Abstract
Natural killer (NK) cells are important effectors of innate immunity. In contrast to many studies of interleukin-2 (IL-2)-activated NK cells, the physiologic requirements for stimulating resting NK cells have only recently received attention. Given the emerging variety of dendritic cell (DC) types and their division of labor for stimulating immunity, we compared the capacity of monocyte-derived DCs (moDCs) with that of CD34+ hematopoietic progenitor cell (HPC)-derived dermal-interstitial DCs (DDC-IDCs) and Langerhans cells (LCs) to stimulate resting NK cells. MoDCs, and to a lesser extent CD34+ HPC-derived DDC-IDCs, directly stimulate NK-cell proliferation, CD56 up-regulation, and cytotoxicity. LCs, on the contrary, require exogenous IL-2 or IL-12 to activate NK cells, but they can maintain resting NK-cell viability and sustain NK-cell proliferation induced by moDCs. LCs do not secrete bioactive IL-12p70 but do produce significantly higher concentrations of IL-15 and IL-18 than either of the other 2 DC types. Despite secretion of IL-15, LCs lack IL-15R-α for surface presentation of IL-15. This together with the deficiency of IL-12p70 undermines any direct NK-cell activation by LCs. Hence, the principal myeloid DCs differ in critical ways regarding the stimulation of NK and T lymphocytes and could be used or targeted accordingly in DC-based immunotherapies. (Blood. 2005;105:266-273)
Introduction
Natural killer (NK) cells were originally identified by their spontaneous cytotoxicity against immature hematopoietic cells, tumor cells, some normal fresh cells, and most cultured cell lines.1-6 Investigators now understand that the sum of positive and negative signals transmitted by activating and inhibitory receptors on NK cells determines NK-cell cytotoxicity.7-9
The ligands of NK-cell inhibitory receptors are mainly classical and nonclassical major histocompatibility complex (MHC) class I molecules. The ability of NK cells to sense loss of class I MHC expression by the absence of signaling through inhibitory NK receptors constitutes the “missing-self” hypothesis.10-12 This in turn fails to inhibit NK-cell function, resulting in NK-cell-mediated lysis. NK cells also have activating receptors that require engagement to develop lytic activity. These include the natural cytotoxicity receptors (NCRs), the specific ligands for which are unknown. Another activating receptor is NKG2D, the ligands for which include nonclassical MHC molecules, such as MHC class I chain-related gene A (MICA) and MICB, and MHC-related molecules such as the human cytomegalovirus UL16-binding proteins (ULBPs).13-16
Microbial products stimulate activation and maturation of dendritic cells (DCs), and mature antigen-presenting DCs appear in draining lymph nodes as early as 4 to 6 hours after localized viral (eg, herpes simplex virus 1 [HSV-1]) infection.17 Because lymph nodes and other secondary lymphoid organs are reservoirs for NK cells,18,19 early NK-cell activation could occur through DCs in these sites. In addition, peripheral blood NK cells infiltrate inflamed tissues and interact with DCs at these sites.20 NK-cell responses thereby execute innate immune responses before the acquisition of MHC-restricted cellular immunity.21,22 This still begs the question, however, as to how and which DC subtypes activate NK cells independently of T-cell activation and secretion of effector cytokines.
Recent studies have shown that at least monocyte-derived DCs (moDCs) can directly and rapidly activate NK-cell proliferation, cytotoxicity, and cytokine secretion.23-26 These moDC-activated NK cells can, in turn, mature DCs at low NK/DC ratios, probably via tumor necrosis factor-α (TNF-α) secretion.25,26 Activated NK cells can also kill immature DCs at high NK/DC ratios via the NCR NKp30,24,26 whereas mature moDCs escape NK-cell lysis by up-regulating class I MHC.24
We have recently found that peptide-pulsed CD34+ hematopoietic progenitor cell (HPC)-derived Langerhans cells (LCs) are more potent on a cell-for-cell basis in stimulating antigen (Ag)-specific, MHC-restricted cytolytic T cells in vitro than are dermal-interstitial dendritic cells (DDC-IDCs) or moDCs.27 This is mediated at least in part by LC secretion of interleukin-15 (IL-15) and apparently by less need for helper epitopes when LCs present only class I MHC-restricted Ag. MoDCs, but neither LCs nor DDC-IDCs, secrete bioactive IL-12p70. IL-15 is an important cytokine not only for Ag-specific memory cytotoxic T lymphocytes (CTLs)28-30 but also for the development and survival of NK cells.29,31,32 DC stimulation of T cells also results in IL-2 secretion, which can activate NK cells. To reconcile these various potential contributions to the activation of NK cells, we separately generated moDCs as well as CD34+ HPC-derived LCs and DDC-IDCs, and we assessed both direct and indirect stimulation of resting NK cells in vitro in the context of these cytokines.
Materials and methods
Cytokines
Sterile recombinant lipopolysaccharide (LPS)-, pyrogen-, and mycoplasma-free recombinant human cytokines for the development of DCs included granulocyte-macrophage colony-stimulating factor (GM-CSF) (originally Immunex, Seattle, WA; now Berlex, Montville, NJ) and FMS-like tyrosine kinase 3 (FLT-3) ligand, c-kit ligand or stem cell factor, IL-4, TNF-α, and tumor growth factor-β (TGF-β) (all R&D Systems, Minneapolis, MN). All cytokines were supplied carrier-free by the manufacturer but were reconstituted using human serum albumin (1% final HSA; 25% human serum albumin, NDC 63546-251-05, pharmaceutical grade; manufactured by Swiss Red Cross, distributed by Alpine Biologics, Orangeburg, NY) in phosphate-buffered saline. Human IL-12 (R&D Systems), human IL-15 (R&D Systems), and human IL-2 (Chiron, Emeryville, CA) were used in the NK-cell cultures as indicated.
Dendritic cell generation
Plastic-adherent mononuclear cell precursors of moDCs, or CD34+ HPCs for LCs and DDC-IDCs, were obtained respectively from healthy volunteer blood donors or from donors already undergoing marrow or peripheral blood stem cell (PBSC) collections for allogeneic transplantation. Informed consent was obtained for research specimen collection using protocols approved by the Institutional Review and Privacy Board of Memorial Hospital, Memorial Sloan-Kettering Cancer Center, New York. These 3 types of myeloid DCs were separately generated in vitro exactly as described.27 Terminal DC maturation was accomplished from days 6 to 8 for moDCs and from days 12 to 14 for LCs and DDC-IDCs using only a combination of inflammatory cytokines that included IL-1-β (2 ng/mL), IL-6 (1000 IU/mL), TNF-α (10 ng/mL or 2-fold the original dose) (all from R&D), and prostaglandin E2 (PGE2) (1 μg/mL; Calbiochem, San Diego, CA).27,33
NK-cell isolation and NK/DC coculture
NK cells were purified with the NK-cell isolation kit (Miltenyi Biotec, Auburn, CA) by negative immunomagnetic cell separation. Resulting NK-cell populations were more than 95% CD56+ CD3-. NK cells were cocultured with DCs at a DC/NK-cell ratio of 1:10 for cytotoxicity assays and flow cytometry. Proliferation assays were performed at the indicated DC/NK-cell ratios. Where indicated, IL-12 500 pg/mL or IL-2 80 IU/mL was added. In some experiments allogeneic CD4+ T cells, purified from the same PBMCs as the NK cells by positive immunomagnetic selection (anti-CD4 MACS beads; Miltenyi Biotec), were irradiated (30 Gy [3000 rad]; 137cesium [137Cs]) and added to the NK/DC cocultures.
Proliferation assay
NK-cell proliferation was measured as previously described.24 A total of 105 NK cells were incubated with decreasing numbers of stimulatory DCs at the indicated ratios for 5 days in RPMI 1640 with 5% pooled human AB+ serum in 96-well round-bottom microtiter plates; 37 kBq (1 μCi) 3H-thymidine was added to each well for the last 12 to 18 hours of the 6-day culture. Cells were harvested with a Harvester Mach IIIM (Tomtec, Hamden, CT) and counted in a 1450 MicroBeta TriLux (Wallac, Turku, Finland). NK cells incorporated 3H-thymidine (3H-TdR) in direct proportion to their proliferation, reflecting the stimulatory capacity of a particular type of DC. The measured counts per minute (cpm) represented mean values of duplicate or triplicate NK/DC microwell cultures.
Cytotoxicity assay
51Chromium (51Cr) release assays were performed as described.24 A total of 106 LCL721.221 target cells were incubated with 3.7 MBq (100 μCi)
Flow cytometry
Maturation phenotype of all DC cultures was confirmed by cytofluorography (FACSort or FACSCalibur, Becton Dickinson, San Jose, CA). The proportion of cells that exhibited large forward scatter (FSC), HLA-DRbright, CD14-, CD11c+, CD86bright, and CD83+ profiles (all using directly fluorescein isothiocyanate [FITC]- or phycoerythrin [PE]-conjugated monoclonal antibodies [mAbs] against the indicated epitopes, BD Pharmingen, San Diego, CA, or Immunotech [anti-CD83], Marseille, France) determined DC dosing for NK-cell stimulation. NK-cell cultures were stained with anti-CD16-FITC (BD Pharmingen), anti-CD56-PE (BD Pharmingen), and anti-CD3-allophycocyanin (BD Pharmingen). Autofluorescing cells were excluded in the FL-3 channel. FSC, side scatter (SSC), CD16, and CD56 levels were evaluated on CD3- lymphocytes. IL-15R-α was stained indirectly with the goat anti-IL-15R-α polyclonal antibody (R&D Systems) and donkey anti-goat immunoglobulin G (IgG)-PE secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). The control for IL-15R-α polyclonal goat antibody staining was polyclonal goat anti-mouse IgG antibody (Jackson ImmunoResearch Laboratories) with the same secondary reagent.
Cytokine ELISAs
Supernatants from DC cultures were collected and normalized to 2 × 105 DCs per milliliter. IL-12 and IL-18 were measured directly from the supernatants. For IL-15 detection, supernatants were concentrated 10-fold by ultrafiltration (Vivaspin, 10 kDa exclusion size; Vivaspin, Goettingen, Germany). Total IL-12 (p40 and p70; Pierce Endogen, Rockford, IL), IL-12p70 (Cell Sciences, Norwood, MA), IL-15 (Quantikine; R&D Systems, Minneapolis, MN), and IL-18 (CellSciences) were quantified with commercial enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions.
Results
DC subtypes differ in their ability to stimulate NK-cell proliferation and induce NK-cell-mediated cytotoxicity
We first compared CD34+ HPC-derived LCs and DDC-IDCs with the known capacity of moDCs to stimulate NK-cell proliferation and cytotoxicity. Each of the 3 mature DC populations was used in comparable numbers based on flow cytometric confirmation of similarly high expression of class II MHC and up-regulation of CD83, CD80, CD86, and CD40.27 In the absence of any exogenous cytokines or adjuvants, mature DDC-IDCs stimulated resting NK cell proliferation but required roughly 1- to 1.5-log greater numbers than moDCs to effect comparable expansion (Figure 1A). NK-cell numbers increased 2- to 5-fold over 7 days' stimulation. Both DDC-IDCs and moDCs also stimulated NK-cell cytotoxicity against class I MHC-negative “classical” NK-cell targets (LCL721.22124,34 ) but with less disparity between their respective activities than for NK-cell proliferation (Figure 1B). LCs used alone were poorly stimulatory for both NK-cell proliferation and cytotoxicity.
DC subtypes differ in their ability to activate NK cells. (A) Proliferation of purified peripheral blood NK cells was assessed by 3H-thymidine incorporation (Y-axis; note log scale) after culture with monocyte-derived dendritic cells (moDC) or CD34+ HPC-derived Langerhans cells (LC) or dermal-interstitial dendritic cells (DDC-IDC) for 6 days. Data points for the indicated NK-cell-dendritic cell ratio (X-axis; NK/DC from 3:1 to 300:1) represent the average of duplicate microwell cultures and their standard deviation. The data are representative of 10 independent experiments. (B) LCs, DDC-IDCs, and moDCs were cocultured with peripheral blood NK cells for 7 days at an NK/DC ratio of 10:1. NK cytotoxicity of the cultures was determined by 51Cr release assay against the MHC class I-negative target LCL721.221. Radiolabeled LCL721.221 targets were exposed for 4 to 6 hours to the NK-cell effectors, which were the responder lymphocytes from the original or primary DC/NK cocultures. The effectors were added as total lymphocytes, without adjustment for specific numbers of NK-cell blasts, to ascertain differences between the different DCs in stimulating NK cells in the primary cultures. The effector-target ratio is indicated along the X-axis, and specific lysis is plotted against the Y-axis. The data are representative of 10 independent experiments.
DC subtypes differ in their ability to activate NK cells. (A) Proliferation of purified peripheral blood NK cells was assessed by 3H-thymidine incorporation (Y-axis; note log scale) after culture with monocyte-derived dendritic cells (moDC) or CD34+ HPC-derived Langerhans cells (LC) or dermal-interstitial dendritic cells (DDC-IDC) for 6 days. Data points for the indicated NK-cell-dendritic cell ratio (X-axis; NK/DC from 3:1 to 300:1) represent the average of duplicate microwell cultures and their standard deviation. The data are representative of 10 independent experiments. (B) LCs, DDC-IDCs, and moDCs were cocultured with peripheral blood NK cells for 7 days at an NK/DC ratio of 10:1. NK cytotoxicity of the cultures was determined by 51Cr release assay against the MHC class I-negative target LCL721.221. Radiolabeled LCL721.221 targets were exposed for 4 to 6 hours to the NK-cell effectors, which were the responder lymphocytes from the original or primary DC/NK cocultures. The effectors were added as total lymphocytes, without adjustment for specific numbers of NK-cell blasts, to ascertain differences between the different DCs in stimulating NK cells in the primary cultures. The effector-target ratio is indicated along the X-axis, and specific lysis is plotted against the Y-axis. The data are representative of 10 independent experiments.
DDC-IDCs and LCs were always derived from CD34+ HPCs of the same donor, while moDCs sometimes were not. NK cells were uniformly allogeneic to all DC populations used in this and all other experiments. In our previous study,24 as well as in the experiments represented in Figure 1 (n = 10), there have been no dramatic differences in NK-cell responses with respect to the allogeneic or autologous source or between different allogeneic sources of the stimulatory DC populations. We conclude that moDCs are superior to DDC-IDCs in NK-cell stimulation and that LCs lack direct NK-cell stimulatory capacity.
MoDCs, and to a lesser extent DDC-IDCs, induce CD56bright NK-cell blasts
Most NK cells circulating in blood constitutively express CD16 and low levels of CD56, exerting cytotoxicity as their principal effector function.35 These cells remain CD16+ and up-regulate CD56 upon activation36 to levels similar to those of CD16-CD56bright NK cells, which predominate in secondary lymphoid tissue and secrete cytokines as their principal effector function.18,19
Purified NK cells were stimulated separately by each of the DC types under study for 7 days. As shown in Figure 2, moDCs stimulated robust NK-cell blast transformation, based on increased forward scatter, as well as increased CD56 expression by approximately 50% of the NK cells, most of which remained CD16+ as well. The kinetics of the moDC-stimulated response are also much more robust over about the first 3 days, after which many of the responding NK cells undergo activation-induced cell death, reflected by the large proportion of events with high SSC and low FSC.24 The changes induced by DDC-IDCs were much more modest. LCs were even less active, based on the combined criteria of minimal to no blast transformation and the lack of any substantial CD56 expression. LCs remained more viable (Figure 2, top right corner of each FSC/SSC dot plot; high FSC/SSC) in these cocultures with NK cells, however, than did either DDC-IDCs or moDCs.
MoDCs, but not LCs, activate NK cells directly, whereas DDC-IDCs are intermediate in activity. Size (forward scatter [FSC]), granularity (side scatter [SSC]), and surface expression of the CD56 and CD16 epitopes were quantified by flow cytometry. FSC and SSC were measured on purified NK cells stimulated for 7 days by Langerhans cells (LC), dermal-interstitial DCs (DDC-IDC), and monocyte-derived DCs (moDC), all at an NK-cell-DC ratio of 10:1. CD56 and CD16 expression was quantified on gated CD3- lymphocytes, which lacked autofluorescence in channel FL-3 and were located in the FSC/SSC lymphocyte gate (dotted line). Percentages indicate NK blasts, CD16-CD56bright, CD16+CD56bright, and CD16+CD56dim cells. The phenotype of NK cells directly isolated from blood before DC stimulation is shown at the far left. This could not be shown at 7 days without DC stimulation, because NK cells die when cultured at high purity without stimulation or cytokines. Note that most peripheral blood NK cells are CD56dim but not CD56- directly after isolation from PBMCs. LCs remained more viable (high FSC/SSC; top right corner of dot plot) in these cocultures with NK cells than did either DDC-IDCs or moDCs. The data are representative of 7 independent experiments.
MoDCs, but not LCs, activate NK cells directly, whereas DDC-IDCs are intermediate in activity. Size (forward scatter [FSC]), granularity (side scatter [SSC]), and surface expression of the CD56 and CD16 epitopes were quantified by flow cytometry. FSC and SSC were measured on purified NK cells stimulated for 7 days by Langerhans cells (LC), dermal-interstitial DCs (DDC-IDC), and monocyte-derived DCs (moDC), all at an NK-cell-DC ratio of 10:1. CD56 and CD16 expression was quantified on gated CD3- lymphocytes, which lacked autofluorescence in channel FL-3 and were located in the FSC/SSC lymphocyte gate (dotted line). Percentages indicate NK blasts, CD16-CD56bright, CD16+CD56bright, and CD16+CD56dim cells. The phenotype of NK cells directly isolated from blood before DC stimulation is shown at the far left. This could not be shown at 7 days without DC stimulation, because NK cells die when cultured at high purity without stimulation or cytokines. Note that most peripheral blood NK cells are CD56dim but not CD56- directly after isolation from PBMCs. LCs remained more viable (high FSC/SSC; top right corner of dot plot) in these cocultures with NK cells than did either DDC-IDCs or moDCs. The data are representative of 7 independent experiments.
All DC subtypes can activate NK cells via alloreactive T-cell stimulation
IL-2, a cytokine secreted mainly by T cells, has been widely used to activate NK cells and study their function. We therefore tested whether different DCs, stimulating adaptive immune responses by CD4+ T cells, could indirectly activate NK cells by the CD4+ T-cell secretion of IL-2, which ranges from 50 to 100 IU/mL IL-2.37,38
We confirmed comparable stimulatory activity by LCs, DDC-IDCs, and moDCs for allogeneic T cells contained in a population of PBMC responders (Figure 3A).27 We then stimulated allogeneic NK cells with LCs, DDC-IDCs, or moDCs to which we added irradiated CD4+ T cells autologous to the NK cells. These CD4+ T cells could secrete cytokines but could not proliferate themselves after irradiation in response to DC stimulation. We further verified that the cultures had no residual allogeneic CD4+ T cells by the time of the proliferative and cytotoxicity assays at days 6 and 7, respectively, based on the presence of fewer than 5% CD3+ T cells by flow cytometry.
MoDCs, LCs, and DDC-IDCs are equally capable of activating NK cells via IL-2 secretion by DC-activated alloreactive CD4+ T cells. (A) Proliferation of PBMCs was assessed by 3H-thymidine incorporation (y-axis; note log scale) after culture with monocyte-derived dendritic cells (moDC; ▾) or CD34+ HPC-derived Langerhans cells (LC; •) or dermal-interstitial dendritic cells (DDC-IDC; ○) for 6 days at the responder-stimulator ratios (10:1 to 1000:1) indicated along the X-axis. Data points represent the average of duplicate microwell cultures and their standard deviation. The data are representative of 3 independent experiments. (B) Proliferation of purified peripheral blood NK cells was assessed by 3H-thymidine incorporation (Y-axis; note log scale) after culture with monocyte-derived dendritic cells (moDC) or CD34+ HPC-derived Langerhans cells (LC) or dermal-interstitial dendritic cells (DDC-IDC) for 6 days. Data points for the indicated NK-cell-dendritic cell ratio (X-axis; NK/DC from 3:1 to 1000:1) represent the average of duplicate microwell cultures and their standard deviation. NK-cell/DC cultures were supplemented with 80 IU/mL rIL-2 (DC + IL-2 ▾), irradiated allogeneic CD4+ T cells (DC + CD4; NK/T-cell ratio = 1:1 ○), or nothing (DC only •). The data are representative of 3 independent experiments. (C) LCs, DDC-IDCs, and moDCs were cocultured with peripheral blood NK cells for 7 days at an NK-cell-DC ratio of 10:1. These primary cultures were supplemented with 80 IU/mL rIL-2 (DC + IL-2 ▾), irradiated allogeneic CD4+ T cells (DC + CD4; NK/T-cell ratio = 1:1 ○), or nothing (DC only •). NK cytotoxicity of the cultures was determined by 51Cr release assay against the MHC class I-negative target LCL721.221. Radiolabeled LCL721.221 targets were exposed for 4 to 6 hours to the NK-cell effectors, which were the responder lymphocytes from the original or primary DC/NK cocultures. The effectors were added as total lymphocytes, without adjustment for specific numbers of NK-cell blasts, to ascertain differences between the different DCs in stimulating NK cells in the primary cultures. The effector-target ratio is indicated along the X-axis, and specific lysis is plotted against the Y-axis. The data are representative of 3 experiments.
MoDCs, LCs, and DDC-IDCs are equally capable of activating NK cells via IL-2 secretion by DC-activated alloreactive CD4+ T cells. (A) Proliferation of PBMCs was assessed by 3H-thymidine incorporation (y-axis; note log scale) after culture with monocyte-derived dendritic cells (moDC; ▾) or CD34+ HPC-derived Langerhans cells (LC; •) or dermal-interstitial dendritic cells (DDC-IDC; ○) for 6 days at the responder-stimulator ratios (10:1 to 1000:1) indicated along the X-axis. Data points represent the average of duplicate microwell cultures and their standard deviation. The data are representative of 3 independent experiments. (B) Proliferation of purified peripheral blood NK cells was assessed by 3H-thymidine incorporation (Y-axis; note log scale) after culture with monocyte-derived dendritic cells (moDC) or CD34+ HPC-derived Langerhans cells (LC) or dermal-interstitial dendritic cells (DDC-IDC) for 6 days. Data points for the indicated NK-cell-dendritic cell ratio (X-axis; NK/DC from 3:1 to 1000:1) represent the average of duplicate microwell cultures and their standard deviation. NK-cell/DC cultures were supplemented with 80 IU/mL rIL-2 (DC + IL-2 ▾), irradiated allogeneic CD4+ T cells (DC + CD4; NK/T-cell ratio = 1:1 ○), or nothing (DC only •). The data are representative of 3 independent experiments. (C) LCs, DDC-IDCs, and moDCs were cocultured with peripheral blood NK cells for 7 days at an NK-cell-DC ratio of 10:1. These primary cultures were supplemented with 80 IU/mL rIL-2 (DC + IL-2 ▾), irradiated allogeneic CD4+ T cells (DC + CD4; NK/T-cell ratio = 1:1 ○), or nothing (DC only •). NK cytotoxicity of the cultures was determined by 51Cr release assay against the MHC class I-negative target LCL721.221. Radiolabeled LCL721.221 targets were exposed for 4 to 6 hours to the NK-cell effectors, which were the responder lymphocytes from the original or primary DC/NK cocultures. The effectors were added as total lymphocytes, without adjustment for specific numbers of NK-cell blasts, to ascertain differences between the different DCs in stimulating NK cells in the primary cultures. The effector-target ratio is indicated along the X-axis, and specific lysis is plotted against the Y-axis. The data are representative of 3 experiments.
The presence of irradiated allogeneic CD4+ T cells enhanced the NK-cell proliferative responses stimulated by all 3 DC types, especially by LCs (Figure 3B). The effect was greater for DDC-IDCs and moDCs at lower DC stimulator doses. To mimic part of the contribution of CD4+ T cell-derived cytokines, 80 IU/mL recombinant human IL-2 (rhIL-2) was added directly to DC/NK-cell cultures. This further enhanced the NK-cell proliferative responses to all 3 types of DCs but obscured any dose effect because the amount of IL-2 no longer depended on the number of DCs stimulating CD4+ T-cell cytokine secretion (Figure 3B). In contrast to NK-cell proliferative responses, NK-cell-mediated cytotoxicity induced by DDC-IDCs or moDCs did not benefit further from the addition of either irradiated allogeneic CD4+ T cells or rhIL-2. LC-stimulated NK-cell cytotoxicity did improve, however. From these data we conclude that LCs, DDC-IDCs, and moDCs stimulate alloreactive T cells to secrete IL-2 with similar efficiency, which in turn supports the indirect activation of NK cells by each of these DC types. LCs are especially dependent on the added contribution of CD4+ T-cell cytokines or IL-2 for stimulating NK-cell cytotoxicity as well as proliferation.
IL-2 and IL-12 alone induce NK-cell activation and cytotoxicity, but only IL-2 sustains independent NK-cell proliferation
We next exposed purified peripheral blood NK cells to 500 pg/mL IL-12 or 80 IU/mL IL-2 in the absence of any DCs. These cytokine concentrations were selected based on comparable amounts secreted by maturing DDC-IDCs and moDCs39 or in DC/allogeneic T-cell cultures,37,38 respectively. Both cytokines induced NK-cell blast formation and CD56 up-regulation, whereas NK cells alone without cytokines or DC stimulators underwent cell death (Figure 4A). IL-12 increased CD56 expression, especially among the NK cells that coexpressed CD16. Moreover, both IL-2 and IL-12 dramatically increased NK-cell cytotoxicity against the class I MHC-negative target LCL721.221 (Figure 4B). In contrast, IL-2 independently induced NK-cell expansion, whereas IL-12 could not support this in the absence of DCs (Figure 4C). Stimulation with IL-12 alone led to massive cell death and no expansion in the NK-cell cultures (data not shown). This suggests that IL-12 activates NK cells but requires a DC-derived factor (Figures 3 and 5) to sustain expansion and/or survival.
In the absence of DC stimulation, IL-2 and IL-12 induce NK-cell activation and cytotoxicity but differ in their capacity to promote NK-cell proliferation. Size (forward scatter [FSC]), granularity (side scatter [SSC]), and surface expression of the CD56 and CD16 epitopes were quantified by flow cytometry. FSC and SSC were measured on purified NK cells cultured without (NK only) or with 80 IU/mL rIL-2 (NK + IL-2) or 500 pg/mL rIL-12 (NK + IL-12) for 7 days. CD56 and CD16 expression was quantified on gated CD3- lymphocytes, which lacked autofluorescence in channel FL-3 and were located in the FSC/SSC lymphocyte gate (dotted line). Percentages indicate NK-cell blasts, CD16-CD56bright, CD16+CD56bright, and CD16+CD56dim cells. Note that most of the peripheral blood NK cells are CD56dim but not CD56- directly after isolation from PBMCs. The data are representative of 3 independent experiments. (B) Purified peripheral blood NK cells were cultured without cytokines (NK only •) or with 80 IU/mL rIL-2 (NK + IL-2 ○) or 500 pg/mL rIL-12 (NK + IL-12 ▾) for 7 days. NK cytotoxicity of the cultures was determined by 51Cr release assay against the MHC class I-negative target LCL721.221. Radiolabeled LCL721.221 targets were exposed for 4 to 6 hours to the NK-cell effectors, which were the responder lymphocytes from the original or primary DC/NK cocultures. The effectors were added as total lymphocytes, without adjustment for specific numbers of NK-cell blasts, to ascertain differences between the different DCs in stimulating NK cells in the primary cultures. The effector-target ratio is indicated along the X-axis, and specific lysis is plotted against the Y-axis. The data are representative of 3 independent experiments. (C) Proliferation of purified peripheral blood NK cells was determined by 3H-thymidine incorporation after 6 days of culture alone (0) or with 80 IU/mL rIL-2 (+ IL-2) or 500 pg/mL IL-12 (+ IL-12). Data points represent the average of triplicate microwells and their standard deviation. These data are representative of 3 independent experiments.
In the absence of DC stimulation, IL-2 and IL-12 induce NK-cell activation and cytotoxicity but differ in their capacity to promote NK-cell proliferation. Size (forward scatter [FSC]), granularity (side scatter [SSC]), and surface expression of the CD56 and CD16 epitopes were quantified by flow cytometry. FSC and SSC were measured on purified NK cells cultured without (NK only) or with 80 IU/mL rIL-2 (NK + IL-2) or 500 pg/mL rIL-12 (NK + IL-12) for 7 days. CD56 and CD16 expression was quantified on gated CD3- lymphocytes, which lacked autofluorescence in channel FL-3 and were located in the FSC/SSC lymphocyte gate (dotted line). Percentages indicate NK-cell blasts, CD16-CD56bright, CD16+CD56bright, and CD16+CD56dim cells. Note that most of the peripheral blood NK cells are CD56dim but not CD56- directly after isolation from PBMCs. The data are representative of 3 independent experiments. (B) Purified peripheral blood NK cells were cultured without cytokines (NK only •) or with 80 IU/mL rIL-2 (NK + IL-2 ○) or 500 pg/mL rIL-12 (NK + IL-12 ▾) for 7 days. NK cytotoxicity of the cultures was determined by 51Cr release assay against the MHC class I-negative target LCL721.221. Radiolabeled LCL721.221 targets were exposed for 4 to 6 hours to the NK-cell effectors, which were the responder lymphocytes from the original or primary DC/NK cocultures. The effectors were added as total lymphocytes, without adjustment for specific numbers of NK-cell blasts, to ascertain differences between the different DCs in stimulating NK cells in the primary cultures. The effector-target ratio is indicated along the X-axis, and specific lysis is plotted against the Y-axis. The data are representative of 3 independent experiments. (C) Proliferation of purified peripheral blood NK cells was determined by 3H-thymidine incorporation after 6 days of culture alone (0) or with 80 IU/mL rIL-2 (+ IL-2) or 500 pg/mL IL-12 (+ IL-12). Data points represent the average of triplicate microwells and their standard deviation. These data are representative of 3 independent experiments.
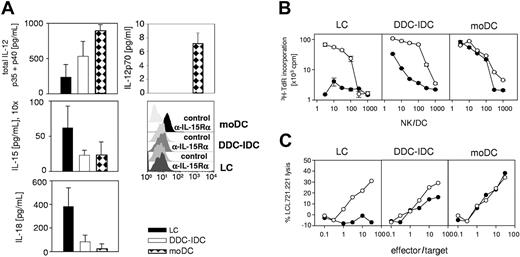
LCs do not secrete sufficient amounts of IL-12p70 to activate NK cells. (A) IL-12p40, IL-12p70, IL-15, and IL-18 secreted during maturation by 2 × 105 LCs, DDC-IDCs, and moDCs/mL were measured by ELISA. The culture supernatants required 10-fold concentration for detection of IL-15, but IL-12 and IL-18 could be measured directly. The values represent the averaged means ± SEMs from separate ELISAs for IL-12p40 (n = 3), IL-12p70 (n = 5), IL-18 (n = 3), and IL-15 (n = 5), in which duplicates were tested for each data point in each assay. In addition, surface expression of IL-15R-α was evaluated on mature moDCs, LCs, and IDC-DDCs. One representative of 3 independent experiments is shown. (B) Proliferation of purified peripheral blood NK cells was assessed by 3H-thymidine incorporation (Y-axis; note log scale) after culture with monocyte-derived dendritic cells (moDC) or CD34+ HPC-derived Langerhans cells (LC) or dermal-interstitial dendritic cells (DDC-IDC) for 6 days. Data points for the indicated NK-cell-dendritic cell ratio (X-axis; NK/DC from 3:1 to 1000:1) represent the average of duplicate microwell cultures and their standard deviation. NK-cell/DC cultures were supplemented with 500 pg/mL rIL-12 (DC + IL-12 ○) or not (DC only •). The data are representative of 5 independent experiments. (C) LCs, DDC-IDCs, and moDCs were cocultured with peripheral blood NK cells for 7 days at an NK/DC ratio of 10:1. These primary cultures were supplemented with 500 pg/mL rIL-12 (DC + IL-12 ○) or not (DC only •). NK cytotoxicity of the cultures was determined by 51Cr release assay against the MHC class I-negative target LCL721.221. Radiolabeled LCL721.221 targets were exposed for 4 to 6 hours to the NK-cell effectors, which were the responder lymphocytes from the original or primary DC/NK cocultures. The effectors were added as total lymphocytes, without adjustment for specific numbers of NK-cell blasts, to ascertain differences between the different DCs in stimulating NK cells in the primary cultures. The effector-target ratio is indicated along the X-axis, and specific lysis is plotted against the Y-axis. The data are representative of 5 independent experiments.
LCs do not secrete sufficient amounts of IL-12p70 to activate NK cells. (A) IL-12p40, IL-12p70, IL-15, and IL-18 secreted during maturation by 2 × 105 LCs, DDC-IDCs, and moDCs/mL were measured by ELISA. The culture supernatants required 10-fold concentration for detection of IL-15, but IL-12 and IL-18 could be measured directly. The values represent the averaged means ± SEMs from separate ELISAs for IL-12p40 (n = 3), IL-12p70 (n = 5), IL-18 (n = 3), and IL-15 (n = 5), in which duplicates were tested for each data point in each assay. In addition, surface expression of IL-15R-α was evaluated on mature moDCs, LCs, and IDC-DDCs. One representative of 3 independent experiments is shown. (B) Proliferation of purified peripheral blood NK cells was assessed by 3H-thymidine incorporation (Y-axis; note log scale) after culture with monocyte-derived dendritic cells (moDC) or CD34+ HPC-derived Langerhans cells (LC) or dermal-interstitial dendritic cells (DDC-IDC) for 6 days. Data points for the indicated NK-cell-dendritic cell ratio (X-axis; NK/DC from 3:1 to 1000:1) represent the average of duplicate microwell cultures and their standard deviation. NK-cell/DC cultures were supplemented with 500 pg/mL rIL-12 (DC + IL-12 ○) or not (DC only •). The data are representative of 5 independent experiments. (C) LCs, DDC-IDCs, and moDCs were cocultured with peripheral blood NK cells for 7 days at an NK/DC ratio of 10:1. These primary cultures were supplemented with 500 pg/mL rIL-12 (DC + IL-12 ○) or not (DC only •). NK cytotoxicity of the cultures was determined by 51Cr release assay against the MHC class I-negative target LCL721.221. Radiolabeled LCL721.221 targets were exposed for 4 to 6 hours to the NK-cell effectors, which were the responder lymphocytes from the original or primary DC/NK cocultures. The effectors were added as total lymphocytes, without adjustment for specific numbers of NK-cell blasts, to ascertain differences between the different DCs in stimulating NK cells in the primary cultures. The effector-target ratio is indicated along the X-axis, and specific lysis is plotted against the Y-axis. The data are representative of 5 independent experiments.
Lack of IL-12p70 secretion and IL-15R-α expression by Langerhans cells impairs their capacity for direct stimulation of NK cells
IL-12, IL-15, IL-15R-α, and IL-18 have all been implicated in the development, activation, and/or survival of NK cells.29,31,40-42 We therefore tested production of these molecules coincident with DC maturation. DC cultures were adjusted to 2 × 105/mL, which was the highest DC density used in the DC/NK-cell proliferation assays. LCs secreted substantially higher amounts of IL-15 and IL-18 than did DDC-IDCs or moDCs (Figure 5A, middle and bottom left panels). In contrast, while all 3 DCs secreted some measurable amount of total IL-12 (Figure 5A, top left), only moDCs secreted the bioactive IL-12p70 heterodimer (7.2 ± 1.5 pg/mL in 5 independent experiments; Figure 5A, top right). As previously reported, neither LCs nor DDC-IDCs secreted IL-12p70 (Figure 5A, top right).27 The level of IL-12p70 secretion by moDCs after cytokine maturation was around 100-fold lower than can be achieved by bacterial stimuli or cellbound CD40L39 but was apparently sufficient to support NK-cell activation (Figure 5B-C, far right). Maturation of LCs or DDC-IDCs with either CD40L or LPS did not rescue IL-12p70 secretion by these DCs (data not shown and Ratzinger et al27 ).
Because total IL-12 secretion paralleled the NK-cell stimulatory function of the DC types under study, we explored IL-12 further as the missing factor for direct stimulation of NK cells by LCs. We therefore supplemented all DC/NK cultures with 500 pg/mL IL-12 (Figure 5B-C), an amount reached or exceeded by moDC preparations after maturation with bacteria and cellbound CD40L.39 While NK-cell proliferation and cytotoxicity induced by DDC-IDCs and moDCs was only minimally increased, LCs supplemented with exogenous IL-12 could now stimulate some NK-cell proliferation and cytotoxicity (Figure 5B-C). In the presence of a constant dose of IL-12, LC-stimulated NK-cell proliferation was dose dependent, just as it was for DDC-IDCs and moDCs (Figure 5B).
This indicates that additional, as yet unidentified factor(s) provided by each of these DC types support NK-cell proliferation and/or survival. In fact, 500 pg/mL IL-12 alone was unable to sustain NK proliferation without DCs (see Figure 4C). LCs plus IL-12 induced NK-cell cytotoxicity against the class I MHC-negative target LCL721.221 similar to that stimulated by DDC-IDCs. Both DC types stimulated less overall cytotoxicity (especially at E/T 10:1 and lower) than did moDCs, which were the only DCs to secrete bioactive IL-12p70 (Figure 5A,C). These data indicate that LCs secrete insufficient IL-12 to activate NK cells directly and that all DCs must provide an additional factor that supports NK-cell proliferation and/or survival.
When we assessed IL-15R-α expression by flow cytometry, only mature moDCs expressed this receptor, even though they secreted very little IL-15. LCs, which secreted the most IL-15, and DDC-IDCs both failed to up-regulate this receptor (Figure 5A). Neither IL-15 nor IL-18 secretion by mature LCs could apparently compensate for the low amounts of secreted IL-12 and IL-15R-α expression in order to rescue NK-cell activation. In fact, for secreted IL-15, we determined the concentration that would promote NK-cell proliferation and cytotoxicity. Induction of NK-cell proliferation and cytotoxicity required 1 ng/mL soluble recombinant IL-15, while 100 pg/mL, which exceeds the amount recovered even from mature LCs, proved insufficient (data not shown). We conclude that the levels of soluble IL-15 recovered from DC culture supernatants are insufficient to activate NK cells. This is consistent with previous reports using activated monocytes, which indicated that the relevant, active IL-15 is presented by IL-15R-α at the cell-surface interface or immunologic synapse between antigen-presenting cells and NK cells.43 Our data support a role for surface-presented IL-15 in direct NK-cell activation by DCs, because we could detect IL-15R-α only on the NK-cell stimulatory moDCs but not on LCs or DDC-IDCs.
Hence, within the limitations imposed by using primary human cell populations, we identified 2 deficiencies of LCs for molecules involved in NK-cell development and activation. Lack of IL-12p70 production and failed expression of the surface receptor for IL-15 could thereby account for the inability of LCs to stimulate NK cells. Activated LCs, however, could theoretically provide IL-15 in a paracrine manner in vivo to the IL-15R-α-expressing, activated, mature moDCs.
Langerhans cells supplemented with allogeneic CD4+ T cells, IL-2, or IL-12 induce NK-cell blast transformation and up-regulation of CD56
We compared the different supplements that increased LC stimulation of NK-cell proliferation and cytotoxicity for the concomitant induction of blast transformation and CD56 up-regulation (Figure 6). While both IL-2 and IL-12 induced blast transformation, only IL-12 substantially increased CD56 expression, especially among the NK cells coexpressing CD16 whose main effector function is cytotoxicity. Irradiated allogeneic CD4+ T cells provided some enhancement over baseline, and rhIL-2 further enhanced both blast transformation and CD56 up-regulation. On balance, the addition of IL-12 to LCs rendered these DCs similar to moDCs in NK-cell stimulation and activation.
LC/NK cultures supplemented with allogeneic CD4+ T cells, IL-2, or IL-12 are able to activate NK cells. Size (forward scatter [FSC]), granularity (side scatter [SSC]), and surface expression of the CD56 and CD16 epitopes were quantified by flow cytometry. FSC and SSC were measured on purified NK cells stimulated for 7 days by Langerhans cells (LC) at an NK-cell-DC ratio of 10:1. Cultures were supplemented with irradiated allogeneic CD4+ T cells at an NK/CD4+ T-cell ratio of 1:1 (LC + CD4), 80 IU/mL rIL-2 (LC + IL-2), 500 pg/mL IL-12 (LC + IL-12), or nothing (LC). CD56 and CD16 expression was quantified on gated CD3- lymphocytes, which lacked autofluorescence in channel FL-3 and were located in the FSC/SSC lymphocyte gate (dotted line). Percentages indicate NK-cell blasts, CD16-CD56bright, CD16+CD56bright, and CD16+CD56dim cells. LCs maintained NK-cell viability very well (bottom left panel) despite the absence of activation and blast transformation without supplements. Note that most of the peripheral blood NK cells are CD56dim but not CD56- directly after isolation from PBMCs or after coculture with LCs. The data are representative of 3 independent experiments.
LC/NK cultures supplemented with allogeneic CD4+ T cells, IL-2, or IL-12 are able to activate NK cells. Size (forward scatter [FSC]), granularity (side scatter [SSC]), and surface expression of the CD56 and CD16 epitopes were quantified by flow cytometry. FSC and SSC were measured on purified NK cells stimulated for 7 days by Langerhans cells (LC) at an NK-cell-DC ratio of 10:1. Cultures were supplemented with irradiated allogeneic CD4+ T cells at an NK/CD4+ T-cell ratio of 1:1 (LC + CD4), 80 IU/mL rIL-2 (LC + IL-2), 500 pg/mL IL-12 (LC + IL-12), or nothing (LC). CD56 and CD16 expression was quantified on gated CD3- lymphocytes, which lacked autofluorescence in channel FL-3 and were located in the FSC/SSC lymphocyte gate (dotted line). Percentages indicate NK-cell blasts, CD16-CD56bright, CD16+CD56bright, and CD16+CD56dim cells. LCs maintained NK-cell viability very well (bottom left panel) despite the absence of activation and blast transformation without supplements. Note that most of the peripheral blood NK cells are CD56dim but not CD56- directly after isolation from PBMCs or after coculture with LCs. The data are representative of 3 independent experiments.
Langerhans cells promote NK-cell survival
We noted that NK cells maintained viability and the CD16+CD56dim phenotype typical of circulating NK cells in peripheral blood when cocultured with LCs. This was in contrast to their gradual death over 7 to 9 days when cultured alone (Figures 2, 4A, and 6) and the rapid activation-induced NK-cell death when cultured with moDCs alone (Figure 2, upper far right dot plot [moDC]; population of high SSC and low FSC cells). We therefore cultured purified peripheral blood NK cells in the presence or absence of LCs for 9 days and analyzed CD16 and CD56 expression by flow cytometry (Figure 7A, upper panels). LCs maintained CD16 and CD56 expression by purified NK cells, while NK cells died and lost expression of these surface markers without LCs. In 4 experiments, only 13.9% ± 26.1% of NK cells retained viable CD16 and/or CD56 expression without LCs, whereas 78% ± 24.6% retained viable expression in the presence of LCs (Figure 7A, lower panel). This was further confirmed by direct hemacytometer inspection of cultured cells using trypan blue exclusion (data not shown). Furthermore, we demonstrated that LCs but not DDC-IDCs supported viable NK-cell proliferation induced by moDCs (Figure 7B), with less activation-induced cell death than occurred in NK-cell/moDC cocultures without LCs (Figure 2). Therefore, addition of LCs benefits survival of both resting and activated NK cells.
LCs support survival of resting and DC-activated NK cells. (A) CD56 and CD16 expression was quantified on gated CD3- lymphocytes by flow cytometry. Percentages indicate CD16-CD56bright (LUQ), CD16+CD56bright (RUQ), and CD16+CD56dim (RLQ) cells. Note that most of the peripheral blood NK cells are CD56dim but not CD56- directly after isolation from PBMCs or after coculture with LCs. One of 4 independent experiments is shown. Percent surviving NK cells in the lower panel indicates the average sum of the percentages for CD16-CD56bright, CD16+CD56bright, and CD16+CD56dim cells in the 4 experiments and their standard deviation. (B) Proliferation of purified peripheral blood NK cells was assessed by 3H-thymidine incorporation after culture with (left panel) monocyte-derived dendritic cells (moDC; •) or with CD34+ HPC-derived Langerhans cells (LC; ○) or dermal-interstitial dendritic cells (DDC-IDC; ▾) or (right panel) with 1:1 mixtures of moDCs plus moDCs (moDC+moDC; •), LCs (moDC+LC; ○), or DDC-IDCs (moDC+DDC-IDC; ▾) for 6 days. Data points for the indicated NK-cell-dendritic cell ratio (X-axis; NK/DC from 3:1 to 1000:1) represent the average of duplicate microwell cultures and their standard deviation. The data are representative of 3 independent experiments.
LCs support survival of resting and DC-activated NK cells. (A) CD56 and CD16 expression was quantified on gated CD3- lymphocytes by flow cytometry. Percentages indicate CD16-CD56bright (LUQ), CD16+CD56bright (RUQ), and CD16+CD56dim (RLQ) cells. Note that most of the peripheral blood NK cells are CD56dim but not CD56- directly after isolation from PBMCs or after coculture with LCs. One of 4 independent experiments is shown. Percent surviving NK cells in the lower panel indicates the average sum of the percentages for CD16-CD56bright, CD16+CD56bright, and CD16+CD56dim cells in the 4 experiments and their standard deviation. (B) Proliferation of purified peripheral blood NK cells was assessed by 3H-thymidine incorporation after culture with (left panel) monocyte-derived dendritic cells (moDC; •) or with CD34+ HPC-derived Langerhans cells (LC; ○) or dermal-interstitial dendritic cells (DDC-IDC; ▾) or (right panel) with 1:1 mixtures of moDCs plus moDCs (moDC+moDC; •), LCs (moDC+LC; ○), or DDC-IDCs (moDC+DDC-IDC; ▾) for 6 days. Data points for the indicated NK-cell-dendritic cell ratio (X-axis; NK/DC from 3:1 to 1000:1) represent the average of duplicate microwell cultures and their standard deviation. The data are representative of 3 independent experiments.
Discussion
Resting NK cells respond to the composite activating and inhibitory signals delivered by their target cells. Investigators now recognize that this NK-cell reactivity is augmented by accessory cells at the onset of innate immune responses. NK-cell activation not only reduces Ag load early in an immune response but also in turn helps promote the subsequent development of acquired immunity. Recently, investigators have found that DCs play an important role in activating resting NK cells.23-26 Here we show that different types of classical DCs vary in their capacity to stimulate NK cells. Human moDCs remain the most potent in stimulating not only NK-cell proliferation but also cytotoxicity against class I MHC-negative targets. CD34+ HPC-derived LCs lack sufficient IL-12p70 secretion and IL-15R-α expression to induce NK-cell activation. Once activated by addition of IL-12, however, LCs provide additional, as yet unidentified factors that promote NK-cell proliferation and survival. DDC-IDCs are intermediate in stimulating NK-cell proliferation, cytotoxicity, and CD56 up-regulation.
The importance of DC secretion of the bioactive p70 form of IL-12 for the induction of NK cytotoxicity has been previously established. Anti-IL-12 antibodies can completely inhibit LPS-matured moDCs from inducing NK cytotoxicity against K562 targets.44 Our data show that IL-12 can additionally support proliferation of activated NK cells, but only when DCs are present, including otherwise poorly stimulatory LCs (compare Figure 4C with Figure 5B).
Of note, we measured both total IL-12 and the bioactive p70 form. Neither DDC-IDCs nor LCs secrete appreciable amounts of IL-12p70, whether matured by inflammatory cytokines or CD40L (Figure 5A and Ratzinger et al27 ). All DCs secrete IL-12p40, however, and DDC-IDCs secrete more than LCs. This IL-12p40 may in turn form a heterodimer with the unique IL-23p19 to yield bioactive IL-23, which is another member of the IL-12 heterodimer family with functions overlapping those of IL-12 itself.40,45,46 Although the dominant effects of IL-23 seem to be on the DCs themselves and favor Ag-specific Th1-acquired immunity, this cytokine could also account for some of the NK-cell stimulatory activity exerted by DDC-IDCs at higher stimulator doses as well as some of the activity of moDCs not solely contributed by IL-12p70.
Furthermore, even though LCs do not secrete sufficient IL-12 to initiate NK-cell activation, our results indicate that LCs provide additional factors to sustain NK-cell proliferation and survival after activation. Leading candidates include IL-18 and IL-15, which LCs secrete at higher levels than do DDC-IDCs or moDCs (Figure 5A and Ratzinger et al27 ). IL-15 is a crucial cytokine in NK-cell survival, and IL-15- as well as IL-15R-α-deficient mice lack NK cells entirely.29,32 Indeed, we could demonstrate a crucial function for IL-15 presented via surface IL-15R-α, for DC-induced NK-cell proliferation.47
Apart from secreted cytokines, NK-cell activation also depends on cell-to-cell contact, because transwell cultures separating mouse DCs from NK cells do not result in NK-cell activation.23 The molecules mediating this effect, however, remain largely uncharacterized. IFN-α-treated DCs represent an exception, where the nonclassical class I MHC molecules, MICA and MICB, mediate cell contact-dependent NK activation.44 MICA and MICB also mediate NKG2D-dependent killing of target cells by activated NK cells.15 The activating NK receptors NKG2D and NCRs (natural cytotoxicity receptors) mediate the lysis of most tumor cell lines by NK cells.13 While activated NK cells recognize moDCs via their NCR NKp30, this interaction does not support DC-mediated activation of resting NK cells.24 Hence, data from a number of studies, including our own, indicate that interactions for target recognition by activated NK cells and interactions for activation of resting NK cells are fundamentally different except under selected conditions such as the above-mentioned IFN-α-treated DCs.
We evaluated LCs matured and activated in vitro, which should be akin to those that have migrated to the T-cell-rich zones of secondary lymphoid organs48,49 but not representative of the resident immature LCs in epidermal and epithelial surfaces in the steady state. LCs normally patrol the epidermis as immature DCs uniquely suited for Ag capture and thereby report antigens to the immune system that invade superficially or are deposited on epithelial surfaces.50 In contrast, DDC-IDCs colonize the dermis and interstitia of solid organs. MoDCs originate from the bloodstream where circulating blood monocytes may be rapidly differentiated and activated to become DCs under inflammatory conditions.51,52 Both of these DC types may therefore be especially sensitive to early inflammatory pertubations. The observed functional differences between LCs, DDC-IDCs, and moDCs might explain why these DCs are differently equipped to activate NK cells. Because cytotoxic NK cells can cause major tissue damage, it would be in the best interest of the host to activate NK cells only if a pathogen has invaded the body systemically in blood or at least into the deeper layers of the skin or interstitia of solid organs. On the other hand, it should be beneficial to ignore superficial pathogen deposits at least with respect to recruitment and activation of NK cells.
DCs are increasingly finding roles for the initiation of acquired immunity as well as for the onset of innate immunity and may provide a critical link between the two. Similar to the divisions of labor between different DC types for eliciting immunogenic or tolerogenic responses by MHC-restricted, Ag-specific T cells,53,54 so also do DCs exhibit different activities for stimulation of NK-cell responses. MoDCs remain most potent in this respect, whereas CD34+ HPC-derived LCs are comparatively impotent without supplemental NK-cell-activating stimuli. CD34+-derived DDC-IDCs exert intermediate activity. Apart from IL-12 or other cytokines in the IL-12 family, IL-15 would seem to be another critical cytokine for NK-cell activation by DCs. High levels of soluble IL-15 that exceed the soluble amounts recoverable from mature DCs, as well as studies using other models,43 suggest that IL-15 presented by surface IL-15R-α in the immunologic synapse between DCs and NK cells may be the more relevant interaction. We indeed found that NK-cell-stimulatory moDCs express IL-15R-α on their surface, while IL-15 secreted by LCs might only be sufficient to mediate NK-cell survival. In vivo, where mixed populations of DCs would also intereact, LC-secreted IL-15 may act in a paracrine fashion to support NK-cell stimulation and activation by IL-15R-α-expressing moDCs.
These data additionally provide a rationale to broaden approaches focusing exclusively on the generation of T-cell immunity by one DC type to include other DC types to stimulate NK-cell activity or even support each other in NK and T-cell stimulation. Our results suggest that a combination of LCs and moDCs would enhance both NK-cell as well as T-cell activation (this study and Ratzinger et al27 ). Furthermore, therapeutic NK-cell activation could also mediate DC activation in situ for better priming and Th1 polarization of T-cell responses.55,56 The combination of DC subsets that stimulates both NK cells and T cells could therefore harness 2 valuable immune effector cell compartments. It could also optimize the interactions between innate and adaptive immunity for more efficient immune control of tumors, viruses, and other pathogens.
Prepublished online as Blood First Edition Paper, August 26, 2004; DOI 10.1182/blood-2004-06-2492.
Supported by Special Fellowship LLS-3286-02 from the Leukemia and Lymphoma Society, Speaker's Fund for Public Health Research from the New York Academy of Medicine, and R01 CA108609 from the National Cancer Institute, National Institutes of Health (C.M.); Associazione Italiana per la Ricerca sul Cancro (AIRC) and Ministero della Salute, Italy (G.F.); PR2001-0336 from the Secretaria de Estado de Educacion y Universidades, Spanish Ministry of Education and Science (M.A.de C.); R01 CA 83070, P01 CA 23766, and P01 CA 59350 from the National Cancer Institute, National Institutes of Health, and LLS 6124-99 from the Leukemia and Lymphoma Society (J.W.Y.). Equipment support was provided by William H. Goodwin and Alice Goodwin, the Commonwealth Cancer Foundation, and the Experimental Therapeutics Center of Memorial-Sloan-Kettering Cancer Center (J.W.Y.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the nurses and physicians of the Allogeneic Bone Marrow and Stem Cell Transplantation Service, Memorial Sloan-Kettering Cancer Center, as well as the Allogeneic Transplant and Cytotherapy Laboratory staff for assistance with sample procurement and processing.


![Figure 2. MoDCs, but not LCs, activate NK cells directly, whereas DDC-IDCs are intermediate in activity. Size (forward scatter [FSC]), granularity (side scatter [SSC]), and surface expression of the CD56 and CD16 epitopes were quantified by flow cytometry. FSC and SSC were measured on purified NK cells stimulated for 7 days by Langerhans cells (LC), dermal-interstitial DCs (DDC-IDC), and monocyte-derived DCs (moDC), all at an NK-cell-DC ratio of 10:1. CD56 and CD16 expression was quantified on gated CD3- lymphocytes, which lacked autofluorescence in channel FL-3 and were located in the FSC/SSC lymphocyte gate (dotted line). Percentages indicate NK blasts, CD16-CD56bright, CD16+CD56bright, and CD16+CD56dim cells. The phenotype of NK cells directly isolated from blood before DC stimulation is shown at the far left. This could not be shown at 7 days without DC stimulation, because NK cells die when cultured at high purity without stimulation or cytokines. Note that most peripheral blood NK cells are CD56dim but not CD56- directly after isolation from PBMCs. LCs remained more viable (high FSC/SSC; top right corner of dot plot) in these cocultures with NK cells than did either DDC-IDCs or moDCs. The data are representative of 7 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-06-2492/6/m_zh80010571820002.jpeg?Expires=1767847640&Signature=ndA7PVNjFcFpntHA358ayICtojmQSnAjjJkT0mVCeG9b82YySv8ukG0lCxXP4tadcWdmq4iE0~kCcHpjsJNfEFWAO6273iWk8fUp07PIVaE10yiuF-fhdtV4cQClrs8570T1IRlOU5H2eypYmkGIygUCp8hAR0pH0FkxTkV2anCvm-EKnjrTLuJ0syy1ZdMydJWZWRJpFG~s5~kJhoo0z4NnYrLQNMjl-bmv5ox8kYUV53LCdEkXPfKVKO6zHvrtP7XbPReKU-KkA-gBO34GsZZdPGSg-lbGNpubTWQmGwrlIi0wBzpojKDyNzDuo-AVQpZi~UO3raOL3jBDhWVtkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. In the absence of DC stimulation, IL-2 and IL-12 induce NK-cell activation and cytotoxicity but differ in their capacity to promote NK-cell proliferation. Size (forward scatter [FSC]), granularity (side scatter [SSC]), and surface expression of the CD56 and CD16 epitopes were quantified by flow cytometry. FSC and SSC were measured on purified NK cells cultured without (NK only) or with 80 IU/mL rIL-2 (NK + IL-2) or 500 pg/mL rIL-12 (NK + IL-12) for 7 days. CD56 and CD16 expression was quantified on gated CD3- lymphocytes, which lacked autofluorescence in channel FL-3 and were located in the FSC/SSC lymphocyte gate (dotted line). Percentages indicate NK-cell blasts, CD16-CD56bright, CD16+CD56bright, and CD16+CD56dim cells. Note that most of the peripheral blood NK cells are CD56dim but not CD56- directly after isolation from PBMCs. The data are representative of 3 independent experiments. (B) Purified peripheral blood NK cells were cultured without cytokines (NK only •) or with 80 IU/mL rIL-2 (NK + IL-2 ○) or 500 pg/mL rIL-12 (NK + IL-12 ▾) for 7 days. NK cytotoxicity of the cultures was determined by 51Cr release assay against the MHC class I-negative target LCL721.221. Radiolabeled LCL721.221 targets were exposed for 4 to 6 hours to the NK-cell effectors, which were the responder lymphocytes from the original or primary DC/NK cocultures. The effectors were added as total lymphocytes, without adjustment for specific numbers of NK-cell blasts, to ascertain differences between the different DCs in stimulating NK cells in the primary cultures. The effector-target ratio is indicated along the X-axis, and specific lysis is plotted against the Y-axis. The data are representative of 3 independent experiments. (C) Proliferation of purified peripheral blood NK cells was determined by 3H-thymidine incorporation after 6 days of culture alone (0) or with 80 IU/mL rIL-2 (+ IL-2) or 500 pg/mL IL-12 (+ IL-12). Data points represent the average of triplicate microwells and their standard deviation. These data are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-06-2492/6/m_zh80010571820004.jpeg?Expires=1767847640&Signature=wx~oalR8Cm5S2VXJWS~9DzScF7IZWoJchIV6FCTfLUI1ZwOwEaCHmjebRQIuh~lHZ2orEDidkKwDsLGfWwTT5WYEHxUn~eXg~AkLTGHvBQ4XLN03LZPqPrEoTuEiMac2AI1xgzOy163QewM496QmMhiQIb4jEb1cSEadwkRPPpA5wtZ7nEq6XJSjTKYmOJqq-pWPSYjvMl2jV-GDSKTQ7hiuSdo5XhCgMloLUuafOGShGUrE14MS8XYsQzNlAh3D-kb5w6CLQ5roadM3TczqL9HzdT46wmji4hcvk~i88j~gaJgfbQpNnGUyCBrVZpHx0oOJlQI9ZD0nJ5~lf8UJsQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. LC/NK cultures supplemented with allogeneic CD4+ T cells, IL-2, or IL-12 are able to activate NK cells. Size (forward scatter [FSC]), granularity (side scatter [SSC]), and surface expression of the CD56 and CD16 epitopes were quantified by flow cytometry. FSC and SSC were measured on purified NK cells stimulated for 7 days by Langerhans cells (LC) at an NK-cell-DC ratio of 10:1. Cultures were supplemented with irradiated allogeneic CD4+ T cells at an NK/CD4+ T-cell ratio of 1:1 (LC + CD4), 80 IU/mL rIL-2 (LC + IL-2), 500 pg/mL IL-12 (LC + IL-12), or nothing (LC). CD56 and CD16 expression was quantified on gated CD3- lymphocytes, which lacked autofluorescence in channel FL-3 and were located in the FSC/SSC lymphocyte gate (dotted line). Percentages indicate NK-cell blasts, CD16-CD56bright, CD16+CD56bright, and CD16+CD56dim cells. LCs maintained NK-cell viability very well (bottom left panel) despite the absence of activation and blast transformation without supplements. Note that most of the peripheral blood NK cells are CD56dim but not CD56- directly after isolation from PBMCs or after coculture with LCs. The data are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/1/10.1182_blood-2004-06-2492/6/m_zh80010571820006.jpeg?Expires=1767847640&Signature=ZmjS1I1fyQMc~DmKZx9xclygTUYQHEspN4cSp01OGzQSddxWV0lIp2vP5kDXjPFrm4CoCQOhEGa1PxHrQDYOxJD09FGl5xH~0DZym73PXRkiI8uMolbBGrsdeN95XjEEbcUCyG9y3jM9lb3P17Gpe6mm5qxSZ5zlqJOXE6hMw31Oxji9NnqWXPFprE~6rY8oWK0ygSTa2AWtLYLyulgstdjDu231kqG0szD6fKmHze96V71LUvnEg1HkmmMWr2CUat1CxlNGGgnViwz8pd~wXbCtTjTwl9VAPwRr-xVOKiG1Z3Xh9owY1Z1Wv2P4FHx4~Ljcv5uH43mkuhIEmPxUtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal