Abstract
Although the chromosomal localization (9q34) of the gene encoding the human form of ADAMTS13 (a disintegrin-like and metalloproteinase with thrombospondin type-1 motifs 13) and its exclusive expression in the liver have been established, the cells that produce this enzyme are yet to be determined. We investigated the expression of ADAMTS13 mRNA and protein in fresh frozen specimens obtained during liver biopsies of 8 patients with liver diseases. In situ hybridizations to localize ADAMTS13 mRNA showed positive signals exclusively in perisinusoidal cells with irregularly elongated dendritic processes extending between hepatocytes. Furthermore, ADAMTS13 was detected immunohistochemically in perisinusoidal cells, whereas no staining was observed in hepatocytes. The positive cells varied in shape from unipolar to dendritic with irregularly elongated cytoplasmic processes, features common to hepatic stellate cells (HSCs). Double-labeling experiments revealed that the ADAMTS13-positive cells also expressed α-smooth muscle actin, confirming that these cells were activated HSCs. These results suggest that HSCs may be major cells producing ADAMTS13 in human liver.
Introduction
ADAMTS13 (a disintegrin-like and metalloproteinase with thrombospondin type-1 motifs 13) is a metalloproteinase that specifically cleaves the multimeric von Willebrand factor (VWF) between Tyr1605 and Met1606 within the VWF A2 domain.1-4 VWF is synthesized in vascular endothelial cells, and released into the plasma as unusually large VWF multimers (UL-VWFMs).5,6 Usually, UL-VWFMs are rapidly degraded into smaller VWF multimers by ADAMTS13. Deficiency of ADAMTS13 caused either by mutations of the ADAMTS13 gene1,7 or by inhibitory autoantibodies against ADAMTS138,9 increases the plasma levels of UL-VWFMs, which leads to platelet clumping and/or thrombi under high shear stress, resulting in thrombotic thrombocytopenic purpura (TTP).5-9
Northern blot analysis indicated that the 4.6-kilobase ADAMTS13 mRNA was exclusively expressed in the liver, and a 2.4-kilobase ADAMTS13 mRNA was also expressed in placenta and skeletal muscle.2 In situ hybridization analysis revealed that the mRNA signals were expressed exclusively in the perisinusoidal cells,10 but without addressing the type of cells expressing ADAMTS13. Moreover, a substantial decrease of plasma ADAMTS13 activity in patients with chronic liver disease has been associated with its disease progression, but not always with the serum levels of enzymes produced by hepatocyte.11 Thus, specification and/or localization of the cells that produce this enzyme in the liver should have clinical importance, and may help elucidate the pathogenesis of sinusoidal microcirculatory disturbances and/or thrombotic complications in patients with liver diseases.
In this study, we have clearly shown that ADAMTS13 is produced specifically in hepatic stellate cells, formerly called Ito cells, by both in situ hybridization techniques and immunohistochemical analysis using 2 novel mouse monoclonal antibodies specific for ADAMTS13.
Study design
Patients
This study examined 8 patients with liver disease (6 women and 2 men; mean age, 54.6 years; range, 43-72 years) including 4 patients with hepatitis C virus (HCV)–related chronic hepatitis, one patient with hepatitis B virus–related chronic hepatitis, one patient with primary biliary cirrhosis, one patient with autoimmune hepatitis, and one patient with a drug-induced liver injury who had undergone laparoscopies or percutaneous needle biopsies. Laboratory findings of these patients showed well-preserved functional liver capacity and platelet counts (mean, 18.3 × 104/mm3; range, 8.8-32.3 × 104/mm3). Informed consent was provided by the patients and their families before the biopsies. The protocol used in this study was approved by the Nara Medical University Hospital Ethics Committee, Nara, Japan.
Production and characterization of anti-ADAMTS13 murine monoclonal antibodies
Full-length wild-type recombinant (r) ADAMTS13 that was purified by anti-FLAG (fludarabine, cytarabine, and granulocyte colony–stimulating factor) M2 agarose affinity chromatography (Sigma, Saint Louis, MO) was used as an immunogen12 to produce monoclonal antibodies (A10 and C7) against ADAMTS13 in mice following standard procedures.13 The immunoglobulin subclasses of A10 and C7 were IgG2b-κ and IgG1-κ, respectively. These antibodies were purified with a Protein A column (Amersham Biosciences, Uppsala, Sweden). These 2 monoclonal antibodies were able to detect endogenous plasma ADAMTS13 as a 190-kDa/180-kDa doublet band by Western blot analysis under nonreducing conditions. The epitopes recognized by the A10 and C7 antibodies were determined to reside in the disintegrin-like domain, and the seventh and eighth thrombospondin type-1 domains, respectively. Detailed characterizations of these antibodies are shown as supplementary data (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article).
Immunohistochemistry
Fresh liver specimens obtained from the 8 patients were fixed in 4% paraformaldehyde solution before frozen sections were prepared. The sections were incubated with primary antibodies (A10 and C7) overnight at 4°C. Bound primary antibodies were subsequently immunodetected using a standard avidin-biotin-peroxidase complex technique. To simultaneously detect A10- and anti–α-smooth muscle actin (α-SMA) immunoreactivity, sequential incubations using the following reagents were performed: A10 antibodies and anti–α-SMA antibodies (DAKO, Kyoto, Japan) followed by Alexa 488–conjugated anti–mouse immunoglobulin G (Invitrogen, Carlsbad, CA) and Alexa 546–conjugated anti–rabbit IgG (Molecular Probes). These labeled sections were observed with a Nikon Labphoto-2 fluorescent microscope and imaged with an MRC-600 confocal laser-scanning microscope system (Bio Rad Laboratories, Tokyo, Japan). Figures were assembled using Confocal Assistant software (Bio Rad Laboratories).
In situ hybridization
The cDNA encoding human ADAMTS13 was kindly provided by Dr Kenji Soejima (Chemo-Thero-Therapeutic Institute, Kumamoto, Japan). Digoxygenin (DIG)–labeled cRNA probes (sense and antisense) were transcribed using either T3 (sense) or T7 (antisense) RNA polymerase and a plasmid with an insert corresponding to nucleotides 3710-4237 of the full-length human ADAMTS13 transcript (Genbank accession no. AB069 698). All prehybridization procedures have been previously described.14 To visualize the DIG-labeled probes, the sections were incubated with alkaline-phosphatase–conjugated anti-DIG antibodies (Roche Diagnostics KK, Tokyo, Japan) followed by 4-nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl-phosphate solution (Roche).
Results and discussion
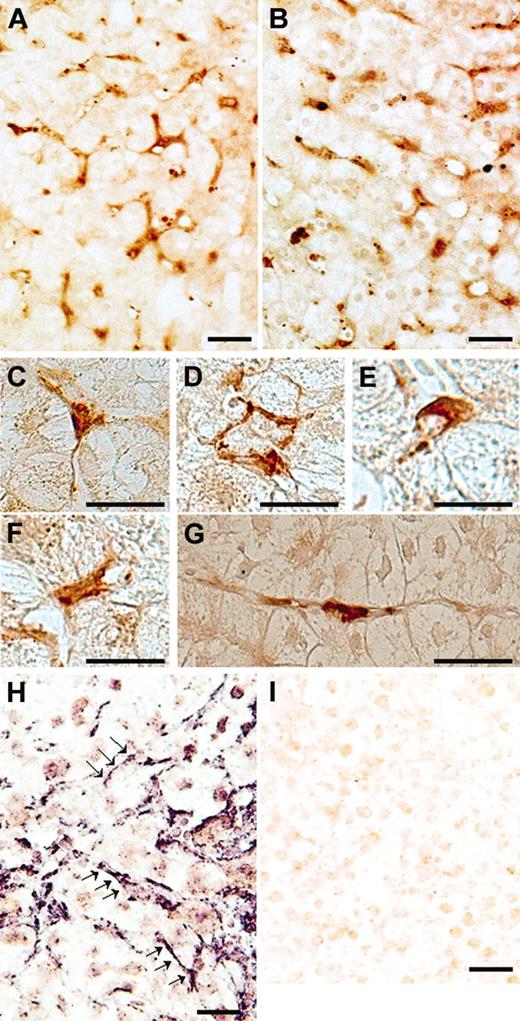
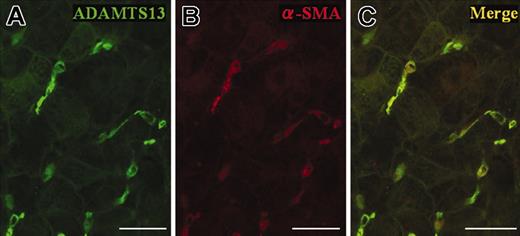
When immunostaining was performed with A10 antibodies on liver specimens from a patient with HCV-related chronic hepatitis, dense brown staining was observed in perisinusoidal cells inside the lobule (Figure 1A). Furthermore, when the same liver section was stained with C7 antibodies, staining patterns were similar to those of A10 antibodies (Figure 1B). The varied morphologic features of these positive cells were consistent with those previously described for perisinusoidal stellate cells (Figure 1C-G).15,16 Control specimens treated with unspecific mouse IgG instead of primary antibodies displayed no significant staining (data not shown). In situ hybridizations using antisense probes for ADAMTS13 revealed strongly positive labeling only in perisinusoidal cells with irregularly elongated cytoplasmic processes extending between hepatocytes (Figure 1H), whereas control specimens treated with sense probes showed no significant staining (Figure 1I). These ADAMTS13 mRNA–positive cells resembled HSCs morphologically, suggesting that HSCs produce ADAMTS13. In order to evaluate whether the ADAMTS13-positive cells truly would be HSCs, we performed double immunofluorescence immunohistochemistry with A10 anti-ADAMTS13 antibodies and anti–α-SMA antibodies. The microfilament protein α-SMA has been recognized as a specific marker for stellate cells17 ; activated stellate cells in pathologic livers are strongly α-SMA–positive, whereas in normal adult human liver, some stellate cells are α-SMA–positive.18 Immunofluorescence labeling with anti-ADAMTS13 antibodies showed intense green fluorescence in perisinusoidal cells that were irregular in shape with spotty, oval, unipolar, and bipolar cytoplasmic processes extending between hepatocytes (Figure 2A). α-SMA antibodies revealed that these cells were also positive for the HSC marker (Figure 2B). Colocalization of ADAMTS13 and α-SMA in single cells clearly indicate that stellate cells produce ADAMTS13 in the liver (Figure 2C). Considering that a similar staining pattern was obtained with both A10 and C7 antibodies against ADAMTS13 using the same liver sections (Figure 1A-B), it would seem that the HSCs may produce full-length ADAMTS13 protein.
ADAMTS13 protein and mRNA expression in frozen sections of a liver specimen from a patient with hepatitis C–related chronic hepatitis. Immunostaining using ADAMTS13-specific monoclonal antibodies (A10) showed dense brown staining in perisinusoidal cells, but not in hepatocytes, inside the lobule (A). When the same liver section was stained with C7 antibodies, staining patterns were similar to those of A10 antibodies (B). The positive cells had a variety of morphologic forms. Examples shown here include a tripolar cell with long processes extending between hepatocytes (C), combined cells surrounding a hepatocyte (D), a dome-shaped cell (E), a bipolar cell with short processes (F), and a unipolar cell with long processes (G). Panels A-B: original magnification, × 200; panels C-G: original magnification, × 400. ADAMTS13 mRNA expression was examined using in situ hybridization. Strongly positive labeling was seen only in perisinusoidal cells, which were unipolar to dendritic in shape with irregularly elongated cytoplasmic processes extending between hepatocytes (H, arrows; original magnification, × 200). When sense probes were used, we observed no significant staining (I; original magnification, × 200). (Bar = 30 μM in A-I.)
ADAMTS13 protein and mRNA expression in frozen sections of a liver specimen from a patient with hepatitis C–related chronic hepatitis. Immunostaining using ADAMTS13-specific monoclonal antibodies (A10) showed dense brown staining in perisinusoidal cells, but not in hepatocytes, inside the lobule (A). When the same liver section was stained with C7 antibodies, staining patterns were similar to those of A10 antibodies (B). The positive cells had a variety of morphologic forms. Examples shown here include a tripolar cell with long processes extending between hepatocytes (C), combined cells surrounding a hepatocyte (D), a dome-shaped cell (E), a bipolar cell with short processes (F), and a unipolar cell with long processes (G). Panels A-B: original magnification, × 200; panels C-G: original magnification, × 400. ADAMTS13 mRNA expression was examined using in situ hybridization. Strongly positive labeling was seen only in perisinusoidal cells, which were unipolar to dendritic in shape with irregularly elongated cytoplasmic processes extending between hepatocytes (H, arrows; original magnification, × 200). When sense probes were used, we observed no significant staining (I; original magnification, × 200). (Bar = 30 μM in A-I.)
Confocal laser-scanning immunofluorescence microscopy using frozen sections of a liver specimen from a patient with hepatitis C–related chronic hepatitis. Immunofluorescence labeling with anti-ADAMTS13 antibodies showed intense green fluorescence in the perisinusoidal cells, which were irregular in shape with spotty, oval, unipolar, and bipolar cytoplasmic processes extending between the hepatocytes (A). Red fluorescence indicated α-SMA immunoreactivity, reflecting the fact that these cells were activated HSCs (B). Colocalization of ADAMTS13 (A) and α-SMA (B) in single cells yielded yellow color in the merged figure (C). (Original magnification, × 400; bar = 30 μM in A-C.)
Confocal laser-scanning immunofluorescence microscopy using frozen sections of a liver specimen from a patient with hepatitis C–related chronic hepatitis. Immunofluorescence labeling with anti-ADAMTS13 antibodies showed intense green fluorescence in the perisinusoidal cells, which were irregular in shape with spotty, oval, unipolar, and bipolar cytoplasmic processes extending between the hepatocytes (A). Red fluorescence indicated α-SMA immunoreactivity, reflecting the fact that these cells were activated HSCs (B). Colocalization of ADAMTS13 (A) and α-SMA (B) in single cells yielded yellow color in the merged figure (C). (Original magnification, × 400; bar = 30 μM in A-C.)
HSCs have many functions, including vitamin A storage, liver fibrogenesis, and regulation of sinusoidal blood flow. These cells are also rich sources of bioactive mediators for maintaining homeostasis in the microenvironment of the hepatic sinusoid.17 HSCs are located in the space of Disse adjacent to endothelial cells. It is, therefore, of particular interest that HSCs produce ADAMTS13. In patients with liver cirrhosis, a remarkably high level of plasma VWF has been noted.11,19 Immunostaining with anti-VWF antibodies has shown the presence of this protein in the sinusoidal lining cells and at the scar-parenchyma interface in cases of liver cirrhosis.20 This is particularly evident in the sinusoids of patients at the early stages of alcoholic liver diseases,21 indicating the capillarization of the sinusoidal endothelial cells. Considering that ADAMTS13 is synthesized in the HSCs and its substrate, UL-VWFM, is produced in transformed vascular endothelial cells, the deficiency of plasma ADAMTS13 activity in liver diseases may play an important role in sinusoidal microcirculatory disturbances and subsequent development of liver injury. It will be necessary to clarify the intralobular heterogeneity of ADAMTS13 expression in HSCs associated with the activity of plasma ADAMTS13 in different stage of liver diseases.
Prepublished online as Blood First Edition Paper, April 26, 2005; DOI 10.1182/blood-2005-01-0152.
Supported in part by research grants from the Japanese Ministry of Education, Culture, and Science (Y.F. and M.M.) and from the Ministry of Health and Welfare of Japan for Blood Coagulation Abnormalities H14-02 (Y.F.).
M.U., K.T., and A.W. performed immunostaining and in situ hybridization and prepared this manuscript. T. Matsuyama, M.I., and M.F. collected liver specimens. M.M., T.I., and T. Mori prepared murine anti-ADAMTS13 monoclonal antibody and characterized it. H.F. directed this study. Y.F. designed and directed this study throughout.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal