similar responses in the tubule cells. PACAP was as effective as dexamethasone in all assays examined. Glucocorticoid binds to a specific receptor (GR) to form the activated receptor, thereby influencing NFκB activity. GR-mediated transcriptional interference is achieved by several important mechanisms,28 including physical interaction with the p65 subunit of NFκB with formation of an inactive complex by blocking degradation of IκBα via enhanced synthesis of IL-10 and others. PACAP and its paralog VIP also stimulate expression of IL-10.29
Signaling cascades modulated by VIP/PACAP in immune cells have been studied.8,9 Both VIP and PACAP inhibit TNFα production from lipopolysaccharide-stimulated macrophages. Although activated cells expressed mRNA for all 3 PACAP receptors, agonist and antagonist studies indicate that the major receptor involved is VPAC1 receptor. VIP/PACAP inhibit TNFα gene expression by affecting both NFκB binding and the composition of the cAMP-responsive element binding complex. Two transduction pathways, a cAMP-dependent and a cAMP-independent pathway, are involved in the inhibition of TNFα gene expression and appear to differentially regulate the transcriptional factors involved in contrast to macrophages in the human renal tubule cells, PAC1 receptors appeared to be the major receptor involved for suppression of TNFα production stimulated by light chains, although VPAC1 receptors also participate to a significant extent. In immune cells, the cAMP/cAMP-dependent protein kinase pathway mediates the effects on CREB-binding protein, whereas a cAMP-independent pathway is the major transducer for the effects on p65 NFκB subunit nuclear translocation.9 In the renal tubule cells, the p50 NFκB subunit, but not p65 NFκB subunit, was activated by the light chain, and the activated p50 NFκB subunit component was suppressed by PACAP as well as dexamethasone. The p65 NFκB subunit was already activated in the cultured tubule cells at the basal state before addition of the light chain, and PACAP and dexamethasone suppressed both activated p50 and p65 subunits of NFκB. In the renal tubule cells, p38 MAPK signaling is involved for suppression of light chain–stimulated cytokine production by PACAP, and the suppression of p38 appears to be mediated by a cAMP-independent pathway. These findings suggest that the mechanisms of inhibitory effect of PACAP on expression of proinflammatory cytokines in immune cells and tubule cells of the kidney are not necessarily identical. These differences may render PACAP a more selective anti-inflammatory agent in the kidney.
Most important, PACAP38 suppressed myeloma cell growth via its direct action, probably through interaction with PAC1 and VPAC1 receptors expressed on these cells. Many signaling pathways relevant to tumor growth control exist. A main stimulatory mechanism involves activation of various MAPK pathways. In pancreatic carcinomas,30 pheochromocytoma,31 and neuroblastoma,32 PACAP stimulates cell proliferation or differentiation through MAPK-dependent pathways. How, then, does PACAP stimulate tumor growth in certain types and inhibit growth in others? That the mitogenic versus antimitogenic effects of PACAP in cultured embryonic cortical neuroblasts can be determined by expression of PACAP-receptor splice isoforms, and the differential coupling of the receptor variants to the phospholipase C (PLC) and PKA pathways has been shown.30 Cortical neuroblasts primarily express the null variant and respond to PACAP with a PKA-mediated decrease in proliferation. When cells were transfected with the hop receptor variant, PACAP activated the PLC pathway and stimulated proliferation. In SCLS cell lines, VIP dose dependently inhibited cell proliferation.33 Because VIP effects on cell proliferation were abolished by the PKA inhibitor KT5720, Maruno and Said33 concluded that a cAMP/PKA pathway mediated the antiproliferative actions.
PAC1-receptor variants derived from alternative splicing have been reported. Within the third intracellular loop, the insertion of 3 different cassettes called hip, hop1, and hop2 may generate 6 different variants called null (no insertion), hip, hop1, or hop2, and either hip-hop1 or hip-hop2 (2 cassettes).34 These variants display differential signal transduction properties. Lu et al35 suggest that the hop form is, in some contexts, a mitogenic variant. Transfection of this variant allowed host cells to proliferate in response to PACAP via a PLC-dependent mechanism, whereas expression of null variants maintained the inhibitory action of PACAP. Subsequently, additional splice variants were reported,36,37 and 10 splice variants have so far been found for PAC1 receptors.
We conclude that these data presented render PACAP38 worth evaluation as a promising candidate for an effective and safe renoprotectant in myeloma kidney, and possibly other nephropathy, and also as a new antitumor agent in multiple myeloma.
Prepublished online as Blood First Edition Paper, October 4, 2005; DOI 10.1182/blood-2005-03-1186.
Supported in part by the Kaken American Foundation and by a Veterans Administration (VA) Merit Review Grant (V.B.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr H. Dundee for his editorial help.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal