Abstract
Cyclin-dependent kinase (CDK) inhibitors have the potential to induce cell-cycle arrest and apoptosis in cancer cells. Seliciclib (CYC202 or R-roscovitine) is a potent CDK inhibitor currently undergoing phase-2 clinical testing in lung and B-cell malignancies. Here we studied the in vitro cytotoxic activity of seliciclib against multiple myeloma (MM) cells. Our data demonstrate that seliciclib has potent cytotoxicity against MM cells that are both sensitive and resistant to conventional therapy as well as primary MM cells from patients. Cell-cycle and Western blot analysis confirmed apoptosis. Importantly, seliciclib triggered a rapid down-regulation of Mcl-1 transcription and protein expression independent of caspase cleavage. Adherence of MM cells to bone marrow stromal cells (BMSCs) induced increased Mcl-1 expression associated with signal transducer and activator of transcription 3 (STAT3) phosphorylation, which was inhibited in a time- and dose-dependent manner by seliciclib. Furthermore, seliciclib inhibited interleukin 6 (IL-6) transcription and secretion triggered by tumor cell binding to BMSCs. Up-regulation of Mcl-1 expression in cocultures was only partially blocked by neutralizing antibody to IL-6, suggesting alternative mechanisms of Mcl-1 modulation by seliciclib. Finally, combination studies of seliciclib with doxorubicin and bortezomib show in vitro synergism, providing the rationale for testing these drug combinations to improve patient outcome in MM.
Introduction
It is estimated that there will be 15 270 new cases of multiple myeloma (MM) diagnosed in the United States and 11 070 deaths attributed to this disease in 2004 alone.1 A major challenge in the treatment of MM is the development of resistance to conventional therapies. This resistance can be attributed to defects in apoptotic signaling, overexpression of multidrug resistance (MDR) genes, cytokines such as interleukin 6 (IL-6) and insulin-like growth factor 1 (IGF-1), and the interaction of MM cells with the bone marrow (BM) microenvironment resulting in cell adhesion–mediated drug resistance (CAM-DR).2-7 Therapies targeting not only the MM cell, but more importantly the BM microenvironment, interfere with these resistance mechanisms and can achieve responses in patients resistant to conventional therapy. Despite several important and exciting advances with novel biologic agents such as bortezomib,8-10 thalidomide,11,12 and CC-501313 in the last few years, MM remains incurable due to the development of relapsed/refractory disease in the majority of patients. Given that novel agents have shown marked antitumor activity as single agents, it is our hypothesis that combining these and other newer classes of drugs will result in enhanced cytotoxicity, abrogate drug resistance, and ultimately improve patient outcome.
In an attempt to overcome drug resistance, we have studied seliciclib (CYC202 or R-roscovitine; Cyclacel, Dundee, United Kingdom), a small-molecule cyclin-dependent kinase (CDK) inhibitor in MM. In the present study, we asked (1) whether the CDK inhibitor seliciclib affects MM cell viability, (2) whether seliciclib overcomes the protective effect of the BM microenvironment, and (3) whether combining seliciclib with other agents enhances cytotoxicity and overcomes drug resistance. This study demonstrates that seliciclib induces apoptosis in MM cells sensitive and resistant to conventional therapy at clinically achievable concentrations. It abrogates growth and survival of MM cells adherent to bone marrow stromal cells (BMSCs) via inhibition of expression of the antiapoptotic protein myeloid cell leukemia 1 (Mcl-1) and phosphorylation of the signal transducer and activator of transcription 3 (STAT3). Furthermore, our data suggest that the modulation of Mcl-1 by seliciclib is, at least in part, due to inhibition of IL-6 secretion in the BM milieu triggered by tumor cell binding to BMSCs. Finally, combination studies with doxorubicin and bortezomib suggest strong in vitro synergism, providing the rationale for clinical trials of these agents in patients with MM.
Materials and methods
Cell culture and reagents
Dexamethasone (Dex)–sensitive (MM.1S) and Dex-resistant (MM.1R) human MM cell lines were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL). Doxorubicin-resistant (Dox-40) and melphalan-resistant (LR5) RPMI 8226 human MM cells were kindly provided by Dr William Dalton (Moffitt Cancer Center, Tampa, FL). The OPM 2 cell line was obtained from Dr Lief Bergsagel (Weill Medical Center, Cornell University, New York, NY) and the U266 cell line was obtained from the American Type Culture Collection (Rockville, MD). All MM cell lines were cultured in RPMI 1640 media (Sigma Chemical, Saint Louis, MO) containing 10% fetal bovine serum, 2 mM l-glutamine (Gibco, Grand Island, NY), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). BM aspirates of patients with MM were obtained after informed consent was obtained per the Declaration of Helsinki and approval by the institutional review board of the Dana Farber Cancer Center (Boson, MA) was granted. The samples were processed by Ficoll Paque gradient and mononuclear cells (MNCs) were separated. MM cells were separated by negative selection as previously described.14 For the generation of BMSCs, MNCs were placed in 25-mm2 culture flasks in RPMI 1640 media (Sigma Chemical) containing 20% fetal bovine serum, 2 mM l-glutamine (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). Once confluent, the cells were trypsinized and passaged as needed. For the experiments, BMSCs were incubated in 96-well culture plates (approximately 5000-10 000 BMSCs/well) for 24 hours or grown to confluence in 6-well plates. The medium was washed off and MM cells were added to the wells (2 × 104 cells/well) and incubated with media or with increasing concentrations of seliciclib for the specified times at 37°C.
Seliciclib (CYC202 or R-roscovitine)
Seliciclib was obtained from Cyclacel (Dundee, United Kingdom). The drug was dissolved in dimethyl sulfoxide (DMSO; Sigma Chemical) at a concentration of 200 mM and stored at -20°C until use; it was diluted in culture medium (1-100 μM, < 0.1% DMSO in the final concentration) immediately before use and was used within 4 hours.
Cell viability and proliferation assays
Colorimetric assays were performed to assay drug activity at increasing concentrations of seliciclib. Cells from 24-hour cultures were pulsed with 10 μL of 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT; Chemicon International, Temecula, CA) to each well; the 96-well plates were incubated at 37°C for 4 hours, followed by 100 μL isopropanol containing 0.04 N HCl. Absorbance was read at a wave length of 570 nm (with correction using readings at 630 nm) on a spectrophotometer (Molecular Devices, Sunnyvale, CA).
Studies with combinations of bortezomib (1-4 nM) and doxorubicin (100-500 nM) were similarly performed in 24-hour cultures, and cytotoxicity was determined by MTT.
DNA synthesis was measured by tritiated thymidine uptake (3H-TdR; Perkin Elmer, Boston, MA) as previously described.15 Briefly, MM cells (2-3 × 104 cells/well) were incubated in 96-well culture plates (Costar, Cambridge, MA) in the presence of media or varying concentrations of seliciclib for 24 hours at 37°C. For evaluation of the effect of growth factors, recombinant IL-6 (10 ng/mL), vascular endothelial growth factor (VEGF; 25 ng/mL), or IGF-1 (50 ng/mL) was added to the wells at the beginning of the incubation to obtain the final concentration indicated. Cells were pulsed with 3H-TdR (0.5 μCi/well; 0.0185 MBq) during the last 8 hours of 24-hour cultures, harvested onto glass filters with an automatic cell harvester (Cambridge Technology, Cambridge, MA), and counted by using the LKB Betaplate scintillation counter (Wallac, Gaithersburg, MD). All experiments were performed in triplicate.
Seliciclib treatment decreases viability of MM cells in a dose-dependent manner. The effect of seliciclib on viability of MM cells was determined by MTT assays. MM cell lines sensitive (MM.1S, OPM2, RPMI, U266) and resistant (Dox-40, LR5, MM1.R) to conventional therapies, as well as patient MM cells, were cultured in the presence of increasing doses of seliciclib (0-100 μM) for 24 hours. Seliciclib resulted in dose-dependent cytotoxicity, with an IC50 ranging from 15 to 25 μM at 24 hours.
Seliciclib treatment decreases viability of MM cells in a dose-dependent manner. The effect of seliciclib on viability of MM cells was determined by MTT assays. MM cell lines sensitive (MM.1S, OPM2, RPMI, U266) and resistant (Dox-40, LR5, MM1.R) to conventional therapies, as well as patient MM cells, were cultured in the presence of increasing doses of seliciclib (0-100 μM) for 24 hours. Seliciclib resulted in dose-dependent cytotoxicity, with an IC50 ranging from 15 to 25 μM at 24 hours.
Cell-cycle analysis and detection of apoptosis
MM cells (1 × 106) were cultured for the specified times in media alone or with 25 μM seliciclib. The cells were harvested, washed with ice-cold phosphate-buffered saline (PBS), fixed with 70% ethanol for 1 hour, and pretreated with 10 μg/mL RNAse (Sigma Chemical) for 1 hour. Cells were stained with propidium iodide (PI; 5 μg/mL; Sigma Chemical), and cell-cycle profile was determined by using the RXP cytomics software on an Epics flow cytometer (Coulter Immunology, Hialeah, FL), as in prior studies. MM cells were also incubated for 24 hours with increasing concentrations of seliciclib, and the cell-cycle profile was determined.
Western blotting
MM cells were cultured with seliciclib (25 μM) for the specified times, harvested, washed, and lysed using lysis buffer (50 mM HEPES [N-2-hydroxyethylpiperazine-N′2-ethanesulfonic acid], pH 7.4, 150 mM NaCl, 1% Triton-X 100, 30 mM sodium pyrophosphate, 5 mM EDTA [ethylenediaminetetraacetic acid, 2 mM Na3VO4, 5 mM NaF, 1 mM phenylmethyl sulfonyl fluoride [PMSF], 5 μg/mL leupeptin, and 5 μg/mL aprotinin). For detection of apoptosis-related proteins, cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membrane, and immunoblotted with antibodies against cleaved poly(adenosine diphosphate [ADP]–ribose) polymerase (PARP) and cleaved caspase 8 (Cell Signaling Technology, Beverly, MA). The membrane was stripped and reprobed with antitubulin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) to ensure equal protein loading.
To characterize molecular mechanisms of action, immunoblotting was done with anti–cyclin A, cyclin B1, cyclin D3, and cyclin H (Santa Cruz Biotechnology) and antiphospho-STAT3 antibodies (Cell Signaling Technology). Antiapoptotic proteins including Mcl-1 (Santa Cruz Biotechnology) and the B-cell lymphoma (BCL) family proteins were similarly probed. Antigen-antibody complexes were detected by using enhanced chemiluminescence (Amersham, Arlington Heights, IL). Blots were stripped and reprobed with antitubulin antibody to ensure equal protein loading. Z-VAD-FMK (N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketane) (25 μM) (Bachem, Bubendorf, Switzerland) was used to pretreat MM cells for 2 hours before addition of seliciclib to block caspase activation. Pretreatment of cells for 6 hours with neutralizing antibody to human IL-6 (10 μg/mL; R&D Systems, Minneapolis, MN) was used to define the role of IL-6.
RT-PCR
Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed on 2 μg total RNA extracted with TRIzol (Invitrogen Life Technologies, Carlsbad, CA) and reverse transcribed by Moloney murine leukemia virus-reverse transcriptase (MMLV-RT; Invitrogen Life Technologies). Then, 1 μg cDNA was amplified using human Mcl-1 primers (5′-CCGCTTGAGGAGATGGAAG-3′ and 5′-CCAACCCGTCGTAAGGTCT-3′; Invitrogen Life Technologies) and human IL-6 (hIL-6) primers (R&D Systems). Human glyceraldehyde-3-phosphate dehydrogenase (hGAPDH) primers were used as control (5′-CCCTCCAAAATCAAGTGGGG-3′ and 5′-CGCCACAGTTTCCCGGAGGG-3′). PCR profiles for human Mcl-1 included one cycle of denaturation at 94°C for 5 minutes, followed by 25 cycles at 94°C for 60 seconds, 58°C (annealing temperature [Ta]) for 60 seconds, and 72°C for 30 seconds. Detection of hIL-6 was set at one cycle of denaturation at 94°C for 4 minutes, followed by 35 cycles at 94°C for 45 seconds, 55°C Ta for 45 seconds, 72°C for 45 seconds, and 72°C for 10 minutes. For hGAPDH control the PCR was set at one cycle of denaturation at 95°C for 90 seconds, followed by 18 cycles at 94°C for 30 seconds, 55°C Ta for 30 seconds, and 72°C for 60 seconds. The PCR products were separated by electrophoresis on 1.5% agarose gels and stained with ethidium bromide. The intensity of bands was measured by a transilluminator and Image J Analysis Software (using the public-domain NIH Image program developed by the US National Institutes of Health; available at http://RSB.info.nih.gov/nih-image) was used to convert band area into pixels.
ELISA
The effect of seliciclib on cytokine secretion by human BMSCs, alone and cocultured with MM cells, was studied using an enzyme-linked immunosorbent assay (ELISA) for IL-6. BMSCs were harvested and cultured in 96-well plates with varying concentrations of seliciclib, with or without MM1.S cells. After a 24-hour incubation, the supernatants were harvested and stored at 70°C until measurement. IL-6 secretion was measured using Duoset ELISA development kits (R&D Systems). All measurements were done in triplicate.
Results
Seliciclib treatment decreases viability of MM cells in a dose-dependent manner
The effect of seliciclib on viability of MM cells was determined by MTT assays. MM cell lines sensitive (MM.1S, OPM2, RPMI, U266) and resistant (Dox-40, LR5, MM1.R) to conventional therapies, as well as MM cells from patients, were cultured in the presence of increasing doses of seliciclib (0-100 μM) for 24 hours. As demonstrated in Figure 1, seliciclib resulted in dose-dependent cytotoxicity with inhibition concentration of 50% (IC50) ranging from 15 to 25 μM at 24 hours. Exposure of MM cells to seliciclib for 48 and 72 hours did not show any added cytotoxicity, suggesting maximum drug effect at 24 hours (data not shown). 3H-TdR uptake studies were also performed on MM cells cultured in the presence of IL-6, VEGF, and IGF-1. Seliciclib was able to induce cytotoxicity against MM cells and overcome the protective effects of all 3 cytokines (data not shown).
Seliciclib treatment induces apoptosis of MM cells in a time- and dose-dependent manner
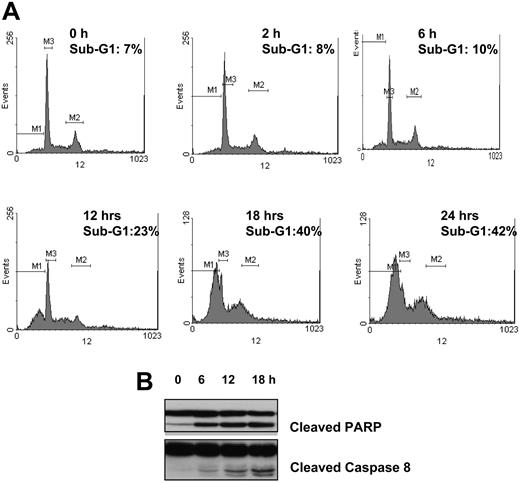
To further characterize the cytotoxic effect of seliciclib on MM cells, we performed cell-cycle analysis on MM1.S cells cultured with media alone or seliciclib (25 μM) for the specified times. As demonstrated in Figure 2A, seliciclib resulted in an increase in sub-G1 cells at 12 hours with maximal effect noted at 24 hours (42% sub-G1 fraction). This was associated with an increase in caspase 3 (data not shown), PARP, and caspase 8 cleavage (Figure 2B). There was no evidence of growth arrest, even at very early time points.
Seliciclib treatment down-regulates Mcl-1, associated with inhibition of phosphorylation of STAT3 and independent of caspase cleavage
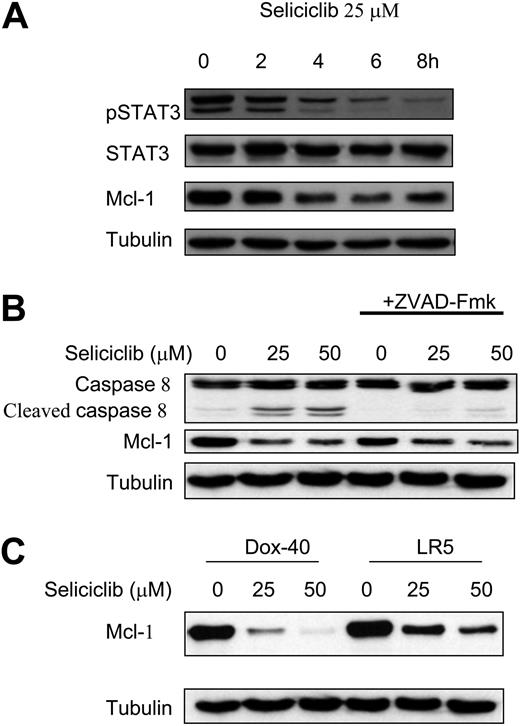
We next examined the molecular mechanisms by which seliciclib triggers apoptosis in MM cells. MM1.S cells were incubated with seliciclib (25 μM) for 2, 4, 6, and 8 hours; cell lysates were prepared as previously described.15 Seliciclib treatment resulted in down-regulation of Mcl-1, associated with a time-dependent inhibition of phosphorylated STAT3 (pSTAT3) (Figure 3A). To confirm that Mcl-1 down-regulation was prior to caspase cleavage, we treated cells with Z-VAD-FMK (25 μM) for 2 hours before treatment with increasing concentrations of seliciclib (6 hours). As demonstrated in Figure 3B, seliciclib resulted in down-regulation of Mcl-1 despite blocking caspase activity, suggesting that down-regulation of Mcl-1 was not due to caspase cleavage. We next examined the effect of seliciclib on resistant MM cells. As shown in Figure 3C, increasing doses of seliciclib decreased expression of Mcl-1 in doxorubicin-resistant Dox-40 and melphalan-resistant LR5 MM cells at 6 hours. The effect of seliciclib on the expression of various cyclins was evaluated. Seliciclib inhibited cyclin A, B1, and D3 in a time-dependent manner, without any effect on cyclin H (data not shown).
Seliciclib overcomes the protective effects conferred by binding of MM cells to BMSCs
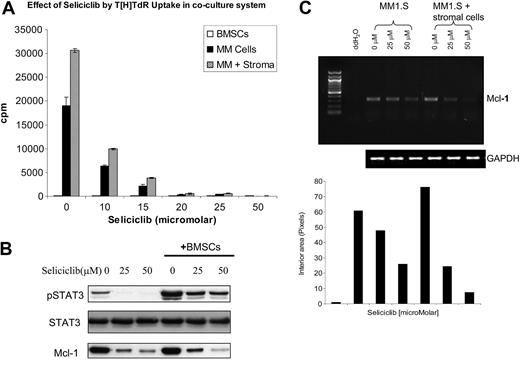
We next examined the effects of seliciclib on MM cells cultured in the presence of BMSCs. As shown in Figure 4A, seliciclib inhibited DNA synthesis of adherent MM cells at 24 hours in a dose-dependent manner, which was further confirmed by MTT assays (data not shown). Mcl-1 transcription and protein expression, as well as phosphorylation of STAT3, were up-regulated in the cocultures at 6 hours; importantly, increasing doses of seliciclib for 6 hours inhibited Mcl-1 transcription and decreased both Mcl-1 protein expression and phosphorylation of STAT3 (Figure 4B-C).
Seliciclib treatment induces apoptosis of MM cells in a time- and dose-dependent manner. Cell-cycle analysis by PI staining was performed on MM1.S cells cultured with media alone or seliciclib (25 μM) for the specified time points. Seliciclib resulted in an increase in sub-G1 fraction as early as 12 hours, with 42% of the cells in sub-G1 phase at 24 hours (A). This was associated with an increase in PARP and caspase 8 cleavage (B). M1 indicates sub-G1 gate; M2, G2M gate; and M3, G1 gate.
Seliciclib treatment induces apoptosis of MM cells in a time- and dose-dependent manner. Cell-cycle analysis by PI staining was performed on MM1.S cells cultured with media alone or seliciclib (25 μM) for the specified time points. Seliciclib resulted in an increase in sub-G1 fraction as early as 12 hours, with 42% of the cells in sub-G1 phase at 24 hours (A). This was associated with an increase in PARP and caspase 8 cleavage (B). M1 indicates sub-G1 gate; M2, G2M gate; and M3, G1 gate.
Seliciclib treatment results in down-regulation of Mcl-1, associated with inhibition of STAT3 phosphorylation and independent of caspase cleavage, in MM cells sensitive and resistant to conventional therapies. MM1.S cells were incubated with seliciclib (25 μM) for 2, 4, 6, and 8 hours; seliciclib treatment resulted in down-regulation of Mcl-1, associated with time-dependent inhibition of pSTAT3 (A). Pretreatment of MM cells with Z-VAD-FMK followed by seliciclib for 6 hours resulted in down-regulation of Mcl-1 despite blocking caspase activity (B), suggesting that Mcl-1 down-regulation is independent of caspase cleavage. Mcl-1 protein expression was also down-regulated in resistant cell lines (Dox-40 and LR5) by seliciclib treatment for 6 hours (C).
Seliciclib treatment results in down-regulation of Mcl-1, associated with inhibition of STAT3 phosphorylation and independent of caspase cleavage, in MM cells sensitive and resistant to conventional therapies. MM1.S cells were incubated with seliciclib (25 μM) for 2, 4, 6, and 8 hours; seliciclib treatment resulted in down-regulation of Mcl-1, associated with time-dependent inhibition of pSTAT3 (A). Pretreatment of MM cells with Z-VAD-FMK followed by seliciclib for 6 hours resulted in down-regulation of Mcl-1 despite blocking caspase activity (B), suggesting that Mcl-1 down-regulation is independent of caspase cleavage. Mcl-1 protein expression was also down-regulated in resistant cell lines (Dox-40 and LR5) by seliciclib treatment for 6 hours (C).
Seliciclib inhibits Mcl-1 transcription, at least in part, by inhibiting IL-6 transcription and secretion in the BM microenvironment
To further characterize the molecular mechanism of Mcl-1 down-regulation in MM cells triggered by seliciclib, we studied the effects of seliciclib on IL-6. Seliciclib was able to inhibit tumor cell adhesion–induced IL-6 secretion, as demonstrated by ELISA, at 24 hours (Figure 5A). IL-6 transcripts were down-regulated, as shown by RT-PCR in MM 1.S cells alone, as well as in the presence of BMSCs, by increasing doses of seliciclib for 6 hours (Figure 5B). Addition of exogenous IL-6 (10 ng/mL) resulted in up-regulation of Mcl-1, which was inhibited by seliciclib (25 μM for 6 hours; Figure 5C). Addition of IL-6 neutralizing antibody (10 μg/mL) only partially down-regulated Mcl-1 expression, suggesting alternative mechanisms of Mcl-1 modulation by seliciclib (Figure 5D).
Seliciclib overcomes the protective effects conferred by BMSCs on MM cells via down-regulation of Mcl-1 transcription. MM 1.S cells were cultured in the presence of BMSCs. (A) Seliciclib resulted in inhibition of DNA synthesis of MM cells in the presence of BMSCs at 24 hours in a dose-dependent manner. Data represent the mean ± standard deviation of triplicate cultures. (B) Mcl-1 and pSTAT3 protein expression were both up-regulated in the coculture system and were inhibited by increasing doses of seliciclib for 6 hours. (C) Repression of Mcl-1 transcription was similarly noted by RT-PCR after 6 hours of exposure to seliciclib.
Seliciclib overcomes the protective effects conferred by BMSCs on MM cells via down-regulation of Mcl-1 transcription. MM 1.S cells were cultured in the presence of BMSCs. (A) Seliciclib resulted in inhibition of DNA synthesis of MM cells in the presence of BMSCs at 24 hours in a dose-dependent manner. Data represent the mean ± standard deviation of triplicate cultures. (B) Mcl-1 and pSTAT3 protein expression were both up-regulated in the coculture system and were inhibited by increasing doses of seliciclib for 6 hours. (C) Repression of Mcl-1 transcription was similarly noted by RT-PCR after 6 hours of exposure to seliciclib.
Seliciclib inhibits Mcl-1 transcription, at least in part, by inhibiting IL-6 transcription in the BM microenvironment. (A) Seliciclib inhibits MM1.S cell adhesion induced IL-6 secretion by BMSCs at 24 hours, assessed by ELISA. Data represent the mean ± standard deviation of triplicate cultures. (B) IL-6 transcripts were also down-regulated, as shown by RT-PCR. (C) Addition of exogenous IL-6 (10 ng/mL) resulted in up-regulation of Mcl-1, which was down-regulated by seliciclib (25 μM for 6 hours). (D) Addition of IL-6 neutralizing antibody (10 μg/mL) partially down-regulated Mcl-1 expression.
Seliciclib inhibits Mcl-1 transcription, at least in part, by inhibiting IL-6 transcription in the BM microenvironment. (A) Seliciclib inhibits MM1.S cell adhesion induced IL-6 secretion by BMSCs at 24 hours, assessed by ELISA. Data represent the mean ± standard deviation of triplicate cultures. (B) IL-6 transcripts were also down-regulated, as shown by RT-PCR. (C) Addition of exogenous IL-6 (10 ng/mL) resulted in up-regulation of Mcl-1, which was down-regulated by seliciclib (25 μM for 6 hours). (D) Addition of IL-6 neutralizing antibody (10 μg/mL) partially down-regulated Mcl-1 expression.
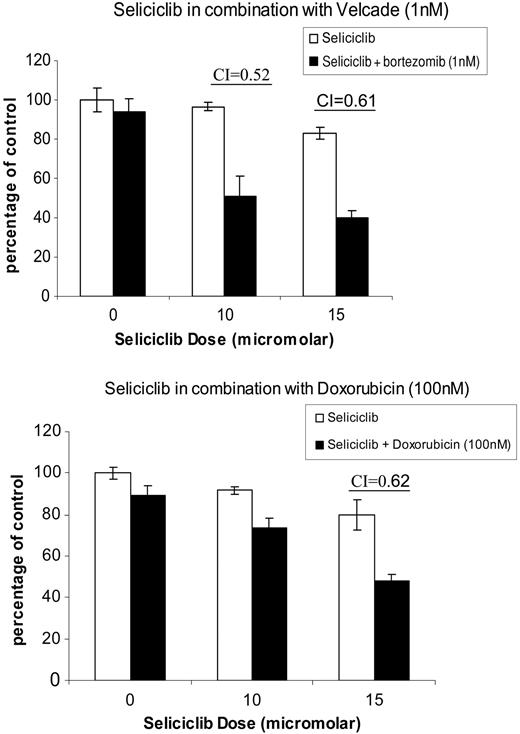
Seliciclib has synergistic anti-MM activity when combined with doxorubicin and bortezomib. Increasing concentrations of seliciclib were added with bortezomib (1-4 nM) and doxorubicin (100-500 nM); MM.1S cytotoxicity was assayed by MTT. Synergism was confirmed by applying the Chou-Talalay method to calculate a CI. Data for bortezomib (1 nM) and doxorubicin (100 nM) are represented, with maximum synergism noted at concentrations of 10 to 15 μM seliciclib and a CI of less than 1. Data represent the mean ± standard deviation of triplicate cultures.
Seliciclib has synergistic anti-MM activity when combined with doxorubicin and bortezomib. Increasing concentrations of seliciclib were added with bortezomib (1-4 nM) and doxorubicin (100-500 nM); MM.1S cytotoxicity was assayed by MTT. Synergism was confirmed by applying the Chou-Talalay method to calculate a CI. Data for bortezomib (1 nM) and doxorubicin (100 nM) are represented, with maximum synergism noted at concentrations of 10 to 15 μM seliciclib and a CI of less than 1. Data represent the mean ± standard deviation of triplicate cultures.
Seliciclib has synergistic anti-MM activity with doxorubicin and bortezomib
We next examined seliciclib in combination protocols with bortezomib and doxorubicin, based on the rationale that low doses of both drugs result in growth arrest and that combining them with seliciclib might result in induction of apoptosis. Increasing concentrations of seliciclib were added to MM1.S cells with bortezomib (1-4 nM) and doxorubicin (100-500 nM) and MM cell cytotoxicity was assayed by MTT (Figure 6). Synergism was confirmed by applying the Chou-Talalay method to calculate a combination index (CI), as previously described.15,16 Data for bortezomib (1 nM) and doxorubicin (100 nM) are represented in Figure 6 with maximum synergism noted at concentrations of 10 to 15 μM seliciclib, with a CI of less than 1.
Discussion
Genetic heterogeneity and the lack of a single pathognomonic genetic lesion makes targeted treatment a challenge in MM. It has been hypothesized that at least one of the cyclins, D1, D2, or D3, is overexpressed in hyperdiploid MM cells, accounting for approximately 30% of patients with MM. Up-regulation of the cyclin genes renders MM cells more responsive to proliferative stimuli and thereby susceptible to additional genetic events, leading to a more malignant phenotype. This postulated critical role of cyclin D dysregulation in MM pathogenesis makes the cyclins attractive therapeutic targets.7 Seliciclib is a small-molecule CDK inhibitor currently undergoing phase-2 clinical testing in lung and B-cell hematologic cancers including MM. Seliciclib or R-roscovitine is a purine analog that competes with adenosine triphosphate (ATP) for its binding site on CDKs. It induces apoptosis in a wide variety of tumor cell lines. Based on in vitro kinase assays, it has specificity against CDKs, acting on CDK2/cyclin A, CDK2/cyclin E, CDK7/cyclin H, and CDK9/cyclinT1.17-23 Its activity against several CDKs may be one of the mechanisms by which it preferentially results in apoptosis, as opposed to growth arrest, of cancer cells. Here we study the in vitro effects and molecular mechanisms of action of seliciclib against MM cells alone and bound to BMSCs. Importantly, our studies show that seliciclib induces apoptosis of MM cells in the BM milieu.
Our data demonstrate that seliciclib inhibits transcription and protein expression of Mcl-1 in MM cells. Mcl-1 is an important antiapoptotic protein belonging to the Bcl-2 family and is present in most MM cell lines and patient cells.24,25 It differs from the other Bcl-2 family proteins due to its short half-life and protects cells from cytotoxic stimuli by conferring drug resistance. The Mcl-1 gene is located on chromosome 1q21, a region frequently involved in genetic abnormalities in a wide range of cancers including MM. Its regulation by cytokines such as IL-6, tumor necrosis factor α (TNF-α), and VEGF have been previously described, and gene expression profiling studies have identified it as a potential target in MM.24-27 Moreover, specific inhibition of Mcl-1 by antisense oligonucleotides has resulted in apoptosis of MM cells28 ; it plays a critical role in modulation of mitochondrial apoptotic events, such as cytochrome c release and caspase activation.29,30 Down-regulation of transcription, as well as protein expression of Mcl-1, by seliciclib was a primary event in our studies; it was not secondary to caspase cleavage and apoptosis. The survival advantage conferred by Mcl-1 in MM cells was therefore lost, resulting in caspase-mediated apoptosis. Importantly, seliciclib down-regulated Mcl-1 and decreased MM cell viability, even in MM cells resistant to doxorubicin (Dox-40) and melphalan (LR5).
MM cells home to the BM and adhere to the BMSCs, extracellular matrix (ECM) proteins, and other cells within the BM milieu.7 This interaction triggers a cascade of pleiotropic signaling events mediating growth, survival, and migration of MM cells. Specifically, adhesion of MM cells to ECM proteins results in CAM-DR.3 Our data demonstrate an increase in the transcription and protein expression of Mcl-1 induced by adherence of MM cells to BMSCs, which was down-regulated in a dose- and time-dependent fashion by seliciclib.
IL-6 activates the Janus kinase (JAK)/STAT pathway, which plays an important role in MM cell survival.31,32 In particular, STAT3-binding motifs are present in the promoter region of Mcl-1, which therefore is regulated by IL-6.25 Our studies demonstrate that the inhibition of Mcl-1 by seliciclib was associated with down-regulation of pSTAT3, similar to inhibition of other STAT family proteins described in other cell systems.33 Because IL-6 activates the JAK/STAT pathway and also modulates Mcl-1, we next studied a possible role for IL-6 mediating Mcl-1 modulation by seliciclib. We demonstrate that seliciclib down-regulates IL-6 transcription and cytokine production triggered by MM cell binding to BMSCs. However, neutralizing antibody to IL-6 did not completely abrogate this down-regulation of Mcl-1, suggesting additional effects of seliciclib on Mcl-1 regulation. This is not surprising, given the heterogeneity of mechanisms modulating Mcl-1.24,25,34-36 Furthermore. CDK inhibition with other agents, such as flavopiridol, is also associated with Mcl-1 repression in MM.37 Specifically, Gojo et al37 demonstrated that flavopiridol treatment resulted in a dose-dependent inhibition of phosphorylation of the RNA polymerase II COOH-terminal domain (CTD), thus blocking transcription elongation and suggesting that Mcl-1 transcriptional repression may reflect a more global effect on transcription. Similarly, it has been previously demonstrated that seliciclib inhibits CDK7 and CDK9 thereby preventing RNA-polymerase II CTD phosphorylation, thus reducing transcription efficiency.38,39 Ongoing gene expression profiling studies are underway to address these alternate mechanisms of Mcl-1 modulation by seliciclib.
We studied seliciclib in combination with bortezomib and doxorubicin, based on our hypothesis that combining these and other newer classes of drugs would result in enhanced cytotoxicity and abrogate drug resistance. Both bortezomib and doxorubicin at low doses result in growth arrest; therefore combining these agents at low doses with seliciclib should result in enhanced cytotoxicity and apoptosis. In the present study, we have demonstrated marked synergism when low doses of both bortezomib and doxorubicin are combined with seliciclib. The synergism noted with seliciclib and bortezomib is reminiscent of previous studies demonstrating synergism of proteasome inhibitors with CDK inhibitors including flavopiridol and roscovitine in leukemia cell lines.40 Ongoing in vivo studies are underway to determine the exact sequence of administration of these drugs to maximize efficacy and minimize toxicity.
Our studies, therefore, demonstrate a molecular mechanism of antitumor activity of seliciclib against MM cells in the BM microenvironment. Our data support evaluation of seliciclib, alone and in combination with doxorubicin and bortezomib, to improve patient outcome in MM.
Prepublished online as Blood First Edition Paper, April 12, 2005; DOI 10.1182/blood-2005-01-0320.
Supported by the National Institutes of Health (NIH) Grant Specialized Programs of Research Excellence (SPORE) grants IP50 CA10070-01, PO-1 78378, and RO-1 CA 50947; Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.); Cure for Myeloma Research Fund (K.C.A.); and Veterans Administration Merit Review (N.C.M.). S.R.G. is an employee of Cyclacel Ltd, whose proprietary product, seliciclib, was used in this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal