Abstract
Semaphorin 3A (Sema3A) is a secreted disulfide-bound homodimeric molecule that induces growth cone collapse and repulsion of axon growth in the nervous system. Recently, it has been demonstrated that Sema3A is produced by endothelial cells and inhibits integrin function in an autocrine fashion. In this study, we investigated the effects of Sema3A on platelet function by using 2 distinct human Sema3A chimera proteins. We detected expression of functional Sema3A receptors in platelets and dose-dependent and saturable binding of Sema3A to platelets. Sema3A dose-dependently inhibited activation of integrin αIIbβ3byall agonists examined including adenosine diphosphate (ADP), thrombin, convulxin, phorbol 12-myristate 13-acetate, and A23187. Sema3A inhibited not only platelet aggregation induced by thrombin or collagen but also platelet adhesion and spreading on immobilized fibrinogen. Moreover, Sema3A impaired αIIbβ3-independent spreading on glass coverslips and aggregation-independent granular secretion. Sema3A inhibited agonist-induced elevation of filamentous action (F-actin) contents, phosphorylation of cofilin, and Rac1 activation. In contrast, Sema3A did not affect the levels of cyclic nucleotides or agonist-induced increase of intracellular Ca2+ concentrations. Thus, the extensive inhibition of platelet function by Sema3A appears to be mediated, at least in part, through impairment of agonist-induced Rac1-dependent actin rearrangement.
Introduction
Platelets play a crucial role not only in a hemostatic plug formation but also in a pathologic thrombus formation, particularly within atherosclerotic arteries subjected to high shear stress.1,2 As an initial step in thrombogenesis, platelets adhere to altered vascular surfaces or exposed subendothelial extracellular matrices, then become activated and aggregate each other. These processes are primarily mediated by platelet surface glycoproteins such as GPIb-IX-V, integrin α2β1, GPVI, and integrin αIIbβ3.3,4 Especially, integrin αIIbβ3 plays an essential part in aggregate formation and adhesive spreading of platelets during hemostasis.5-7 Pathways that inhibit platelet function are as important as those that activate them. Endothelial cells produce 2 well-documented inhibitors of platelet activation and aggregation, prostaglandin I2 (PGI2) and nitric oxide (NO).8 PGI2 binds to a specific Gs-coupled receptor, thereby activating adenylate cyclase and cyclic adenosine monophosphate (cAMP)–dependent protein kinase or protein kinase A (PKA). NO activates soluble guanylate cyclase and cyclic guanosine monophosphate (cGMP)–dependent kinase or PKG. Ecto–adenosine diphosphatase (ADPase, CD39) located on the luminal surface of endothelial cells also inhibits platelet aggregation by decreasing the local concentration of ADP. Thus, endothelial dysfunction or damage promotes a prothrombotic state and may be involved in the pathogenesis of cardiovascular disorders, including atherosclerosis, diabetes mellitus, essential hypertension, hypercholesterolemia, and hyperhomocysteinemia.8
The semaphorin family comprises soluble and membrane-bound proteins that are defined by the presence of a conserved 500–amino acid semaphorin domain at their amino termini.9 Class 3 semaphorins are secreted disulfide-bound homodimeric molecules, and Sema3A, a prototypic class 3 semaphorin, causes growth cone collapse and provides chemorepulsive guidance for migrating axons.10-12 Cell surface receptor for Sema3A consists of a complex of 2 distinct transmembrane receptors, neuropilin-1 and plexin A (A1-A3).10-13 Neuropilin-1 provides a binding site of Sema3A, while plexin A transduces the Sema3A signals into the cells through its cytoplasmic domain.10-13 Although the intracellular signaling pathways evoked by Sema3A binding are not fully understood, plexins should interact with signaling molecules to regulate actin reorganization, since growth cone collapse is accompanied by rapid reorganization of the actin filaments normally present in lamellipodia or filopodia.11,12 In this context, a Rho family small G-protein, Rac, has been identified as a potential regulator of semaphorin-dependent actin cytoskeletal dynamics.11,12
Although Sema3A function on neural development is studied intensively, its function in other organs is poorly understood. The fact that semaphorins are expressed in many different tissues suggests that they also play a role in systems other than the nervous system.12 Indeed, in addition to neural abnormalities, mice lacking a functional Sema3A gene have abnormalities in their heart and visceral tissues, suggesting that Sema3A signaling might be indispensable for normal development in several organs.14,15 Very recently, Serini et al reported that semaphorins are also involved in angiogenesis.16 They showed that endothelial cells generate chemorepulsive autocrine signals of class 3 semaphorins that localize at nascent adhesive sites in spreading endothelial cells.16 Interestingly, Sema3A inhibits the integrin-mediated adhesion to extracellular matrix and impedes their directional motility, which could explain the aberrant vascularization that is observed in Sema3A-deficient mice.16 Others also showed that plexin signaling negatively regulates integrin-based adhesive complexes, which leads to the inhibition of cell adhesion, lamellipodia formation, and cell migration.17
Since integrin αIIbβ3 is essential for platelet function and endothelial cells express Sema3A, we sought to investigate the effects of Sema3A on platelet function. In this study, we demonstrate that Sema3A binds to platelets and inhibits αIIbβ3 activation extensively. Sema3A also inhibits platelet aggregate formation and platelet adhesion and spreading on immobilized fibrinogen. Moreover, Sema3A inhibits αIIbβ3-independent spreading on glass coverslips and aggregation-independent granular secretion. Further investigation of signaling pathways demonstrates that Sema3A markedly impairs agonist-induced Rac1-dependent actin rearrangement.
Materials and methods
Reagents
Recombinant human Sema3A fused to human Fc fragment (Sema3A/Fc) was obtained from R&D Systems (Minneapolis, MN). A construct consisting of the human Sema3A cDNA fused to the catalytic domain of human placental alkaline phosphatase (AP) cDNA was prepared as previously described using the pAP-tag2 expression vector (GenHunter, Nashville, TN).10 The plasmid was transfected to 293T cells by Lipofectamine2000 (Invitrogen, Carlsbad, CA), and recombinant Sema3A/AP was purified from cultured medium using anti–human AP monoclonal antibody–conjugated sepharose beads (clone 8B6) and dialyzed against phosphate-buffered saline (PBS). Human IgG (hIgG) and human placental AP were used for controls of Sema3A/Fc and Sema3A/AP, respectively. Purity of Sema3A/Fc and Sema3A/AP was confirmed by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining (SilverSNAP Stain Kit; Pierce, Rockford, IL). Convulxin was kindly provided by Dr M. Moroi (Department of Protein Biochemistry, Institute of Life Science, Kurume University, Fukuoka, Japan). Fibrinogen was purchased from Calbiochem (San Diego, CA) and was labeled with fluorescein isothiocyanate (FITC), as previously described.18 Type I collagen was obtained from MC Medical (Tokyo, Japan). A hybridoma producing IV.3, a mouse monoclonal antibody specific for human Fcγ-RIIA (CD32), was obtained from American Type Culture Collection (Rockville, MD) and IV.3 Fab fragments were generated as described previously.19 All other reagents were purchased from Sigma (St Louis, MO), unless otherwise indicated.
Platelet preparation
Washed platelets were prepared as described previously.20 In brief, 6 vol freshly drawn venous blood from healthy volunteers was mixed with 1 vol acid-citrate-dextrose and centrifuged at 250g for 10 minutes to obtain platelet-rich plasma (PRP). After a 5-minute incubation with 1 μM prostaglandin E1 (PGE1) and 1 U/mL apyrase, the PRP was centrifuged at 750g for 10 minutes, washed once with citrate buffer containing 1 μM PGE1 and 1 U/mL apyrase, and resuspended in an appropriate buffer. Washed platelets were rested for 30 minutes at 37°C before use in any experiments. In all experiments using Sema3A/Fc, platelet FcγRIIA receptor was blocked by preincubation with 20 μg/mL IV.3 Fab.
Detection of binding of Sema3A to platelets and Sema3A receptors in platelets
For detection of binding of Sema3A/Fc to platelets, 5 × 105 washed platelets in Walsh buffer (137 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, 3.3 mM NaH2PO4, 3.8 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.1% glucose, 0.1% bovine serum albumin [BSA], pH 7.4) were incubated with various concentrations of Sema3A/Fc for 30 minutes at room temperature and washed once with citrate buffer. Then, platelets were resuspended in PBS with FITC-labeled anti–human Fc for 20 minutes, followed by flow cytometric analysis. For detection of the binding of Sema3A/AP, 5 × 106 platelets were incubated with various concentrations of Sema3A/AP for 30 minutes at room temperature. After washing with citrate buffer, AP activity was measured using disodium phenylphosphate as a substrate (Sanko Jun-yaku, Tokyo, Japan). The number of Sema3A binding sites was estimated by the maximum AP activity of Sema3A/AP obtained from standard AP activity. In some experiments, platelets were first incubated with 125 μg/mL Sema3A/Fc or hIgG for 10 minutes. After washing, platelets were incubated with 10 μg/mL Sema3A/AP for another 30 minutes, and AP activity was measured.
Western blotting and flow cytometry of neuropilin-1 were performed with mouse anti–neuropilin-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) as described previously.22,23 Horseradish peroxidase (HRP)–conjugated anti–mouse IgG (New England Biolabs, Beverly, MA) and Alexa488-conjugated anti–mouse IgG (Molecular Probes, Eugene, OR) were used as secondary antibodies for Western blotting and flow cytometry, respectively. Reverse transcriptase–polymerase chain reaction (RT-PCR) for detection of plexin-A1, -A2, and -A3 was performed as described.16 In brief, RNA was extracted by a Trizol reagent (Invitrogen), and cDNA was synthesized using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen). RT products were amplified in a PCR reaction with a Taq polymerase (Takara ExTaq; Takara Bio, Shiga, Japan). Primer sequences and PCR conditions were described previously.16
Activation of αIIbβ3 by various agonists
Activation state of αIIbβ3 was monitored by binding of a ligand-mimetic antibody, PAC-1, or soluble fibrinogen under flow cytometric analysis as described previously.21,22,24 In brief, 5 × 105 platelets in Walsh buffer were preincubated with Sema3A/Fc or Sema3A/AP for 10 minutes, followed by incubation with agonists and FITC-conjugated PAC-1 (BD Biosciences, Franklin Lakes, NJ) or FITC-fibrinogen for 20 minutes at room temperature. Then, platelets were diluted to 500 μL with Walsh buffer and analyzed immediately on flow cytometry (FACScan; BD Japan, Tokyo, Japan).
Platelet aggregation study
Platelet aggregation was monitored using a platelet aggregometer (model 313M; MC Medical) at 37°C with a stirring rate at 1000 rpm, as previously described.20 In brief, Sema3A/AP- or AP-treated platelets were suspended in modified Tyrode buffer containing 1 mM MgCl2 at the concentration of 2 × 105/μL. After addition of CaCl2 at the final concentration of 1 mM and incubation for one minute at 37°C, aggregation was initiated by addition of agonists.
Platelet granular secretion
Granular secretion was monitored by FITC-CD62P (Immunotech, Marseille, France) and phycoerythrin-conjugated CD63 (Immunotech) binding to platelets under flow cytometry as described previously.25
Adhesion to immobilized fibrinogen or glass coverslips
Adhesion of platelets to immobilized fibrinogen was assessed as described previously.26 In brief, a 96-well polystyrene plate (Greiner Japan, Tokyo, Japan) was coated with fibrinogen at the various concentrations in PBS for 16 hours at 4°C. Platelets (1.25 × 106) in Tyrode buffer (137 mM NaCl, 12 mM NaHCO3, 2.6 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, 0.1% glucose, 0.1% BSA, pH 7.4) were incubated with 20 μg/mL Sema3A/Fc or hIgG for 10 minutes at room temperature, and then they were placed on each well followed by incubation for one hour at room temperature. After washing 3 times with PBS to remove nonadherent platelets, adhered platelets were quantified by measuring endogenous cellular acid phosphate activity.27 Relative adhesion to the maximum binding was calculated by dividing the acid phosphatase activity of adherent platelets by that of nontreated platelets adhered on the 10 μg/mL fibrinogen.
Morphologic study of adhered platelets was performed as described previously.28 In brief, glass coverslips were coated with 20 μg/mL fibrinogen for 16 hours at 4°C, and then washed with PBS. After incubation with Sema3A/Fc or hIgG, 2 × 106 platelets in Tyrode buffer were incubated on the fibrinogen-coated coverslips for 45 minutes at 37°C or on the nontreated coverslips for 10 minutes at room temperature. Nonadherent platelets were washed away and adherent cells were stained with tetramethylrhodamine B isothiocyanate–conjugated phalloidin. Platelet spreading was observed under a florescence microscope (PROVIS AX-80; Olympus, Tokyo, Japan).
Quantification of F-actin contents
Filamentous actin (F-actin) content was analyzed by flow cytometry with bodipy-phallacidin as described previously.28 In brief, after incubation with 20 μg/mL Sema3A/Fc or hIgG, platelets in Walsh buffer were stimulated with a 30-second incubation with 30 μM protease-activated receptor 1 (PAR1)–thrombin receptor-activating peptide (TRAP) or 0.5 U/mL thrombin at 37°C. Then, platelets were fixed with 4 vol of 2.6% glutaraldehyde in 5.3 mM EDTA (ethylenediaminetetraacetic acid) for 2 hours at 37°C. After washing twice with PBS, the platelets were resuspended to half their initial volume and incubated at 37°C either with 3.3 μM bodipy-phallacidin (Molecular Probes) or bodipy-phallacidin in the presence of a 300-fold molar excess of unlabeled phallacidin. After 30 minutes, the platelets were washed twice with PBS and platelet fluorescence was analyzed in the fluorescence intensity 1 (FL1) channel of the flow cytometer. Specific phallacidin binding was obtained by subtraction of mean fluorescence intensity of FL1 with unlabeled phallacidin from that of FL1 without unlabeled phallacidin.
Detection of phosphorylation of cofilin and activated Rac1
After incubation with 20 μg/mL Sema3A/Fc or hIgG for 10 minutes at room temperature, 1 × 107 platelets in Walsh buffer were incubated with 0.5 U/mL thrombin for the indicated times at 37°C without stirring. Then, cells were lysed with SDS sample buffer with 5% β-mercaptoethanol (β-ME). Proteins were resolved on a 15% SDS-PAGE gel and transferred to a polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Millipore, Bedford, MA). Phosphorylated cofilin was detected by using anti–phospho-cofilin antibody (Cell Signaling Technology, Beverly, MA). After stripping the membrane with a stripping buffer (Restore Western Blot Stripping Buffer; Pierce), the membrane was rehybridzed with anticofilin antibody (BD Biosciences). Optical density of the bands was measured by National Institutes of Health (NIH) Image software (Bethesda, MD). After calibrating the density of phosphorylated cofilin with that of total cofilin, relative increase of phosphorylated cofilin against that of IgG-treated platelets without agonist stimulation was calculated.
Detection of activated Rac1 was performed using a kit of pull-down assay according to the manufacturer's directions (EZ-Detect Rac1 Activation Kit; Pierce). In brief, Sema3A/Fc- or hIgG-treated platelets in Walsh buffer were incubated with 30 μM PAR1-TRAP for the indicated times at 37°C without stirring. Then, cells were lysed with 0.5% Triton-X100 lysis buffer. Guanosine triphosphate (GTP)–form of Rac1 was pull-downed by incubation with glutathione-S–transferase (GST)–p21-activated kinase 1 (PAK1)–p21-binding domain (PBD) and glutathione beads for one hour at 4°C. After washing with lysis buffer, precipitates were eluted with SDS sample buffer with β-ME, followed by electrophoresis on a 12% SDS-PAGE gel. After transfer to a PVDF membrane, Rac1 was detected by a mouse anti-Rac1–specific antibody. Total Rac1 was detected by electrophoresis of total lysates on an SDS-PAGE gel followed by detection with the Rac1-specific antibody.
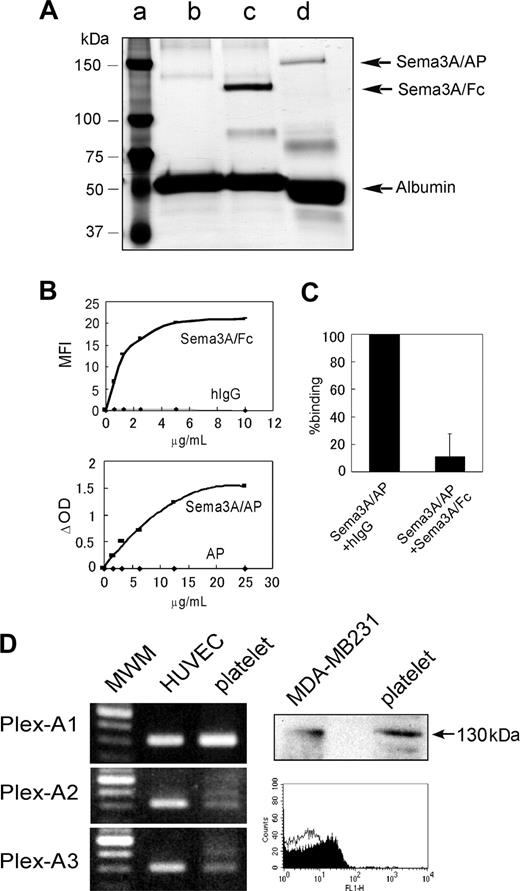
Detection of Sema3A binding to platelets and expression of Sema3A receptors in platelets. (A) Silver stain of purified Sema3A fusion proteins; 0.25 μgof Sema3A/Fc (∼ 125 kDa, lane c) and Sema3A/AP (∼ 150 kDa, lane d) were loaded on a 7.5% SDS-PAGE gel under reducing conditions and silver staining was performed. Sema3A/Fc and Sema3A/AP samples contain BSA as a carrier protein. In lane c, only BSA was loaded. Molecular weight marker was loaded in lane a. (B) Binding of Sema3A/Fc or Sema3A/AP to platelets. Washed platelets (5 × 105) were incubated with Sema3A/Fc or hIgG, followed by incubation with FITC anti–human Fc. Sema3A/Fc binding was detected by flow cytometry, and mean fluorescence intensity (MFI) was plotted in the top panel. Washed platelets (5 × 106) were incubated with Sema3A/AP or AP, and after washing, AP activity was measured using disodium phenylphosphate as a substrate. Change in optical density (ΔOD) was plotted on the bottom panel. Shown are representative results of 3 independent experiments. (C) Inhibition of Sema3A/AP binding by Sema3A/Fc. Washed platelets were first incubated with 125 μg/mL hIgG or Sema3A/Fc. Then, platelets were incubated with 10 μg/mL Sema3A/AP, and AP activity was measured. Shown is mean and SE of relative binding to hIgG-incubated sample of 3 independent experiments. (D) Expression of plexin-A1, -A2, or -A3 in platelets was detected by RT-PCR assay (left). Human umbilical vein endothelial cell (HUVEC) was used as a positive control. Expression of neuropilin-1 in platelets was detected by Western blotting and flow cytometric analysis (right). In Western blotting, neuropilin-1 expression was detected by anti–neuropilin-1 antibody, followed by incubation with HRP anti–mouse IgG. MDA-MB231 was used as a positive control. In flow cytometry, platelets were incubated with mouse monoclonal anti–neuropilin-1 antibody (filled curve) or control antibody (MOPC21; open curve), followed by incubation with Alexa488-conjugated anti–mouse IgG. MWM indicates molecular weight marker.
Detection of Sema3A binding to platelets and expression of Sema3A receptors in platelets. (A) Silver stain of purified Sema3A fusion proteins; 0.25 μgof Sema3A/Fc (∼ 125 kDa, lane c) and Sema3A/AP (∼ 150 kDa, lane d) were loaded on a 7.5% SDS-PAGE gel under reducing conditions and silver staining was performed. Sema3A/Fc and Sema3A/AP samples contain BSA as a carrier protein. In lane c, only BSA was loaded. Molecular weight marker was loaded in lane a. (B) Binding of Sema3A/Fc or Sema3A/AP to platelets. Washed platelets (5 × 105) were incubated with Sema3A/Fc or hIgG, followed by incubation with FITC anti–human Fc. Sema3A/Fc binding was detected by flow cytometry, and mean fluorescence intensity (MFI) was plotted in the top panel. Washed platelets (5 × 106) were incubated with Sema3A/AP or AP, and after washing, AP activity was measured using disodium phenylphosphate as a substrate. Change in optical density (ΔOD) was plotted on the bottom panel. Shown are representative results of 3 independent experiments. (C) Inhibition of Sema3A/AP binding by Sema3A/Fc. Washed platelets were first incubated with 125 μg/mL hIgG or Sema3A/Fc. Then, platelets were incubated with 10 μg/mL Sema3A/AP, and AP activity was measured. Shown is mean and SE of relative binding to hIgG-incubated sample of 3 independent experiments. (D) Expression of plexin-A1, -A2, or -A3 in platelets was detected by RT-PCR assay (left). Human umbilical vein endothelial cell (HUVEC) was used as a positive control. Expression of neuropilin-1 in platelets was detected by Western blotting and flow cytometric analysis (right). In Western blotting, neuropilin-1 expression was detected by anti–neuropilin-1 antibody, followed by incubation with HRP anti–mouse IgG. MDA-MB231 was used as a positive control. In flow cytometry, platelets were incubated with mouse monoclonal anti–neuropilin-1 antibody (filled curve) or control antibody (MOPC21; open curve), followed by incubation with Alexa488-conjugated anti–mouse IgG. MWM indicates molecular weight marker.
Intracellular Ca2+ mobilization
Intracellular Ca2+ concentrations in fluo-3–loaded platelets were assessed under flow cytometry as described previously.29 In brief, platelets were labeled with 5 μM fluo-3-AM (Wako Pure Chemical, Osaka, Japan) at 37°C for 15 minutes. After incubation with 20 μg/mL Sema3A/Fc or hIgG, 5 × 105 platelets in 200 μL Walsh buffer were subjected to flow cytometry analysis. After the determination for about 10 seconds of baseline fluo-3 fluorescence from the platelet population, cell aspiration into the flow cytometry was briefly paused, and 1:10 volume of 5 U/mL thrombin was added. The acquisition was then resumed, and changes in log fluorescence versus time were recorded. For each plot, rectangular analysis regions were defined over the time axis, and mean florescence intensity was calculated with CellQuest software (BD Japan).
Quantification of platelet cyclic nucleotide levels
For cAMP quantification, 1.6 × 106 platelets in Walsh buffer were incubated with 20 μg/mL Sema3A/Fc or hIgG for 10 minutes at room temperature. Iloprost (20 μg/L; Cayman Chemical, Ann Arbor, MI) was used as an agonist for activation of adenylate cyclase. ADP (5 μM) was added to the platelet samples and incubated for 2 minutes at room temperature to study inhibition of adenylate cyclase. After lysing platelets, cAMP contents were measured by an enzyme immunoassay kit according to the manufacturer's directions (Biotrak cAMP EIA System; Amersham, Piscataway, NJ). For cGMP quantification, 3.6 × 106 platelets in Walsh buffer were incubated with 20 μg/mL Sema3A/Fc or hIgG for 10 minutes at room temperature, and cGMP contents were measured by an EIA kit (Biotrak cGMP EIA System; Amersham).
Statistical analysis
Experimental differences over the controls were analyzed by the Student t test. Probability values of P less than .05 were considered significant.
Results
Binding of Sema3A to platelets and expression of Sema3A receptors in platelets
We used 2 distinct Sema3A chimera proteins in this study: recombinant human Sema3A fused to human Fc fragment (Sema3A/Fc) or to the catalytic domain of human placental alkaline phosphatase (Sema3A/AP) (Figure 1A). We first investigated the binding of Sema3A to platelets. As shown in Figure 1B (upper), Sema3A/Fc bound to platelets in a dose-dependent and saturable manner. Sema3A/AP also bound to the platelets in basically the same manner as Sema3A/Fc, although it needed about 2-fold concentrations, compared with Sema3A/Fc, to saturate the binding to platelets (Figure 1B lower). About 90% of the Sema3A/AP binding was inhibited by preincubation with excess amounts of Sema3A/Fc, confirming the specificity of Sema3A binding to platelets (Figure 1C). We estimated the binding sites of Sema3A were approximately 8000 (7980 ± 500, n = 4) per platelet.
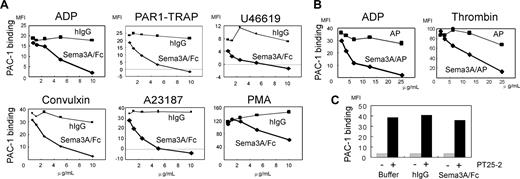
Inhibition of αIIbβ3 activation by Sema3A. (A) Washed platelets preincubated with the indicated concentrations of Sema3A/Fc (♦ and bold lines) or hIgG (▪ and thin lines) were activated with ADP (5 μM), PAR1-TRAP (15 μM), U46619 (2 μM), convulxin (5 ng/mL), A23187 (2.5 μM), or PMA (200 nM). Activated αIIbβ3 was detected by binding of FITC–PAC-1. Shown are representative results of 3 to 5 independent experiments. (B) Washed platelets were preincubated with Sema3A/AP (♦ and bold lines) or AP (▪ and thin lines) and activated by ADP (5 μM) or thrombin (0.5 U/mL), and then FITC–PAC-1 binding was detected. Shown are representative results of 3 independent experiments. (C) PBS-, hIgG-, or Sema3A/Fc-treated platelets were incubated with or without an αIIbβ3-activating antibody, PT25-2, and PAC1 binding was examined. Shown are representative results of 3 independent experiments.
Inhibition of αIIbβ3 activation by Sema3A. (A) Washed platelets preincubated with the indicated concentrations of Sema3A/Fc (♦ and bold lines) or hIgG (▪ and thin lines) were activated with ADP (5 μM), PAR1-TRAP (15 μM), U46619 (2 μM), convulxin (5 ng/mL), A23187 (2.5 μM), or PMA (200 nM). Activated αIIbβ3 was detected by binding of FITC–PAC-1. Shown are representative results of 3 to 5 independent experiments. (B) Washed platelets were preincubated with Sema3A/AP (♦ and bold lines) or AP (▪ and thin lines) and activated by ADP (5 μM) or thrombin (0.5 U/mL), and then FITC–PAC-1 binding was detected. Shown are representative results of 3 independent experiments. (C) PBS-, hIgG-, or Sema3A/Fc-treated platelets were incubated with or without an αIIbβ3-activating antibody, PT25-2, and PAC1 binding was examined. Shown are representative results of 3 independent experiments.
Next, we examined expression of Sema3A receptors in platelets. Western blotting and flow cytometric analysis revealed that neuropilin-1 was expressed in platelets (Figure 1C). Plexin expression was examined by RT-PCR assay, using platelet samples in which the contaminated leukocytes were removed by a leukocyte removal filter. As shown in Figure 1C, plexin-A1 and low levels of plexin-A2 and plexin-A3 were expressed in platelets. These results suggest that platelets express functional Sema3A receptors.
Effects of Sema3A on αIIbβ3 activation by various agonists and platelet aggregation
Since Sema3A inhibits integrin function in endothelial cells,16 we examined the effects of Sema3A on integrin αIIbβ3 activation using a ligand-mimetic antibody, PAC-1. Sema3A/Fc dose-dependently inhibited PAC-1 binding induced by all agonists examined, including agonists that act via G-protein–coupled receptors (ie, ADP, thrombin, and U46619) and convulxin, which acts via G-protein–uncoupled receptor, GPVI (Figure 2A; Table 1). Sema3A/Fc inhibited A23187- and phorbol 12-myristate 13-acetate (PMA)–induced PAC-1 binding, suggesting that Sema3A inhibits αIIbβ3 activation mainly downstream of intracellular calcium mobilization and protein kinase C activation. Sema3A/AP also inhibited αIIbβ3 activation by thrombin and ADP (Figure 2B), indicating that the inhibitory effects were caused by the Sema3A domain, not by the fused Fc or AP domain. Sema3A/Fc inhibited a physiologic ligand, soluble fibrinogen binding to platelets after ADP and PAR1-TRAP stimulation, as well as PAC-1 binding (data not shown). PAC-1 binding with PT25-2, an anti-αIIbβ3 antibody that induces activated conformation of αIIbβ3 without intracellular signaling, was unaffected by preincubation with Sema3A (Figure 2C), indicating that Sema3A does not disturb PAC-1 binding competitively to its receptor. Since activation of αIIbβ3 leads to ligand binding and platelet aggregate formation, we studied the effects of Sema3A on platelet aggregation. Sema3A/AP impaired aggregate formation in low concentrations of collagen and thrombin (Figure 3), although it was hard to detect the inhibitory effects of Sema3A on platelet aggregation in high concentrations of the agonists.
Inhibition of agonist-induced PAC1 binding by Sema3A
Agonist . | Concentration (% inhibition) . |
|---|---|
| ADP, μM | 5 (90.4 ± 12.1) |
| PAR1-TRAP, μM | 15 (115.2 ± 10.2) |
| Thrombin, U/mL | 0.5 (97.4 ± 3.3) |
| U46619, μM | 2 (112.5 ± 9.3) |
| Convulxin, ng/mL | 5 (94.7 ± 6.0) |
| A23187, μM | 2.5 (106 ± 5.5) |
| PMA, nM | 200 (58.1 ± 14.3) |
Agonist . | Concentration (% inhibition) . |
|---|---|
| ADP, μM | 5 (90.4 ± 12.1) |
| PAR1-TRAP, μM | 15 (115.2 ± 10.2) |
| Thrombin, U/mL | 0.5 (97.4 ± 3.3) |
| U46619, μM | 2 (112.5 ± 9.3) |
| Convulxin, ng/mL | 5 (94.7 ± 6.0) |
| A23187, μM | 2.5 (106 ± 5.5) |
| PMA, nM | 200 (58.1 ± 14.3) |
Platelets preincubated with 10 μg/mL Sema3A/Fc were activated with indicated agonists, and FITC-PAC1 binding was detected as demonstrated in Figure 2. Percent inhibition of mean fluorescent intensity against hIgG-treated platelets was calculated. Data represent mean ± SE of at least 3 independent experiments.
Effects of Sema3A on granular secretion
We examined effects of Sema3A binding to platelets on granular secretion after ADP and thrombin stimulation. Surface expression of CD62P and CD63 was used for monitoring the secretion of alpha granule and dense or lysosome granule, respectively.30 Sema3A/Fc dose-dependently inhibited surface expression of both CD62P and CD63 after ADP and thrombin stimulation without stirring, indicating that Sema3A inhibits aggregation-independent granule secretion induced by platelet agonists (Figure 4).
Inhibition of platelet aggregation by Sema3A. Washed platelets preincubated with 20 μg/mL Sema3A/AP or AP were activated with the indicated concentrations of collagen (left column) or thrombin (right column). Platelet aggregation was monitored using a platelet aggregometer. Arrow indicates the addition of agonists. Shown are representative results of 3 independent experiments.
Inhibition of platelet aggregation by Sema3A. Washed platelets preincubated with 20 μg/mL Sema3A/AP or AP were activated with the indicated concentrations of collagen (left column) or thrombin (right column). Platelet aggregation was monitored using a platelet aggregometer. Arrow indicates the addition of agonists. Shown are representative results of 3 independent experiments.
Inhibition of agonist-induced granular secretion by Sema3A. Washed platelets were preincubated with the indicated concentrations Sema3A/Fc, and then activated with ADP (5 μM) or thrombin (0.5 U/mL). Granular secretion was assessed by FITC-CD62P and PE-CD63 binding to platelets, and percent binding against hIgG-treated platelets was calculated. Shown are mean ± SE of percent binding of 3 independent experiments. *P < .05; **P < .01.
Inhibition of agonist-induced granular secretion by Sema3A. Washed platelets were preincubated with the indicated concentrations Sema3A/Fc, and then activated with ADP (5 μM) or thrombin (0.5 U/mL). Granular secretion was assessed by FITC-CD62P and PE-CD63 binding to platelets, and percent binding against hIgG-treated platelets was calculated. Shown are mean ± SE of percent binding of 3 independent experiments. *P < .05; **P < .01.
Effects of Sema3A on platelet adhesion and spreading
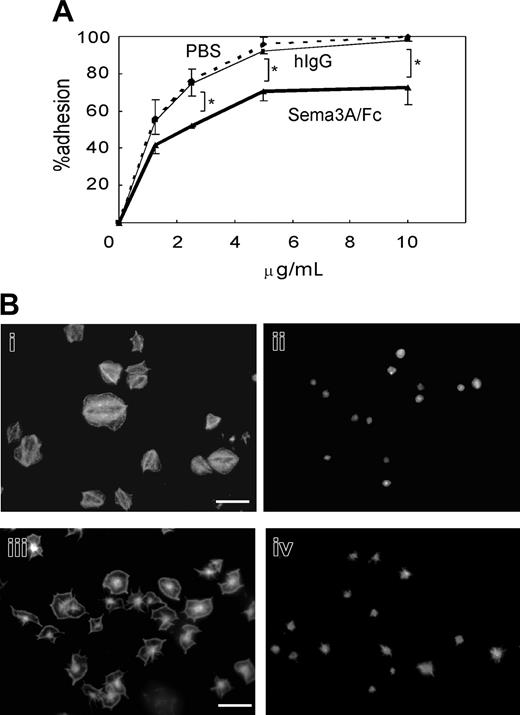
We next examined the effects of Sema3A on platelet adhesion to immobilized fibrinogen or nonspecific glass coverslips under static conditions. Quantification of adhered platelets by acid phosphatase method showed that preincubation with 20 μg/mL Sema3A/Fc led to about 20% reduction in platelet adhesion at every concentration of fibrinogen examined (Figure 5A). Microscopic examination demonstrated that after 45 minutes of incubation on 20 μg/mL fibrinogen, more than 80% of platelets showed full spreading (Figure 5Bi). In sharp contrast, spreading of Sema3A-treated platelets was markedly impaired (Figure 5Bii). The inhibition of platelet spreading by Sema3A was not αIIbβ3 specific, since Sema3A also inhibited platelet spreading on noncoated glass coverslips (Figure 5Biii-iv).
Effects of Sema3A on agonist-induced cytoskeleton rearrangement of platelets
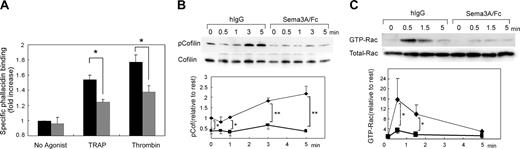
The remarkable inhibition of platelet spreading by Sema3A suggests that Sema3A affects cytoskeletal rearrangement of platelets. To address the hypothesis, we quantified F-actin contents in platelets using bodipy-phallacidin and flow cytometry. Thrombin and PAR1-TRAP induced elevation of F-actin as demonstrated,28 and Sema3A significantly impaired the elevation of agonist-induced F-actin elevation (Figure 6A). Cofilin is a protein that promotes severing and depolymerization of F-actin,31,32 and involvement of cofilin in Sema3A signaling has been demonstrated.31 Therefore, we next examined phosphorylation of cofilin after PAR1-TRAP stimulation. Sema3A decreased the level of phosphorylated cofilin in both resting and PAR1-TRAP–stimulated platelets, suggesting that Sema3A may keep cofilin in the dephosphorylated, activated state and increase severing of F-actin (Figure 6B). Since phosphorylation of cofilin is regulated by LIM kinase,31,32 an effecter of Rac-PAK signaling pathway,33 and the involvement of Rac in semaphorin signaling is well demonstrated,11,12 we examined the effects of Sema3A on Rac1 activation by PAR1-TRAP. Consistent with previous reports,34,35 PAR1-TRAP induced rapid activation of Rac1 in platelets at the maximum in 30 seconds, and Sema3A almost completely inhibited the Rac1 activation induced by PAR1-TRAP (Figure 6C). These results suggest that Sema3A inhibits agonist-induced actin rearrangement via Rac1-dependent pathway including phosphorylation of cofilin.
Effects of Sema3A on platelet adhesion and spreading. (A) Washed platelets were incubated with 20 μg/mL Sema3A/Fc (bold line) or hIgG (thin solid line), or PBS (dashed line), and then placed on the various concentrations of immobilized fibrinogen for one hour. After washing with PBS to remove nonadherent platelets, adhered platelets were quantified by acid phosphatase method. Mean and SE of percent adhesion of 3 independent experiments was plotted. *P < .05. (B) Sema3A-treated platelets (ii,iv) or hIgG-treated platelets (i,iii) were placed on fibrinogen-coated (i-ii) or nontreated (iii-iv) glass coverslips. Adhered platelets were stained with TRICT (tetramethylrhodamine-5(and 6)-isothiocyanate)–phalloidin. Images were captured with a CCD camera (DP-70; Olympus) mounted on an Olympus AX-80 fluorescence microscope equipped with a 100 ×/1.30 oil immersion objective lens. Images were acquired with Olympus DP Controller software and processed with Adobe Photoshop Elements 2.0 (Adobe Systems, San Jose, CA). Original magnification × 1000, and bar in panel Bi represents 10 μM.
Effects of Sema3A on platelet adhesion and spreading. (A) Washed platelets were incubated with 20 μg/mL Sema3A/Fc (bold line) or hIgG (thin solid line), or PBS (dashed line), and then placed on the various concentrations of immobilized fibrinogen for one hour. After washing with PBS to remove nonadherent platelets, adhered platelets were quantified by acid phosphatase method. Mean and SE of percent adhesion of 3 independent experiments was plotted. *P < .05. (B) Sema3A-treated platelets (ii,iv) or hIgG-treated platelets (i,iii) were placed on fibrinogen-coated (i-ii) or nontreated (iii-iv) glass coverslips. Adhered platelets were stained with TRICT (tetramethylrhodamine-5(and 6)-isothiocyanate)–phalloidin. Images were captured with a CCD camera (DP-70; Olympus) mounted on an Olympus AX-80 fluorescence microscope equipped with a 100 ×/1.30 oil immersion objective lens. Images were acquired with Olympus DP Controller software and processed with Adobe Photoshop Elements 2.0 (Adobe Systems, San Jose, CA). Original magnification × 1000, and bar in panel Bi represents 10 μM.
Effects of Sema3A on Ca2+ and cyclic nucleotide signaling in platelets
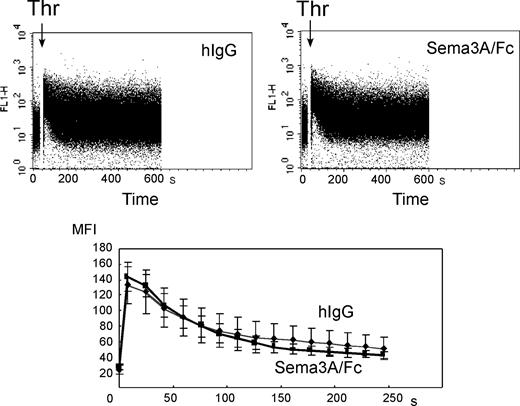
To examine whether Sema3A may affect Ca2+ signaling, fluo-3–loaded platelets were stimulated with thrombin and intracellular Ca2+ concentrations were monitored under flow cytometry. Thrombin induced rapid increase in intracellular Ca2+ concentrations in control platelets as described,20 and Sema3A/Fc did not affect the thrombin-induced increase in intracellular Ca2+ concentrations (Figure 7).
Since the best characterized platelet inhibitory signaling pathways are cyclic nucleotide pathways,38 we finally examined the effects of Sema3A on cyclic nucleotides in platelets. Sema3A did not increase the basal cAMP level in nonstimulated platelets per se (Table 2). Stable prostacyclin, iloprost, elevates intracellular cAMP, and addition of ADP impairs the iloprost-induced cAMP elevation by inhibiting adenylate cyclase.39 Again, Sema3A treatment did not change cAMP contents in iloprost- and ADP-treated platelets (Table 2). Sema3A also had no effects on basal cGMP contents, whereas sodium nitroprusside, a stimulator of NO/protein kinase G pathway, induced elevation of cGMP contents (Table 3). Moreover, a nitric oxide synthase (NOS) inhibitor, L-nitroarginine methyl ester, or a NO-donor, L-arginine, had no effects on the inhibition of αIIbβ3 activation by Sema3A (data not shown).40 These results suggest that neither cAMP nor cGMP is involved in inhibition of platelet function by Sema3A.
Effects of Sema3A on cAMP
. | cAMP, pmol/108 platelets . |
|---|---|
| hIgG, 20 μg/mL | 1.06 ± 0.19* |
| Sema3A/Fc, 20 μg/mL | 1.00 ± 0.68* |
| Iloprost, 20 μg/L | 45.94 ± 5.31 |
| Iloprost, 20 μg/L + ADP, 5 μM + hIgG, 20 μg/mL | 7.34 ± 0.47† |
| Iloprost, 20 μg/L + ADP, 5 μM + Sema3A/Fc, 20 μg/mL | 5.66 ± 0.90† |
. | cAMP, pmol/108 platelets . |
|---|---|
| hIgG, 20 μg/mL | 1.06 ± 0.19* |
| Sema3A/Fc, 20 μg/mL | 1.00 ± 0.68* |
| Iloprost, 20 μg/L | 45.94 ± 5.31 |
| Iloprost, 20 μg/L + ADP, 5 μM + hIgG, 20 μg/mL | 7.34 ± 0.47† |
| Iloprost, 20 μg/L + ADP, 5 μM + Sema3A/Fc, 20 μg/mL | 5.66 ± 0.90† |
Data represent the mean ± SE of 3 independent experiments.
P = .94.
P = .24.
Effects of Sema3A on cGMP
. | cGMP, pmol/108 platelets . |
|---|---|
| hIgG, 20 μg/mL | 0.83 ± 0.08* |
| Sema3A/Fc, 20 μg/mL | 0.86 ± 0.04* |
| Sodium nitroprusside, 1mM | 5.56 ± 0.83 |
. | cGMP, pmol/108 platelets . |
|---|---|
| hIgG, 20 μg/mL | 0.83 ± 0.08* |
| Sema3A/Fc, 20 μg/mL | 0.86 ± 0.04* |
| Sodium nitroprusside, 1mM | 5.56 ± 0.83 |
Data represent the mean ± SE of 3 independent experiments.
P = .70.
Effects of Sema3A on F-actin contents, cofilin phosphorylation, and Rac1 activation. (A) Sema3A/Fc-(gray bars) or hIgG-treated (black bars) platelets were activated by 30 μM PAR1-TRAP or 0.5 U/mL thrombin at 37°C for 30 seconds without stirring. After fixation, F-actin was stained with bodipy-phallacidin. Specific phallacidin binding was obtained by subtraction of FL1 fluorescence with a 300-fold more excess of unlabeled phallacidin from FL1 fluorescence without unlabeled phallacidin, and fold increase against fluorescence of no agonist sample was calculated. Data represent mean and SE of 3 independent experiments. *P < .05. (B) Sema3A/Fc- or hIgG-treated platelets were activated with 30 μM PAR1-TRAP for the indicated time at 37°C without stirring. Then, cells were lysed and SDS-PAGE was performed. Phospho-cofilin was detected by anti–phospho-cofilin–specific antibody. After stripping, total cofilin was detected by anticofilin antibody. Optical density of the bands was measured by NIH Image software, and relative increase against phospho-cofilin in IgG-treated platelets without thrombin was calculated. Mean and SE of 3 independent experiments was plotted in bottom panel. *P < .05; **P < .01. (C) Sema3A/Fc- or hIgG-treated platelets were activated with 30 μM PAR1-TRAP for the indicated time at 37°C without stirring. GTP-form of Rac1 was precipitated by incubation with GST-PAK1-PBD and glutathione beads. After SDS-PAGE electrophoresis, Rac1 was detected by a Rac1-specific antibody. Total Rac was detected by electrophoresis of total lysates on an SDS-PAGE gel followed by detection with the same antibody. Optical density of the bands was measured by NIH Image software, and relative increase against GTP-Rac in IgG-treated platelets without thrombin was calculated. Sema3A/Fc is indicated by ▪ and bold lines; hIgG, by ♦ and thin lines. Mean and SE of 3 independent experiments was plotted in lower panel. *P < .05.
Effects of Sema3A on F-actin contents, cofilin phosphorylation, and Rac1 activation. (A) Sema3A/Fc-(gray bars) or hIgG-treated (black bars) platelets were activated by 30 μM PAR1-TRAP or 0.5 U/mL thrombin at 37°C for 30 seconds without stirring. After fixation, F-actin was stained with bodipy-phallacidin. Specific phallacidin binding was obtained by subtraction of FL1 fluorescence with a 300-fold more excess of unlabeled phallacidin from FL1 fluorescence without unlabeled phallacidin, and fold increase against fluorescence of no agonist sample was calculated. Data represent mean and SE of 3 independent experiments. *P < .05. (B) Sema3A/Fc- or hIgG-treated platelets were activated with 30 μM PAR1-TRAP for the indicated time at 37°C without stirring. Then, cells were lysed and SDS-PAGE was performed. Phospho-cofilin was detected by anti–phospho-cofilin–specific antibody. After stripping, total cofilin was detected by anticofilin antibody. Optical density of the bands was measured by NIH Image software, and relative increase against phospho-cofilin in IgG-treated platelets without thrombin was calculated. Mean and SE of 3 independent experiments was plotted in bottom panel. *P < .05; **P < .01. (C) Sema3A/Fc- or hIgG-treated platelets were activated with 30 μM PAR1-TRAP for the indicated time at 37°C without stirring. GTP-form of Rac1 was precipitated by incubation with GST-PAK1-PBD and glutathione beads. After SDS-PAGE electrophoresis, Rac1 was detected by a Rac1-specific antibody. Total Rac was detected by electrophoresis of total lysates on an SDS-PAGE gel followed by detection with the same antibody. Optical density of the bands was measured by NIH Image software, and relative increase against GTP-Rac in IgG-treated platelets without thrombin was calculated. Sema3A/Fc is indicated by ▪ and bold lines; hIgG, by ♦ and thin lines. Mean and SE of 3 independent experiments was plotted in lower panel. *P < .05.
Discussion
In this report, we demonstrated for the first time the binding of Sema3A on platelets and extensive inhibitory effects of Sema3A on platelet function. As reported in endothelial cells,16 Sema3A inhibited integrin-mediated function in platelets (ie, inhibition of αIIbβ3 activation and platelet aggregate formation, and adhesion and spreading on immobilized fibrinogen). However, Sema3A also inhibited αIIbβ3-independent adhesion and spreading on nontreated glass coverslips and aggregation-independent granular secretion. Although the most potent platelet inhibitory pathways are cyclic nucleotide pathways,38 we did not detect any effect on cAMP and cGMP contents by Sema3A treatment. Thrombin-induced Ca2+ signaling was also unaffected by Sema3A treatment.
Effects of Sema3A on thrombin-induced increase of intracellular Ca2+concentrations. Fluo-3–labeled platelets were incubated with 20 μg/mL Sema3A/Fc or hIgG. After the determination for about 10 seconds of baseline fluo-3 fluorescence from the platelet population, cell aspiration into the flow cytometry was briefly paused, and 1:10 volume of 5 U/mL thrombin (Thr) was added. The acquisition was then resumed, and changes in log fluorescence versus time were recorded (top panels). For each plot, rectangular analysis regions were defined over the time axis, and mean fluorescence intensity was calculated. Mean ± SE of 3 independent experiments was plotted in bottom panel. Bold and thin lines represent Sema3A/Fc and hIgG, respectively.
Effects of Sema3A on thrombin-induced increase of intracellular Ca2+concentrations. Fluo-3–labeled platelets were incubated with 20 μg/mL Sema3A/Fc or hIgG. After the determination for about 10 seconds of baseline fluo-3 fluorescence from the platelet population, cell aspiration into the flow cytometry was briefly paused, and 1:10 volume of 5 U/mL thrombin (Thr) was added. The acquisition was then resumed, and changes in log fluorescence versus time were recorded (top panels). For each plot, rectangular analysis regions were defined over the time axis, and mean fluorescence intensity was calculated. Mean ± SE of 3 independent experiments was plotted in bottom panel. Bold and thin lines represent Sema3A/Fc and hIgG, respectively.
Sema3A markedly impaired αIIbβ3-independent as well as αIIbβ3-dependent platelet spreading. We demonstrated that Sema3A inhibited the increase of F-actin contents after thrombin or PAR1-TRAP stimulation. Thus, Sema3A inhibited adhesion-induced and agonist-induced actin rearrangement. Furthermore, Sema3A inhibited phosphorylation of cofilin and Rac1 activation after PAR1-TRAP stimulation. Several reports revealed that Rac1 activation is necessary for platelet actin assembly and lamellipodia formation after agonist stimulation.34,35,41 Therefore, marked impairment of Rac1 activation is very likely to account for the Sema3A-induced impairment of actin rearrangement and spreading in platelets. There were 2 major downstream effectors of Rac1 identified, PAK and WAVEs ([Wiskott-Aldrich syndrome protein] WASP family Verprolin-homologous proteins).42 Several PAK substrates or binding partners have been implicated in the effects of PAK, including filamin, LIM kinase, myosin, and paxillin.43 Among them, LIM kinase phosphorylates and inactivates cofilin, a protein that promotes severing and depolymerization of F-actin.31,32 Consistent with the inhibition of Rac1 activation, Sema3A inhibited phosphorylation of cofilin in both resting and activated platelets, suggesting that Sema3A increases severing and depolymerization of F-actin by keeping cofilin in the activated state. Rac1 inhibition by Sema3A might affect the activation of another major downstream effector of Rac1, WAVEs. WAVEs, also known as Scar, belong to the WASP family and activate actin-related protein 2 and 3 (Arp2/3) complex, resulting in nucleating actin polymerization.43 Others and we have demonstrated that platelets contain WAVE isoforms and may regulate lamellipodia formation.44,45 Therefore, it is also likely that Sema3A may inhibit actin rearrangement via inhibition of WAVE-dependent initiation of actin polymerization.
In contrast to our results, it has been demonstrated that Rac1 activation is essential for Sema3A-induced growth cone collapse in neural cells,46,47 and Sema3A-induced phosphorylation of cofilin is necessary for the process.48 However, in these reports, the authors analyzed direct signaling induced by the binding of Sema3A. In this study, we analyzed the effects of Sema3A binding on agonist-induced signaling in platelets. Interestingly, Aizawa et al also found that phosphorylated cofilin was subsequently dephosphorylated within 5 minutes at the neural growth cone and the phosphorylated level of cofilin decreased to 0.16-fold of that of untreated growth cone,48 which is consistent with our observation that cofilin is dephosphorylated in Sema3A-treated platelets. Signaling pathways from semaphorin receptors to Rac have not been fully understood even in neural cells.11,12 Human plexin-B1, a receptor for Sema4D, and fly plexin B interact with activated Rac directly, and it has been suggested that these plexins sequester activated Rac and antagonize its signaling pathway.49-51 Very recently Turner et al reported the association of activated Rac and the cytoplasmic tail of plexin-A1,52 although others failed to detect the interaction.50,53 Further studies are necessary to reveal the mechanism of regulation of Rac by Sema3A in platelets.
Is the impairment of actin rearrangement via inhibition of Rac1 responsible for Sema3A-induced extensive negative regulation of platelet function other than platelet spreading? To investigate the role of actin rearrangement on platelet function, effects of cytochalasins or latrunculin A, inhibitors of actin polymerization, have been studied.54-58 There are some discrepancies in these reports, mainly because of the differences in experimental conditions; some reports demonstrated that high concentrations of cytochalasins inhibited agonist-induced αIIbβ3 activation and platelet aggregation, indicating that de novo actin polymerization affects activation of αIIbβ3,54,55,58 whereas low concentrations of cytochalasin D and latrunculin A activated αIIbβ3.57 Integrin activating inside-out signaling consists of 2 aspects: conformational change that regulates integrin affinity and integrin clustering that regulates its avidity.5,7 αIIbβ3 clustering may be promoted by actin cytoskeletal rearrangement, although conformational change seems to be the dominant way in αIIbβ3 activation.59 Moreover, recent reports revealed that talin binding to integrin cytoplasmic tails is essential for integrin activation.60,61 Since talin links integrin to actin filaments in clustering of integrins into adhesion complexes,62,63 defects of actin polymerization may impair broad aspects of integrin signaling. However, impairment of actin rearrangement does not appear to be the sole mechanism of Sema3A inhibition of platelet function, since, in contrast to Sema3A, cytochalasins have no inhibitory effects on granular secretion.55,58 Rac1 regulates many cellular activities besides cytoskeletal rearrangement, such as cell polarity and vesicle trafficking in other cells.42 Moreover, Sema3A may act via Rac1-independent pathways (eg, the collapsin response mediator protein (CRMP)–mediated pathway).12 These hypotheses remain to be determined.
It has been well documented that endothelial cells negatively regulate platelet function by secreted PGI2, NO, and membrane-bound ecto-ADPase.8 Since Sema3A is also produced in endothelial cells and inhibits platelet function extensively, Sema3A may contribute to maintain blood flow in normal, injured, or newly synthesized vessels by keeping platelets in the resting state. Moreover, since Sema3A appears to inhibit platelet function via unique Rac1-dependent pathway, modulation of Sema3A-inducing signaling pathway may be a new target of antiplatelet therapy.
In conclusion, we demonstrated that Sema3A binds to platelets and inhibits platelet function extensively. The inhibition of platelet function by Sema3A appeared to be mediated, at least in part, through impairment of agonist-induced Rac1-dependent actin rearrangement. We believe that these results reveal a new Sema3A function on thrombosis and hemostasis, and a unique inhibitory signaling pathway evoked by Sema3A binding to platelets.
Prepublished online as Blood First Edition Paper, April 14, 2005; DOI 10.1182/blood-2004-10-4092.
Supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture in Japan; the Yamanouchi Foundation for Research and Metabolic Disorders, Tsukuba, Japan; and Mitsubishi Pharma Research Foundation, Osaka, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr M. Moroi (Department of Protein Biochemistry, Institute of Life Science, Kurume University, Fukuoka, Japan) for providing us with convulxin.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal