Abstract
Hepatic transdifferentiation of bone marrow cells has been previously demonstrated by intravenous administration of donor cells, which may recirculate to the liver after undergoing proliferation and differentiation in the recipient's bone marrow. In the present study, to elucidate which cellular components of human bone marrow more potently differentiate into hepatocytes, we fractionated human bone marrow cells into mesenchymal stem cells (MSCs), CD34+ cells, and non-MSCs/CD34- cells and examined them by directly xenografting to allylalcohol (AA)-treated rat liver. Hepatocyte-like cells, as revealed by positive immunostaining for human-specific alpha-fetoprotein (AFP), albumin (Alb), cytokeratin 19 (CK19), cytokeratin 18 (CK18), and asialoglycoprotein receptor (AGPR), and by reverse transcription-polymerase chain reaction (RT-PCR) for expression of AFP and Alb mRNA, were observed only in recipient livers with MSC fractions. Cell fusion was not likely involved since both human and rat chromosomes were independently identified by fluorescence in situ hybridization (FISH). The differentiation appeared to follow the process of hepatic ontogeny, reprogramming of gene expression in the genome of MSCs, as evidenced by expression of the AFP gene at an early stage and the albumin gene at a later stage. In conclusion, we have demonstrated that MSCs are the most potent component in hepatic differentiation, as revealed by directly xenografting into rat livers. (Blood. 2005;106:756-763)
Introduction
Evidence has been accumulated to indicate that certain compartments of bone marrow cells are capable of differentiating into hepatocytes in vitro.
Schwartz et al1 demonstrated that multipotent adult progenitor cells (MAPCs)2,3 from the bone marrow of humans as well as mice and rats, when cultured with fibroblast growth factor-4 (FGF-4) and hepatocyte growth factor (HGF) in matrigel, secreted albumin, expressed P450, took up low-density lipoprotein (LDL), and stored glycogen.3 Lee et al reported that mesenchymal stem cells (MSCs) from human bone marrow and umbilical cord blood differentiated into hepatocyte-like cells with the use of HGF and oncostatin M.4,5 Most recently, Jang et al reported that hematopoietic stem cells (HSCs) from mice devoid of progenitors and selected for unique properties displayed a plasticity by which they became liver cells when cocultured with injured liver separated by a barrier.6 Thus, the origin of cells which may undergo hepatic differentiation as revealed by in vitro experiments were quite diverse, reflecting the sources, human or rodent, and methods of isolation.
The differentiation of bone marrow or umbilical cord blood derived cells into hepatocytes has also been demonstrated by in vivo transplantation procedures. In most of these transplantation studies, either isolated7-12 or clonally defined13-21 HSCs of donor origin, though their characteristics were not equally specified in each study, were found to induce hepatocytes in recipient liver, suggesting the differentiation potency of HSCs.
However, in these studies HSCs were generally introduced intravenously and were surmised to reside once in bone marrow where they may undergo proliferation and differentiation. Therefore, the cells distributed to the liver via bone marrow may not necessarily represent the original HSCs themselves.
In fact, some recent studies have disclosed that hepatocytes with apparent donor characters were the result of fusion of donor myelomonocytic cells differentiated from HSCs with host hepatocytes.20,22
Further, the results of these studies with HSCs do not exclude the possibility that other cell types in bone marrow such as MSCs are also capable of undergoing hepatic differentiation. Thus, issues still remained to be clarified as to which cellular component of bone marrow is most suitable to bring about hepatic regeneration in consideration of future clinical application. It also remains to be solved if any bone marrow components indeed differentiate in vivo without fusion.
In the present investigation, we attempted to examine the differentiation ability of fractionated human bone marrow components—MSCs, CD34+ cells, and non-MSCs/CD34- cells—into hepatocytes in vivo by directly inoculating them into rat livers which had sustained chronic damage by allylalcohol (AA) treatment,23 without allowing them to reside in bone marrow. The results obtained clearly indicated that MSCs were more potent than 2 other components in differentiating into hepatocytes without any evidence of fusion.
Materials and methods
Isolation of human MSCs, CD34+ cells, and non-hMSCs/CD34- cells from bone marrow
Isolation of human MSCs (hMSCs) was performed as previously described.24,25 Briefly, human bone marrow (BM) cells were obtained by aspiration from the posterior iliac crest of healthy adult volunteers (n = 6) after informed consent. Informed consent admitted by the Sapporo Medical University institutional review board was provided according to the Declaration of Helsinki. BM mononuclear cells were isolated with a density gradient (Percoll; 1.073 g/mL; Pharmacia, Peapack, NJ) and resuspended in complete culture medium: Dulbecco modified Eagle medium (DMEM; Gibco BRL, Rockville, MD), 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine (Gibco BRL).
For preparation of CD34+ cells, BM mononuclear cells from these volunteers were positively selected by means of a MACS Direct CD34 Progenitor Cell Isolation Kit (Miltenyi Biotech, Bergish-Gladbach, Germany) according to the manufacturer's instructions. More than 95% of the enriched cells were CD34+ as confirmed by flow cytometric analysis, and 1.1 plus or minus 0.2 × 106 CD34+ cells were recovered from each volunteer. CD34+ cells from all volunteers were mixed and then divided into 6 parts, of which 3 parts were injected into each of 3 rats in a group in the first set of experiments. The remaining 3 parts of CD34+ cells were used for 3 rats in the second set of experiments.
The remainder of the nucleated cells from 6 volunteers were plated in 20 mL medium in a 180-cm2 culture dish and incubated at 37°C with 5% CO2 for 24 hours. Then nonadherent cells (non-hMSCs/CD34- cells) from all volunteers were mixed in DMEM and divided into 6 parts, of which 3 parts were injected into each of 3 rats in a group in the first set of experiments and the other 3 parts were injected to each of 3 rats in a group in the second set of experiments. Adherent cells (hMSCs) from 6 volunteers were thoroughly washed twice with phosphate-buffered saline (PBS). Fresh complete medium was added and replaced every 3 or 4 days. After 10 to 14 days in culture, on reaching confluence, they were harvested and cryopreserved as primary hMSCs or replated at a density of 1 × 106 per 180-cm2 tissue culture dish (passage 1) until processing for injection. With each treatment of trypsin-EDTA (ethylenediaminetetraacetic acid; Sigma, St Louis, MO) and replating, the passage number was increased and represented approximately 3 population doublings (PDs). At the end of the fifth passage, hMSCs from 6 volunteers were mixed and divided into 20 parts, and then each part was injected into each rat in the first set of experiments (n = 10) and in second set of experiments (n = 10). Viability of CD34+ cells, non-hMSCs/CD34- cells, or hMSCs was more than 90%, as estimated by the trypan blue exclusion test.
Immunophenotyping of hMSCs, CD34+ cells, or non-hMSCs/CD34- cells
Flow cytometric analysis of hMSCs, CD34+ cells, and non-hMSCs/CD34- cells were performed on a FACScalibur flow cytometer (Becton Dickinson, Mountain View, CA) after labeling with respective antibodies directly conjugated with fluoroscein isothiocyanate (FITC) or phycoerythrin (PE) according to the manufacturer's recommendation. These included CD34, CD44, CD45 (Immunotech, Marseille, France), CD105 (SH-2; Ancell, Bayport, MN), CD117 (Immunotech), CD14, human leukocyte antigen (HLA) class I (Dako, Kyoto, Japan), CD133 (Miltenyi Biotech, Auburn, CA) and CD166 (ALCAM; Antigenix America, Huntington, NY). For indirect assays, cells were immunolabeled with anti-human CD73 (SH-3; Alexis Biochemicals, San Diego, CA). As the secondary antibody, goat anti-mouse immunoglobulin G (IgG)-PE (Immunotech) was used. As an isotype-matched control, mouse IgG1-FITC and IgG1-PE (Immunotech) was used.
Evaluation of the mesenchymal lineage differentiation of hMSCs
The differentiation potential of hMSCs (passage 5) into osteocytes, adipocytes, or chondrocytes was evaluated using differentiation-induction media purchased from Bio-Whittaker (Walkersville, MD) according to provided protocols.
Treatment of animals
Four- to 5-week-old female Sprague Dawley rats (Charles River, Tokyo, Japan) were used. All animal procedures were approved by the Sapporo Medical College Institutional Animal Care and Use Committee. In this experiment, chronic liver injury was induced in the rats. At day 0, the rats were administered intraperitoneal injections of AA diluted in normal saline at a dose of 0.62 mM/kg body weight. This dosage produces a uniform amount of acceptable periportal liver injury that is well tolerated (causing no deaths) by the rats.26 On the subsequent day (day 1), CD34+ cells, non-hMSCs/CD34- cells or culture-expanded (passage 5) hMSCs (approximately 1 × 106 cells suspended in 200 μL of DMEM) were directly transplanted into the left lateral lobe by intrahepatic injection using a 26-gauge syringe after median laparotomy under anesthesia. We then marked the point of injected lesion of the liver using a marking pen.At day 3, the rats were further administered intraperitoneal injections of AA at half doses (0.31 mM/kg) every third day until killed. All rats were immunosuppressed with a daily intraperitoneal injection of cyclosporin A (10 mg/kg/d), from 24 hours before the transplantation to the day of death. The animals were anesthetized with inhaled diethylether (Fisher Scientific, Fairlawn, NJ).
Immunohistochemical detection of liver-specific markers
Rat livers harvested at the indicated time points after transplantation and the liver surrounding the marked sites were immediately excised, and transversal slices were placed at 4°C in a tube with 4% paraformaldehyde, washed, dehydrated in 30% sucrose, then cryopreserved in optimal cutting temperature (OCT). Tissue sections (7 μm) were incubated with the following mouse monoclonal antibodies (mAbs): mAbs against human albumin (clone HAS-11, 1:1 000; Sigma, St Louis, MO); CK19 (clone A53-B/A2, 1:50; Sigma); cytokeratin 18 (CK18, clone RCK106, 1:50; PROGEN, Heidelberg, Germany); alpha-fetoprotein (AFP, clone C3, 1:500, Sigma); asialoglycoprotein receptor (AGPR, clone 30201, 1:50);27 CD68 (clone PG-M1, 1:100, Dako, Carpinteria, CA); and CD31 (clone JC/70A, 1:25, LabVision, Fremont, CA). These antibodies show no cross-reaction with rat liver in immunohistochemistry. For each staining, a negative control was performed either by addition of an appropriate nonimmune serum or by removal of the primary antibody from the protocol. After unbound primary antibodies were washed off with PBS, the sections were stained using the Dako LSAB2 peroxidase-based kit (Dako) specific for rat tissue, according to the manufacturer's instructions followed by the Dako Liquid DAB (diaminobenzidine) Substrate-Chromagen System kit. Finally, the tissue was counterstained with hematoxylin. Photomicrographs were obtained using an Axioscop microscope equipped with a PlanNEOFLUAR objective lens and an AxioCam color digital camera (Zeiss, Oberkochen, Germany). KS 400 version 3.0 software (Zeiss) was used to acquire images, and Adobe Photoshop version 7.0.1 (Adobe Systems, San Jose, CA) was used for imaging process.
Estimation of cell numbers
We estimated the number of hepatocytes in the sections based on the method described by Wang et al.28 Briefly, it was assumed that (1) the hepatocyte nodules are spheres; and (2) the number of cells found in the section of the largest clones in a sample represents a cross-section of the middle of that nodule. We then roughly estimated the number of cells in the clonal hepatocyte nodule based on these assumptions extrapolated from the cells at the maximal cross-section of nodules in the serial sections. For example, the maximal cross-section area of a 256-cell spherical nodule at the equator is 40.8 times larger than that of single cell (A =πr2), and the sphere diameter is 6.4 times larger than the diameter of single cell (V = 4/3πr3).
ELISA for human specific albumin
Plasma was collected for the human serum albumin assays, and enzyme-linked immunosorbent assay (ELISA) with an amplified biotin/streptavidin detection system that allows detection of human albumin in serum at less than 200 pg/mL was used according to the manufacturer's instructions (Cygnus Technologies, Plainville, MA).
Characterization of bone marrow-derived hMSCs, CD34+ cells, or non-hMSCs/CD34- cells. (A) FACS profiles of hMSCs, CD34+ cells, or non-hMSCs/CD34- cells stained with CD73 (PE-conjugated mAbs) and CD34 (FITC-conjugated mAbs). (B) Expression of surface antigens on hMSCs. (Left) The x-axis indicates CD45 expression labeled with FITC-conjugated mAbs; the y-axis indicates CD14 expression labeled with PE-conjugated mAbs. (Right) The x-axis indicates HLA class I expression labeled with PE-conjugated mAbs; the y-axis indicates CD105 expression labeled with FITC-conjugated mAbs. Numbers indicate the percentage of cells in each quadrant. (C) Expression of surface antigens on non-hMSCs/CD34- cells. The x-axis indicates CD45 expression labeled with FITC-conjugated mAbs; the y-axis indicates CD14 expression labeled with PE-conjugated mAbs. Cells were gated based on forward and side light scatter to exclude debris. Positivity for a surface antigen was defined using the isotype control monoclonal antibody. (D) Immunophenotype of hMSCs. Cells were harvested at passage 5, labeled with the antibodies specific for the indicated human surface antigens or negative controls, and analyzed by flow cytometry. Bar indicates positive reactivity with the indicated antigens. (E) hMSCs at passage 5 are induced to differentiate into osteoblasts and stain positive for alkaline phosphatase. Under adipogenic conditions, hMSCs differentiate into adipocytes, which are positively stained by Oil-Red staining. Under chondrogenic conditions, hMSCs differentiate into chrondrocyte-like cells and stained positively by Alcian blue. Original magnification, × 100. The results shown are representative of 2 experiments that had similar results.
Characterization of bone marrow-derived hMSCs, CD34+ cells, or non-hMSCs/CD34- cells. (A) FACS profiles of hMSCs, CD34+ cells, or non-hMSCs/CD34- cells stained with CD73 (PE-conjugated mAbs) and CD34 (FITC-conjugated mAbs). (B) Expression of surface antigens on hMSCs. (Left) The x-axis indicates CD45 expression labeled with FITC-conjugated mAbs; the y-axis indicates CD14 expression labeled with PE-conjugated mAbs. (Right) The x-axis indicates HLA class I expression labeled with PE-conjugated mAbs; the y-axis indicates CD105 expression labeled with FITC-conjugated mAbs. Numbers indicate the percentage of cells in each quadrant. (C) Expression of surface antigens on non-hMSCs/CD34- cells. The x-axis indicates CD45 expression labeled with FITC-conjugated mAbs; the y-axis indicates CD14 expression labeled with PE-conjugated mAbs. Cells were gated based on forward and side light scatter to exclude debris. Positivity for a surface antigen was defined using the isotype control monoclonal antibody. (D) Immunophenotype of hMSCs. Cells were harvested at passage 5, labeled with the antibodies specific for the indicated human surface antigens or negative controls, and analyzed by flow cytometry. Bar indicates positive reactivity with the indicated antigens. (E) hMSCs at passage 5 are induced to differentiate into osteoblasts and stain positive for alkaline phosphatase. Under adipogenic conditions, hMSCs differentiate into adipocytes, which are positively stained by Oil-Red staining. Under chondrogenic conditions, hMSCs differentiate into chrondrocyte-like cells and stained positively by Alcian blue. Original magnification, × 100. The results shown are representative of 2 experiments that had similar results.
FISH and immunofluorescence analysis
We performed incubation with the primary antibody before FISH to preserve the epitope and prevent damage during FISH pretreatment. Cryostat sections 7 μm in thickness were fixed 3 times, 10 minutes each, in Carnoy fixative. The slides were incubated with mouse anti-human albumin antibody (clone HAS-11, 1:500). After the primary antibody was washed off, the slides were pretreated at 37°C for 30 minutes in preheated 2 × standard saline citrate (SSC) buffer, pH 7.0. Serial ethanol dehydration was performed (1.5 minutes each), and the slides were air-dried at room temperature. Tissue was digested with proteinase K (10 μg/mL; Sigma) for 5 minutes at 37°C, washed with water and then rinsed in 2 × SSC for 3 minutes, air-dried and transferred through ice-cold 70%, 85%, and 100% ethanol, and air-dried again. Sections were denatured at 85°C for 2 minutes in preheated 70% formamide and 2 × SSC buffer, pH 7.0, and were then “quenched” with ice-cold 70% ethanol for 1.5 minutes. Serial ethanol dehydration was performed again. A mixture of rat 12-chromosome probe labeled with FITC (Rat Y/12 probe, Y Cy 3/12 FITC; we used a female rat, so only the 12 FITC probe reacted, STAR*FISH; Cambio, Cambridge, United Kingdom) and the human Y chromosome probe labeled with Cy 3 (STAR*FISH; Cambio) was heated to 72°C for 5 minutes, placed in a 37°C water bath for 3 hours to preanneal, and then applied to the sections at 45°C. The sections were coverslipped and sealed with rubber cement for incubation overnight in a hydrated slide box at 42°C. The next day, the coverslips were carefully removed in preheated 2 × SSC buffer, pH 7.0, at 45°C. The sections were washed twice in preheated 50% formamide in 2 × SSC buffer for 5 minutes each at 45°C and were then gently washed twice in preheated 0.1 SSC buffer for 5 minutes each at 45°C. The slides were then incubated with the anti-human albumin antibody (clone HAS-11; Sigma) again at a 1:500 dilution and subsequently with Alexa Fluor 488 (Molecular Probes, Eugene, OR). After washing, slides were mounted in a 4′, 6-diamidino-2-phenylindole (DAPI) antifade. Multicolored fluorescent staining of tissues were analyzed by confocal laser scanning microscopy using a Radiance2100 Rainbow (Bio-Rad, Hercules, CA) equipped with a Zeiss Axioskop 2 fluorescent microscope (Carl Zeiss MicroImaging, Thornwood, NY). Images were captured using the appropriate light absorption and emission filters supplied by the manufacturer of the microscope. Images were recorded using Lazer-Sharp-OS2 software (Bio-Rad), and data were subsequently processed using Adobe Photoshop (Adobe Systems). Counting of FISH signal-positive nuclei was accomplished by systematically examining the FISH-stained tissue, field by field, under a × 630 or × 1000 magnification.
Results
Surface antigens of the expanded hMSCs, CD34+ cells, or non-hMSCs/CD34- cells
Fluorescence-activated cell-sorting (FACS) analyses on a dot plot showed that the CD34+ cells were positive for CD34 and negative for the MSC marker CD73 (Figure 1A). The hMSCs (passage 5) were positive for CD73 and negative for CD34 (Figure 1A) and showed no contamination of CD45+ cells for HSCs, CD14+ cells for macrophages, monocytes, or granulocytes, and CD105 or HLA class I-negative cells for MAPC1,2 (Figure 1B). The non-hMSCs/CD34- cells were both negative for CD34 and CD73 (Figure 1A) and were positive for CD45 and CD14 (Figure 1C). The hMSCs (passage 5) expressed CD105 (SH2), CD 73 (SH3), CD29, CD44, CD166, and HLA class I, but not hematopoietic cells markers such as CD45, CD34, CD14, CD133, and CD117, in good agreement with the expression pattern of surface antigens on classical hMSCs that have been previously reported (Figure 1D).29-33
Differentiation potentiality of hMSCs into various mesenchymal lineage cells
Differentiation potentiality of the expanded hMSCs (passage 5) into classical mesenchymal lineage cells including osteoblasts, chondrocytes, or adipocytes was verified by using previously reported methods (Figure 1E).
Immunohistochemical and immunofluorescent staining patterns for hAFP and hAlb in the multiple AA-treated rat liver that received a transplant of human bone marrow cells. (A) Representative hematoxylin-eosin (HE) staining of multiple AA-treated rat liver. Mild periportal necroinflammation and fibrosis characterized by mild portal expansion were observed at day 28. Severe septal fibrosis and swelling of hepatocytes is diffusely observed at day 56. (B) Sections of injured rat liver (day 28) from rats not given transplants were not stained at all with antibodies specific for hAFP and hAlb. Rats were treated with multiple AA and underwent transplantation with hMSCs (C) or CD34+ cells or non-hMSCs/CD34- cells (D) by means of direct injection into their liver. Serial cryosections were immunostained using monoclonal antibodies specific for hAFP (Ci-x) or hAlb (Cxi-xx). (vi-x) and (xvi-xx) are magnified images of corresponding squared areas of (i-v) and (xi-xv), respectively. Similar results were obtained in 2 independent experiments. (E) Immunofluorescent staining patterns for hAlb and hAFP in rat-injected hMSCs. Rats were treated with multiple AA. Fourteen days after intrahepatic injection of hMSCs, immunofluorescent images of liver sections were obtained using a confocal laser microscope (Bio-Rad, Radiance 2100) with the Alexa 568 (Molecular Probes)-labeled anti-human AFP antibody (clone C3, 1:500; Sigma) (red) and with the Alexa 488 (Molecular Probes)-labeled anti-human Alb antibody (clone HAS-11, 1: 500; Sigma) (green). Merged immunofluorescence images of hAlb and hAFP are also presented. Scale bars represent 20 μm. Original magnifications, × 100 (A-B, E); × 200 (Ci-v, xi-xv, and D; and × 400 (Cvi-x, xvi-xx).
Immunohistochemical and immunofluorescent staining patterns for hAFP and hAlb in the multiple AA-treated rat liver that received a transplant of human bone marrow cells. (A) Representative hematoxylin-eosin (HE) staining of multiple AA-treated rat liver. Mild periportal necroinflammation and fibrosis characterized by mild portal expansion were observed at day 28. Severe septal fibrosis and swelling of hepatocytes is diffusely observed at day 56. (B) Sections of injured rat liver (day 28) from rats not given transplants were not stained at all with antibodies specific for hAFP and hAlb. Rats were treated with multiple AA and underwent transplantation with hMSCs (C) or CD34+ cells or non-hMSCs/CD34- cells (D) by means of direct injection into their liver. Serial cryosections were immunostained using monoclonal antibodies specific for hAFP (Ci-x) or hAlb (Cxi-xx). (vi-x) and (xvi-xx) are magnified images of corresponding squared areas of (i-v) and (xi-xv), respectively. Similar results were obtained in 2 independent experiments. (E) Immunofluorescent staining patterns for hAlb and hAFP in rat-injected hMSCs. Rats were treated with multiple AA. Fourteen days after intrahepatic injection of hMSCs, immunofluorescent images of liver sections were obtained using a confocal laser microscope (Bio-Rad, Radiance 2100) with the Alexa 568 (Molecular Probes)-labeled anti-human AFP antibody (clone C3, 1:500; Sigma) (red) and with the Alexa 488 (Molecular Probes)-labeled anti-human Alb antibody (clone HAS-11, 1: 500; Sigma) (green). Merged immunofluorescence images of hAlb and hAFP are also presented. Scale bars represent 20 μm. Original magnifications, × 100 (A-B, E); × 200 (Ci-v, xi-xv, and D; and × 400 (Cvi-x, xvi-xx).
Expression of hAFP and hAlb examined by immunohistochemistry in the livers of rats treated with multiple AA and injected with hMSCs, CD34+ cells, or non-hMSCs/CD34- cells
To investigate which cellular components of bone marrow are capable of undergoing hepatic differentiation, we injected hMSCs, CD34+ cells, or non-hMSCs/CD34- cells into the livers of rats treated with multiple AA for 56 days. Rats were killed at days 7, 10, 14, 28, and 56 after transplantation, and liver specimens taken from the area previously marked to indicate cell injection site (“Materials and methods”) were examined for their expression of hAFP and hAlb by serial sections of immunohistochemistry. Administration of AA was continued until the rats were killed. The chronic hepatitis lesions induced by AA at days 28 and 56 are shown in Figure 2A. In Figure 2C, representative immunohistochemical staining patterns of 1 of 2 independent examinations for hAFP and hAlb are shown. At day 7, in 1 speciment that received hMSCs, staining for hAFP became weakly and spottily positive, and that for hAlb also became positive but more faintly. At day 10, staining intensity and number of staining-positive cells became evident for both hAFP and hAlb. At day 14, both hAFP+ and hAlb+ cells appeared to form a cluster with stronger staining intensity. At day 28, staining for hAFP became almost negative, showing a single spotty positivity stain in the center of the observation area, while with hAlb staining, essentially all the cells in the observation area became positive. At day 56, albumin-positive clusters that were smaller than those from day 28 and negative for AFP were observed. By contrast, no positive staining for either hAlb or hAFP was observed at days 14, 28, or 56 in the specimens of transplanted human CD34+ cells or non-hMSCs/CD34- cell-transplanted specimens (Figure 2D). mRNA expression of hAlb and hAFP by reverse transcription-polymerase chain reaction (RT-PCR) using specific primers for each was consistent with the immunohistochemical results (data not shown). To identify the hAFP- and hAlb-producing cells, we performed immunofluorescent staining on specimens from rats killed on day 14. Essentially the same staining patterns for both proteins, which almost completely merged, were observed (Figure 2E).
Human albumin production in the serum of rats given hMSCs assessed by ELISA
To further confirm our findings, we employed an ELISA specific for hAlb (Figure 3). Normal rat serum was tested and showed no cross-reaction with the ELISA reagents.
The ELISA could detect normal human serum albumin in rat serum in a dose-dependent fashion. In the serum of rats treated with multiple AA and given hMSCs, hAlb was clearly detected (1.2 ± 0.4 ng/mL) at day 14, and its concentration increased to 1.8 plus or minus 0.6 ng/mL at day 28 and decreased to 0.6 plus or minus 0.4 ng/mL at day 56. In contrast, hAlb was detectable in neither the liver of rats given transplants of CD34+ cells nor non-hMSCs/CD34- cells.
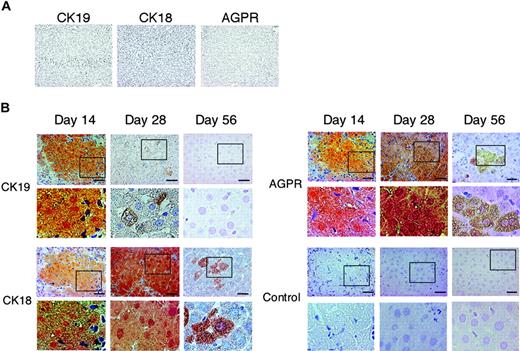
Immunohistochemical staining of differentiated hepatocytes for CK19, CK18, and AGPR
To verify hepatic differentiation, we examined by serial sections of immunohistochemical staining the expression of liver specific markers other than hAFP or hAlb: CK18, a marker for hepatocytes; AGPR, of which distribution is mainly found on hepatocytes;34 and CK19, a marker for hepatic progenitor or cholangiocytes (Figure 4). At day 14, all 3 markers were stained diffusely in cytoplasm; at day 28, CK19 was barely detectable, but the intensity for CK18 and AGPR rather increased. At day 56, CK19 became negative and CK18 and AGPR remained as small clusters with diffuse staining intensity.
Human albumin production in the serum of rats receiving hMSCs, CD34+ cells, or non-hMSCs/CD34- cell transplantation as evidenced by ELISA. Three elevated doses of purified human albumin (included in the kit) were mixed to the normal rat serum to test the sensitivity of detecting human albumin in rat serum. Rat plasma was collected and human serum albumin was assayed with ELISA at days 14, 28, and 56. Two rats were used in each group for CD34+ cells and non-hMSC/CD34- cell transplantation, respectively. Four rats were used in each group for hMSCs. We included in the ELISA 5 different concentrations of human albumin to create a standard curve from which to extrapolate the absorbance reading for each test sample. A triplicate sample of diluent alone was used to set the zero point for the ELISA. The final absorbance obtained in our test samples was calculated after subtracting the zero point absorbance. Error bars indicate standard deviations (SDs). N.D. indicates not determined.
Human albumin production in the serum of rats receiving hMSCs, CD34+ cells, or non-hMSCs/CD34- cell transplantation as evidenced by ELISA. Three elevated doses of purified human albumin (included in the kit) were mixed to the normal rat serum to test the sensitivity of detecting human albumin in rat serum. Rat plasma was collected and human serum albumin was assayed with ELISA at days 14, 28, and 56. Two rats were used in each group for CD34+ cells and non-hMSC/CD34- cell transplantation, respectively. Four rats were used in each group for hMSCs. We included in the ELISA 5 different concentrations of human albumin to create a standard curve from which to extrapolate the absorbance reading for each test sample. A triplicate sample of diluent alone was used to set the zero point for the ELISA. The final absorbance obtained in our test samples was calculated after subtracting the zero point absorbance. Error bars indicate standard deviations (SDs). N.D. indicates not determined.
Nonfusion origin of human hepatocytes revealed by FISH analysis
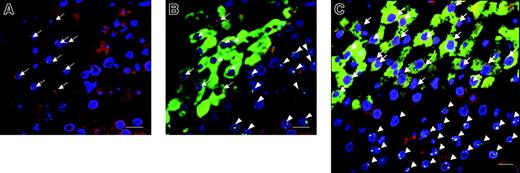
To exclude the possibility that hepatic differentiation was the result of spontaneous cell fusion, we analyzed the presence of rat and human specific chromosome DNA by the FISH method using human Y chromosome (red: Cy3) and rat 12-chromosome DNA probes (green: FITC) on the specimen of the livers from multiple AA rats receiving hMSC transplantations. We also performed immunofluorescent staining for hAlb on the same specimen. In Figure 5, representative images at day 28 are shown. In the area where hAlb was positively stained with Alexa 488 (green)-conjugated antibodies, only red fluorescent dots for human chromosomes were detected within the nuclei identified with blue fluorescence of DAPI staining, while green fluorescent dots for rat chromosome were found in the nuclei that were not associated with hAlb-positive cytoplasm. The results of such analyses on 3 specimens, 1 from each of 3 different rat livers, are summarized in Table 1. A total of 840 hybridization signals for either human or rat chromosome probes was identified in 1440 nuclei, suggesting truncation of nuclei during sectioning, focal plane restriction, or incomplete hybridization. Of 840 hybridization signals, 510 were those for human Y chromosomes and 330 were those for rat 12 chromosomes, and no colocalized signals of these chromosomes were visualized. Further, all the human Y chromosome signals (418) were found in the nuclei of hAlb-positive cells.
FISH analysis for hMSC-derived hepatocytes in rat liver
Rat . | No. total nuclei . | No. FISH signal+ nuclei (% of total nuclei) . | No. human FISH+ nuclei (% of FISH+ total nuclei) . | No. rat FISH+ nuclei (% of total FISH+ nuclei) . | No. human and rat FISH+ nuclei (% of total FISH+ nuclei) . | No. albumin+ cells . | No. human FISH+ nuclei (% of albumin+ cells) . | No. rat FISH+ nuclei (% of albumin+ cells) . |
|---|---|---|---|---|---|---|---|---|
| No. 1 | 480 | 228 (47.5) | 122 (53.5) | 106 (46.5) | 0 (0.0) | 200 | 122 (61.0) | 0 (0.0) |
| No. 2 | 600 | 360 (60.0) | 220 (61.1) | 140 (38.9) | 0 (0.0) | 240 | 220 (91.7) | 0 (0.0) |
| No. 3 | 360 | 252 (70.0) | 168 (66.7) | 84 (33.3) | 0 (0.0) | 76 | 76 (100.0) | 0 (0.0) |
| Total | 1440 | 840 (58.3) | 510 (60.7) | 330 (39.3) | 0 (0.0) | 516 | 418 (81.0) | 0 (0.0) |
Rat . | No. total nuclei . | No. FISH signal+ nuclei (% of total nuclei) . | No. human FISH+ nuclei (% of FISH+ total nuclei) . | No. rat FISH+ nuclei (% of total FISH+ nuclei) . | No. human and rat FISH+ nuclei (% of total FISH+ nuclei) . | No. albumin+ cells . | No. human FISH+ nuclei (% of albumin+ cells) . | No. rat FISH+ nuclei (% of albumin+ cells) . |
|---|---|---|---|---|---|---|---|---|
| No. 1 | 480 | 228 (47.5) | 122 (53.5) | 106 (46.5) | 0 (0.0) | 200 | 122 (61.0) | 0 (0.0) |
| No. 2 | 600 | 360 (60.0) | 220 (61.1) | 140 (38.9) | 0 (0.0) | 240 | 220 (91.7) | 0 (0.0) |
| No. 3 | 360 | 252 (70.0) | 168 (66.7) | 84 (33.3) | 0 (0.0) | 76 | 76 (100.0) | 0 (0.0) |
| Total | 1440 | 840 (58.3) | 510 (60.7) | 330 (39.3) | 0 (0.0) | 516 | 418 (81.0) | 0 (0.0) |
Data are derived from 3 different livers from day-28 rats that underwent hMSC transplantatin and treatment with multiple AA.
Immunohistochemical staining patterns for CK19, CK18, and AGPR in the multiple AA-treated rat liver receiving hMSC transplantation. Rats were treated with multiple AA and hMSCs were transplanted into the rat by means of direct injection into their liver. (A) Sections of injured rat liver (day 28) from a rat that did not receive a transplant were not stained at all with antibodies specific for human CK19, CK18, and AGPR. (B) Serial cryosections obtained from the samples as shown in Figure 2C were immunostained using monoclonal antibodies specific for human CK19 (i-vi), CK18 (vii-xii), and AGPR (xii-xviii). Panels Biv-vi, x-xii, xvi-xviii, and xxii-xxiv are magnified images of corresponding squared areas of panels Bi-iii, vii-ix, xiii-xv, and xix-xxi, respectively. Similar results were obtained in 2 independent experiments. Scale bars represent 20 μm. Original magnifications: × 100 (A); × 200 (Bi-iii, vii-ix, xiii-xv, and xix-xxi); and × 400 (Biv-vi, x-xii, xvi-xviii, and xxii-xxiv).
Immunohistochemical staining patterns for CK19, CK18, and AGPR in the multiple AA-treated rat liver receiving hMSC transplantation. Rats were treated with multiple AA and hMSCs were transplanted into the rat by means of direct injection into their liver. (A) Sections of injured rat liver (day 28) from a rat that did not receive a transplant were not stained at all with antibodies specific for human CK19, CK18, and AGPR. (B) Serial cryosections obtained from the samples as shown in Figure 2C were immunostained using monoclonal antibodies specific for human CK19 (i-vi), CK18 (vii-xii), and AGPR (xii-xviii). Panels Biv-vi, x-xii, xvi-xviii, and xxii-xxiv are magnified images of corresponding squared areas of panels Bi-iii, vii-ix, xiii-xv, and xix-xxi, respectively. Similar results were obtained in 2 independent experiments. Scale bars represent 20 μm. Original magnifications: × 100 (A); × 200 (Bi-iii, vii-ix, xiii-xv, and xix-xxi); and × 400 (Biv-vi, x-xii, xvi-xviii, and xxii-xxiv).
Number of hAlb-positive or CK19+ cells in rats given transplants of hMSCs
In order to assess the efficiency of differentiation, we estimated the number of cells expressing hAlb or CK19 in the loci where 1 × 106 hMSCs were injected (Table 2). The estimation was based on the method described by Wang et al.28 Two weeks after hMSC injection, a hAlb-positive cell cluster was estimated to consist of 354 plus or minus 205 cells. Thus, the differentiation efficiency at day 14 was to be 0.035% plus or minus 0.021% (354 ± 205/1 × 106). By 4 weeks after hMSC injection, the estimated hAlb-positive cell number increased to 4990 plus or minus 1362, a differentiation efficiency of 0.49% plus or minus 0.136% (4990 ± 1362/1 × 106). At 8 weeks after hMSC injection, a hAlb-positive cell cluster was estimated to consist of 156 plus or minus 97 cells. Thus, the differentiation efficiency at day 56 was 0.016% plus or minus 0.010% (156 ± 97/1 × 106). Two weeks after hMSC injection, a cluster of cells positive for CK19, which is known to be a marker of cholangiocytes, was estimated to consist of 375 ± 211 cells. Thus the differentiation efficiency at day 14 was 0.038% plus or minus 0.021% (375 ± 211/1 × 106). At day 28 after injection, the differentiation efficiency was very low, as evidenced by small, spotty staining. At day 56 after injection, CK19 became negative (Table 2).
Human albumin- or CK19-expressing cell numbers in the sections from AA-treated rat liver injected with hMSCs
Time after transplantation, d . | No. positive cells at equator, mean ± SD . | . | No. positive cells in nodule, mean ± SD . | . | hAlb+ or CK19+ cells/injected hMSCs, %*, mean ± SD . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | Albumin . | CK19 . | Albumin . | CK19 . | Albumin . | CK19 . | |||
| 14 | 50 ± 24 | 52 ± 18 | 354 ± 205 | 375 ± 211 | 0.035 ± 0.02 | 0.038 ± 0.021 | |||
| 28 | 292 ± 51 | 7 ± 3 | 4990 ± 1362 | ND | 0.49 ± 0.136 | ND | |||
| 56 | 29 ± 11 | 0 ± 0 | 156 ± 97 | ND | 0.016 ± 0.01 | ND | |||
Time after transplantation, d . | No. positive cells at equator, mean ± SD . | . | No. positive cells in nodule, mean ± SD . | . | hAlb+ or CK19+ cells/injected hMSCs, %*, mean ± SD . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | Albumin . | CK19 . | Albumin . | CK19 . | Albumin . | CK19 . | |||
| 14 | 50 ± 24 | 52 ± 18 | 354 ± 205 | 375 ± 211 | 0.035 ± 0.02 | 0.038 ± 0.021 | |||
| 28 | 292 ± 51 | 7 ± 3 | 4990 ± 1362 | ND | 0.49 ± 0.136 | ND | |||
| 56 | 29 ± 11 | 0 ± 0 | 156 ± 97 | ND | 0.016 ± 0.01 | ND | |||
Data shown are from 4 rats from 2 experiments. ND indicates not done; the number of CK19+ cells was not tested since they did not form apparent nodules. hAlb indicates human albumin.
Differentiation efficiency is expressed by dividing the total number of hALb- or CK19-expressing cells by the number of injected hMSCs.
A representative image series from combined FISH for human and rat specific chromosomes and immunofluorescence for human albumin in the multiple AA-treated rat liver-transplanted hMSCs. (A) When viewed through a rhodamine and ultraviolet filter, positive human Y signals appear as red dots at the periphery of the nuclei stained with DAPI, a chromosomal marker that shows up as blue fluorescence. For ease of reference, the positive Y signals are highlighted by arrows. (B) The same field as in panel A is shown when overlaying the immunofluorescence images viewed through the FITC filter to demonstrate the immunostaining for the human albumin positive cytoplasm (green staining), and the rat-12 chromosome is represented by the green fluorescent dots (arrowheads). Nonhuman albumin-expressing cells which show background level of autofluorescence in injured rat liver tissue are seen surrounding the albumin-expressing cells. (C) Another representative image from different rat livers was made from overlaying the images seen through the 3 separate filters to show all the colors. Scale bars represent 20 μm. Original magnification, × 630.
A representative image series from combined FISH for human and rat specific chromosomes and immunofluorescence for human albumin in the multiple AA-treated rat liver-transplanted hMSCs. (A) When viewed through a rhodamine and ultraviolet filter, positive human Y signals appear as red dots at the periphery of the nuclei stained with DAPI, a chromosomal marker that shows up as blue fluorescence. For ease of reference, the positive Y signals are highlighted by arrows. (B) The same field as in panel A is shown when overlaying the immunofluorescence images viewed through the FITC filter to demonstrate the immunostaining for the human albumin positive cytoplasm (green staining), and the rat-12 chromosome is represented by the green fluorescent dots (arrowheads). Nonhuman albumin-expressing cells which show background level of autofluorescence in injured rat liver tissue are seen surrounding the albumin-expressing cells. (C) Another representative image from different rat livers was made from overlaying the images seen through the 3 separate filters to show all the colors. Scale bars represent 20 μm. Original magnification, × 630.
Discussion
Here we showed that hMSCs were more potent in undergoing hepatic differentiation than other human bone marrow components, CD34+ cells, or non-MSCs/CD34- cells, when they were directly xenografted to the livers of rats which had been treated with multiple AA injections.
Although the possibility that other types of cells, particularly MAPCs, which might have been copurifying with MSCs, are actually responsible for the hepatic differentiation cannot be completely ruled out, this possibility must be quite rare, we believe, since in the cultivation conditions for our hMSCs, 10% fetal calf serum (FCS) and a cell density greater than 2 × 104/cm,2 MAPCs, which require a longer cultivation period under more strict conditions (less than 2% FCS and a cell density less than 104/cm2) than MSCs1,35 readily lose their properties. Furthermore, unlike our hMSCs, MAPCs do not express HLA class I, CD44, or CD105, which have been reported to be some of the key differences between MSCs and MAPCs (Figure 1B, D).1-3 Contamination by hematopoietic lineage cells is also considered unlikely on the basis of both a previous report demonstrating that cultivation conditions for MSCs eliminate these cells,30 including monocytes and macrophages,36,37 and our present finding showing that neither CD45+ nor CD14+ cells were present in our hMSC preparations (Figure 1B).
Hepatic differentiation of human CD34+ cells intravenously injected into mice has been previously reported.19 We, in the present experiment, found that human CD34+ cells were not capable of differentiation into hepatocytes when injected into the liver. Thus, taken together with the previous reports, it is highly plausible that either priming or differentiation in bone marrow is requisite for human CD34+ cells to undergo hepatic differentiation after they recirculate to the liver.
Successful hepatic differentiation of cells with hematopoietic totipotential cells other than CD34+ cells has also been demonstrated in several investigations.16,20,22 Since in these studies the cells were generally intravenously injected into the recipient, transiently residing in bone marrow may be an essential step in their hepatic differentiation, as with CD34+ cells. In our study, the fraction of non-MSCs/CD34- cells also did not differentiate into hepatocytes. Since this fraction should include myelomonocytic cells, our results apparently contradict those by Willenbring et al22 and Camargo et al,20 who showed that hepatocyte regeneration occurs through cell fusion of hepatocytes with HSC progeny cells such as granulocytes or macrophages. This contradiction may be explained by either the species difference in cell sources or by the fact that their myelomonocytic cells had been primed in bone marrow, thereby acquiring some potency to fuse with resident hepatocytes, though the possibility that treatment with immunosuppressive agents may result in an absence of signals needed to activate cells of the myelomonocytic lineage to participate in the formation of hepatocytes by fusion cannot be completely ruled out.
With regard to the issue of whether MSCs that had been expanded in vitro in the present study indeed represent those present in bone marrow in vivo, definite elucidation may be difficult. However, as far as phenotypic expression revealed by FACS is concerned, our hMSCs at the fifth passage were quite comparable with those after the first passage (data not shown), suggesting that the properties of the original hMSCs must have been largely maintained after expansion. Nonetheless, the superiority of hMSCs in hepatic differentiation ability was evident in the present in vivo study, compatible with results by Lee et al, who found that human MSCs are capable of differentiating into hepatocyte-like cells in vitro.4,5
Another critical issue of hepatic differentiation of bone marrow cells is the “fusion” between donor cells and recipient cells.12,17,18,38-40 Our present results support a nonfusion theory6,11,12,41 because FISH analysis with human and rat chromosome probes excluded spontaneous fusion between hMSCs and rat hepatocytes as the cause for the generation of any of hepatocytes examined (Table 1, Figure 5). Although there still remains the possibility that during cell division, interspecies hybrids will “kick out” or loosen chromosomes of one or the other species because the chromosome complement of interspecies hybrids is not stable,42 it is not likely that this “kick out” phenomenon occurred in all the hAlb-positive cells, and therefore fusion may not be the primary mechanism for hepatocyte differentiation.
One of the most interesting observations in the present study was that the differentiation followed the ontogenic sequence; that is, the appearance of AFP at day 7 after transplantation followed by expression of albumin at day 10 and subsequent disappearance of the former by day 28, when expression of albumin became evident. Since the staining of AFP merged with that of albumin as revealed by an immunofluorescence study (Figure 2E), it was surmised that cells producing both proteins were identical and that their genetic resetting to acquire a phenotype of hepatic progenitor cells and then to undergo ontogenic differentiation must have occurred by their interaction with damaged donor liver. This ontogenic differentiation was further supported by the observation that CK19, a marker for hepatic progenitor cells as well as that for biliary ductal epithelium, was transiently expressed at day 14 and barely detectable by day 28, paralleling the expression of AFP. Incidentally, the staining pattern for CK19 in hepatic progenitors is different from that for biliary ductules: diffuse in the former and intense in the latter.43 The diffuse, cytoplasmic staining pattern of CK19 at day 14 in the current study was also indicative of a hepatic progenitor theory.
There was no evidence in this study of differentiation into cellular compartments other than hepatocytes such as endothelial cells (CD31) or kupffer cells (CD68) as revealed by immunostaining on day 28 (late stage ontogeny; data not shown). With regard to the differentiation into biliary ductules, which are another essential constituent of matured liver, since CK19-expressing cells almost disappeared by day 28, evidence was also poor. This may be due to their representing only approximately 2% of the cellular mass of the liver or that their relative turnover is low compared with hepatocytes.44
A lack of correlation between induction levels of systemic human albumin (about 2-fold induction) and the proportion of human albumin-expressing cells (about 10-fold improvement) in the rat livers shown between day 14 and day 28 could be due to nonuniform distribution of the human cells in the livers, as mentioned by previous investigators.19
It is generally accepted that differentiation of bone marrow cells requires chronic damage of the donor tissues to be transplanted. In experiments dealing with hepatic differentiation, tissue damage is also requisite, and previous studies have successfully facilitated such damage in several ways.6,10,14,20,22,45,46 We, in the present study, considering future clinical applications and the potential activation of periductual liver stem cells, selected the chronic AA administration model.23,26,47
The efficiency of differentiation of hMSCs in the present experiment was unexpectedly low, despite the fact that direct intrahepatic injections were employed. One plausible explanation for this low efficiency is the heterogeneity of hMSCs; that is, the percentage of the hMSC population which is capable of undergoing differentiation. Moreover, due to the xenogeneic nature of the transplantation, a considerable number of the inoculated hMSCs might have been rejected, even in an immunosuppressive state brought about by cyclosporin A administration. Further, the possibility that not only recipient hepatocytes but also differentiated donor hepatocytes were damaged by exposure to AA should be taken into consideration. In this context, the fact that human hepatic differentiation was observed only transiently may be rationalized by the assumption that during the maturation process from fetal phenotype to adult phenotype, donor cells are relatively resistant to AA compared with recipient rat hepatocytes, and once hepatic differentiation has been completed, donor hepatocytes become more susceptible to AA than rat hepatocytes. In fact, a previous study demonstrated that although both human livers and rat livers are damaged by AA, the latter was less susceptible to AA-induced toxicity than the former.48 Elucidation of these possibilities is a pivotal task to improve the low differentiation efficiency.
The ultimate goal of differentiation studies is the amendment of damaged tissue by cellular transplantation. In our present study, we have not examined the therapeutic effect of hMSC transplantation to damaged liver simply because of our method employing a xenograft model; human tissue in rat organs is considered to be unsuitable for assessment of the effects. However, since the hepatic differentiation of hMSCs was an indisputable phenomenon, we believe that recovery of damaged liver may be clearly attained if a syngeneic model on a larger scale of transplantation is used. In conclusion, our results indicate that hMSCs from bone marrow indeed acquired the phenotype and functional characteristics of hepatocytes under conditions of chronic liver damage without any evidence of fusion with recipient liver components in a xenograft model.
Prepublished online as Blood First Edition Paper, April 7, 2005; DOI 10.1182/blood-2005-02-0572.
Y.S. and H.A. contributed equally to this work.
Supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
We thank Kevin S. Litton for assistance in the preparation of this manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal