Abstract
In erythroid cells the vast majority of iron (Fe) released from endosomes must cross both the outer and the inner mitochondrial membranes to reach ferrochelatase that inserts Fe into protoporphyrin IX. In the present study, we developed a method whereby a cohort of 59Fe-transferrin (Tf)-laden endosomal vesicles were generated, from which we could evaluate the transfer of 59Fe into mitochondria. Iron chelators, dipyridyl or salicylaldehyde isonicotinoyl hydrazone (SIH), were able to bind the 59Fe when they were present during a 37°C incubation; however, addition of these agents only during lysis at 4°C chelated virtually no 59Fe. Bafilomycin A1 (which prevents endosome acidification) and succinylacetone (an inhibitor of 5-aminolevulinate dehydratase) prevented endosomal 59Fe incorporation into heme. Importantly, both the myosin light chain kinase inhibitor wortmannin and the calmodulin antagonist, N-(6-aminohexyl)-5-chloro-1-naphthalene-sulfonamide (W-7), caused significant inhibition of 59Fe incorporation from 59Fe-Tf-labeled endosomes into heme, suggesting that myosin is required for Tf-vesicle movement. Our results reaffirm the astonishing efficiency of Tf-derived Fe utilization in hemoglobin (Hb)-producing cells and demonstrate that very little of this Fe is present in a chelatable pool. Collectively, these results are congruent with our hypothesis that a transient endosome-mitochondrion interaction mediates iron transfer between these organelles. (Blood. 2005;105:368-375)
Introduction
Normal hemoglobinization of immature erythroid cells requires iron uptake from transferrin (Tf), mediated by Tf receptors, whose high levels are essential for maintaining the exceptionally rapid rates of heme synthesis. On a per-cell basis the rate of heme synthesis in immature erythroid cells is at least an order of magnitude higher than in the liver, the second highest heme producer in the organism.1 Following the binding of Fe(III)2-Tf to Tf receptors on the erythroid cell membrane, the Tf-receptor complexes are internalized by endocytosis, and iron is then released from Tf by a process involving endosomal acidification.1-3 The transporter, Nramp2 (also known as DMT14 or DCT15 ), has been shown to be likely responsible for the egress of iron from the endosome.6-8 This protein is encoded by a gene that belongs to the “natural resistance-associated macrophage protein” (Nramp) family of genes identified by Gros and his coworkers9 and transports Fe(II).5 Therefore, reduction of Fe(III) must occur in endosomes; however, nothing is known about the mechanism of this process. It is generally believed that following its release from endosomes, iron enters the cytosol where it equilibrates with a low molecular weight labile iron pool.10,11 However, the extraordinary efficiency of Fe utilization in erythroid tissues, as well as the apparent targeting of Fe to mitochondria (as discussed in the next paragraph), may be inconsistent with such a model.
In hemoglobin-synthesizing cells, the vast majority of iron released from endosomes must cross both the outer and the inner mitochondrial membranes to reach ferrochelatase.1 It is remarkable that in these cells iron acquired from Tf continues to flow into mitochondria, even when the synthesis of protoporphyrin IX is markedly compromised in vitro (by isonicotinic acid hydrazide [INH] or succinylacetone [SA])12-16 or in vivo (patients with erythroid specific 5-aminolevulinate synthase deficiency17 ). A significant proportion of nonheme iron that accumulates in mitochondria under these circumstances is in a form readily available for heme synthesis when protoporphyrin IX formation is restored.12,13,16 Interestingly, when heme synthesis is inhibited in definitive erythroid cells, very little13,16 or no18 iron accumulates in cytosolic ferritin. In contrast, it is well established that in normal nonerythroid cells, iron in excess of metabolic needs ends up in ferritin. Thus, it seems highly likely that in erythroid cells the transport of iron into mitochondria is controlled differently than in nonerythroid cells.
Because Tf-bound iron is extremely efficiently used for hemoglobin synthesis, because iron is targeted into erythroid mitochondria, and because no low molecular weight cytoplasmic iron transport intermediate has been identified in reticulocytes, a new hypothesis of intracellular iron transport has been suggested.1,16 This model proposes that, after iron is released from Tf in the endosome, it is passed directly from endosomal protein to mitochondrial protein until it reaches ferrochelatase in the mitochondrion, thus bypassing the cytosol.
In the present study we developed a method to specifically label reticulocyte endosomes with 59Fe and showed that the use of this 59Fe for heme synthesis requires endosomal acidification and active porphyrin synthesis. Additionally, we showed that 59Fe flux from endosomes into heme can be intercepted by the membrane permeable chelators dipyridyl (DP) and salicylaldehyde isonicotinoyl hydrazone (SIH), but the 59Fe-chelates can be formed only in metabolically active cells. We also found that myosin light chain kinase inhibitors, wortmannin (WT) and 1-5(-chloronaphthalene-1-sulfonyl)-1h-hexahydro-1,4-diazepine (ML-9), as well as the calmodulin antagonist, N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W-7), caused significant inhibition of 59Fe incorporation from 59Fe-Tf-labeled endosomes into heme. All these agents are known to inhibit the microfilament motor, myosin, which thus appears to be involved in intracellular movement of endosomes. Collectively, these results support the hypothesis that intracellular translocation of Fe involves a transient interaction between endocytic vesicles and mitochondria.
Materials and methods
Materials
SA, bafilomycin A1 (Baf), INH, nocodazole, vinblastine, taxol, cytochalasin D (CD), WT, ML-9, W-7, 2,3-butanedione monoxime (BDM), antimycin A1, apo-Tf, pronase, and bovine serum albumin (BSA) were obtained from Sigma (St Louis, MO). α,α′-dipyridyl (DP) was obtained from Fisher Scientific (Fair Lawn, NJ). 59FeCl3 was purchased from Amersham (Buckinghamshire, United Kingdom) and 125I from ICN (Irvine, CA). SIH was synthesized as previously described.19 Apo-Tf was labeled with 59Fe using 59Fe-citrate as described,20 yielding approximately 80% saturated Tf (the other 20% being a mixture of monoferric and apo-transferrin).
Reticulocyte preparation
CD1 mice were injected with neutralized phenylhydrazine (intraperitoneally) at a dose of 50 mg/kg/d for 3 continuous days. On the 3rd or 4th day following the last injection blood was taken from the heart under ether anesthesia using heparin as anticoagulant. After 3 washes with ice-cold phosphate-buffered saline (PBS), the cells (about 45% reticulocytes, as determined by new methylene blue staining) were resuspended in the incubation medium (Minimum Essential Media [MEM] containing 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 10 mM NaHCO3, pH 7.4, and 1% BSA) and referred to as “reticulocytes.”
Preparation of reticulocytes containing 59Fe-labeled endosomes and measurement of 59Fe transfer into mitochondria
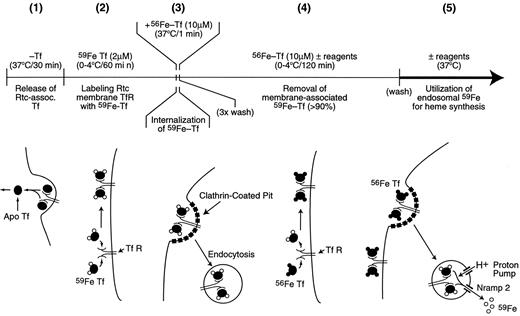
To eliminate the possible effects of various reagents on endocytosis, rates of 59Fe-heme synthesis were measured from internalized 59Fe-Tf present within endosomes. The protocol to specifically label reticulocyte endosomes with 59Fe is illustrated in Figure 1. Briefly, reticulocytes depleted of endogenous Tf (period 1) were incubated (0-4°C/60 minutes) with 2 μM 59Fe-Tf to saturate membrane Tf receptors with 59Fe-Tf (period 2). To obtain a “cohort” of 59Fe-containing endosomes, samples were warmed up at 37°C for 1 minute by adding 4 volumes of prewarmed medium containing excess 56Fe-Tf (10 μM final concentration) to allow endocytosis of 59Fe-Tf (period 3). After 3 washes with cold PBS the reticulocyte pellet was resuspended in incubation medium containing 56Fe-Tf (10 μM final concentration) and incubated on ice for an additional 2 hours to exchange remaining membrane-associated 59Fe-Tf with 56Fe-Tf (period 4), followed by a wash with cold incubation medium in which the cells were incubated during period 5. Measurements of cellular heme and non-heme 59Fe were performed either by an acid precipitation method12 or by acid methyl ethyl ketone extraction21 ; because both methods yielded virtually identical results, the acid precipitation method was used for convenience. The radioiron measurements were carried out in a LKB Compugamma Counter (LKB Instruments, Pleasant Hill, CA). At the beginning of period 5 (Figure 1) reticulocytes contained up to 40% of 59Fe in their heme, indicating a remarkably efficient transfer of radioactive iron into heme during period 3; this can be prevented by heme synthesis inhibitors (INH, SA) or chelators such as SIH and DP (data not shown). Additionally, preliminary experiments indicated that there was no incorporation of cell-associated 59Fe into heme during any of the 4°C incubations (data not shown). During incubation of reticulocytes at 37°C (period 5), 59Fe radioactivity in heme rapidly increased, reaching 90% within 20 minutes; generally, effects of various agents were assessed during this period. (This method was used in experiments presented in Figures 2, 3, 4, 5, 6, 7 and Table 1 [“Endosomal 59Fe incorporation into heme”].)
Protocol to prepare reticulocytes containing 59Fe-labeled endosomes.
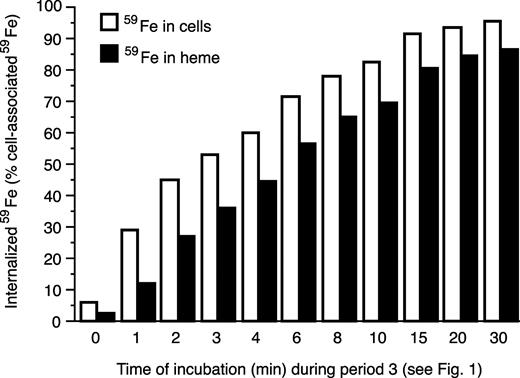
Internalization of 59Fe from membrane-associated 59Fe-Tf and its incorporation into heme. After saturating the membrane TfR with 59Fe-Tf (see Figure 1, the end of period 2), internalization was initiated by incubating cells at 37°C in the presence of 5-fold excess of 56Fe-Tf. At the indicated times, samples were collected and washed, and membrane-bound 59Fe-Tf was removed by incubating the cells with 56Fe-Tf (Figure 1, period 4). Radioactivity in cells and that in heme was then evaluated. The values are displayed as the percentage of the original cell associated 59Fe as it was before displacement by 56Fe-Tf (Figure 1, beginning of period 4).
Internalization of 59Fe from membrane-associated 59Fe-Tf and its incorporation into heme. After saturating the membrane TfR with 59Fe-Tf (see Figure 1, the end of period 2), internalization was initiated by incubating cells at 37°C in the presence of 5-fold excess of 56Fe-Tf. At the indicated times, samples were collected and washed, and membrane-bound 59Fe-Tf was removed by incubating the cells with 56Fe-Tf (Figure 1, period 4). Radioactivity in cells and that in heme was then evaluated. The values are displayed as the percentage of the original cell associated 59Fe as it was before displacement by 56Fe-Tf (Figure 1, beginning of period 4).
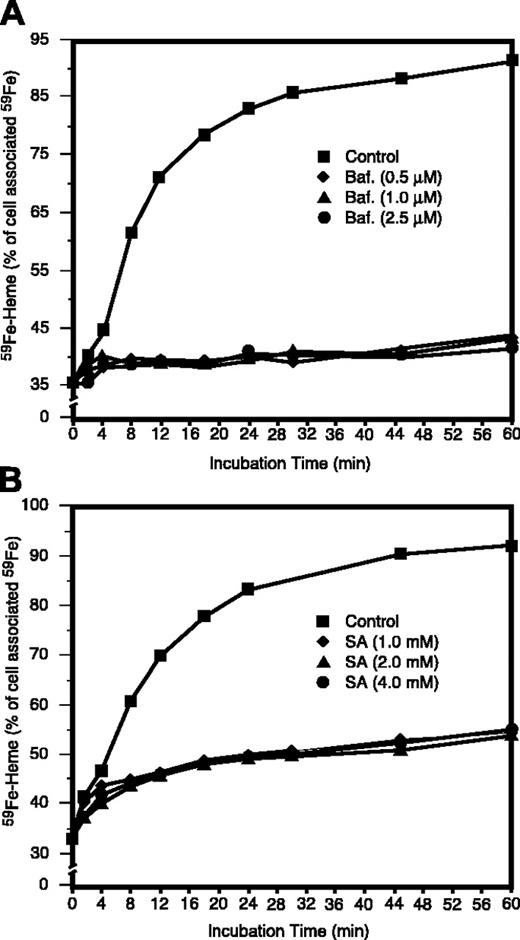
Effects of Baf and SA on the use of endosomal 59Fe for heme synthesis. Endosomal vesicles were loaded with 59Fe as described in Figure 1, and the cells were incubated at 37°C for the indicated time intervals. Baf (A) or SA (B) was included during the last 30 minutes of period 4 (Figure 1) and the entire period 5 (Figure 1) where indicated. The values are displayed as the percentage of the total cellular 59Fe as it was after the membrane-associated radioiron was displaced by 56Fe-Tf (Figure 1, end of period 4).
Effects of Baf and SA on the use of endosomal 59Fe for heme synthesis. Endosomal vesicles were loaded with 59Fe as described in Figure 1, and the cells were incubated at 37°C for the indicated time intervals. Baf (A) or SA (B) was included during the last 30 minutes of period 4 (Figure 1) and the entire period 5 (Figure 1) where indicated. The values are displayed as the percentage of the total cellular 59Fe as it was after the membrane-associated radioiron was displaced by 56Fe-Tf (Figure 1, end of period 4).
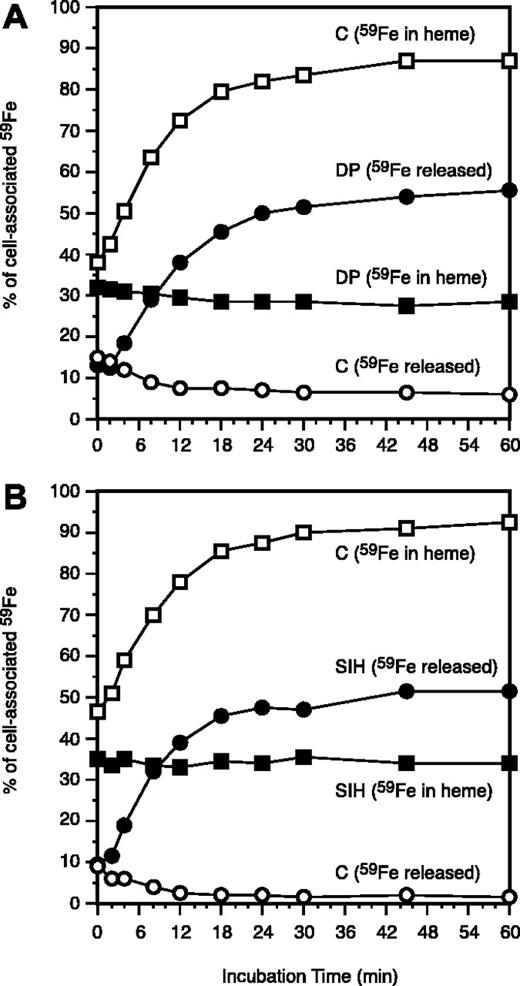
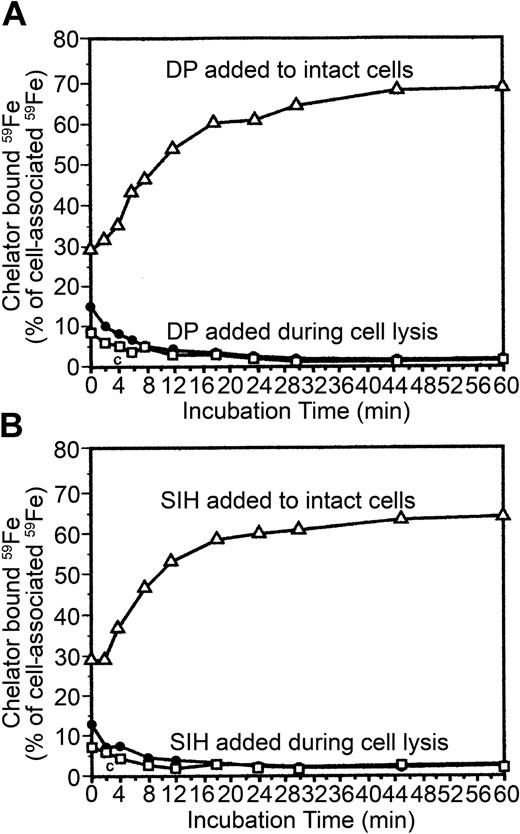
Effects of dipyridyl (DP) and salicylaldehyde isonicotinoyl hydrazone (SIH) on the use of endosomal 59Fe for heme synthesis and release of 59Fe from reticulocytes. One mM (A) or 0.1 mM SIH (B) was included during periods 4 and 5 (Figure 1). At the indicated intervals (during period 5), 2 samples were collected: one was used for measurements of 59Fe radioactivity in heme (▪, DP or SIH; □, control), while the other was transferred to 4 mL ice-cold PBS and centrifuged and the 59Fe radioactivity in the supernatant measured (•, DP or SIH; ○, control). The values are displayed as the percentage of the total cellular 59Fe as it was after the membrane-associated radioiron was displaced by 56Fe-Tf (Figure 1, end of period 4).
Effects of dipyridyl (DP) and salicylaldehyde isonicotinoyl hydrazone (SIH) on the use of endosomal 59Fe for heme synthesis and release of 59Fe from reticulocytes. One mM (A) or 0.1 mM SIH (B) was included during periods 4 and 5 (Figure 1). At the indicated intervals (during period 5), 2 samples were collected: one was used for measurements of 59Fe radioactivity in heme (▪, DP or SIH; □, control), while the other was transferred to 4 mL ice-cold PBS and centrifuged and the 59Fe radioactivity in the supernatant measured (•, DP or SIH; ○, control). The values are displayed as the percentage of the total cellular 59Fe as it was after the membrane-associated radioiron was displaced by 56Fe-Tf (Figure 1, end of period 4).
Effects of decreasing metabolic activity (4°C) on mobilization of vesicular 59Fe. Chelators (1 mM DP or 0.1 mM SIH) were included either during periods 4 and 5 (▵) or dissolved in the H2O used to lyse 59Fe-reticulocytes (•). Following incubations of 59Fe-reticulocytes with the chelators, radioactivity was measured in the media, and that value was added to that of the EtOH soluble fraction. This sum represents the total chelator-bound 59Fe. The data are expressed as the percentage of the total cellular radioactivity as it was after the membrane-associated radioiron was displaced by 56Fe-Tf (Figure 1, end of period 4). As a control, the EtOH soluble 59Fe was extracted from untreated, 59Fe-labeled cells (□).
Effects of decreasing metabolic activity (4°C) on mobilization of vesicular 59Fe. Chelators (1 mM DP or 0.1 mM SIH) were included either during periods 4 and 5 (▵) or dissolved in the H2O used to lyse 59Fe-reticulocytes (•). Following incubations of 59Fe-reticulocytes with the chelators, radioactivity was measured in the media, and that value was added to that of the EtOH soluble fraction. This sum represents the total chelator-bound 59Fe. The data are expressed as the percentage of the total cellular radioactivity as it was after the membrane-associated radioiron was displaced by 56Fe-Tf (Figure 1, end of period 4). As a control, the EtOH soluble 59Fe was extracted from untreated, 59Fe-labeled cells (□).
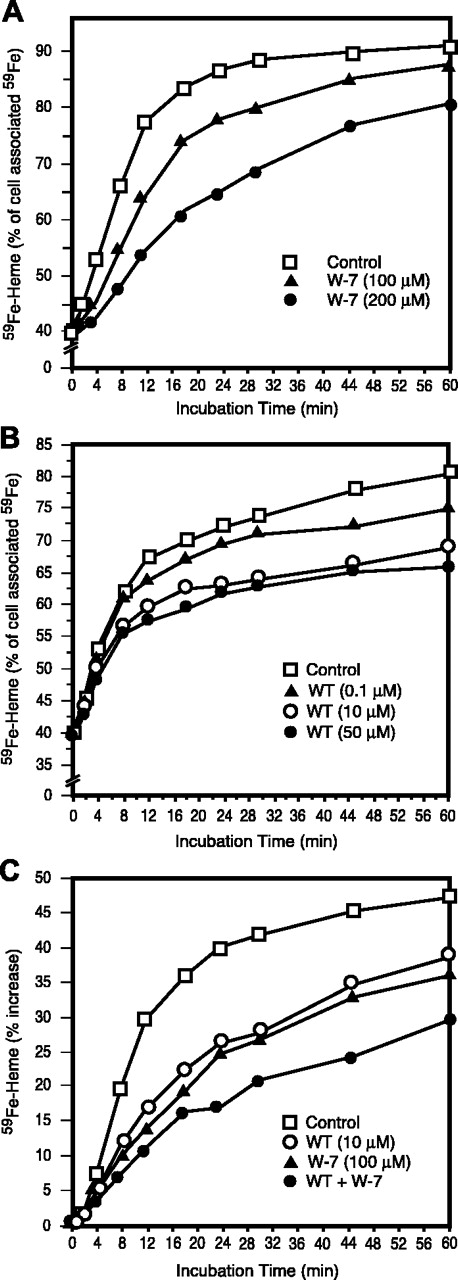
Effects of wortmannin (WT) and N-(6-aminohexyl)-5-chloro-1-naphthalene sulfonamide (W-7) and their combination on the use of endosomal 59Fe for heme synthesis. WT (A) and W-7 (B) were included during the last 30 minutes of period 4 and all of period 5 (Figure 1); the data are displayed as in the previous 3 figures. (C) The results of incubation WT or W-7 (as in panels A and B) or the combination of WT and W-7 are presented as the percentage of increase in 59Fe-heme content from time = 0 (ie, at the beginning of period 5).
Effects of wortmannin (WT) and N-(6-aminohexyl)-5-chloro-1-naphthalene sulfonamide (W-7) and their combination on the use of endosomal 59Fe for heme synthesis. WT (A) and W-7 (B) were included during the last 30 minutes of period 4 and all of period 5 (Figure 1); the data are displayed as in the previous 3 figures. (C) The results of incubation WT or W-7 (as in panels A and B) or the combination of WT and W-7 are presented as the percentage of increase in 59Fe-heme content from time = 0 (ie, at the beginning of period 5).
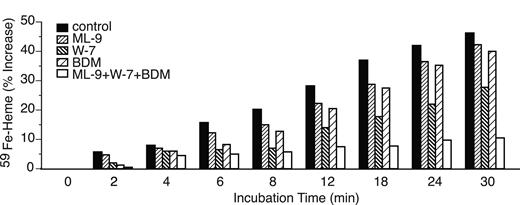
Effects of 1-5(-chloronaphthalene-1-sulfonyl)-1h-hexahydro-1,4-diazepine (ML-9; 100 μM), W-7 (100 μM) and 2,3-butanedione monoxine (BDM) (20 mM), or their combination, on the use of endosomal 59Fe for heme synthesis. These reagents were included during the last 30 minutes of period 4 and all of period 5. The results are presented as in Figure 6C (ie, percentage of increase in 59Fe-heme from the beginning of period 5).
Effects of 1-5(-chloronaphthalene-1-sulfonyl)-1h-hexahydro-1,4-diazepine (ML-9; 100 μM), W-7 (100 μM) and 2,3-butanedione monoxine (BDM) (20 mM), or their combination, on the use of endosomal 59Fe for heme synthesis. These reagents were included during the last 30 minutes of period 4 and all of period 5. The results are presented as in Figure 6C (ie, percentage of increase in 59Fe-heme from the beginning of period 5).
Effects of microfilament and microtubule inhibitors on Iron metabolism and Tf cycle in reticulocytes
. | . | . | . | Incorporation of 59Fe into* . | . | . | . | |
|---|---|---|---|---|---|---|---|---|
| Reagent . | Concentration, μM . | Tf internalization, % (max. time)† . | Tf release, % (max. time)† . | Cells, %‡ . | Heme, %‡ . | Endosomal 59Fe incorporated into heme, %‡ . | Mitochondrial 59Fe incorporated into heme, %‡ . | |
| WT | ||||||||
| 0.1 | 97.1 (30) | 90.7 (6) | 92.9 | 90.5 | 89.6 (8) | 97.5 | ||
| 1.0 | 94.3 (30) | 90.6 (6) | 72.3 | 65.5 | 76.4 (8) | 95.4 | ||
| 10.0 | 76.6 (30) | 90.6 (6) | 53.9 | 40.2 | 73.0 (8) | 95.4 | ||
| 50.0 | 60.5 (30) | 87.7 (6) | ND | ND | 61.8 (8) | ND | ||
| ML-9 | ||||||||
| 50.0 | 56.9 (3) | 59.4 (2) | 67.2 | 65.7 | 74.3 (4) | ND | ||
| 100.0 | 40.6 (3) | 48.6 (2) | 61.1 | 52.2 | 66.6 (4) | ND | ||
| 200.0 | 32.5 (3) | 35.2 (2) | 30.5 | 13.2 | 61.5 (4) | ND | ||
| W-7 | ||||||||
| 10.0 | ND | ND | 96.8 | 95.6 | ND | |||
| 50.0 | 83.4 (2) | 46.8 (2) | 78.3 | 74.2 | 77.9 (4) | ND | ||
| 100.0 | 69.2 (2) | 33.6 (2) | 55.5 | 48.6 | 53.0 (4) | 96.5 | ||
| 200.0 | 37.4 (2) | 12.8 (2) | 32.5 | 22.6 | 30.4 (4) | 94.5 | ||
| BDM | ||||||||
| 10 000.0 | 88.4 (4) | 95.9 (2) | 85.5 | 87.0 | 54.6 (4) | 88.3 | ||
| 20 000.0 | 72.6 (4) | 95.5 (2) | 65.2 | 61.1 | 44.9 (4) | 84.8 | ||
| Nocodazole | ||||||||
| 10.0 | 112.7 (10) | ND | 114.2 | 118.4 | 114.2 (8) | ND | ||
| 50.0 | 114.7 (10) | ND | 122.7 | 128.5 | 112.8 (8) | ND | ||
| 100.0 | 118.9 (30) | ND | 117.7 | 122.0 | 112.7 (8) | ND | ||
. | . | . | . | Incorporation of 59Fe into* . | . | . | . | |
|---|---|---|---|---|---|---|---|---|
| Reagent . | Concentration, μM . | Tf internalization, % (max. time)† . | Tf release, % (max. time)† . | Cells, %‡ . | Heme, %‡ . | Endosomal 59Fe incorporated into heme, %‡ . | Mitochondrial 59Fe incorporated into heme, %‡ . | |
| WT | ||||||||
| 0.1 | 97.1 (30) | 90.7 (6) | 92.9 | 90.5 | 89.6 (8) | 97.5 | ||
| 1.0 | 94.3 (30) | 90.6 (6) | 72.3 | 65.5 | 76.4 (8) | 95.4 | ||
| 10.0 | 76.6 (30) | 90.6 (6) | 53.9 | 40.2 | 73.0 (8) | 95.4 | ||
| 50.0 | 60.5 (30) | 87.7 (6) | ND | ND | 61.8 (8) | ND | ||
| ML-9 | ||||||||
| 50.0 | 56.9 (3) | 59.4 (2) | 67.2 | 65.7 | 74.3 (4) | ND | ||
| 100.0 | 40.6 (3) | 48.6 (2) | 61.1 | 52.2 | 66.6 (4) | ND | ||
| 200.0 | 32.5 (3) | 35.2 (2) | 30.5 | 13.2 | 61.5 (4) | ND | ||
| W-7 | ||||||||
| 10.0 | ND | ND | 96.8 | 95.6 | ND | |||
| 50.0 | 83.4 (2) | 46.8 (2) | 78.3 | 74.2 | 77.9 (4) | ND | ||
| 100.0 | 69.2 (2) | 33.6 (2) | 55.5 | 48.6 | 53.0 (4) | 96.5 | ||
| 200.0 | 37.4 (2) | 12.8 (2) | 32.5 | 22.6 | 30.4 (4) | 94.5 | ||
| BDM | ||||||||
| 10 000.0 | 88.4 (4) | 95.9 (2) | 85.5 | 87.0 | 54.6 (4) | 88.3 | ||
| 20 000.0 | 72.6 (4) | 95.5 (2) | 65.2 | 61.1 | 44.9 (4) | 84.8 | ||
| Nocodazole | ||||||||
| 10.0 | 112.7 (10) | ND | 114.2 | 118.4 | 114.2 (8) | ND | ||
| 50.0 | 114.7 (10) | ND | 122.7 | 128.5 | 112.8 (8) | ND | ||
| 100.0 | 118.9 (30) | ND | 117.7 | 122.0 | 112.7 (8) | ND | ||
ND indicates not determined.
Reticulocytes (20% suspension) were incubated with 59Fe-Tf (10 μM, in terms of transferrin concentration) for 60 minutes, following which radioactivity in washed cells and extracted heme was evaluated.
Maximum effects at time intervals indicated in parentheses (minutes).
Results are expressed as percentages of corresponding controls.
Measurement of chelator-bound 59Fe
59Fe-reticulocytes (50 μL), obtained after experimental manipulations indicated in specific experiments, were lysed with 200 μL cold water, and proteins were precipitated with 1 mL cold 95% ethanol. The mixture was then spun to yield an ethanol soluble fraction containing 59Fe bound to low molecular weight chelators (in the majority of experiments, DP or SIH), and an ethanol precipitate fraction containing protein-bound 59Fe.22 A control experiment demonstrated that 95% ethanol precipitated 59Fe bound to Tf or incorporated into ferritin or hemoglobin, whereas chelator-bound 59Fe was shown to be ethanol soluble. (This method was used in experiments presented in Figure 5.)
The assessment of the utilization of mitochondrial non-heme 59Fe for heme synthesis
Reticulocyte mitochondria were loaded with non-heme 59Fe by incubating (30 minutes) the cells with INH (10 mM), an inhibitor of 5-aminolevulinate synthase, following which the samples were supplemented with 59Fe-Tf and incubated for an additional 60 minutes. Unbound 59Fe-Tf was removed by a thorough washing in cold buffer. As compared with control reticulocytes that contained more than 80% 59Fe in heme, INH-treated reticulocytes contained less than 10% of 59Fe in heme.23 Following washing and second incubation (1 hour) without the heme synthesis inhibitor, non-heme 59Fe accumulated in mitochondria is used for the synthesis of heme that contains 60% or more of cell-associated 59Fe.23 All the reagents that were shown to inhibit the incorporation of endosomal-59Fe for heme synthesis were also tested for their effect on the use of mitochondrial non-heme 59Fe for heme synthesis. (This method was used in experiments presented in Table 1, last column.)
125I-Tf uptake and release
Reticulocytes were preincubated with tested reagents (60 minutes/4°C) following which 125I-Tf (2 μM) was added and 125I-Tf internalization was initiated by warming up at 37°C; the tested reagents were present during incubation at 37°C. Samples were taken in duplicates at different time intervals and immediately transferred to ice-cold PBS to terminate the Tf internalization. After 3 washes, the cell pellet was resuspended in 400 μL 0.25% pronase and incubated on ice for 30 minutes. Following centrifugation, 125I-Tf radioactivity of reticulocytes (pronase resistant, ie, internalized 125I-Tf) was measured. To examine 125I-Tf release, reticulocytes were first incubated with 125I-Tf (2 μM) at 37°C for 30 minutes. After 3 washes with PBS (4°C), cells were preincubated with tested reagents in an ice-bath. 125I-Tf release was initiated by warming up at 37°C in the presence of 56Fe2-Tf (2 μM); tested reagents were also present during this incubation. Samples were collected in duplicates, immediately transferred into ice-cold PBS and centrifuged to separate cellular 125I-Tf from 125I-Tf released into the medium. (This method was used in experiments presented in Table 1, first 2 columns.)
Data analysis
All of the results presented are averages of duplicate data (variability < 3%) points from 1 experiment which is representative of at least 4 experiments.
Results
A “pulse-chase” system to investigate 59Fe transfer from endosomes to heme
Figure 2 shows that following a 1-hour incubation (4°C) of Tf-depleted reticulocytes with 59Fe2-Tf (the end of period 2, Figure 1), about 95% of cell-associated 59Fe represents noninternalized 59Fe-Tf that is available for exchange with nonradioactive, 56Fe2-Tf (Figure 2, 0 minute). As expected, increasing 37°C incubation time led to increased internalization of radioiron; by 30 minutes 96% of the cell-associated 59Fe could not be displaced by excess extracellular 56Fe2-Tf. Interestingly, the incorporation of internalized 59Fe into heme occurred very rapidly so that at 2 minutes, 4 minutes, and 15 minutes of incubation 59Fe-heme represented 42%, 74%, and 90% of internalized 59Fe, respectively (Figure 2). It was somewhat surprising to find that even after only 1 minute, some 59Fe was already detected in heme, representing about 40% of internalized 59Fe. Since the purpose of this experiment was to establish an incubation time interval at period 3 (Figure 1) that would generate a cohort of 59Fe2-Tf labeled endosomes, the iron from which could be “chased” into heme, we chose this 1-minute interval for subsequent experiments.
Vesicular 59Fe utilization requires a vacuolar proton-translocating adenosine triphosphatase (V-ATPase) proton pump and intact heme synthesis
We next examined (Figure 3) whether 59Fe translocation into heme during period 5 (“chase”) required endosomal acidification and intact porphyrin synthesis. Figure 3 shows that in control 59Fe-labeled reticulocytes, the radioactivity of 59Fe in heme increased from 36% to about 90% within 45 to 60 minutes. A specific V-ATPase inhibitor, bafilomycin A1,24 almost totally inhibited the use of 59Fe for heme synthesis during period 5 (Figure 3A). This strongly suggests that at the end of period 3 59Fe is associated with Tf within endosomes. As expected, SA, a specific inhibitor of 5-aminolevulinate dehydratase,13 the second enzyme of heme synthesis, prevented endosomal-59Fe incorporation into heme (Figure 3B).
Iron chelators intercept 59Fe only in metabolically active reticulocytes
The ferrous iron chelator, DP, can intercept iron following its release from Tf and mobilize it from endosomes,14,25 whereas the ferric iron chelator, SIH, can readily oxidize ferrous iron26 and efficiently mobilize iron from both endosomes and mitochondria.19 As expected both DP and SIH promoted 59Fe release from 59Fe-labeled reticulocytes and prevented the use of endosomal-59Fe for heme synthesis (Figure 4). Approximately the same fraction of endosomal 59Fe that is normally used for heme synthesis (Figure 4) can be detected as being bound to the chelators (a sum of the 59Fe released from the cells plus the chelator-bound 59Fe within the cells; Figure 5). Importantly, the chelators can capture 59Fe only when they are present during the incubation of the reticulocytes at 37°C. When reticulocytes are incubated without chelators, which are then added solely during cell lysis (4°C), only a very small fraction of 59Fe is available for binding by either DP or SIH (ie, ethanol-soluble 59Fe; Figure 5). These experimental results indicate that the chelators can intercept 59Fe during its translocation from 59Fe-endosomes to ferrochelatase only in metabolically active reticulocytes.
Inhibitors of microfilament, but not microtubule, function abrogate the use of endosomal 59Fe for heme synthesis
There is increasing evidence that microfilaments and microtubules, and their corresponding motors, are involved not only in endocytosis but also in subsequent endosome trafficking.27-33 Hence, the following experiments were designed to examine whether the disruption of microtubule or microfilament function affects the use of endosomal 59Fe for heme synthesis. We found that inhibitors of microtubule function, nocodazole34-36 and taxol37,38 (not shown), when added during period 5, at concentrations known to inhibit the aforementioned functions, did not block the use of endosomal 59Fe for heme synthesis. In fact, nocodazole slightly stimulated the incorporation of 59Fe from endosomes into heme (see Table 1). A microfilament depolymerizer, cytochalasin D,39 did not block endosomal-59Fe incorporation into heme (not shown). However, cytochalasin D is able to depolymerize microfilaments only when they are in a dynamic state40,41 ; microfilaments are extremely stable at the latest stages of erythroid differentiation.42 Therefore, we examined the effects of inhibitors of microfilament motors, myosins. We found that the myosin light chain kinase inhibitor WT43,44 inhibited 59Fe incorporation from 59Fe-Tf-labeled endosomes into heme (Figure 6A). Similar inhibition was seen (see Table 1) using another myosin light chain kinase inhibitor, ML-9.45,46 Moreover, a general myosin ATPase inhibitor, BDM47,48 (see Table 1), as well as a calmodulin antagonist, W-749,50 (Figure 6B), also caused significant inhibition of 59Fe incorporation from 59Fe-endosomes into heme. It should be pointed out that the effects of WT and W-7 were additive (Figure 6C), and the combined presence of ML-9, W-7, and BDM during period 5 almost completely blocked the utilization of endosomal-59Fe for the synthesis of heme (Figure 7). Importantly, none of the above listed reagents, except for BDM (see Table 1), inhibited the use of mitochondrial non-heme 59Fe for heme synthesis, indicating that these reagents neither interfere with porphyrin biosynthesis nor inhibit ferrochelatase. Additionally, BDM inhibits endosomal 59Fe utilization more significantly than mitochondrial 59Fe incorporation into heme (see Table 1), suggesting that the former effect is primarily responsible for the effects of BDM shown in Figure 7.
To further elucidate the nature of these reagents' inhibitory properties, we examined their effects on the uptake and release of 125I-Tf as well as on the incorporation of 59Fe from 59Fe-Tf into reticulocytes and heme (Table 1). All the agents, except for nocodazole, slowed down Tf cycle and inhibited incorporation of 59Fe from 59Fe-Tf into reticulocytes and heme. Nocodazole, somewhat surprisingly, increased the rate of Tf internalization and slightly stimulated cellular uptake of 59Fe and its incorporation into heme.
Discussion
In reticulocytes, the delivery of Tf-borne iron to hemoglobin occurs with extremely high efficiency since after only a 3-minute incubation of reticulocytes with 59Fe2-Tf about 80% of cell-associated 59Fe can be found in heme.16 This remarkably effective utilization of iron in erythroid cells can be explained, at least in part, by a specific targeting of iron toward mitochondria where ferrochelatase inserts Fe2+ into protoporphyrin IX. Although ferritin has been postulated to act as an intermediate for heme synthesis in erythroid cells, several studies failed to show that 59Fe from 59Fe-ferritin could be incorporated into hemoglobin51 (reviewed in Richardson and Ponka2 ). Moreover, erythroleukemia cells overexpressing H-ferritin subunits show significantly inhibited synthesis of heme,52 suggesting that excess ferritin traps intracellular iron and curtails its availability for heme synthesis.
As pointed out above, in hemoglobin-synthesizing cells iron seems to be very specifically channeled to the site where heme is synthesized. Earlier work suggested an association of Tf with mitochondria in erythroid cells,53-55 leading to the proposal1,16 that a highly efficient use of Tf-borne iron for heme synthesis may require a direct interaction of endosomes, bearing Fe2-Tf, with mitochondria, followed by the transfer of iron through organellar membrane-associated proteins to ferrochelatase. The main purpose of our study was to further investigate this hypothesis.
Using an incubation protocol exploiting 59Fe-Tf and 56Fe-Tf (Figure 1) and the temperature dependence of endocytosis, we specifically labeled mouse reticulocyte endosomes with 59Fe bound to Tf. We demonstrated that a specific V-ATPase inhibitor, bafilomycin A1, almost totally blocked the use of endosomal 59Fe for heme synthesis (Figure 3A), indicating that this 59Fe was bound to Tf within the vesicles. The transfer of endosomal 59Fe into heme was very rapid and required an unrestrained supply of protoporphyrin IX (Figure 3B). Approximately the same amount of endosome-derived 59Fe that normally appears in heme (Figures 3, 4), can be intercepted by ferrous or ferric chelators when they are added to the 59Fe-reticulocytes during their incubation at 37°C. Some of the chelator-bound 59Fe can be released from the cells (Figure 4) while a fraction of 59Fe-DP or 59Fe-SIH remains trapped in the reticulocytes (Figure 5). However, very little intracellular 59Fe can be acquired by the chelators when they are added during lysis at 4°C of 59Fe-reticulocytes. In other words, at any given time the vast majority of 59Fe is bound to components that bind 59Fe with a stronger affinity than DP or SIH. The only exceptions are very short incubation time intervals (up to 4 minutes) when 59Fe is probably just being released from Tf within endosomes (Figure 5).
In the following experiments we examined whether agents that may disturb intracellular trafficking of endosomes inhibit the use of endosomal 59Fe for heme synthesis. Neither the specific microtubule depolymerizer, nocodazole, nor the microtubule stabilizer, taxol, inhibited Tf and iron uptake by reticulocytes or 59Fe incorporation from endosomes into heme. This result is in agreement with previous observations that microtubule inhibitors do not block Tf endocytosis and iron uptake by reticulocytes.56 On the contrary, we observed that nocodazole slightly stimulated Tf and iron uptake by reticulocytes (Table 1). Although the mechanism of this stimulation is unclear, it is pertinent to mention that nocodazole also modestly increased incorporation of endosomal 59Fe into heme (Table 1).
Myosins, some types of which are present in erythroid cells,42,57-59 are molecular motors involved in the transport of vesicles at specific locations in cells.60 We showed that myosin light chain kinase inhibitors, WT and ML-9, calmodulin antagonist, W-7, and a general myosin heavy chain ATPase inhibitor, BDM, inhibited endosomal 59Fe incorporation into heme (Figures 6, 7). WT, which is also an inhibitor of phosphatidylinositol 3′-kinase,61 was previously shown to inhibit Tf receptor recycling,62 but it is unknown whether this inhibition is due to WT's effect on the kinase. The inhibitory effects of WT and W-7 are additive (Figure 6C), a finding that suggests different mechanisms of action of these 2 agents. It should be pointed out that calmodulin, a regulator of myosin light chain kinase, was previously shown to play a role in Tf recycling and exocytic Tf release in rat reticulocytes.63 All these inhibitors also inhibited Tf internalization and release as well as the uptake of iron by reticulocytes and its utilization for heme synthesis (Table 1). These results strongly suggest that attenuated cycling of Tf is associated with impaired mobility of endosomes within reticulocytes that, in turn, results in an inefficient utilization of iron for heme synthesis. If iron, following its release from endosomes, entered a “freely diffusible” Fe pool in the cytosol, the inhibition of intracellular endosome trafficking would not be expected to interfere with iron transport toward mitochondrial ferrochelatase. The concept that iron bypasses the cytosol would seem to be in conflict with studies11 that attempt to estimate iron concentrations in the cytosolic “labile iron pool” (LIP). However, these measurements are based on the use of a chelator, calcein, that can be expected to intercept iron upon its release from endosomes. This conclusion is strongly supported by the observation that iron concentration in the LIP increases up to 10-fold immediately following the exposure of K562 cells to Fe2+-Tf.64 In fact, as we have demonstrated in the current study, virtually all of the chelator accessible iron in reticulocytes, that are not actively directing iron to mitochondria, is probably the small amount (< 5% at time = 45 minutes, Figure 5) that has been released from Tf within the vesicle, as opposed to the majority (about 70% at time = 45 minutes, Figure 5) of the cell-associated iron that bound when the chelators are added at 37°C. It is important to note that those studies measuring the LIP with fluorescent chelators, calcein or phen green SK, load cells with these reagents for as many as 30 minutes at 37°C.11,64,65 Thus, most of the reported measurements of this compartment may not reflect cytoplasmic iron, rather iron that has been captured during its active transfer to more tightly binding ligands, such as ferrochelatase/heme or ferritin. It needs to be also pointed out that the calcein-based LIP measurements were done only with non-erythroid cells. Since this method requires fluorescence measurements, it may not be appropriate for hemoglobin-synthesizing cells which contain high levels of fluorescent porphyrins.
Collectively, these results support the hypothesis that in erythroid cells a transient mitochondria-endosome interaction may be involved in iron translocation to ferrochelatase. As pointed out in the “Introduction,” the substrate for the endosomal transporter, Nramp2/DMT1, is Fe2+, the redox form of iron in which it is also translocated across the mitochondrial membrane toward ferrochelatase.66 These facts make the above hypothesis attractive, since ferrous ions would bypass an oxygen-rich cytosol in hemoglobin-synthesizing cells. In this connection it is pertinent to mention that interactions of organelles in other cell types have been characterized. Desjardin et al67 described a contact of endosomes with phagosomes in J-774 macrophages, and Mannella et al68 showed that rat liver mitochondria interact with the endoplasmic reticulum. Another study demonstrated the internalization of apo-Tf from the basolateral membrane of an intestinal cell line (Caco-2) and colocalization of that protein with vesicles containing apical membrane-derived DMT1, indicating that the transfer of nutritional iron to Tf may bypass the enterocyte cytosol.69
Scott and Eaton70 showed that trace amounts of “free” iron in combination with cellular reducing equivalents oxidize hemoglobin. This report strongly suggests that a pool of free iron is capable of establishing a self-amplifying and self-propagating redox reaction with hemoglobin that can eventually lead to red cell destruction. Hence, the chaperonelike function of endosomes may be one of the mechanisms that keeps the concentration of reactive iron at extremely low levels. Moreover, apart from the mitochondrion, it is possible that the endosome may interact with other membranes/organelles to deliver iron to newly synthesized proteins that require iron.71 The intricate and highly organized structure of the intracellular matrix,72 together with the extremely rapid incorporation of iron into hemoglobin, argues against freely diffusible and potentially toxic cytosolic iron pool and suggests a more tightly coordinated and efficient mechanism. It is also pertinent to mention that specific chaperones keep free cellular copper at extremely low levels; in fact intracellular concentration of free copper is limited to less than 1 free copper ion per cell.73 Hence, it is tempting to speculate that general principles controlling intracellular trafficking of iron and copper, 2 essential but potentially toxic transition metals, are similar.
Prepublished online as Blood First Edition Paper, August 26, 2004; DOI 10.1182/blood-2004-06-2226.
Supported by a grant from the Canadian Institutes of Health Research (MT-14100).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Joseph Rifkind for very useful discussion on the interaction of transient metals with hemoglobin, and Sandy Fraiberg for excellent editorial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal