Abstract
Low-density lipoprotein (LDL) receptor (LDLR) and LDLR-related protein (LRP) are members of the LDLR family of endocytic receptors. LRP recognizes a wide spectrum of structurally and functionally unrelated ligands, including coagulation factor VIII (FVIII). In contrast, the ligand specificity of LDLR is restricted to apolipoproteins E and B-100. Ligand binding to the LDLR family is inhibited by receptor-associated protein (RAP). We have previously reported that, apart from LRP, other RAP-sensitive mechanisms contribute to the regulation of FVIII in vivo. In the present study, we showed that the extracellular ligand-binding domain of LDLR interacts with FVIII in vitro and that binding was inhibited by RAP. The physiologic relevance of the FVIII–LDLR interaction was addressed using mouse models of LDLR or hepatic LRP deficiency. In the absence of hepatic LRP, LDLR played a dominant role in the regulation and clearance of FVIII in vivo. Furthermore, FVIII clearance was accelerated after adenovirus-mediated gene transfer of LDLR. The role of LDLR in FVIII catabolism was not secondary to increased plasma lipoproteins or to changes in lipoprotein profiles. We propose that LDLR acts in concert with LRP in regulating plasma levels of FVIII in vivo. This represents a previously unrecognized link between LDLR and hemostasis.
Introduction
Low-density lipoprotein (LDL) receptor (LDLR) plays a pivotal role in the metabolism of large cholesterol-rich lipoproteins from the circulation in a process known as receptor-mediated endocytosis.1 This process is dependent on the high-affinity interaction between LDLR and its protein ligands, apolipoprotein E (apoE) and apoB-100, both of which are present at the surfaces of lipoprotein particles in plasma.1,2 The importance of LDLR is illustrated by the fact that genetic defects within the LDLR gene are the underlying cause of familial hypercholesterolemia.3 These patients display elevated plasma LDL cholesterol concentrations and increased risk for atherosclerosis and coronary artery disease.
LDLR belongs to the LDLR family of cell-surface endocytic receptors that also includes apoE-receptor-2, very low-density lipoprotein (VLDL) receptor, megalin, and LDLR-related protein (LRP).2,4 The extracellular ligand-binding domains of these receptors are composed of 1 to 4 homolog clusters of complement-type repeats. The chaperone receptor–associated protein (RAP) blocks all ligand binding to these clusters.5 Disruption of the LRP gene in LDLR knockout mice accumulates apoE-rich VLDL/LDL-sized lipoproteins in plasma, indicating that LDLR works in concert with LRP in lipoprotein metabolism in vivo.6 Unlike LDLR, however, LRP also recognizes a broad spectrum of lipoprotein-unrelated ligands.4
In the past few years, LRP has been established to interact with coagulation factor VIII (FVIII) and to mediate its cellular uptake into the lysosomal degradation pathway.7,8 Within the blood coagulation cascade, the cofactor activity of FVIII is indispensable for appropriate hemostasis.9 Deficiency or dysfunction of FVIII is associated with the bleeding disorder hemophilia A, whereas elevated plasma FVIII markedly increases the risk for arterial and venous thrombosis.9-11 The cofactor circulates in plasma in complex with its carrier protein von Willebrand factor (VWF).12
Using conditional hepatic LRP-deficient mice, we recently demonstrated that LRP contributes to the removal of FVIII from the circulation.13 These mice display not only elevated plasma FVIII levels but also prolonged FVIII half-life. Adenovirus-mediated overexpression of RAP in LRP-deficient mice further increases plasma FVIII, indicating that RAP-sensitive determinants other than hepatic LRP also contribute to the regulation of plasma FVIII.13 FVIII has been reported to comprise multiple binding sites for the RAP-sensitive VLDL receptor in vitro.14 However, we have recently demonstrated that VLDL receptor does not cooperate with LRP in FVIII clearance in vivo.15 In the present study, we investigated the physiologic role of LDLR in the catabolism of FVIII. We took advantage of unique conditional knockout mouse models for LDLR, LRP, and the combination thereof. By this approach, we identified a novel role for LDLR as a receptor that contributes to the catabolism of coagulation FVIII in vivo.
Materials and methods
Materials
CNBr-Sepharose 4B and Mono Q were from Amersham Pharmacia Biotech (Uppsala, Sweden). Microtiter plates (Microlon, Greiner bio-one), cell culture flasks, OptiMem I medium, penicillin, and streptomycin were from Life Technologies (Breda, The Netherlands).
Proteins
Purification of the anti-FVIII,16 anti-VWF,17 and anti-FIX18 monoclonal antibodies CLB-CAg 12, CLB-CAg 117, CLB-CAg 69, CLB-RAg 56, CLB-FIX 11, plasma-derived FVIII heterodimer,19 and FIX18 have been described previously. Human VWF was purified from human VWF concentrate (von Willebrand factor-SD; Regional Center of Blood Transfusion, Lille, France). This concentrate was first depleted for FVIII by immunoaffinity chromatography using antibody CLB-CAg 117. VWF was further purified by Mono Q chromatography, using 100 mM NaCl and 50 mM Tris (tris(hydroxymethyl)aminomethane) (pH 7.4) for washing and a salt gradient from 100 mM to 1 M NaCl in the same buffer for elution. The VWF preparation was dialyzed to 150 mM NaCl, 5 mM CaCl2, and 50 mM Tris (pH 7.4) and was stored at 4°C. An estimated average molecular mass of 200 kDa was used to calculate the concentration of FVIII heterodimer. The concentration of VWF refers to the concentration of VWF monomers (Mr = 220 kDa). RAP was expressed in Escherichia coli strain DH5α and was purified using glutathione–Sepharose, as described.20 LRP cluster 2 was purified by affinity chromatography using RAP-coupled CNBr-Sepharose 4B.18 LDL (1.019 g/mL < d < 1.063 g/mL) and high-density lipoprotein (HDL) (1.063 g/mL < d < 1.120 g/mL) fractions were isolated from fresh human plasma, as described,21 and were dialyzed to 150 mM NaCl, 5 mM CaCl2, and 50 mM Tris (pH 7.4). Protein was quantified using the Bradford method22 with human serum albumin (HSA) (Sanquin Plasma Products, Amsterdam, The Netherlands) as a standard.
Construction, expression, and purification of sLDLRCR1-7
Construction of the adenoviral-plasmid containing full-length human LDLR cDNA has been described previously23,24 and was used as a template to amplify the cDNA of the entire extracellular ligand-binding domain of LDLR (sLDLRCR1-7), comprising amino acids 22 to 317, by polymerase chain reaction. Oligonucleotide primers 5′-ATTCTCGAGGCAGTGGGCGACAGATGT-3′ (sense) and 5′-TAAACTAGTTTCGTTGGTCCCGCACTC-5′ (antisense) were used. This cDNA fragment was purified, digested with XhoI and SpeI, ligated into the XhoI/SpeI-digested pZEN vector,25 and sequenced to verify the construct. The resultant plasmid contains a 16-amino acid tag (KKEDFDIYDEDENQSP) for purification, which represents the epitope of monoclonal antibody CLB-CAg 69,16 followed by the sLDLRCR1-7 coding sequence. The construct was transfected into baby hamster kidney cells as described.25 Stable cell lines that express sLDLRCR1-7 were obtained by limiting dilution, and protein was expressed in OptiMem I medium (Life Technologies) supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin. After harvesting of the medium, benzamidine (10 mM) and CaCl2 (10 mM) were added. Purification of sLDLRCR1-7 was performed by affinity-chromatography using a column of CLB-CAg 69 coupled to CNBr-Sepharose 4B as affinity matrix. This matrix was washed with 100 mM NaCl, 5 mM CaCl2, and 50 mM Tris (pH 7.4) and was eluted with 2 M NaCl, 5 mM CaCl2, and 50 mM Tris (pH 7.4). The obtained sLDLRCR1-7 protein was subsequently concentrated by Mono Q chromatography using 100 mM NaCl, 5 mM CaCl2, and 50 mM Tris (pH 7.4) for washing and 2 M NaCl, 5 mM CaCl2, and 50 mM Tris (pH 7.4) for elution. The preparation was dialyzed to 150 mM NaCl, 5 mM CaCl2, 50% (vol/vol) glycerol, and 20 mM Tris (pH 7.4) and was stored at –20°C. Purified sLDLRCR1-7 was subjected to 10% (vol/vol) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (5 μg protein/lane) under reducing conditions and was stained with Coomassie brilliant blue. For immunoblotting, SDS-PAGE–separated sLDLRCR1-7 (0.5 μg protein/lane) was transferred to a nitrocellulose membrane, blocked for 30 minutes in phosphate-buffered saline (pH 7.4), 0.1% (vol/vol) Tween 20, and 4% (wt/vol) milk powder; this was followed by a 90-minute incubation of a rabbit polyclonal antibody directed against CR4 of human LDLR (Research Diagnostics, Flanders, New Jersey) in the same buffer. Bound IgG was detected with peroxidase-conjugated goat antirabbit antibody followed by staining with 3,3-diaminobenzidine.
Solid-phase binding assay
Purified recombinant sLDLRCR1-7 or LRP cluster 2 was adsorbed onto microtiter wells (0.5 μg/well) in 50 mM NaHCO3 (pH 9.8), in a volume of 50 μL for 16 hours at 4°C. Wells were blocked with 150 mM NaCl, 5 mM CaCl2, 2.5% (wt/vol) HSA, and 50 mM Tris (pH 7.4) in a volume of 200 μL for 2 hours at 37°C. Subsequently, purified human FVIII, VWF, or FIX was incubated in 150 mM NaCl, 5 mM CaCl2, 1% (wt/vol) HSA, 0.1% (vol/vol) Tween 20, and 50 mM Tris (pH 7.4), in a volume of 50 μL for 2 hours at 37°C. Bound ligand was detected by incubation with peroxidase-conjugated monoclonal antibody CLB-CAg 12, CLB-RAg 56, or CLB-FIX 11, respectively, in the same buffer for 10 minutes at 37°C. In competition experiments, immobilized sLDLRCR1-7 or immobilized LRP cluster 2 was incubated with purified FVIII at 150 nM or 20 nM, respectively, in the absence or presence of serial dilutions of competitor. For RAP-, LDL-, and HDL-binding experiments, these proteins were adsorbed onto microtiter wells (1 μg/well) in 150 mM NaCl, 5 mM CaCl2, and 50 mM Tris (pH 7.4) in a volume of 100 μL for 16 hours at 4°C. Wells were blocked with 150 mM NaCl, 5 mM CaCl2, 2.5% (wt/vol) HSA, and 50 mM Tris (pH 7.4) in a volume of 200 μL for 2 hours at 37°C. Subsequently, sLDLRCR1-7 (250 nM) was incubated in 150 mM NaCl, 5 mM CaCl2, 1% (wt/vol) HSA, and 50 mM Tris (pH 7.4), in a volume of 100 μL for 2 hours at 37°C. Bound ligand was detected by incubation with peroxidase-conjugated monoclonal antibody CLB-CAg 69 in the same buffer for 10 minutes at 37°C. All data were corrected for binding to control microtiter wells lacking immobilized sLDLRCR1-7, RAP, LDL, or HDL, which was less than 10% relative to binding to wells containing these immobilized proteins.
Transgenic animals
Mice carrying loxP sites within the LRP gene (LRPflox/flox), LDLR-deficient mice (LDLR–/–), VLDLR-deficient mice (VLDLR–/–), and apoE-deficient mice (ApoE–/–) were generated by homologous recombination in embryonic stem cells, as described previously.23,26-29 Mice transgenic for the MX1cre expression construct (MX1Cre+) were generated by pronuclear injection of hybrid (SJL X C57BL/6J) mice, as described.6 Combining MX1Cre+, LRPflox/flox, LDLR–/–, VLDLR–/–, and ApoE–/– genotypes resulted in the following 7 genetically distinct knockout strains used in this study: LRPflox/flox (control), MX1Cre+LRPflox/flox (LRP–), LDLR–/– LRPflox/flox (LDLR–/–), LDLR–/–MX1Cre+LRPflox/flox (LDLR–/–LRP–), VLDLR–/–LDLR–/–MX1Cre+LRPflox/flox (VLDLR–/–LDLR–/–LRP–), ApoE–/–LDLR–/–LRPflox/flox (ApoE–/–LDLR–/–), and ApoE–/–LDLR–/– MX1Cre+LRPflox/flox (ApoE–/–LDLR–/–LRP–). All mice were genotyped for LRPflox/flox, MX1cre, LDLR, VLDLR, or apoE status by polymerase chain reaction, as described.6,28,29 Mice were injected with polyinosinic: polycytidylic-ribonucleic acid (pI:pC), as described.13 Noninduced MX1Cre+LRPflox/flox, noninduced LRPflox/flox, and pI:pC-induced LRPflox/flox control animals displayed similar plasma levels of FVIII, VWF, FV, FIX, tissue-type plasminogen activator (t-PA), total cholesterol, and triglycerides, indicating that neither MX1Cre status nor pI:pC alone affected these plasma protein levels in our mice. C57BL6/J mice homozygous for the human APOCI transgene (huAPOCI) and nontransgenic littermate controls were generated, as described.30 For experiments, male mice 8 to 12 weeks of age were used (each weighing 20-25 g). Mice were housed under standard conditions in conventional cages and given free access to food (standard rodent chow diet; Hope Farms, Woerden, The Netherlands) and water. The institutional committees on animal welfare of TNO Prevention and Health approved all animal experiments.
Quantification of mouse plasma FVIII, FV, FIX, VWF, t-PA, cholesterol, and triglycerides
Blood was obtained by tail bleeding into polypropylene Eppendorf tubes containing 0.1 vol of 3.2% (wt/vol) trisodium citrate. Plasma was prepared by centrifugation of blood at 2000g for 10 minutes at 4°C, immediately snap-frozen in liquid nitrogen, and stored at –80°C before analysis. Mouse plasma FVIII activity, FV activity, FIX zymogen activity, VWF antigen, t-PA activity, total cholesterol, and triglyceride levels were determined as described previously.13,14 Plasma FVIII, FV, FIX, VWF, and t-PA levels were expressed in murine plasma-equivalent units per milliliter. Unless stated otherwise, control LRPflox/flox pooled plasma was used as a reference. Lipoprotein distribution was determined by fast performance liquid chromatography (FPLC) size fractionation.28
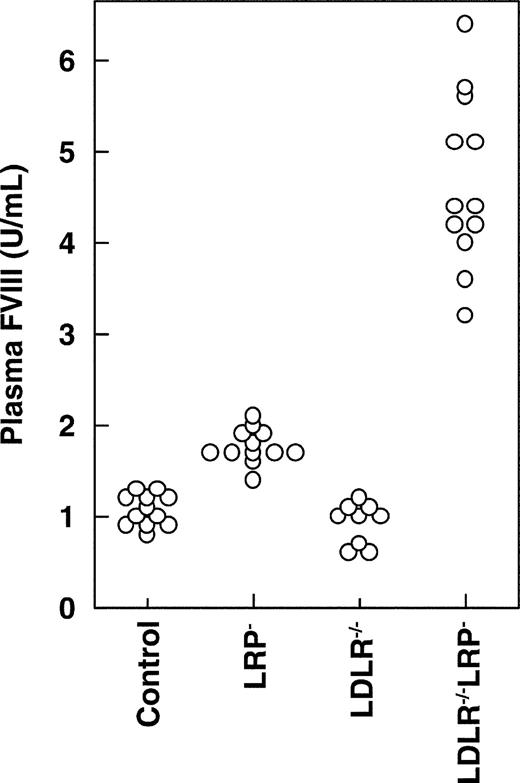
Plasma FVIII levels in mice that lack LRP, LDLR, or both. Four weeks after the final pI:pC injection, blood was drawn from control (n = 12), LRP– (n = 12), LDLR–/–(n = 9), and LDLR–/–LRP– mice (n = 12). Plasma samples were analyzed for FVIII and are plotted as individual values.
Plasma FVIII levels in mice that lack LRP, LDLR, or both. Four weeks after the final pI:pC injection, blood was drawn from control (n = 12), LRP– (n = 12), LDLR–/–(n = 9), and LDLR–/–LRP– mice (n = 12). Plasma samples were analyzed for FVIII and are plotted as individual values.
Clearance of human FVIII
Human immunopurified plasma-derived FVIII (Aafact; Sanquin Plasma Products, Amsterdam, The Netherlands) (20 IU in 200 μL) was injected into the tail veins of weight-matched male mice. At indicated time points, individual mice were serially monitored for human plasma FVIII antigen levels, as described previously.13 The amount of FVIII recovered in the plasma 1 minute after injection was 90% to 100%. Values are expressed as the percentage of FVIII remaining in the circulation, considering the amount of FVIII present at 1 minute after injection as 100%. Pharmacokinetic parameters were calculated using a model-independent (noncompartmental) approach.31 A double-exponential fit was used to calculate the standard parameters area under the curve and mean residence time (MRT).
Recombinant adenovirus transduction
Recombinant adenovirus containing the human LDLR cDNA (Ad-CMV-LDLR) or β-galactosidase cDNA (Ad-CMV-β-Gal) under the control of the cytomegalovirus (CMV) promotor was generated and purified, as described previously.23,32 Normal male C57BL/6J, LDLR–/– or LDLR–/–LRP– mice received 2 × 109 plaque-forming units of Ad-CMV-LDLR or Ad-CMV-β-Gal in 200 μL physiological saline into the tail vein. Blood samples (100 μL) were withdrawn by tail bleeding before and 5 days after adenovirus injection. FVIII levels were assayed using pooled C57BL/6J plasma as a reference. Five days after adenovirus administration, normal C57BL/6J or LDLR–/–LRP– mice were intravenously injected with human FVIII (20 IU), and the removal of FVIII from plasma was monitored as described.
Detection of LDLR in mouse liver
Mouse liver membrane extracts13 (50 μg/lane) were subjected to nonreducing 4% to 15% (vol/vol) SDS-PAGE analysis and transferred to nitrocellulose membranes, and LDLR was detected as described, with the exception that bound immunoglobulin G (IgG) was stained using the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Uppsala, Sweden).
Statistical analysis
In vitro data were represented as mean plus or minus standard deviation (SD). In vivo data were represented as geometric means and 68% confidence intervals (CIs), which represented 1 SD from the geometric mean if a log normal distribution was assumed. Data were analyzed by means of the Mann-Whitney U test. P less than .05 was regarded as statistically significant.
Results
Role of LDLR in regulating plasma FVIII in vivo
To address the physiologic role of LDLR in regulating plasma FVIII, we measured plasma FVIII levels and its carrier protein, VWF, in control, LRP–, LDLR–/–, and LDLR–/–LRP– double-knockout mice (Figure 1; Table 1). Plasma FVIII and, to a lesser extent, VWF levels were increased in LRP– mice approximately 1.6-fold and 1.3-fold, respectively, which is in agreement with our previous findings.13 In contrast, LDLR–/– mice that expressed functional LRP had normal plasma FVIII and VWF levels. Interestingly, LDLR–/–LRP– double-knockout mice demonstrated a 4.2-fold increase of plasma FVIII, which was more pronounced, as expected, from plasma FVIII in mice that lacked LRP or LDLR alone. This synergistic effect of plasma FVIII in mice that lacked LRP and LDLR coincided with a 3.3-fold increase in plasma VWF level. In contrast to FVIII and VWF, disruption of LRP, LDLR, or both did not affect the plasma levels of the other coagulation-related plasma proteins, FV and FIX (Table 1). For the LRP ligand t-PA,26 we found that the induction of hepatic LRP deficiency resulted in an increase of its plasma level. However, this LRP-dependent increase of plasma t-PA was not affected by LDLR status (Table 1). These data demonstrate that LDLR knockout mice have normal plasma FVIII and VWF levels. In contrast, in the absence of hepatic LRP, LDLR deficiency further increased plasma FVIII and VWF levels.
Plasma parameters in mice that lack LRP, LDLR, or both
Plasma component . | Control . | LRP- . | LDLR-/- . | LDLR-/-LRP- . |
|---|---|---|---|---|
| FVIII, U/mL | 1.1 (0.9-1.2) | 1.8 (1.6-2.0)* | 0.9 (0.7-1.2) | 4.6 (3.7-5.6)† |
| VWF, U/mL | 1.0 (0.9-1.3) | 1.3 (1.1-1.5)* | 0.9 (0.7-1.2) | 3.3 (2.7-4.1)† |
| FV, U/mL | 1.0 (0.9-1.1) | 1.1 (1.0-1.3) | 1.1 (1.0-1.2) | 1.1 (1.0-1.2) |
| FIX, U/mL | 1.1 (0.9-1.2) | 1.0 (0.9-1.1) | 1.1 (0.9-1.3) | 1.1 (1.0-1.3) |
| TPA, U/mL | 1.8 (1.5-2.0) | 2.4 (1.9-3.1)* | 2.0 (1.8-2.1) | 2.3 (1.9-2.7) |
| Cholesterol, mM | 1.7 (1.5-1.9) | 1.7 (1.5-1.9) | 7.0 (6.1-8.1)* | 19.5 (17.2-22.3)† |
| Triglycerides, mM | 0.2 (0.1-0.3) | 0.2 (0.1-0.2) | 0.8 (0.7-1.0)* | 3.0 (2.5-3.7)† |
Plasma component . | Control . | LRP- . | LDLR-/- . | LDLR-/-LRP- . |
|---|---|---|---|---|
| FVIII, U/mL | 1.1 (0.9-1.2) | 1.8 (1.6-2.0)* | 0.9 (0.7-1.2) | 4.6 (3.7-5.6)† |
| VWF, U/mL | 1.0 (0.9-1.3) | 1.3 (1.1-1.5)* | 0.9 (0.7-1.2) | 3.3 (2.7-4.1)† |
| FV, U/mL | 1.0 (0.9-1.1) | 1.1 (1.0-1.3) | 1.1 (1.0-1.2) | 1.1 (1.0-1.2) |
| FIX, U/mL | 1.1 (0.9-1.2) | 1.0 (0.9-1.1) | 1.1 (0.9-1.3) | 1.1 (1.0-1.3) |
| TPA, U/mL | 1.8 (1.5-2.0) | 2.4 (1.9-3.1)* | 2.0 (1.8-2.1) | 2.3 (1.9-2.7) |
| Cholesterol, mM | 1.7 (1.5-1.9) | 1.7 (1.5-1.9) | 7.0 (6.1-8.1)* | 19.5 (17.2-22.3)† |
| Triglycerides, mM | 0.2 (0.1-0.3) | 0.2 (0.1-0.2) | 0.8 (0.7-1.0)* | 3.0 (2.5-3.7)† |
At 4 weeks after the final pI:pC injection, blood was drawn from control, LRP-, LDLR-/-, and LDLR-/-LRP- mice. Plasma samples were analyzed for FVIII, VWF, FV, FIX, t-PA cholesterol, and triglycerides, as described in “Materials and methods.” Data represent geometric mean values with 68% confidence intervals in parentheses. n = 12 for all groups except LDLR-/- (n = 9).
P < .05, significantly different from control mice; Mann-Whitney U test.
P < .05, significantly different from LRP-, LDLR-/-, and control mice; Mann-Whitney U test.
FVIII interacts with LDLR in vitro
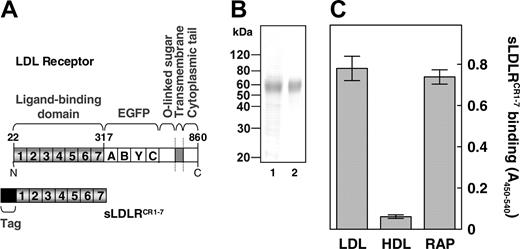
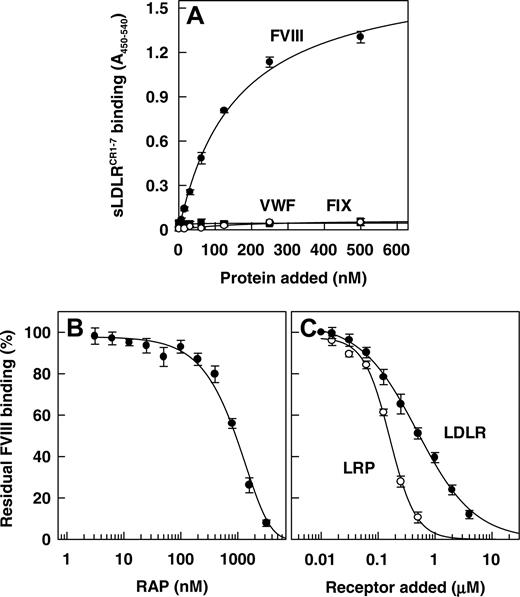
It has been well established that LRP comprises multiple binding sites for FVIII.25,33 We now investigated whether LDLR also recognizes FVIII as a ligand. To this end, we constructed a soluble recombinant LDLR fragment that contains the entire extracellular ligand-binding domain, comprising complement-type repeats 1 to 7 (CR 1-7) (amino acid residues 22-317) (Figure 2A). Using SDS-PAGE and immunoblotting with an antibody directed against CR4 of human LDLR, this fragment (referred to as sLDLRCR1-7) migrated at a single band with the expected molecular mass34 (Figure 2B). Purified sLDLRCR1-7 efficiently bound to the archetypal LDLR ligand apoB-100 containing LDL1,2 and the universal LDLR family antagonist RAP, whereas binding to immobilized HDL was only minor (Figure 2C). A dose-dependent increase in binding occurred on incubation of increasing concentrations of FVIII with immobilized sLDLRCR1-7, with half-maximum binding at a FVIII concentration of 156 ± 6 nM (Figure 3A). In contrast, FIX and the FVIII carrier protein VWF did not show any interaction with immobilized sLDLRCR1-7 (Figure 3A). The specificity of FVIII binding to LDLR was addressed using the LDLR-antagonist RAP. Indeed, FVIII binding to sLDLRCR1-7 decreased in a dose-dependent manner in the presence of increasing concentrations of RAP (Figure 3B). Half-maximum inhibition (Ki) occurred at a RAP concentration of approximately 0.9 μM, which is close to the affinity of RAP for full-length LDLR.35 Previously, we demonstrated that FVIII interacts with the ligand-binding cluster 2 of LRP.33 Binding of FVIII to immobilized purified recombinant LRP cluster 2 was observed with half-maximum binding at a FVIII concentration of 20 plus or minus 2 nM (data not shown). To address the affinity of FVIII for LDLR relative to LRP, we used competition experiments between sLDLRCR1-7 and LRP cluster 2 in the interaction with FVIII. Both sLDLRCR1-7 and LRP cluster 2 competed for binding of FVIII to immobilized LRP cluster 2, with Ki values of 0.51 ± 0.10 μM and 0.15 ± 0.01 μM, respectively (Figure 3C). This indicates that FVIII binds to LRP with approximately 3.4-fold higher affinity compared with the FVIII-LDLR complex assembly. Taken together, these data demonstrate that FVIII is a ligand of LDLR in vitro.
Characterization of recombinant sLDLRCR1-7. (A) Domain organization of full-length LDLR (top) and of the recombinant soluble ligand-binding fragment sLDLRCR1-7 (bottom). Ligand-binding domain that harbors complement-type repeats 1 to 7, EGF precursor (EGFP) domain that contains EGF-like modules (A-C) and a beta-propeller domain comprising 6 YWTD motifs (Y), O-linked sugar domain, transmembrane domain, cytoplasmic tail, immunopurification tag, and amino acid residue numbers are indicated. (B) Coomassie brilliant blue–stained 10% (vol/vol) SDS-PAGE analysis of the purified recombinant sLDLRCR1-7 (lane 1). Immunoblotting of the purified recombinant sLDLRCR1-7 using an anti–human LDLR antibody followed by 3,3-diaminobenzidine staining (lane 2). Molecular weight standard is indicated. (C) Purified sLDLRCR1-7 (250 nM) was incubated with immobilized isolated human LDL, HDL, and recombinant RAP (1 μg/well). Binding was detected using the peroxidase-conjugated monoclonal antibody CLB-CAg 69. Data represent the mean ± SD of 3 separate experiments.
Characterization of recombinant sLDLRCR1-7. (A) Domain organization of full-length LDLR (top) and of the recombinant soluble ligand-binding fragment sLDLRCR1-7 (bottom). Ligand-binding domain that harbors complement-type repeats 1 to 7, EGF precursor (EGFP) domain that contains EGF-like modules (A-C) and a beta-propeller domain comprising 6 YWTD motifs (Y), O-linked sugar domain, transmembrane domain, cytoplasmic tail, immunopurification tag, and amino acid residue numbers are indicated. (B) Coomassie brilliant blue–stained 10% (vol/vol) SDS-PAGE analysis of the purified recombinant sLDLRCR1-7 (lane 1). Immunoblotting of the purified recombinant sLDLRCR1-7 using an anti–human LDLR antibody followed by 3,3-diaminobenzidine staining (lane 2). Molecular weight standard is indicated. (C) Purified sLDLRCR1-7 (250 nM) was incubated with immobilized isolated human LDL, HDL, and recombinant RAP (1 μg/well). Binding was detected using the peroxidase-conjugated monoclonal antibody CLB-CAg 69. Data represent the mean ± SD of 3 separate experiments.
Binding of FVIII, VWF, and FIX to recombinant sLDLRCR1-7. (A) Immobilized purified recombinant sLDLRCR1-7 (0.5 μg/well) was incubated with purified FVIII (•) (0-500 nM), VWF (○) (0-500 nM), or FIX (▪) (0-500 nM). Bound FVIII, VWF, or FIX was detected by incubation with peroxidase-conjugated monoclonal antibodies CLB-CAg 12, CLB-RAg 56, and CLB-FIX 11, respectively. (B) Immobilized purified recombinant sLDLRCR1-7 (0.5 μg/well) was incubated with purified FVIII (150 nM) in the absence or presence of increasing concentrations of recombinant RAP (0-3 μM). (C) Immobilized purified recombinant LRP cluster 2 (0.5 μg/well) was incubated with FVIII (20 nM) in the absence or presence of increasing concentrations of LRP cluster 2 (○) (0-0.5 μM) or sLDLRCR1-7 (•) (0-4 μM). Residual FVIII binding was detected as described. Data represent the mean ± SD of 3 separate experiments.
Binding of FVIII, VWF, and FIX to recombinant sLDLRCR1-7. (A) Immobilized purified recombinant sLDLRCR1-7 (0.5 μg/well) was incubated with purified FVIII (•) (0-500 nM), VWF (○) (0-500 nM), or FIX (▪) (0-500 nM). Bound FVIII, VWF, or FIX was detected by incubation with peroxidase-conjugated monoclonal antibodies CLB-CAg 12, CLB-RAg 56, and CLB-FIX 11, respectively. (B) Immobilized purified recombinant sLDLRCR1-7 (0.5 μg/well) was incubated with purified FVIII (150 nM) in the absence or presence of increasing concentrations of recombinant RAP (0-3 μM). (C) Immobilized purified recombinant LRP cluster 2 (0.5 μg/well) was incubated with FVIII (20 nM) in the absence or presence of increasing concentrations of LRP cluster 2 (○) (0-0.5 μM) or sLDLRCR1-7 (•) (0-4 μM). Residual FVIII binding was detected as described. Data represent the mean ± SD of 3 separate experiments.
Adenovirus-mediated overexpression of human LDLR in mice accelerates FVIII clearance
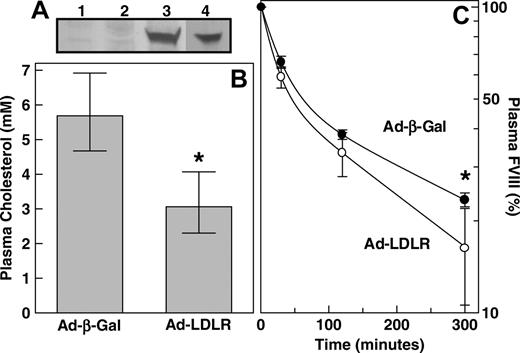
The ability of FVIII to bind to LDLR in vitro (Figure 3) prompts the question whether LDLR is able to clear FVIII from the circulation in vivo. To this end, we used adenovirus-mediated gene transfer to induce hepatic overexpression of human LDLR. We injected an adenovirus containing the human LDLR cDNA (Ad-CMV-LDLR) or control adenovirus encoding the β-galactosidase cDNA (Ad-CMV-β-Gal). It was verified that human LDLR was indeed expressed in the livers of mice that received Ad-CMV-LDLR (Figure 4A). As expected,23 Ad-CMV-LDLR completely reversed the hypercholesterolemic effects in LDLR–/– mice (P = .002) (Figure 4B). Five days after adenovirus administration, plasma FVIII levels were 1.0 U/mL (68% CI, 0.8-1.2 U/mL) and 0.9 U/mL (68% CI, 0.6-1.3 U/mL) in Ad-CMV-β-Gal– and Ad-CMV-LDLR–treated C57BL/6J mice, respectively (P = 1.000). Subsequently, the same mice received intravenous bolus injections of human FVIII, and its clearance rate from plasma was monitored. The plasma removal of FVIII in Ad-CMV-LDLR–treated mice was slightly accelerated compared with removal in control animals that received Ad-CMV-β-Gal (Figure 4C). The MRT of FVIII was calculated to be 205 minutes in control Ad-CMV-β-Gal–treated mice and 141 minutes in mice that received Ad-CMV-LDLR, with 68% CIs of 194 to 217 and 109 to 183 minutes, respectively (P = .01). These data indicate that LDLR has the potential to contribute to the clearance of FVIII from plasma in vivo.
FVIII clearance after adenovirus-mediated overexpression of human LDLR in mice. (A) Liver membrane extracts of wild-type mice that received Ad-CMV-β-Gal (n = 2) (lanes 1-2) or Ad-CMV-LDLR (n = 2) (lanes 3-4) were subjected to 4% to 15% SDS-PAGE analysis under nonreducing conditions. Human LDLR expression was detected by immunoblotting, using a rabbit anti–human LDLR antibody and the ECL system. (B) LDLR–/– mice were intravenously injected with 2 × 109 plaque-forming units Ad-CMV-β-Gal (n = 5) or Ad-CMV-LDLR (n = 5). Five days after adenovirus injection, blood was drawn and plasma was analyzed for plasma cholesterol. (C) Wild-type C57BL/6J mice were intravenously injected with 2 × 109 plaque-forming units Ad-CMV-β-Gal (•) (n = 4) or Ad-CMV-LDLR (○) (n = 6). Five days after adenovirus injection, animals were intravenously injected with purified human FVIII (20 IU), and its plasma removal was monitored at indicated time points. Data represent geometric mean values and 68% confidence intervals. *P < .05, significantly different from that of control Ad-CMV-β-Gal–treated mice; Mann-Whitney U test.
FVIII clearance after adenovirus-mediated overexpression of human LDLR in mice. (A) Liver membrane extracts of wild-type mice that received Ad-CMV-β-Gal (n = 2) (lanes 1-2) or Ad-CMV-LDLR (n = 2) (lanes 3-4) were subjected to 4% to 15% SDS-PAGE analysis under nonreducing conditions. Human LDLR expression was detected by immunoblotting, using a rabbit anti–human LDLR antibody and the ECL system. (B) LDLR–/– mice were intravenously injected with 2 × 109 plaque-forming units Ad-CMV-β-Gal (n = 5) or Ad-CMV-LDLR (n = 5). Five days after adenovirus injection, blood was drawn and plasma was analyzed for plasma cholesterol. (C) Wild-type C57BL/6J mice were intravenously injected with 2 × 109 plaque-forming units Ad-CMV-β-Gal (•) (n = 4) or Ad-CMV-LDLR (○) (n = 6). Five days after adenovirus injection, animals were intravenously injected with purified human FVIII (20 IU), and its plasma removal was monitored at indicated time points. Data represent geometric mean values and 68% confidence intervals. *P < .05, significantly different from that of control Ad-CMV-β-Gal–treated mice; Mann-Whitney U test.
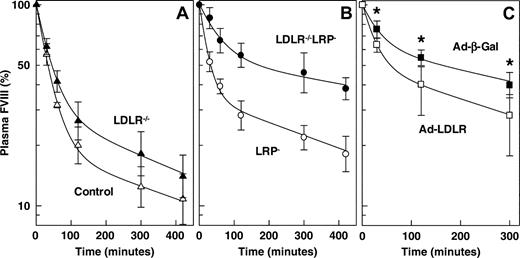
FVIII clearance in mice that lack LRP, LDLR, or both. At 4 weeks after the final pI:pC injection, purified human FVIII (20 IU) was intravenously administered into (A) control mice (▵) (n = 5) and LDLR–/– mice (▴) (n = 5) and into (B) LRP– mice (○) (n = 5) and LDLR–/– LRP– mice (•) (n = 5). Human FVIII removal from plasma was monitored at indicated time points. (C) LDLR–/–LRP– mice were intravenously injected with 2 × 109 plaque-forming units Ad-CMV-β-Gal (▪) (n = 8) or Ad-CMV-LDLR (□) (n = 8). Five days after adenovirus injection, animals were intravenously injected with purified human FVIII (20 IU), and its plasma removal was monitored at indicated time points. *P < .01, significantly different from that of control Ad-CMV-β-Gal–treated mice; Mann-Whitney U test. Data represent geometric mean values and 68% confidence intervals.
FVIII clearance in mice that lack LRP, LDLR, or both. At 4 weeks after the final pI:pC injection, purified human FVIII (20 IU) was intravenously administered into (A) control mice (▵) (n = 5) and LDLR–/– mice (▴) (n = 5) and into (B) LRP– mice (○) (n = 5) and LDLR–/– LRP– mice (•) (n = 5). Human FVIII removal from plasma was monitored at indicated time points. (C) LDLR–/–LRP– mice were intravenously injected with 2 × 109 plaque-forming units Ad-CMV-β-Gal (▪) (n = 8) or Ad-CMV-LDLR (□) (n = 8). Five days after adenovirus injection, animals were intravenously injected with purified human FVIII (20 IU), and its plasma removal was monitored at indicated time points. *P < .01, significantly different from that of control Ad-CMV-β-Gal–treated mice; Mann-Whitney U test. Data represent geometric mean values and 68% confidence intervals.
FVIII clearance in LDLR and LRP knockout mice
To investigate whether the increased plasma FVIII levels in LDLR–/–LRP– mice resulted from a slower rate of FVIII elimination from the circulation, we studied the plasma disappearance of intravenously injected human FVIII in control, LRP–, LDLR–/–, and LDLR–/–LRP– double-knockout mice. FVIII clearance was slightly, but not statistically significantly, slower in LDLR–/– mice (1.25-fold) than in control mice (Figure 5A). The MRT of FVIII was calculated to be 160 minutes in control mice and 200 minutes in LDLR–/– mice, with 68% CIs of 117 to 218 and 154 to 259 minutes, respectively (P = .222). The MRT of infused FVIII in LRP– mice was prolonged approximately 1.6-fold from 160 minutes (68% CI, 117-218 minutes) in control mice to 263 minutes (68% CI, 206-336 minutes) in LRP– mice (P = .032) (Figure 5B). In contrast, combined receptor deficiency resulted in an approximately 4.8-fold prolongation of the MRT compared with control mice (P = .008) (Figure 5B). The MRT in LDLR–/–LRP– mice was calculated to be 760 minutes, with a 68% CI of 691 to 836 minutes. This is compatible with the observed FVIII plasma levels in the different genotypes (Figure 1; Table 1). If LDLR contributes to FVIII catabolism, one would expect that the delayed clearance of FVIII in LDLR–/–LRP– mice is rescued after reintroduction of LDLR by adenovirus-mediated gene transfer. Indeed, whereas FVIII clearance in LDLR–/–LRP– mice was not affected by Ad-CMV-β-Gal, administration of Ad-CMV-LDLR to these mice significantly accelerated FVIII clearance, almost resembling the observed FVIII clearance pattern in LRP-deficient mice (Figure 5C). These data indicate that, in the absence of hepatic LRP, LDLR plays a predominant role in the clearance of FVIII from the circulation.
No relation between plasma FVIII and cholesterol levels in mice
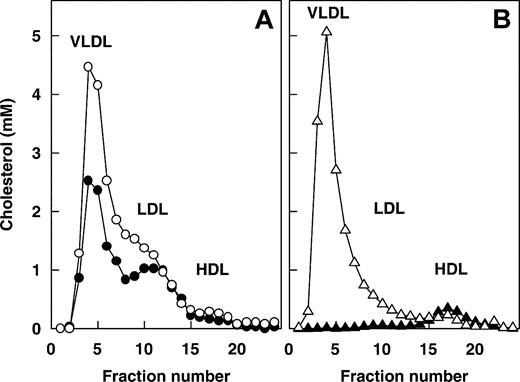
It has been well established that LDLR and LRP are of major physiologic importance in plasma lipoprotein metabolism.6 This is illustrated by increased plasma cholesterol and triglyceride levels in mice that lack LDLR, which becomes more evident when LRP and LDLR are absent in combination (Table 1), leading to the question whether the increase in plasma FVIII levels in LDLR–/– LRP– double-knockout mice is secondary to increased lipoprotein plasma levels. To address this possibility, we used well-described mice models of hyperlipidemia. These include mice that genetically lack apoE, either on the LDLR–/– or the LDLR–/– LRP– background,36,37 and mice that constitutively overexpress human apoC1.30 The hyperlipidemic lipoprotein distribution profiles of ApoE–/–LDLR–/– and ApoE–/–LDLR–/–LRP– mice are comparable in that both genotypes display elevated VLDL and LDL cholesterol (Figure 6A).37 Plasma cholesterol and triglyceride levels were clearly increased in ApoE–/–LDLR–/–LRP– and ApoE–/– LDLR–/– mice (Table 2). In contrast, plasma FVIII was elevated in ApoE–/–LDLR–/–LRP– mice but not in the ApoE–/–LDLR–/– mice (Table 2). Additionally, plasma FVIII levels in ApoE–/–LDLR–/–LRP– mice (Table 2) were not different (P = .699) from those of LDLR–/–LRP– mice (Table 1), indicating that the apoE status does not affect FVIII levels in plasma. To further investigate the relation between FVIII and lipoproteins, we used human apoC1 transgenic mice (huAPOCI) that have increased plasma levels of cholesterol and triglyceride-rich VLDL (Figure 6B).30 Although huAPOCI mice had increased cholesterol and triglyceride levels, plasma FVIII levels were lower in huAPOCI mice than in nontransgenic controls (Table 2). Taken together, these data support our observation that LDLR–/– mice showed elevated plasma levels of mainly apoB/E-containing LDL cholesterol and triglycerides,6,23 whereas FVIII levels remained unaffected (Table 1). We conclude that elevated plasma FVIII in LDLR–/–LRP– double-knockout mice is not secondary to increases in apoE, apoB, cholesterol, or triglyceride levels or to changes in lipoprotein distribution but that it is directly attributed to LDLR deficiency.
No relation between plasma FVIII and cholesterol or triglyceride levels in mice
Mice . | No. . | Cholesterol, mean mM (68% CI) . | Triglycerides, mean mM (68% CI) . | FVIII, mean U/mL (68% CI) . |
|---|---|---|---|---|
| ApoE-/-LDLR-/-LRP- | 8 | 16.2 (11.5-22.9) | 1.0 (0.7-1.6) | 4.2 (3.3-5.5) |
| ApoE-/-LDLR-/- | 10 | 23.3 (17.6-31.0)* | 2.1 (1.4-3.1)* | 1.6 (1.4-1.8)* |
| Nontransgenic controls | 6 | 2.1 (1.9-2.2) | 0.3 (0.3-0.4) | 1.0 (0.9-1.1) |
| HuAPOCI | 6 | 19.5 (16.4-23.2)† | 36.9 (27.4-45.4)† | 0.5 (0.3-0.8)† |
Mice . | No. . | Cholesterol, mean mM (68% CI) . | Triglycerides, mean mM (68% CI) . | FVIII, mean U/mL (68% CI) . |
|---|---|---|---|---|
| ApoE-/-LDLR-/-LRP- | 8 | 16.2 (11.5-22.9) | 1.0 (0.7-1.6) | 4.2 (3.3-5.5) |
| ApoE-/-LDLR-/- | 10 | 23.3 (17.6-31.0)* | 2.1 (1.4-3.1)* | 1.6 (1.4-1.8)* |
| Nontransgenic controls | 6 | 2.1 (1.9-2.2) | 0.3 (0.3-0.4) | 1.0 (0.9-1.1) |
| HuAPOCI | 6 | 19.5 (16.4-23.2)† | 36.9 (27.4-45.4)† | 0.5 (0.3-0.8)† |
Blood was drawn from ApoE-/-LDLR-/-LRP-, ApoE-/-LDLR-/-, huAPOCI transgenic, and nontransgenic control mice. Plasma samples were analyzed for cholesterol, triglycerides, and FVIII, as described in “Materials and methods.” FVIII levels of huAPOCI transgenic and nontransgenic control mice were assayed using pooled C57BL/6J plasma as a reference, whereas those of ApoE-/-LDLR-/-LRP- and ApoE-/-LDLR-/- mice were expressed against pooled LRPflox/flox plasma. Data represent geometric mean values with 68% confidence intervals in parentheses.
P < .05, significantly different from ApoE-/-LDLR-/-LRP- mice; Mann-Whitney U test.
P < .05, significantly different from nontransgenic controls; Mann-Whitney U test.
Discussion
In the present study, we show that the level of coagulation FVIII in the circulation is regulated by a dual-receptor system that involves not only LRP but also LDLR. This is compatible with our previous observation that RAP-sensitive determinants other than hepatic LRP also contribute to the catabolism of FVIII in vivo.13 Our findings that LDLR interacts with FVIII in vitro (Figure 3) and that LDLR plays a role in the clearance of FVIII from the circulation (Figures 4, 5) are remarkable because thus far only 2 ligands in plasma have been identified for this receptor, apoE and apoB-100.1,2 This is in marked contrast to LRP, for which more than 35 ligands have been identified to date.2,4 The identification of FVIII as a novel ligand of LDLR suggests that in addition to its well-established function in lipoprotein metabolism, this receptor may play a previously unrecognized role in modulating hemostasis.
Lipoprotein distribution. Blood was drawn from (A) ApoE–/–LDLR–/–LRP– (•) and ApoE–/–LDLR–/– (○) mice and from (B) huAPOCI transgenic (▵) and nontransgenic control (▴) mice. Lipoproteins were size fractionated by FPLC. Individual fractions were analyzed for cholesterol.
Lipoprotein distribution. Blood was drawn from (A) ApoE–/–LDLR–/–LRP– (•) and ApoE–/–LDLR–/– (○) mice and from (B) huAPOCI transgenic (▵) and nontransgenic control (▴) mice. Lipoproteins were size fractionated by FPLC. Individual fractions were analyzed for cholesterol.
The importance of LDLR and LRP in FVIII catabolism is illustrated by our observation that combined deficiency of this dual-receptor system in mice resulted in an approximately 4.2-fold increase of FVIII levels in plasma (Table 1). The relative contribution of both receptors to FVIII catabolism remains unclear. Strikingly, mice that lack LDLR or LRP alone displayed FVIII levels that were normal or that were elevated no more than 1.6-fold, respectively (Table 1). This implies that LDLR can functionally compensate for LRP deficiency and vice versa. The same view emerges from the pharmacokinetic experiments in these mice (Figure 5). Whereas the MRT of intravenously administered FVIII was 4.8-fold prolonged in LDLR–/–LRP– double-knockout mice, the effects of single-receptor deficiencies were relatively minor, providing additional evidence that the elimination of FVIII from the circulation involves the concerted action of LRP and LDLR.
The in vivo catabolism of FVIII shows some intriguing dissimilarities compared with that of lipoproteins.6,23,32 First, LRP-deficient mice have slightly elevated plasma FVIII levels but normal cholesterol-rich lipoprotein levels, whereas LDLR knockout mice display the opposite (Table 1). Second, though VLDLR plays a major role in the metabolism of postprandial lipoproteins in vivo,38 this FVIII-binding receptor does not contribute to the clearance of FVIII from the circulation alone15 or in concert with LDLR and LRP. This latter conclusion is based on our observations that LDLR–/–LRP– mice (Table 1; Figure 5) display similar plasma FVIII and FVIII clearance rates compared with VLDLR–/–LDLR–/–LRP– mice. These triple-knockout mice had FVIII plasma levels of 4.6 U/mL (68% CI, 3.7-5.7 U/mL) (n = 12) and a FVIII MRT of 674 minutes (68% CI, 587-774 minutes) (n = 5). Apparently, members of the LDLR family of endocytic receptors display differential specificity for their ligands in vivo. FVIII catabolism involves a complex multistage process in which circulating FVIII is first captured by heparan sulfate proteoglycans (HSPGs) onto the cell surface.39 This is compatible with a model in which HSPG-mediated enrichment of FVIII on the plasma membrane results in its subsequent internalization by LRP and LDLR. A similar catabolic route has been proposed for apoE.40 Possibly, differences in affinity of FVIII, apoE, and apoB for LDLR, LRP, VLDLR, and HSPG constitute an important determinant for the specificity of LDLR receptor family members in vivo.7,8,39,41 This might further be related to the markedly distinct endocytosis rates reported for LDLR family members (LRP greater than LDLR greater than VLDLR).42
Another new observation from this study is that elevated plasma FVIII levels in LRP-deficient and LDLR–/–LRP– double-knockout mice coincide with increased levels of its carrier protein VWF (Table 1). Because VWF has been reported not to be a ligand for LRP7,8 or LDLR (Figure 3) in vitro, it remains difficult to explain why VWF is increased in these mice. Although in humans an increase of the VWF concentration in plasma often results in a concomitant increase of FVIII, this is not the case in mice.13,43,44 One might conclude that the observed increases in VWF levels are the direct result of elevated plasma FVIII levels. However, further research is required to elucidate the molecular mechanism behind the elevated levels of plasma VWF in mice that lack hepatic LRP and LDLR.
High levels of FVIII in plasma (greater than 1.5 U/mL) constitute a major risk factor for arterial and venous thrombosis in humans.10,11 Our observation that the up-regulation of hepatic LDLR protein expression in mice by gene transfer accelerated FVIII clearance from the circulation (Figures 4 and 5) may be of therapeutic interest for patients who have elevated plasma FVIII levels. In humans, the up-regulation of LDLR protein is achieved by treatment with 3-hydroxy-3-methylglutaryl co-enzyme A (HMG-CoA) reductase inhibitors, also called statins.3 Statins are widely recognized in the treatment of hypercholesterolemia in humans.3 It would be interesting to study whether statins have the potential to lower the elevated levels of plasma FVIII in humans, with the goal of reducing the risk for thrombotic events.
Prepublished online as Blood First Edition Paper, April 19, 2005; DOI 10.1182/blood-2004-11-4230.
Supported by a fellowship from the Royal Netherlands Academy of Art and Sciences (B.J.M.v.V.) and by grant 0104 from the Landsteiner Foundation for Blood Transfusion Research (K.M., B.J.M.v.V.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Mrs M. Voskuilen, Dr S.M.S. Espirito Santo, and Dr J.F.P. Berbée for technical assistance. We thank Dr K. Willems van Dijk for providing the adenovirus encoding human LDLR and Dr P.C.N. Rensen for access to the huAPOCI transgenic mouse model.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal