Abstract
γδ T cells localize to target tissues of graft-versus-host disease (GVHD) and therefore we investigated the role of host γδ T cells in the pathogenesis of acute GVHD in several well-characterized allogeneic bone marrow transplantation (BMT) models. Depletion of host γδ T cells in wild-type (wt) B6 recipients by administration of anti-T-cell receptor (TCR) γδ monoclonal antibody reduced GVHD, and γδ T-cell-deficient (γδ-/-) BM transplant recipients experienced markedly improved survival compared with normal controls (63% vs 10%, P < .001). γδ T cells were responsible for this difference because reconstitution of γδ-/- recipients with γδ T cells restored GVHD mortality. γδ-/- recipients showed decreased serum levels of tumor necrosis factor α (TNF-α), less GVHD histopathologic damage, and reduced donor T-cell expansion. Mechanistic analysis of this phenomenon demonstrated that dendritic cells (DCs) from γδ-/- recipients exhibited less allostimulatory capacity compared to wt DCs after irradiation. Normal DCs derived from BM caused greater allogeneic T-cell proliferation when cocultured with γδ T cells than DCs cocultured with medium alone. This enhancement did not depend on interferon γ (IFN-γ), TNF-α, or CD40 ligand but did depend on cell-to-cell contact. These data demonstrated that the host γδ T cells exacerbate GVHD by enhancing the allostimulatory capacity of host antigen-presenting cells. (Blood. 2005;106:749-755)
Introduction
Allogeneic bone marrow transplantation (BMT) is a widely performed therapy for many hematologic malignancies. However, acute graft-versus-host disease (GVHD) remains the most important complication of allogeneic BMT. The pathophysiology of GVHD is complex. The target organs of acute GVHD include the gastrointestinal tract, skin, and liver. Several convergent lines of experimental data have demonstrated that donor T cells and host antigen-presenting cells (APCs) are critical for the induction of GVHD.1-3 In addition, a growing body of data also suggest that several other cellular subsets such as host macrophages,4,5 donor natural killer (NK) cells,6,7 and donor NKT cells8 are involved in the pathogenesis of GVHD.
γδ T cells represent a distinct lineage of T cells that evolved early in vertebrate phylogeny in conjunction with αβ T cells and B cells. Antigen recognition by γδ T-cell receptors (TCRs) occurs in a fundamentally different manner from that of αβ T cells. γδ T cells can recognize molecules other than peptides, do not require professional APCs for activation, and use their TCRs to recognize patterns associated with foreign pathogens or tissue injury.9 These cells serve as sentinels to monitor their environment for signs of stress caused by infection, inflammation, and transformation. γδ T cells modulate immune responses by secreting cytokines and by regulating the function of other immune cells. For example, γδ T cells produce either interferon γ (IFN-γ) or interleukin 4 (IL-4) in response to certain pathogens and can influence T-cell function during the development of an immune response.10 By releasing proinflammatory cytokines, γδ T cells can also facilitate activation of other host cells such as macrophages11,12 and NK cells12-14 in the early phases of infection.
Several recent studies have focused on the role played by donor γδ T cells in GVHD,15-21 but the role of host γδ T cells is not known. Using several mouse models, we have found that host γδ T cells exacerbate acute GVHD by enhancing the allostimulatory capacity of host APCs.
Materials and methods
Mice
Female C57BL/6 (B6, H-2b), BALB/c (H-2d), B6.C-H2bm12 (bm12), B6.C-H2bm1 (bm1), B6.129S7-Ifngtm1Ts/J (IFN-γ-/-; IFN-γ deficient), B6.129P2-Tcrbtm1Mom/J (αβ-/-; αβ T-cell deficient), and B6.129P2-Tcrdtm1Mom/J (γδ-/-; γδ T-cell deficient) mice22,23 (both TCR-deficient mice were backcrossed onto C57BL/6 genetic background) were purchased from The Jackson Laboratory (Bar Harbor, ME). B6.SJL-Ptprca/BoAiTac-Abbtm1N13 (II-/-, B6.Ly-5a-background, major histocompatibility antigen (MHC) class II deficient) mice were purchased from Taconic Farms (Germantown, NY). B6.129P2-Tnfrsf5tm1Kik/J (CD40-/-, CD40 deficient) mice were kindly provided by Dr D. Keith Bishop. The age of mice used for experiments ranged between 8 and 12 weeks.
Bone marrow transplantation
Mice received 1100 cGy total body irradiation (TBI; 137Cs source). Bone marrow cells (5 × 106) plus 2 × 106 nylon wool-purified (NWP) splenic CD3+ T cells from respective allogeneic or syngeneic donors were resuspended in 0.25 mL Leibovitz L-15 media (Gibco BRL, Grand Island, NY) and injected intravenously into recipients on day 0. Bone marrow cells plus 1 × 106 CD4+ T cells or 2 × 106 CD8+ T cells isolated by immunomagnetic selection (Miltenyi Biotec, Bergisch Gladbach, Germany) were injected into recipients in MHC class II or I mismatched mouse model, respectively.
Clinical and histopathologic analysis of GVHD
Survival was monitored daily, and GVHD clinical scores were assessed weekly by a scoring system incorporating 5 clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity, as described.24 Individual mice were ear-tagged and graded weekly on a scale from 0 to 2 for each criterion (maximum score 10).
Formalin-preserved small (ileum) intestines were embedded in paraffin, cut into 5-μm thick sections, and stained with hematoxylin and eosin for histologic examination. Slides were coded without reference to prior treatment and examined in a blinded fashion by a pathologist (C.L.). A semiquantitative scoring system was used to assess the following abnormalities known to be associated with GVHD:25 villous blunting, crypt regeneration, loss of enterocyte brush border, luminal sloughing of cellular debri, crypt cell apoptosis, crypt destruction, and lamina propria lymphocytic infiltrate. The scoring system denoted 0 as normal, 0.5 as focal and rare, 1.0 as focal and mild, 2.0 as diffuse and mild, 3.0 as diffuse and moderate, and 4.0 as diffuse and severe. Scores were added to provide a total score for each specimen. After scoring the codes were broken and data compiled.
Cytokine ELISA
Concentrations of IFN-γ, IL-2 (BD Pharmingen, San Diego, CA), and TNF-α (Quantikine; R&D Systems, Minneapolis, MN) were measured by sandwich enzyme-linked immunosorbent assay (ELISA) as described.26 To determine serum levels of lipopolysaccharide (LPS), the limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD) was performed according to the manufacturer's protocol. All units expressed are relative to US reference standard EC-6.
In vivo depletion of γδ T cells
Five days before BMT, mice were depleted of γδ T cells as previously described27 by intraperitoneal injection of 500 μg hamster anti-TCR γδ monoclonal antibody (mAb) (IgG, UC7-13D5; LigoCyte Pharmaceutical, Bozeman, MT) in a volume of 0.25 mL Leibovitz L-15 media. Sham depletion was accomplished with hamster Ig (Jackson Laboratories, Bar Harbor, ME).
Adoptive transfer of γδ T-cell experiments
γδ T-cell reconstitution was performed as described previously.28 γδ T cells were obtained from αβ-/- donor mice and αβ T cells were obtained from γδ-/- donors. Briefly, splenocytes from mice were isolated, and red blood cells were lysed. Cells were resuspended in Leibovitz L-15 media. Cells (60 × 106) were injected in the tail vein of each mouse. At 24 hours after transfer, mice received 1100 cGy TBI following BMT for clinical assessment of GVHD.
Monoclonal antibodies and blocking reagents
The following mAbs were used for flow cytometry and blocking experiments: CD3-fluorescein isothiocyanate (FITC), H2Dd-FITC, CD4-FITC, CD45.1-phycoerythrin (PE), I-Ab-PE, CD40-PE, CD86-PE, anti-CD40 ligand (L)-E, anti-TNF-α-PE, anti-IFN-γ-PE, TCR γδ-PE, CD11c-allophycocyanin, CD4-allophycocyanin, CD8-allophycocyanin (all from BD PharMingen, San Diego, CA).
Cell surface phenotype and intracellular cytokine analysis
Fluorescence-activated cell sorting (FACS) analysis was performed as described previously.29 Briefly, cells were incubated with mAb 2.4G2 (rat anti-mouse FcγR mAb) for 15 minutes at 4°C and then with the relevant mAb for 30 minutes at 4°C. Three-color flow cytometry was performed with an EPICS Elite ESP cell sorter (Beckman Coulter, Miami, FL). For intracellular cytokine staining, splenocytes were harvested 7 days after transplantation. Cells were incubated for 4 hours with phorbol 12-myristate 13-acetate (PMA)-ionomycin (BD Pharmingen) and brefeldin A at 37°C. Then, the cells were permeabilized with the Cytofix/Cytoperm Kit from BD Pharmingen and subsequently stained with PE-conjugated IFN-γ mAb. Flow cytometry was conducted by gating for the designated cell populations.
Preparation of DCs and γδ T cells
Splenic dendritic cells (DCs) were isolated as described previously.3 Briefly, after digestion with collagenase D (1 mg/mL; Boehringer Mannheim, Indianapolis, IN), cells were resuspended in 1.035 g/mL Percoll (Pharmacia Biotech, Uppsala, Sweden) and underlaid with an equivalent volume of 1.075 g/mL Percoll. After centrifugation, the resulting band was harvested and washed twice, and DCs were isolated by using CD11c (N418) microbeads and the AutoMACS (Miltenyi Biotec).
Bone marrow-derived DCs were generated as described previously.30 Briefly, bone marrow cells were flushed from tibias and femurs of B6 mice and seeded at 2 × 106 cells in bacteriologic Petri dishes (100-mm diameter; Falcon, NJ) in a volume of 10 mL of complete medium (CM) containing 20 ng/mL granulocyte macrophage-colony-stimulating factor (GM-CSF). On day 3, 10 mL CM containing 20 ng/mL GM-CSF was added to the dishes. On day 6, nonadherent cells were harvested, washed twice, and incubated with anti-CD11c microbeads for magnetic cell separation with an AutoMACS system. The purity of the DC suspension was greater than 90% as determined by dual positivity for MHC class II (I-Ad) and CD11c.
γδ T cells were collected from spleen in αβ-/- mice (as in “Bone marrow transplantation”) or intestinal intraepithelial lymphocytes (iIELs) in wild-type B6 mice. iIELs were separated as described.31 Briefly, Peyer patches were pinched out of the small intestine, which was opened longitudinally and cut into 5-mm pieces. The pieces were washed in 4°C CMF/HEPES (calcium/magnesium free N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and transferred into a 50-mL Erlenmeyer flask with 37°C CMF/HEPES/EDTA (ethylenediaminetetraacetic acid) solution on a magnetic stirrer. After stirring, the supernatant containing iIELs was collected and the iIELs were enriched with a Percoll procedure. Lymphocytes were stained with PE-labeled anti-TCR γδ mAb. γδ+ T cells, and γδ-depleted lymphocyte subset (γδ T cells) was purified by cell sorting, using a FACSVantage (BD Biosciences, San Jose, CA). Purity of γδ+ T cells assessed using flow cytometry was greater than 99%. Contamination of γδ T cells in γδ-depleted lymphocytes subset was less than 0.1%.
T cell-DC cocultures and mixed leukocyte reactions
T cell-DC cocultures and mixed leukocyte reactions were performed as described previously.32 Briefly, bone marrow-derived DCs were cultured in 24-well tissue-culture plates at a concentration of 1 × 106 cells/mL for 36 hours in RPMI complete medium. Additions to certain wells included 10 ng/mL Escherichia coli LPS (Sigma-Aldrich, Poole, United Kingdom), γδ+ T cells (T-cell to DC ratio = 1:1), or γδ- T cells. Anti-IFN-γ (10 μg/mL), anti-TNF-α (20 μg/mL), and anti-CD40L (10 μg/mL) mAbs were used for blocking experiments. In some experiments, DCs were isolated after 36 hours by centrifugation on a 14.5% metrizamide (Sigma Chemical, St Louis, MO) gradient,33 irradiated (2000 cGy), and then used as stimulators for allogeneic NWP responder T cells (T-cell to DC ratio = 10:1) for 3 to 4 days. Splenic DCs isolated from mice were irradiated (1000 cGy) and then used as stimulators. At 48 and 72 hours, supernatants were collected for cytokine analysis. During the final 12 hours of culture, cells were pulsed with 1 μCi [3H] thymidine (2 Ci/mmol; New England Nuclear, Shelton, CT) and proliferation was determined on a Top Count NTX (Packard Instruments, Meriden, CT).
Statistical analysis
The Mann-Whitney U test was used for the statistical analysis of cytokine data, LPS levels, clinical scores, weight loss, and histology, whereas the Wilcoxon rank test was used to analyze survival data. P less than .05 was considered statistically significant.
Results
Absence of host γδ T cells reduces GVHD severity
The role of host γδ T cells on the severity of GVHD was evaluated by using a well-characterized experimental model of GVHD, BALB/c (H2d) → B6 (H2b). Wild-type B6 and γδ-/- mice were irradiated with 1100 cGy TBI and underwent transplantation with 5 × 106 BM cells and 2 × 106 splenic CD3+ T cells from either syngeneic B6 or allogeneic BALB/c donors. γδ-/- mice that received allogeneic cells demonstrated significantly better survival than the wild-type allogeneic recipients (P < .001, Figure 1A). Clinical GVHD severity was assessed by a standard scoring system that sums changes in 5 parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity (see “Materials and methods”). Consistent with improved survival, allogeneic B6 γδ-/- BM transplant recipients demonstrated significantly less clinical acute GVHD than wt controls (P < .05, Figure 1B). All surviving transplant recipient mice in both groups displayed complete donor hematopoietic chimerism, ruling out mixed chimerism as a cause for reduced GVHD (data not shown).
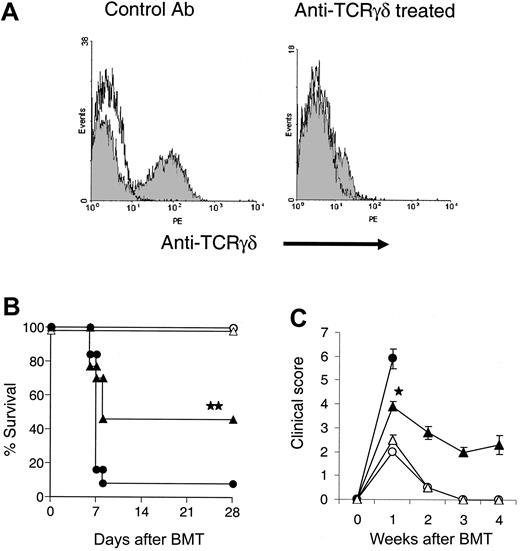
We next depleted γδ T cells in wt B6 mice by administering anti-TCR γδ mAb on day -5 prior to BMT.27 γδ+ splenocytes and iIELs were no longer detectable by FACS 3 days after treatment (Figure 2A). These γδ T-cell-depleted mice were irradiated and underwent transplantation as in Figure 1. γδ T-cell-depleted recipients demonstrated significantly better survival at day 28 (46% vs 8%, P < .01) and reduced clinical severity of acute GVHD on day 7 compared with control Ab recipients (P < .001, Figure 2B-C). These data confirmed that the elimination of host γδ T cells results in reduced GVHD severity.
We next analyzed histopathologic changes of the small intestine using a semiquantitative index for GVHD damage (see “Materials and methods”). As shown in Figure 3A-D, allogeneic B6 γδ-/- BM transplant recipients demonstrated significantly less GVHD in gastrointestinal tract compared with wt controls (P < .05). Serum levels of LPS and TNF-α, biochemical markers that correlate with the severity of intestinal and systemic acute GVHD,25,34 were also significantly reduced (Figure 3E-F). Thus γδ-/- mice showed less acute GVHD compared with wt controls as determined by clinical, histopathologic, and biochemical markers.
To rule out strain-dependent artifacts, we next evaluated the role of host γδ T cells in well-defined GVHD models in which donor and recipient differed at MHC class II or class I only (bm12 → B6 or bm1 → B6, respectively). In the CD4+-mediated, MHC class II-mismatched GVHD model (bm12 → B6), γδ-/- recipients demonstrated significantly better survival at day 35 (73% vs 16%, P < .001) and reduced clinical severity of acute GVHD on day 7 compared with wt mice (3.8 ± 0.2 vs 5.0 ± 0.1, P < .001). In the CD8+-mediated, MHC class I-mismatched model (bm1 → B6), clinical GVHD was also reduced (3.1 ± 0.1 vs 4.8 ± 0.2, P < .001), although there was no significant difference in survival rate at day 50 (94% vs 73%, P = .19). Taken together, these data demonstrate that host γδ T cells regulate CD4+- and CD8+-mediated acute GVHD.
Absence of γδ T cells in allogeneic BM transplant recipients reduces GVHD severity. B6 γδ-/- mice (γδ-/-: ▴, n = 24) or wild-type (wt: •, n = 38) mice received 1100 cGy TBI and underwent transplantation with 5 × 106 BM cells and 2 × 106 T cells from BALB/c or syngeneic B6 donors (syn: ○, n = 21) and evaluated for survival (A) and clinical GVHD score (B) as detailed in “Materials and methods.” Data from 4 similar experiments are combined. Error bars represent standard error. ▴ vs •, **P < .01 by Wilcoxon rank test. ▴ vs •, *P < .05 by Mann-Whitney U test.
Absence of γδ T cells in allogeneic BM transplant recipients reduces GVHD severity. B6 γδ-/- mice (γδ-/-: ▴, n = 24) or wild-type (wt: •, n = 38) mice received 1100 cGy TBI and underwent transplantation with 5 × 106 BM cells and 2 × 106 T cells from BALB/c or syngeneic B6 donors (syn: ○, n = 21) and evaluated for survival (A) and clinical GVHD score (B) as detailed in “Materials and methods.” Data from 4 similar experiments are combined. Error bars represent standard error. ▴ vs •, **P < .01 by Wilcoxon rank test. ▴ vs •, *P < .05 by Mann-Whitney U test.
In vivo depletion of γδ T cells from recipients reduces GVHD severity. Five days before BMT, mice were depleted of γδ T cells by intraperitoneal injection of anti-TCR γδ mAb. Three days later, iIELs were collected from mice treated with anti-TCR γδ mAb or control Ab and stained with PE-anti-γδ (A). γδ T-cell-depleted and sham-depleted mice received transplants from BALB/c (γδ T-cell-depleted: ▴, n = 13; sham-depleted: •, n = 13) or syngeneic B6 donors (γδ T-cell-depleted: ▵, n = 6; sham-depleted: ○, n = 6) as in Figure 1 and evaluated for survival (B) and clinical GVHD score (C). Data from 2 similar experiments are combined. Error bars represent standard error. ▴ vs •, **P < .01 by Wilcoxon rank test. ▴ vs •, *P < .05 by Mann-Whitney U test.
In vivo depletion of γδ T cells from recipients reduces GVHD severity. Five days before BMT, mice were depleted of γδ T cells by intraperitoneal injection of anti-TCR γδ mAb. Three days later, iIELs were collected from mice treated with anti-TCR γδ mAb or control Ab and stained with PE-anti-γδ (A). γδ T-cell-depleted and sham-depleted mice received transplants from BALB/c (γδ T-cell-depleted: ▴, n = 13; sham-depleted: •, n = 13) or syngeneic B6 donors (γδ T-cell-depleted: ▵, n = 6; sham-depleted: ○, n = 6) as in Figure 1 and evaluated for survival (B) and clinical GVHD score (C). Data from 2 similar experiments are combined. Error bars represent standard error. ▴ vs •, **P < .01 by Wilcoxon rank test. ▴ vs •, *P < .05 by Mann-Whitney U test.
Adoptive transfer of host γδ T cells aggravate GVHD
To evaluate whether γδ T cells were unique lymphocyte subsets in their ability to amplify GVHD, we compared the severity of GVHD among recipients that were deficient in either γδ T cells or αβ T cells. As shown in Figure 4A-B, B6 γδ-/- recipients of allogeneic BMT showed significantly reduced GVHD mortality and clinical scores (P < .01), whereas mortality in B6 αβ-/- recipients was similar to wt controls.
To confirm the specificity of γδ T cell-mediated in vivo effects on GVHD severity, we reconstituted γδ-/- mice with either αβ T cells or γδ T cells that were syngeneic to the host, as described in “Materials and methods.” Because NK cells could activate DC function, we determined the number of NK cells in the donor inocula. Contamination of NK cells in splenocytes from both αβ-/- and γδ-/- donors was similar (6.5% + 0.7% vs 5.6% + 0.7%, respectively). Mice reconstituted with γδ T cells showed significantly higher mortality and clinical scores than unreconstituted mice or mice reconstituted with αβ T cells (Figure 4C-D). Taken together, these results demonstrate the unique ability of the host γδ T-cell subset in the host to amplify the severity of GVHD.
γδ T cells induce activation and allostimulatory capacity of DCs
The activation of donor T cells by host DCs is critical to the induction of GVHD.1-3 We first determined the amplitude of donor T-cell expansion and intracellular cytokine expression in the spleens of BM transplant recipients 7 days later in the BALB/c → B6 model. We observed a significant (P < .01) reduction in the numbers of donor CD4+ and CD8+ T cells in γδ-/- recipients compared with allogeneic controls as shown in Figure 5A-B. Consistent with this reduced expansion, the number of CD4+ and CD8+ donor T cells that produced IFN-γ was also significantly reduced (IFN-γ+ CD4+: 1.8 ± 0.5 vs 18.9 ± 5.0 × 105, P < .01; IFN-γ+ CD8+: 0.68 ± 0.15 vs 14.6 ± 4.5 × 105, P < .01).
Absence of γδ T cells in the host reduces acute GVHD pathology. γδ-/- (▥, n = 4), wt (▪, n = 4), and syn BM transplant recipients (□, n = 4) underwent transplantation as in Figure 1 and the gastrointestinal tract was analyzed on day 7 after BMT as described in “Materials and methods.” Damage to syn small bowel was minimal (A), whereas wt exhibited severe villous blunting, crypt destruction changes and atrophy, and increased lymphocytic infiltrates (B). γδ-/- small bowel showed significantly less damage (C). Original magnification, ×100. Images were visualized using an Olympus Bx 40 microscope (Olympus, Melville, NY) equipped with an 10 ×/0.65 aperture objective lens. Image acquisition was performed with a JVC digital camera GC-Qx 5HDU (JVC, Wayne, NJ). Coded slides were scored for pathologic damage (D) as described in “Materials and methods.” Serum was obtained on day 7 after BMT and analyzed for LPS (E) and TNF-α (F). γδ-/- vs wt, *P < .05 (D-F). Error bars represent standard error.
Absence of γδ T cells in the host reduces acute GVHD pathology. γδ-/- (▥, n = 4), wt (▪, n = 4), and syn BM transplant recipients (□, n = 4) underwent transplantation as in Figure 1 and the gastrointestinal tract was analyzed on day 7 after BMT as described in “Materials and methods.” Damage to syn small bowel was minimal (A), whereas wt exhibited severe villous blunting, crypt destruction changes and atrophy, and increased lymphocytic infiltrates (B). γδ-/- small bowel showed significantly less damage (C). Original magnification, ×100. Images were visualized using an Olympus Bx 40 microscope (Olympus, Melville, NY) equipped with an 10 ×/0.65 aperture objective lens. Image acquisition was performed with a JVC digital camera GC-Qx 5HDU (JVC, Wayne, NJ). Coded slides were scored for pathologic damage (D) as described in “Materials and methods.” Serum was obtained on day 7 after BMT and analyzed for LPS (E) and TNF-α (F). γδ-/- vs wt, *P < .05 (D-F). Error bars represent standard error.
We hypothesized that the reduced donor T-cell expansion in γδ-/- recipients was due to a reduced allostimulatory capacity of host DCs and we thus evaluated the ability of host DCs to stimulate allogeneic responder T cells from wt and γδ-/- mice. T cells proliferated less when cocultured with allogeneic DCs from γδ-/- spleens than with DCs from wt spleens 6 hours after irradiation (Figure 5C, P < .05). They also secreted less IL-2 and IFN-γ (Figure 5D-E, P < .05).
The host γδ T cells but not αβ T cells amplify the severity of GVHD. (A,B) αβ-/- mice (▪, n = 11), γδ-/- mice (▴, n = 13), or wt mice (•, n = 10) received transplants from allogeneic BALB/c or syngeneic B6 donors (○, n = 10) as in Figure 1 and were evaluated for survival (A) and clinical GVHD score (B). Data from 2 similar experiments are combined. ▴ vs ▪, **P < .01, ▪ vs •, P = .26 by Wilcoxon rank test. ▴ vs ▪, *P < .05, ▪ vs •, P = .43 by Mann-Whitney U test from weeks 1 to 5. Error bars represent standard error. (C,D) γδ+ splenocytes ( , n = 12), αβ+ splenocytes (
, n = 12), αβ+ splenocytes ( , n = 8) or media (▴ and solid line, n = 8) were injected into γδ-/- (60 × 106 cells/mouse). Wt B6 mice (• and solid line, n = 12) were injected with media alone. Twenty-four hours later, all mice received transplants from allogeneic BALB/c donors as in Figure 1 and were evaluated for survival (C) and clinical GVHD score (D). Data from 2 similar experiments are combined.
, n = 8) or media (▴ and solid line, n = 8) were injected into γδ-/- (60 × 106 cells/mouse). Wt B6 mice (• and solid line, n = 12) were injected with media alone. Twenty-four hours later, all mice received transplants from allogeneic BALB/c donors as in Figure 1 and were evaluated for survival (C) and clinical GVHD score (D). Data from 2 similar experiments are combined.  vs
vs  , **P < .01 by Wilcoxon rank test.
, **P < .01 by Wilcoxon rank test.  vs
vs  , weeks 3 to 5, *P < .001 by Mann-Whitney U test. Error bars represent standard error.
, weeks 3 to 5, *P < .001 by Mann-Whitney U test. Error bars represent standard error.
The host γδ T cells but not αβ T cells amplify the severity of GVHD. (A,B) αβ-/- mice (▪, n = 11), γδ-/- mice (▴, n = 13), or wt mice (•, n = 10) received transplants from allogeneic BALB/c or syngeneic B6 donors (○, n = 10) as in Figure 1 and were evaluated for survival (A) and clinical GVHD score (B). Data from 2 similar experiments are combined. ▴ vs ▪, **P < .01, ▪ vs •, P = .26 by Wilcoxon rank test. ▴ vs ▪, *P < .05, ▪ vs •, P = .43 by Mann-Whitney U test from weeks 1 to 5. Error bars represent standard error. (C,D) γδ+ splenocytes ( , n = 12), αβ+ splenocytes (
, n = 12), αβ+ splenocytes ( , n = 8) or media (▴ and solid line, n = 8) were injected into γδ-/- (60 × 106 cells/mouse). Wt B6 mice (• and solid line, n = 12) were injected with media alone. Twenty-four hours later, all mice received transplants from allogeneic BALB/c donors as in Figure 1 and were evaluated for survival (C) and clinical GVHD score (D). Data from 2 similar experiments are combined.
, n = 8) or media (▴ and solid line, n = 8) were injected into γδ-/- (60 × 106 cells/mouse). Wt B6 mice (• and solid line, n = 12) were injected with media alone. Twenty-four hours later, all mice received transplants from allogeneic BALB/c donors as in Figure 1 and were evaluated for survival (C) and clinical GVHD score (D). Data from 2 similar experiments are combined.  vs
vs  , **P < .01 by Wilcoxon rank test.
, **P < .01 by Wilcoxon rank test.  vs
vs  , weeks 3 to 5, *P < .001 by Mann-Whitney U test. Error bars represent standard error.
, weeks 3 to 5, *P < .001 by Mann-Whitney U test. Error bars represent standard error.
We next analyzed the ability of γδ T cells to enhance the stimulatory function of DCs in vitro. Intestinal intraepithelial lymphocytes (iIELs) were isolated from B6 mice, stained with anti-TCR γδ mAb, and sorted, producing 2 populations: γδ+ T cells (> 99% purity γδ+ by FACS) and γδ- T cells (< 0.1% γδ+ by FACS). Bone marrow-derived DCs from B6 mice were cultured for 36 hours with either LPS, γδ+ T cells, or γδ- T cells at a T-cell to DC ratio of 1:1. After culture, DCs were isolated by centrifugation on a 14.5% metrizamide gradient, irradiated, and then used as stimulators for allogeneic BALB/c responder T cells. DCs cultured in the presence of γδ+ T cells stimulated significantly greater proliferation (Figure 6A, P < .01) of IL-2 and IFN-γ secretion of allogeneic T cells when compared with DCs that had been cultured in γδ- T cells (IL-2; 213 ± 19 pg/mL vs 62 ± 15 pg/mL, P < .01, IFN-γ; 5059 ± 167 pg/mL vs 1350 ± 98 pg/mL, P < .01).
We next evaluated effect of γδ T-cell coculture on the phenotype of DCs. γδ T cells increased the expression of CD40 from 53% to 84% (Figure 6B), whereas γδ-depleted lymphocytes had minimal effects on CD40 (53% vs 64%). Expression of MHC class II and CD80 on DCs was greater than 90% in all cultures and did not change when cocultured with γδ T cells (data not shown).
γδ T cells activate DCs and enhance allogeneic responses in vivo
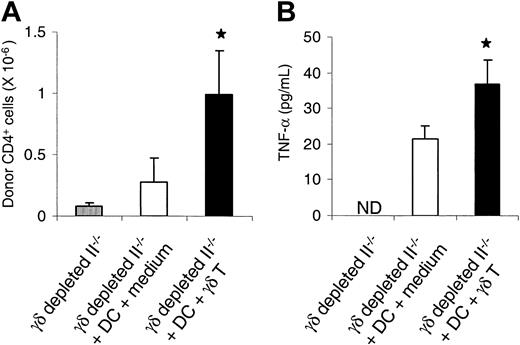
We directly evaluated whether γδ T cells activate DCs and enhance the responses of allogeneic T cells in vivo. γδ T cells were depleted from B6.Ly-5a (CD45.1+) MHC class II-deficient mice (II-/-) by intraperitoneal injection of anti-TCR γδ mAb for 5 days prior to BMT.27 These animals then received 1100 cGy TBI and were injected with 5 × 106 wt B6 DCs that had previously been cocultured either with γδ T cells or with medium. The following day mice received 5 × 106 NWP T cells from bm12 (CD45.1-) donors. Spleens were harvested on day +7 after BMT and analyzed by FACS. The phenotype of donor CD4+ T cells was determined to be CD4+ CD45.1-. Recipients of DCs cocultured with γδ T cells showed a 4-fold increase in donor CD4+ T cells compared with recipients of control DCs (Figure 7A). Serum levels of TNF-α, a biochemical marker of GVHD, were also twice as high in these recipients (P < .05, Figure 7B). We conclude that γδ T cells activate host DCs both in vitro and in vivo and amplify the response of allogeneic donor T cells that cause GVHD.
Absence of host γδ T cells reduces allogeneic donor T-cell responses. (A,B) γδ-/- (▥, n = 4), wt (▪, n = 4), and syn BM transplant recipients (□, n = 4) underwent transplantation as in Figure 1. Splenocytes were harvested on day 7 after BMT, and donor (H2Dd+) CD4+ T-cell (A) and CD8+ T-cell (B) phenotype were determined by FACS analysis as described in “Materials and methods.” Data represent the mean ± SE. wt vs γδ-/-, *P < .05. (C-E) Splenic DCs were isolated from γδ-/- (▴) and wt (•) mice 6 hours after 1100 cGy TBI and were cultured for 3 to 4 days with allogeneic responder BALB/c T cells. During the final 12 hours of a 96-hour culture, cells were pulsed with [3H] thymidine and assayed for proliferation (C). Supernatants were collected at 48 hours and 72 hours and assayed by ELISA for IL-2 (D) and IFN-γ (E), respectively, as described in “Materials and methods.” Each graph represents 1 of 4 similar experiments. wt vs γδ-/-, *P < .05. Error bars represent standard error.
Absence of host γδ T cells reduces allogeneic donor T-cell responses. (A,B) γδ-/- (▥, n = 4), wt (▪, n = 4), and syn BM transplant recipients (□, n = 4) underwent transplantation as in Figure 1. Splenocytes were harvested on day 7 after BMT, and donor (H2Dd+) CD4+ T-cell (A) and CD8+ T-cell (B) phenotype were determined by FACS analysis as described in “Materials and methods.” Data represent the mean ± SE. wt vs γδ-/-, *P < .05. (C-E) Splenic DCs were isolated from γδ-/- (▴) and wt (•) mice 6 hours after 1100 cGy TBI and were cultured for 3 to 4 days with allogeneic responder BALB/c T cells. During the final 12 hours of a 96-hour culture, cells were pulsed with [3H] thymidine and assayed for proliferation (C). Supernatants were collected at 48 hours and 72 hours and assayed by ELISA for IL-2 (D) and IFN-γ (E), respectively, as described in “Materials and methods.” Each graph represents 1 of 4 similar experiments. wt vs γδ-/-, *P < .05. Error bars represent standard error.
Cell-to-cell contact is required for γδ T-cell-mediated DC activation
Because γδ T cells can secrete activating cytokines such as IFN-γ,10,12,13,35,36 we examined a potential role for cytokines in the enhancement of the allostimulatory capacity of DCs caused by these cells. As shown in Figure 6C, the addition of anti-IFN-γ mAb to coculture did not inhibit the activation of DCs by γδ T cells. DCs matured in the presence of γδ T cells from IFN-γ-/- mice stimulated allogeneic T cells to the same degree as DCs from wt mice (Figure 6C). Similar results were observed with anti-TNF-α mAb (data not shown). The percentage of DCs expressing CD40 was increased when cocultured with γδ T cells. CD40L is a costimulatory molecule that is upregulated on CD4+ T cells after exposure to antigen and plays a critical role in DC activation.37-40 However, mAb blockade of CD40L did not prevent DC activation (Figure 6E). Furthermore, DCs from CD40-/- mice were activated to the same degree as DCs from wt mice, confirming that CD40-CD40L interactions are not required for γδ T-cell-mediated DC activation (Figure 6D).
We next determined whether cell-to-cell contact was necessary for the γδ T-cell activation of DCs. DCs were cultured for 36 hours either in direct contact with γδ T cells or separated by a 0.2 μ transwell membrane and were then used as stimulators of allogeneic T cells. Physical separation of DCs and γδ T cells in the transwells completely abrogated DC activation (Figure 6E), demonstrating the requirement for cell-to-cell contact.
γδ T cells activate DCs in vitro and in vivo. Intestinal intraepithelial lymphocytes were sorted into γδ+ and γδ- populations. BM-derived DCs were then cultured with either γδ+ T cells (γδ+), γδ- T cells (γδ-), LPS, or medium. Allogeneic not T-cell proliferation was determined. DCs were isolated on a metrizamide gradient and the expression of CD40 was evaluated by FACS (B). The solid line denotes CD40 expression. The dashed line denotes the IgG negative control. These DCs were irradiated and then cultured with allogeneic BALB/c responder T cells that were assayed for proliferation (A). DCs plus γδ+ vs DCs plus γδ-, *P < .05. Responder BALB/c T cells were cultured for 3 to 4 days with irradiated B6 DCs that had been previously cultured for 36 hours with (C) wt γδ T cells with and without anti-IFN-γ mAb or with γδ T cells from IFN-γ-deficient (IFN-γ-/-) mice; (D) irradiated CD40-deficient (CD40-/-) DCs; (E) wt γδ T cells with and without anti-CD40L mAb (DCs plus γδ T) or separated by a 0.2 μ transwell membrane (DCs/γδ T transwell). During the final 12 hours of culture, cells were pulsed with [3H] thymidine and assayed for proliferation. DCs plus γδ T vs DCs/γδ T transwell, **P < .05. Each graph represents 1 of 3 to 4 similar experiments. Data represent the mean ± SE.
γδ T cells activate DCs in vitro and in vivo. Intestinal intraepithelial lymphocytes were sorted into γδ+ and γδ- populations. BM-derived DCs were then cultured with either γδ+ T cells (γδ+), γδ- T cells (γδ-), LPS, or medium. Allogeneic not T-cell proliferation was determined. DCs were isolated on a metrizamide gradient and the expression of CD40 was evaluated by FACS (B). The solid line denotes CD40 expression. The dashed line denotes the IgG negative control. These DCs were irradiated and then cultured with allogeneic BALB/c responder T cells that were assayed for proliferation (A). DCs plus γδ+ vs DCs plus γδ-, *P < .05. Responder BALB/c T cells were cultured for 3 to 4 days with irradiated B6 DCs that had been previously cultured for 36 hours with (C) wt γδ T cells with and without anti-IFN-γ mAb or with γδ T cells from IFN-γ-deficient (IFN-γ-/-) mice; (D) irradiated CD40-deficient (CD40-/-) DCs; (E) wt γδ T cells with and without anti-CD40L mAb (DCs plus γδ T) or separated by a 0.2 μ transwell membrane (DCs/γδ T transwell). During the final 12 hours of culture, cells were pulsed with [3H] thymidine and assayed for proliferation. DCs plus γδ T vs DCs/γδ T transwell, **P < .05. Each graph represents 1 of 3 to 4 similar experiments. Data represent the mean ± SE.
Discussion
The localization of γδ T cells within GVHD target tissues and their ability to modulate immune responses suggested that these cells might play a role in the pathogenesis of GVHD. In this study we demonstrate that the absence of γδ T cells in allogeneic BM transplant recipients caused significantly less severe acute GVHD as determined by all measurable parameters in several different donor/recipient strain combinations. This finding was also confirmed by depleting γδ T cells in wt recipients by in vivo administration of mAb against γδ TCRs. Reconstitution of host γδ T cells to γδ-/- recipients increased lethal GVHD. Coculture of γδ T cells with DCs enhanced their allostimulatory capacity and increased the proliferation of allogeneic responder T cells in vitro and in vivo. This enhancement was independent of IFN-γ, TNF-α, and CD40L, but depended on cell-to-cell contact. These data demonstrate that host γδ T cells play a critical role in the pathogenesis of GVHD by amplifying the stimulatory function of host DCs.
Donor T cells' interactions with host DCs are essential for the induction of GVHD.1-3 DCs represent a pool of professional APCs whose maturation/activation state is critical to their function. The chemo-radiotherapy used to condition BM transplant recipients induces the secretion of inflammatory cytokine such as IL-1, TNF-α, and IL-6 from damaged host tissues,41 and allows LPS to enter into the systemic circulation.42 Such “danger signals” are critical for the activation of host DCs,43 but Shlomchik44 has reported that recipients deficient in TNFR1/R2, IL-1R, CD40, and Toll-like receptor 4 (essential for LPS-induced activation of DCs) developed GVHD comparable to that seen in wild-type controls. These experiments exclude a role for each of these individual pathways in the activation of host DCs and suggest that either together or through other pathways and/or cell interactions might contribute to the process. Several cell types such as macrophages,5 NK cells,45 CD4+ T,37-40 and CD8+ T46 have been shown to provide maturation and activation signals for DCs.
γδ T cells activate DCs and enhance allogeneic responses. II-/- B6 mice were depleted of γδ T cells, irradiated with 1100 cGy TBI, and injected on day -1 with 5 × 106 B6 DCs that had been cocultured either with γδ T cells or with medium. Mice underwent transplantation with 5 × 106 T cells from bm12 donors on day 0. Splenocytes and serum were harvested on day 5 after BMT (n = 4 per group), and donor CD4+ T cells were phenotyped by FACS. Data represent the mean + SE. (A) Donor CD4+ T cells. ▥ vs ▪, *P < .05. (B) TNF-α. □ vs ▪, *P < .05. ND indicates not detected.
γδ T cells activate DCs and enhance allogeneic responses. II-/- B6 mice were depleted of γδ T cells, irradiated with 1100 cGy TBI, and injected on day -1 with 5 × 106 B6 DCs that had been cocultured either with γδ T cells or with medium. Mice underwent transplantation with 5 × 106 T cells from bm12 donors on day 0. Splenocytes and serum were harvested on day 5 after BMT (n = 4 per group), and donor CD4+ T cells were phenotyped by FACS. Data represent the mean + SE. (A) Donor CD4+ T cells. ▥ vs ▪, *P < .05. (B) TNF-α. □ vs ▪, *P < .05. ND indicates not detected.
Our data demonstrate that murine γδ T cells promote DC activation in a cell-to-cell contact-dependent manner. Leslie et al32 have also demonstrated that human γδ T cells promote DC maturation in a cell-to-cell contact-dependent manner that is restricted by CD1c. Mice do not express CD1c but do express only CD1d, which presents glycolipid antigens to NKT cells.47 Recently, Hashimoto et al48 have demonstrated that absence of CD1d in BM transplant recipients results in a loss of protection from GVHD that is mediated by NKT cells. Thus, while our data demonstrated that γδ T cells exacerbate GVHD, NKT cells can reduce GVHD, suggesting that in mice CD1 probably regulates GVHD through its interaction with NKT cells.
Other studies have focused on the role of γδ T cells in the pathogenesis of GVHD.15-21 One study reported that a clonal population of γδ T cells can induce lethal GVHD,17 whereas Drobyski and Majewski20 and Drobyski et al21 demonstrated that donor γδ T cells alone do not cause acute GVHD. Coadministration of γδ T cells and αβ T cells exacerbated GVHD when compared with αβ T cells alone, suggesting that donor γδ T cells could contribute to GVHD but required the presence of αβ T cells.18 However, the role played by host γδ T cells in the pathogenesis of allogeneic GVHD has not been defined. Sakai et al27 reported that allogeneic BMT recipients depleted of γδ T cells by in vivo administration of mAb against γδ TCRs showed less intestinal damage, but the mechanisms for this phenomenon were not explored. We demonstrate that host γδ T cells amplify systemic GVHD in irradiated GVHD models. Our data suggest that they might function as the “danger signals” and activate the DCs, thus enhancing their allostimulatory capacity.
Although γδ T cells are a minor subpopulation of lymphocytes within lymph nodes and the spleen, they predominate in the epithelium of several principal target organs of GVHD, such as the skin, intestinal tract, and lung.49 γδ T cells respond to infected or transformed epithelial cells and may therefore function as sensors of danger signals that can activate an immune response.43 Recent evidence suggests that γδ IELs express several chemokines such as macrophage inflammatory protein 1α (MIP1α), MIP1β, Regulated or Activation, Normal-T cell Expressed and Secreted (RANTES), and lymphotactin, and therefore play an important role in the rapid recruitment of CCR5+ cells into these epithelial tissues, helping to explain the target organ distribution of GVHD.50,51
γδ T cells may also affect events in the gastrointestinal (GI) tract through their production of keratinocyte growth factor (KGF).52 KGF administration can reduce the severity of GVHD, particularly in the GI tract, and GVHD mortality.53,54 An absence of γδ T cells would be expected to result in reduced endogenous KGF production and enhanced GI tract damage. To examine this possibility we compared the degree of tissue damage on day 7 after lethal irradiation and found no significant difference between wild-type and γδ-/- mice (data not shown). Mice devoid of γδ T cells showed decreased histopathologic damage from GVHD after allogeneic BMT (Figure 3), suggesting that whatever potential benefit γδ T cells might have in GVHD by the secretion of KGF and subsequent promotion of tissue repair, it is overshadowed by the activation of host APCs and a greater donor response of allogeneic T cells.
In summary, we demonstrate a novel role for host γδ T cells in the amplification of GVHD through the activation of host APCs, suggesting that elimination of host γδ T cells might be a novel therapeutic strategy to reduce GVHD.
Prepublished online as Blood First Edition Paper, March 29, 2005; DOI 10.1182/blood-2004-10-4087.
Supported by National Institutes of Health grants K08 AI052 863-01 (P.R.), DK 02 958 (C.L.), and CA 49 542 (J.L.M.F.). J.L.M.F. is a Doris Duke Distinguished Clinical Scientist.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




![Figure 5. Absence of host γδ T cells reduces allogeneic donor T-cell responses. (A,B) γδ-/- (▥, n = 4), wt (▪, n = 4), and syn BM transplant recipients (□, n = 4) underwent transplantation as in Figure 1. Splenocytes were harvested on day 7 after BMT, and donor (H2Dd+) CD4+ T-cell (A) and CD8+ T-cell (B) phenotype were determined by FACS analysis as described in “Materials and methods.” Data represent the mean ± SE. wt vs γδ-/-, *P < .05. (C-E) Splenic DCs were isolated from γδ-/- (▴) and wt (•) mice 6 hours after 1100 cGy TBI and were cultured for 3 to 4 days with allogeneic responder BALB/c T cells. During the final 12 hours of a 96-hour culture, cells were pulsed with [3H] thymidine and assayed for proliferation (C). Supernatants were collected at 48 hours and 72 hours and assayed by ELISA for IL-2 (D) and IFN-γ (E), respectively, as described in “Materials and methods.” Each graph represents 1 of 4 similar experiments. wt vs γδ-/-, *P < .05. Error bars represent standard error.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/2/10.1182_blood-2004-10-4087/6/m_zh80140581420005.jpeg?Expires=1768039752&Signature=RX3jEDC7QKswtaE7xGxgGvCEkTFCfMQq1JAzx79VV-kR1XypjRPjsUdqXZgJi8kmKyFhGiH~JyDXbpV6b1gPek9m-ujhcfCIh~T7z~Gi6t-k5Z0b6dXiQNso3O-ep872pEJ6dpfjwAYk6RG4W7xr~7ox9t2xc0wxwGA8i8TKcmjg4slB7t8Rbl9VrYkVpVlfQvyjZFHXD84U9WAQHHccTKwW2xMxw56jt6S9-MGw12p-cJRAQcHD5U5gMp7csSM1vZNJjykqDc2q6iHqUP~373kK5lAIwYQcVcaFHMNKjuPln6t1aZbZBhXCIhYQd0M12khWAGA2O8NcrseQnj~bkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. γδ T cells activate DCs in vitro and in vivo. Intestinal intraepithelial lymphocytes were sorted into γδ+ and γδ- populations. BM-derived DCs were then cultured with either γδ+ T cells (γδ+), γδ- T cells (γδ-), LPS, or medium. Allogeneic not T-cell proliferation was determined. DCs were isolated on a metrizamide gradient and the expression of CD40 was evaluated by FACS (B). The solid line denotes CD40 expression. The dashed line denotes the IgG negative control. These DCs were irradiated and then cultured with allogeneic BALB/c responder T cells that were assayed for proliferation (A). DCs plus γδ+ vs DCs plus γδ-, *P < .05. Responder BALB/c T cells were cultured for 3 to 4 days with irradiated B6 DCs that had been previously cultured for 36 hours with (C) wt γδ T cells with and without anti-IFN-γ mAb or with γδ T cells from IFN-γ-deficient (IFN-γ-/-) mice; (D) irradiated CD40-deficient (CD40-/-) DCs; (E) wt γδ T cells with and without anti-CD40L mAb (DCs plus γδ T) or separated by a 0.2 μ transwell membrane (DCs/γδ T transwell). During the final 12 hours of culture, cells were pulsed with [3H] thymidine and assayed for proliferation. DCs plus γδ T vs DCs/γδ T transwell, **P < .05. Each graph represents 1 of 3 to 4 similar experiments. Data represent the mean ± SE.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/2/10.1182_blood-2004-10-4087/6/m_zh80140581420006.jpeg?Expires=1768039752&Signature=EAvp8KsITIjVnNX0IquKSlGWHGgFoUwUkfBEp7qg3vJU3qSY1-krmOGTu0JDNacfiGLOLG9z-5xWpxehZGzNIS51h6itnrHvJB3xZrLt5pcu~7GMuppihk~ikZVoiekIJR1VpAG541Vx6DIgwbPj8RhML1aDR0iSIKD58Q2Z0Bum3dvJo85h9cDHjfkYm890BjBdKx1FNe~POkDsN-sS1dnMjAa3E~Xc-FAqwvNkNuTwQfYXFlrK4SDRiUOERDZwFfJ4AEm5rurGvFjcAb6u2nWe89uOCgPL0yPoj1MFdSR1C~wkQKYF2UWBmbnHoPOQfvfW2cJPy-Huo74NyFCtEA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal