Abstract
During B-cell development in the mouse, Bruton tyrosine kinase (Btk) and the adaptor protein SLP-65 (Src homology 2 [SH2] domain-containing leukocyte protein of 65 kDa) limit the expansion and promote the differentiation of pre-B cells. Btk is thought to mainly function by phosphorylating phospholipase Cγ2, which is brought into close proximity of Btk by SLP-65. However, this model was recently challenged by the identification of a role for Btk as a tumor suppressor in the absence of SLP-65 and by the finding that Btk function is partially independent of its kinase activity. To investigate if enzymatic activity is critical for the tumor suppressor function of Btk, we crossed transgenic mice expressing the kinase-inactive K430R-Btk mutant onto a Btk/SLP-65 double-deficient background. We found that K430R-Btk expression rescued the severe developmental arrest at the pre-B-cell stage in Btk/SLP-65 double-deficient mice. Moreover, K430R-Btk could functionally replace wild-type Btk as a tumor suppressor in SLP-65- mice: at 6 months of age, the observed pre-B-cell lymphoma frequencies were approximately 15% for SLP-65- mice, 44% for Btk/SLP-65-deficient mice, and 14% for K430R-Btk transgenic mice on the Btk/SLP-65-deficient background. Therefore, we conclude that Btk exerts its tumor suppressor function in pre-B cells as an adaptor protein, independent of its catalytic activity. (Blood. 2005;105:259-265)
Introduction
Early in B-cell development, productive V(D)J (variable diversity joining) recombination leads to the synthesis of immunoglobulin (Ig) μ H chain protein, which is expressed on the cell surface together with the nonrearranging VpreB and λ5 surrogate light-chain (SLC) proteins, and forms the pre-B-cell receptor (pre-BCR; reviewed in Meffre et al1 and Melchers et al2 ). It appears that the pre-BCR has a ligand-independent signal transducing capacity, inducing phosphorylation of the Ig-α/CD79a and Ig-β/CD79b signaling components and subsequently proliferative expansion and differentiation into large cycling pre-B cells.3,4 Pre-BCR signaling involves the formation of a lipid raft-associated signaling module, which is composed of the tyrosine phosphorylated proteins Syk, the Src-family tyrosine kinase Lyn, Src homology 2 (SH2) domain-containing leukocyte protein of 65 kDa (SLP-65; also known as B-cell linker [BLNK] or B-cell adaptor containing SH2 domain [BASH]), phosphoinositol 3-kinase (PI3K), Bruton tyrosine kinase (Btk), Vav, and phospholipase Cγ2 (PLCγ2).5-7 Activation of PLCγ2 by Btk results in the production of the second messengers inositol trisphosphate (IP3) and diacylglycerol (DAG), which activate calcium signaling and protein kinase C (PKC), respectively.8,9 The pre-BCR is transiently expressed on the cell surface, whereby the signaling molecules SLP-65 and Btk are important for the developmental progression of large cycling into small resting pre-B cells in which Ig L chain rearrangement occurs (reviewed in Hendriks and Middendorp10 ).
By analogy with the BCR in mature B cells, Btk is thought to be phosphorylated by Syk or Lyn upon recruitment to the pre-B-cell membrane through binding of its pleckstrin homology (PH) domain to the PI3K product phosphatidyl-inositol trisphosphate (PIP3).11,12 In this signaling pathway, phosphorylated SLP-65 plays an essential role as it provides docking sites for phosphorylated Btk as well as PLCγ2 and thus brings Btk in close proximity with PLCγ2.13,14 However, this model is challenged by several independent observations.
Firstly, Btk can also act as adaptor molecule, independent of its catalytic activity. Expression of kinase-inactive Btk mutants have been shown to partially or fully reconstitute BCR-induced calcium mobilization in Btk-deficient chicken DT40 and human A20 mature B-cell lines.8,15,16 In addition, phosphorylation of PLCγ2 upon BCR stimulation is apparently unaffected in human Btk-deficient B-cell lines.17 We have recently shown that during in vivo B-cell development, Btk function is in part independent of its catalytic activity, as transgenic expression of the kinase-inactive K430R-Btk mutant partially restored pre-B cell and B-cell defects in Btk-deficient mice.18
Secondly, in mature A20 B cells, Btk is essential for the recruitment of phosphatidylinositol phosphate 5-kinase (PIP5K) to the membrane, whereby enzymatic activity of Btk is not required for its association with PIP5K.15 Activation of PIP5K leads to local synthesis of PIP2, which is a common substrate shared by both PI3K and PLCγ2. As a result, a positive feedback loop is initiated that allows Btk to stimulate the production (by PI3K) of PIP3, which is required for sustained Btk localization to the plasma membrane. At the same time, the shuttling function of Btk also provides substrate for sustained PLCγ2 activity.12,15
Thirdly, Btk/SLP-65 double-deficient mice show an almost complete block in B-cell development at the pre-B-cell stage when compared with the partial block at this stage in SLP-65 or Btk single-mutant mice,19-21 indicating that Btk and SLP-65 partially function in parallel pathways. Both Btk and SLP-65 are essential for the regulation of pre-B-cell development, in particular by limiting pre-B-cell expansion at the transition of large cycling into small pre-B cells.22-26 SLP-65- mice spontaneously develop pre-B-cell lymphomas expressing high levels of the pre-BCR on the cell surface.20,27 Although Btk-deficient mice do not develop pre-B-cell tumors, we recently found that Btk cooperates with SLP-65 as a tumor suppressor because the incidence of pre-B-cell lymphomas was significantly higher in Btk/SLP-65 double-mutant mice when compared with SLP-65 single-deficient mice. Moreover, transgenic expression of the constitutive active E41K-Y223F Btk mutant, which shows enhanced membrane localization,28,29 prevented tumor formation in Btk/SLP-65 double-deficient mice.21
Importantly, the finding of defective SLP-65 expression in approximately 50% of childhood pre-B acute lymphoblastic leukemias (ALLs) indicated that SLP-65 also acts as a tumor suppressor in pre-B cells in humans.30 The loss of SLP-65 protein was apparently due to the incorporation of alternative exons into SLP-65 transcripts, leading to premature stop codons.30 Moreover, in human Bcr-Abl+ pre-B ALL, the activity of the Bcr-Abl1 kinase was found to be linked to defective pre-B signaling and the expression of the same aberrant SLP-65 transcripts.31 It was also observed that a large fraction of childhood pre-B ALL cases manifested aberrant Btk transcripts predicted to encode Btk proteins with a substantial kinase domain deletion.32
The mechanism by which Btk exerts its tumor suppressor function in pre-B cells independent of SLP-65 is currently unknown. By analogy with the findings in mature A20 B cells, Btk may function in pre-B cells as an adaptor molecule to localize PIP5K to the plasma membrane. However, it remains possible that this PIP5K shuttling mechanism that allows Btk to stimulate PLCγ2 is dependent on the presence of SLP-65 (eg, as a scaffold molecule to bring Btk or other tyrosine kinase proteins, such as Syk or Src-family kinases, in close proximity with PLCγ2).
In this study, we investigated whether Btk tumor suppressor function is independent of its kinase activity and at the same time whether SLP-65 is required for adaptor function of Btk. To this end, we crossed transgenic mice expressing the kinase-inactive Btk mutant K430R onto a Btk/SLP-65 double-deficient background and found that kinase-inactive Btk was able to functionally replace wild-type Btk as a tumor suppressor in the absence of SLP-65.
Materials and methods
Mice and genotyping
Btk-deficient mice33 and SLP-65-deficient mice22 were on the C57BL/6 and Balb/c background, respectively. Wild-type and targeted alleles were identified as described previously.21,26 Btk-K430R transgenic mice, in which B-cell-specific expression of human Btk is under the control of the CD19 promoter region, were on a mixed C57Bl/6 × FvB background. The presence of the Btk-K430R transgene was evaluated by polymerase chain reaction (PCR).18
Flow cytometry and Ig ELISA
Standard and intracellular flow cytometry and conjugated monoclonal antibodies (BD Biosciences, Mountain View, CA) have been described previously.26,33 The anti-SLC hybridoma LM3434 was kindly provided by A. Rolink (University of Basel, Basel, Switzerland). Levels of Ig subclasses in serum were measured by sandwich enzyme-linked immunosorbent assay (ELISA).35
Cell culture and Western blotting
Total bone marrow (BM) cells or pre-B-lymphoma cells were cultured in the presence of 100 U/mL interleukin 7 (IL-7; Sigma-Aldrich, St Louis, MO) as described.26 For analysis of Btk phosphorylation, cells were stimulated with 10 μg/mL F(ab′)2 fragment of polyclonal goat antimouse IgM (Jackson Immuno Research, Westgrove, PA) in RPMI1640 at 37°C. Total cell lysates of unstimulated and stimulated cells were immunoprecipitated overnight at 4°C with antiphosphotyrosine (pTyr-100; Cell Signaling Technology, Beverly, MA) and samples were blotted using standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) procedures.36 Western blots were stained with anti-Btk C-20 (SantaCruz Biotechnology, Santa Cruz, CA) to detect Btk protein. Routinely, a small fraction of the total cell lysates were blotted with pTyr-100 to verify pre-BCR stimulation.21
Ca2+ mobilization assay
Cells were loaded with 6 μg/mL indo-1 acetoxymethylester (indo-1 am; Molecular Probes Europe, Leiden, The Netherlands) at 37°C for 45 minutes and subsequently stained with phycoerythrin (PE)-labeled anti-CD4, anti-NK1.1, anti-Ter119, anti-CD11b, and fluorescein isothiocyanate (FITC)-labeled anti-CD8 monoclonal antibodies (BD Biosciences). Cells were stimulated by 20 μg/mL F(ab′)2 fragment of polyclonal goat antimouse IgM (Jackson Immuno Research) and subsequently by 2 μg/mL ionomycin as a positive control. Fluorescence ratios (FL-5/FL-4) were measured on a FACS-VantageSE/DiVa (BD Biosciences) and data for B cells (CD4-CD8NK1.1-Ter119-CD11b-) were analyzed by FlowJo (Tree Star, Ashland, OR) multiparameter flow application.
Results
Kinase-inactive Btk stimulates calcium mobilization in mature B cells
We previously showed that transgenic expression of the kinase-dead Btk mutant K430R partially rescued Btk function in vivo, including the induction of nuclear factor κB (NF-κB) and the expression of Bcl-xL and cyclin D2 in splenic B cells upon BCR stimulation.18 To investigate whether these effects are mediated by the ability of K430R-Btk to potentiate calcium flux, we evaluated calcium mobilization in splenic B cells in response to BCR engagement in K430R-Btk transgenic mice on a Btk-deficient background, in comparison with wild-type and Btk-deficient mice (Figure 1A). Btk-deficient B cells showed a significantly reduced BCR-mediated Ca2+ signal when compared with wild-type B cells. Transgenic K430R-Btk provided complete correction of the Ca2+ response in Btk-deficient B cells, indicating that Btk stimulates calcium flux independent of its catalytic activity.
Kinase domain-independent and SLP-65-independent function of Btk. (A) K430R-Btk stimulates calcium flux in response to BCR activation. Indo-1 AM-loaded splenic B cells of the indicated mice were stimulated with anti-IgM F(ab′)2 fragments and calcium flux was monitored. The plots are representative for 2 to 4 mice of each genotype. (B) Btk phosphorylation in the absence of SLP-65. Cultured cells from 2 different SLP-65- pre-B-cell lymphomas (BL no. 1 and BL no. 2) were either not stimulated or stimulated for 1 or 5 minutes with polyclonal anti-IgM F(ab′)2 fragments. The presence of Btk in antiphosphotyrosine immunoprecipitates from total cellular lysates was analyzed by Western blotting using Btk-specific antibodies.
Kinase domain-independent and SLP-65-independent function of Btk. (A) K430R-Btk stimulates calcium flux in response to BCR activation. Indo-1 AM-loaded splenic B cells of the indicated mice were stimulated with anti-IgM F(ab′)2 fragments and calcium flux was monitored. The plots are representative for 2 to 4 mice of each genotype. (B) Btk phosphorylation in the absence of SLP-65. Cultured cells from 2 different SLP-65- pre-B-cell lymphomas (BL no. 1 and BL no. 2) were either not stimulated or stimulated for 1 or 5 minutes with polyclonal anti-IgM F(ab′)2 fragments. The presence of Btk in antiphosphotyrosine immunoprecipitates from total cellular lysates was analyzed by Western blotting using Btk-specific antibodies.
SLP-65 is not required for pre-BCR-induced tyrosine phosphorylation
If Btk and SLP-65 can function independently in parallel pathways in pre-B cells, this implies that Btk can be activated in the absence of SLP-65. It was previously shown that in SLP-65- pre-B-cell lines, established by long-term IL-7-dependent bone marrow culture, the pre-BCR is signaling competent, whereby pre-BCR stimulation led to phosphorylation of Ig-α, Syk, Lyn, PI3K, and PLCγ2.20 We evaluated phosphorylation of Btk in stable SLP-65-deficient pre-B-cell lines expressing high levels of pre-BCR on the cell surface, which we established from SLP-65- pre-B-lymphoma cells.21 Pre-B cells were stimulated with antibodies specific for Ig μ H chain, tyrosine-phosphorylated proteins were immunoprecipitated from total cellular lysates using pTyr-specific monoclonal antibodies, and Btk protein was identified by blotting with a Btk-specific antibody. As shown in Figure 1B, pre-BCR stimulation in SLP-65-deficient pre-B cells resulted in Btk phosphorylation. Similar results were obtained in pre-BCR stimulation of short-term IL-7-dependent bone marrow-derived cultured pre-B cells from tumor-free SLP-65- mice (data not shown). These findings show that in murine pre-B cells, Btk can be phosphorylated upon pre-BCR stimulation in the absence of SLP-65.
Transgenic K430R-Btk expression partially corrects the defects in B-cell development in Btk/SLP-65 double-mutant mice
Mice deficient for either Btk or SLP-65 have a partial block in B-cell development at the pre-B-cell stage in the BM, whereas Btk/SLP-65 double-deficient mice have an almost complete block at this stage.19,22-26 We have previously shown that expression of transgenic kinase-inactive K430R-Btk is able to partially rescue the mild differentiation defects at the pre-B-cell stage and the more severe block in peripheral B-cell maturation in Btk-deficient mice.18 To investigate to what extent the K430R-Btk transgene can reconstitute the almost complete pre-B-cell differentiation block in Btk/SLP-65 double-deficient mice, we compared the size of the B-cell subpopulations in spleen and BM by flow cytometry in 6 different mouse groups: Btk+, Btk-, or K430R-Btk transgenic on a Btk- background, each of which were either SLP-65+ or SLP-65-.
In agreement with our reported findings,18 reconstitution with K430R-Btk partially overcame the block in peripheral B-cell maturation present in Btk-deficient mice (Table 1; Figure 2A). Also, consistent with previous findings,19,21 the size of the total splenic B-cell population in Btk/SLP-65 double-deficient mice was severely reduced (∼1.7 × 106 cells) when compared with wild-type or each of the single-deficient mice (Table 1). Expression of K430R-Btk in Btk/SLP-65 double-deficient mice increased the size of the total B-cell population in the spleen with a factor of approximately 4 (to 7.1 × 106 cells). This reconstitution was only partial, since the total numbers of splenic B cells in SLP-65- mice were significantly higher (∼22 × 106 cells; Table 1). Nevertheless, the residual B cells present, which failed to differentiate into IgMlowIgDhigh B cells in Btk/SLP-65 double-deficient mice, in part differentiated to IgMlowIgDhigh mature B cells when the K430R-Btk transgene was present (Figure 2A).
Absolute numbers of B-cell subpopulations in spleen and bone marrow
. | . | . | Bone marrow . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | n* . | Spleen† . | Pro-B cells† . | Pre-B cells† . | Immature B cells† . | Mature B cells† . | |||
| Mouse strain | NA | B220+ | B220+cμ− | B220+cμ+lgM− | lgM+lgD− | lgM+lgD+ | |||
| Btk+ | 14 (5) | 61.0 ± 9.3 | 2.6 ± 0.3 | 13.8 ± 1.1 | 3.9 ± 1.3 | 3.7 ± 0.7 | |||
| Btk− | 13 (5) | 11.9 ± 1.3 | 3.0 ± 0.3 | 11.9 ± 1.0 | 3.9 ± 0.3 | 2.0 ± 0.2 | |||
| K430R-Btk | 5 | 23.1 ± 4.5 | 2.8 ± 0.4 | 12.8 ± 2.0 | 3.8 ± 0.8 | 2.0 ± 0.7 | |||
| SLP-65− | 5 | 21.7 ± 5.0 | 3.0 ± 0.5 | 12.8 ± 2.3 | 3.1 ± 0.6 | 1.5 ± 0.4 | |||
| Btk−SLP-65− | 5 | 1.7 ± 0.3 | 3.7 ± 0.3 | 8.2 ± 1.6 | 1.1 ± 0.3 | 0.1 ± 0.0 | |||
| K430R-Btk/SLP-65− | 7 | 7.1 ± 1.6‡ | 2.8 ± 0.3 | 10.4 ± 1.6 | 2.6 ± 0.4 | 0.7 ± 0.2 | |||
. | . | . | Bone marrow . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | n* . | Spleen† . | Pro-B cells† . | Pre-B cells† . | Immature B cells† . | Mature B cells† . | |||
| Mouse strain | NA | B220+ | B220+cμ− | B220+cμ+lgM− | lgM+lgD− | lgM+lgD+ | |||
| Btk+ | 14 (5) | 61.0 ± 9.3 | 2.6 ± 0.3 | 13.8 ± 1.1 | 3.9 ± 1.3 | 3.7 ± 0.7 | |||
| Btk− | 13 (5) | 11.9 ± 1.3 | 3.0 ± 0.3 | 11.9 ± 1.0 | 3.9 ± 0.3 | 2.0 ± 0.2 | |||
| K430R-Btk | 5 | 23.1 ± 4.5 | 2.8 ± 0.4 | 12.8 ± 2.0 | 3.8 ± 0.8 | 2.0 ± 0.7 | |||
| SLP-65− | 5 | 21.7 ± 5.0 | 3.0 ± 0.5 | 12.8 ± 2.3 | 3.1 ± 0.6 | 1.5 ± 0.4 | |||
| Btk−SLP-65− | 5 | 1.7 ± 0.3 | 3.7 ± 0.3 | 8.2 ± 1.6 | 1.1 ± 0.3 | 0.1 ± 0.0 | |||
| K430R-Btk/SLP-65− | 7 | 7.1 ± 1.6‡ | 2.8 ± 0.3 | 10.4 ± 1.6 | 2.6 ± 0.4 | 0.7 ± 0.2 | |||
NA indicates not applicable.
Number of mice analyzed; the numbers in parentheses indicate that the B-cell population in spleen was analyzed in 5 mice per group.
Absolute number as average ± SEM (×106).
The absolute numbers of splenic B220+ cells in K430R-Btk/SLP-65− mice were significantly different from the values in SLP-65− mice (P = .008) and in Btk/SLP-65 double-deficient mice (P = .025).
K430R-Btk partially corrects the defects in B-cell development in Btk/SLP-65 double-mutant mice. Flow cytometric analysis of (A) spleen and (B) bone marrow of Btk+, Btk-, and K430R-Btk mice on either the SLP-65+ or the SLP-65- background. (A) IgM/IgD profiles of total splenic lymphoid cells, (B) IgM/B220, and (C) IgM/IgD profiles of total bone marrow lymphoid cells. Lymphoid cells were electronically gated on the basis of forward and side scatter characteristics. Data are presented as dot plots and the percentages within the indicated quadrants or gates are given. The plots are representative for 5 to 14 mice of each genotype.
K430R-Btk partially corrects the defects in B-cell development in Btk/SLP-65 double-mutant mice. Flow cytometric analysis of (A) spleen and (B) bone marrow of Btk+, Btk-, and K430R-Btk mice on either the SLP-65+ or the SLP-65- background. (A) IgM/IgD profiles of total splenic lymphoid cells, (B) IgM/B220, and (C) IgM/IgD profiles of total bone marrow lymphoid cells. Lymphoid cells were electronically gated on the basis of forward and side scatter characteristics. Data are presented as dot plots and the percentages within the indicated quadrants or gates are given. The plots are representative for 5 to 14 mice of each genotype.
In the bone marrow, the Btk-, K430R-Btk, and SLP-65- mice differed from wild-type mice by an approximately 50% reduction in the size of the mature IgM+IgD+ population of recirculating B cells (Table 1). By contrast, the Btk/SLP-65 double-deficient mice manifested a dramatic reduction in the total numbers of both immature IgM+IgD- and mature IgM+IgD+ B cells. Importantly, the size of the immature B-cell population was similar in K430R-Btk/SLP-65- mice (∼2.6 × 106) and SLP-65- mice (∼3.1 × 106). Thus, in the absence of SLP-65, kinase-inactive K430R-Btk could functionally replace Btk at the developmental progression from pre-B cells into immature B cells. From the analysis of IgM/IgD profiles of the B220+ population (Figure 2C), it is clear that SLP-65- and K430R-Btk/SLP-65- mice contained similar fractions of IgM+IgD- immature B cells (∼12%). Expression of the K430R-Btk transgene only partly restored the reduction of the size of the mature IgM+IgD+ B-cell population observed in Btk/SLP-65- mice (Figure 2B-C; Table 1).
In summary, these flow cytometric analyses show that expression of kinase-inactive Btk can partially correct the severe B-cell differentiation defect in Btk/SLP-65 double-deficient mice, whereby the level of reconstitution is more complete in immature B cells than in mature B cells. Therefore, these results indicate that in SLP-65-independent signaling pathways, Btk kinase activity is not critical for the transition of pre-B cells into immature B cells and is apparently more important for the maturation of peripheral B cells.
Btk kinase activity is specifically required for IgG3 production
To further investigate if the Btk kinase domain is essential for the synergistic role of Btk and SLP-65 in mature B cells, we investigated the serum Ig levels in the 6 mouse groups. When compared with wild-type mice, Btk- and SLP-65- mice have severely decreased levels of IgM and IgG3, whereas other isotypes are generally not affected.22-25,37 We have previously found that kinase-inactive Btk could significantly restore the decreased levels of IgM in serum of Btk-deficient mice, but only a limited correction of serum IgG3 was observed.18 As shown in Figure 3, Btk/SLP-65 double-deficient mice have reduced serum levels of all isotypes when compared with each of the single-mutant littermates. Serum Ig levels in K430R transgenic Btk/SLP-65 double-deficient mice were restored to levels similar to those found in SLP-65 single-deficient mice (Figure 3), except for IgG3. In fact, serum IgG3 levels in Btk/SLP-65 double-deficient and K430R-Btk/SLP-65- mice were very low, close to the lower detection limit of the ELISA.
K430R-Btk restores serum Ig levels in Btk/SLP-65 double-mutant mice. Serum concentrations of IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA, as determined by ELISA, are shown for the indicated mouse strains. Mice were 2 months of age and each symbol represents an individual animal. □ indicates Btk+; ▵, Btk-; ○, K430R-Btk; ▪, Btk+ SLP-65-; ▴, Btk- SLP-65-; and •, K430R-Btk SLP-65-.
K430R-Btk restores serum Ig levels in Btk/SLP-65 double-mutant mice. Serum concentrations of IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA, as determined by ELISA, are shown for the indicated mouse strains. Mice were 2 months of age and each symbol represents an individual animal. □ indicates Btk+; ▵, Btk-; ○, K430R-Btk; ▪, Btk+ SLP-65-; ▴, Btk- SLP-65-; and •, K430R-Btk SLP-65-.
Taken together, these results indicate that, with respect to late B-cell and plasma cell differentiation, in the absence of SLP-65, Btk mainly acts as a scaffolding protein. Only for the differentiation of B cells into IgG3-producing plasma cells is Btk kinase activity essential.
K430R-Btk can functionally replace wild-type Btk in cellular maturation of SLP-65-deficient pre-B cells
We have previously shown that Btk and SLP-65 are required for efficient transition of large cycling into small resting cytoplasmic μ+ pre-B cells.21,26 In Btk or SLP-65 mutant mice, the down-regulation of the pro-B/large pre-B-cell-specific expression of SLC, CD43, and BP-1/6C3 aminopeptidase A and the up-regulation of CD2, CD25, and major histocompatibility complex (MHC) class II on small pre-B cells are impaired. A synergistic role for Btk and SLP-65 in this context is clear from the more pronounced defects in the modulation of these markers in Btk/SLP-65 double-deficient mice.21,26 We also reported that kinase-inactive Btk is able to partially restore the defects at the pre-B-cell stage in Btk-deficient mice.18
To investigate the effects of K430R-Btk on pre-B-cell maturation in the absence of SLP-65, we compared the surface IgM- pro-B/pre-B-cell fraction of Btk+, Btk-, and K430R-Btk transgenic mice, either on an SLP-65+ or an SLP-65- background. We analyzed the expression of various developmentally regulated markers, including SLC, CD25, and MHC class II by flow cytometric analysis. In wild-type cells, only a small proportion of μ+ pre-B cells expressed SLC (∼7%; Figure 4A). As previously reported,18,21 transgenic K430R-Btk was able to restore the impaired down-regulation of SLC expression in Btk-deficient mice, whereas Btk/SLP-65 double-mutant mice manifested a much more severe defect in SLC down-regulation (∼71% SLC+ pre-B cells). Interestingly, the levels of SLC expression in K430R-Btk/SLP-65- and SLP-65- single-deficient pre-B cells were similar (∼43% SLC+ pre-B cells; Figure 4A), indicating that K430R-Btk could completely replace wild-type Btk function with respect to SLC down-regulation. Likewise, the levels of CD25 and MHC class II induction in K430R-Btk/SLP-65- pre-B cells (∼38% and ∼32%, respectively) were comparable to those in SLP-65- pre-B cells (∼56% and ∼21%) and significantly higher than those in Btk/SLP-65 double-mutant mice (∼18% and ∼6%).
K430R-Btk corrects pre-B-cell maturation in Btk/SLP-65 double-mutant mice. Flow cytometric analysis of gated surface IgM-negative B220+ pro-/pre-B cells of Btk+, Btk-, and K430R-Btk mice on either an SLP-65+ or the SLP-65- background. B220+IgM- cells were gated and analyzed for the expression of (A) cytoplasmic Igμ H chain (cμ)/SLC, (B) cμ/MHC, class II, and (C) forward scatter (FSC)/CD25. Data are displayed as dot plots and the percentage of cells within the indicated gates are given. Data shown are representative of 5 to 14 mice analyzed within each group.
K430R-Btk corrects pre-B-cell maturation in Btk/SLP-65 double-mutant mice. Flow cytometric analysis of gated surface IgM-negative B220+ pro-/pre-B cells of Btk+, Btk-, and K430R-Btk mice on either an SLP-65+ or the SLP-65- background. B220+IgM- cells were gated and analyzed for the expression of (A) cytoplasmic Igμ H chain (cμ)/SLC, (B) cμ/MHC, class II, and (C) forward scatter (FSC)/CD25. Data are displayed as dot plots and the percentage of cells within the indicated gates are given. Data shown are representative of 5 to 14 mice analyzed within each group.
In summary, these analyses show that transgenic expression of kinase-inactive Btk is able to reduce the severe pre-B-cell maturation defects in Btk/SLP-65 double-deficient mice to a level that is comparable with SLP-65 single-deficient pre-B cells. Therefore, we conclude that in the absence of SLP-65, Btk signaling contributes to cellular maturation of pre-B cells, independent of its kinase activity.
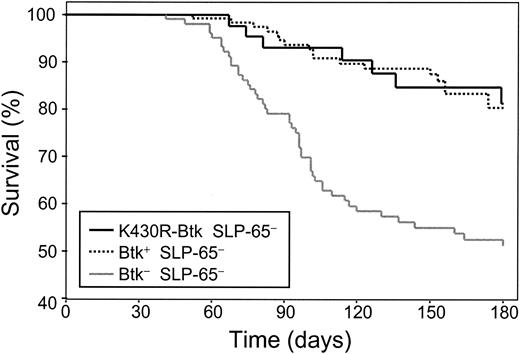
K430R-Btk can functionally replace wild-type Btk as a tumor suppressor in SLP-65- mice
Concomitant deficiency of Btk strongly increases the frequency of pre-B-cell lymphoma formation in SLP-65- mice, demonstrating that Btk cooperates with SLP-65 as a tumor suppressor in pre-B cells.21 To investigate whether the tumor suppressor function of Btk is dependent on its kinase activity, we examined the capacity of K430R-Btk to substitute for Btk by following a panel of K430R-Btk transgenic mice for 180 days. At this age, 18 (∼15%) of 123 SLP-65 single-deficient mice developed a pre-B-cell lymphoma, whereas the Btk/SLP-65 double-deficient mice showed a frequency of approximately 44% (47 of 107). In contrast, only 7 (∼14%) of 51 K430R-Btk/SLP-65-deficient mice developed a pre-B-cell lymphoma (Figure 5). From these results we conclude that transgenic kinase-inactive Btk can substitute for the endogenous wild-type Btk as a tumor suppressor in SLP-65-deficient mice, and therefore that the tumor suppressor function of Btk in pre-B cells is independent of its kinase activity.
Tumor suppressor function of Btk is not dependent on its kinase activity. Kaplan-Meier tumor-free survival estimates for SLP-65- (n = 123), Btk-SLP-65- (n = 107), and K430R-Btk mice on the Btk-SLP-65- background (n = 51). Tumor-free survival in Btk/SLP-65 double-deficient mice was significantly reduced compared with SLP-65- mice (P < .00001, by log-rank) and to K430R-Btk/SLP-65- mice (P < .0008). Tumor-free survival in the SLP-65- and K430R-Btk/SLP-65- groups of mice was not significantly different.
Tumor suppressor function of Btk is not dependent on its kinase activity. Kaplan-Meier tumor-free survival estimates for SLP-65- (n = 123), Btk-SLP-65- (n = 107), and K430R-Btk mice on the Btk-SLP-65- background (n = 51). Tumor-free survival in Btk/SLP-65 double-deficient mice was significantly reduced compared with SLP-65- mice (P < .00001, by log-rank) and to K430R-Btk/SLP-65- mice (P < .0008). Tumor-free survival in the SLP-65- and K430R-Btk/SLP-65- groups of mice was not significantly different.
Discussion
The finding that the block in B-cell differentiation and the associated pre-B-cell lymphoma formation in SLP-65- mice is much more severe when Btk is concomitantly mutated suggested that the 2 proteins do not function only in a common signal transduction pathway. In this study, we demonstrate that during pre-B-cell development in the mouse, Btk functions as an adaptor molecule in an SLP-65-independent pathway.
We found that transgenic expression of the kinase-inactive K430R-Btk mutant was able to rescue the severe defects present in Btk/SLP-65 double-deficient pre-B cells to such an extent that the resulting phenotype was similar to that of SLP-65 single-deficient mice. In particular, we found that in the absence of SLP-65, Btk can act as an adaptor molecule that signals the modulation of SLC, CD25, and MHC class II expression in pre-B cells. Importantly, the Btk kinase activity is also not required for its function as a tumor suppressor in SLP-65-deficient pre-B cells. Expression of the K430R-Btk transgene also restored the severe defects in mature B cells, although total B-cell numbers in the spleen and serum IgG3 levels were still reduced in K430R-Btk transgenic mice on the Btk/SLP-65 double-deficient background compared with SLP-65 single-deficient mice. This partial inability of K430R-Btk to substitute for Btk in mature B cells could reflect their absolute dependence on Btk kinase activity. Alternatively, this might be related to the expression pattern of the K430R-Btk transgene, which is under the control of the CD19 promoter region. While the expression level is in the physiologic range in the bone marrow (1-2 × endogenous levels), this increases significantly as immature B cells differentiate to mature peripheral B cells (∼15 × overexpression). Therefore, it is possible that transgenic K430R-Btk expression partly has dominant-negative effects in vivo, which would be supported by our previous finding of reduced serum IgG3 levels and T-cell-independent IgM responses in K430R-Btk transgenic mice on a Btk wild-type background.18
The mechanism by which Btk functions as an adaptor molecule in pre-B cells is not known, but it is attractive to hypothesize that equivalent to the findings in mature A20 B cells, Btk may associate with PIP5K and recruit this enzyme to the plasma membrane.15 As overexpression of PIP5K was reported to lead to an increase in the sustained calcium response upon BCR activation,12 it is assumed that by providing the substrate PIP2 for PLCγ2, PIP5K recruitment by Btk is sufficient to induce production of IP3 and DAG by PLCγ2. This mechanism would then explain the ability of kinase-inactive Btk to induce calcium mobilization in B cells upon BCR stimulation (Figure 1A). In this model, Btk should be recruited to the membrane and activated in an SLP-65-independent fashion. Although it has been proposed that Btk phosphorylation would be SLP-65 dependent,13 other data including ours favor a model in which Btk activation is SLP-65 independent (Fu et al38 and Chiu et al39 ; Figure 1B).
Taken together, our findings provide the biochemical basis for a parallel SLP-65-independent escape route when Btk is not able to phosphorylate PLCγ2 directly: upon pre-BCR expression, Lyn, Syk, and PI3K are activated, resulting in phosphorylation and membrane translocation of Btk. Activated Btk is able to recruit PIP5K, which provides substrate for both PLCγ2 and PI3K. This mechanism may also explain the phenotype of CD19/SLP-65 double-deficient mice, in which pre-B-cell differentiation was also almost completely blocked,27 quite similar to the phenotype of Btk/SLP-65 double-deficient mice.19,21 From the findings in CD19/SLP-65 double-deficient mice, it was concluded that the pre-B-cell transition, including cell cycle progression, down-regulation of recombination-activating gene 2 (RAG-2) protein, and SLC expression, as well as transcriptional activation of the Ig κ locus, which is principally mediated by SLP-65, can also be (inefficiently) executed by CD19.27 In addition, in reconstituted myeloma cells, CD19 has been shown to be necessary for efficient BCR-mediated activation of Btk,40 most likely through the PI3K pathway and the production of PIP3 in concert with Lyn- or Syk-mediated phosphorylation of Btk.41,42 Therefore, we propose that the SLP-65-independent parallel PLCγ2 activation pathway in pre-B cells requires both Btk (as an adaptor protein) and CD19-dependent PIP3 synthesis by PI3K. In this model, a direct interaction between Btk and PLCγ2 is not required and Btk and PIP5K would interact in the absence of SLP-65.
A possibility remains that SLP-65 deficiency can be compensated by the presence of redundant proteins because the concomitant absence of linker of activated T cells (LAT) in SLP-65- mice resulted in an almost complete block at the large pre-B-cell stage.43 Furthermore, it was shown that upon pre-BCR engagement, LAT recruits PLCγ2 to the pre-BCR by association with Ig-α and the SLP-65 homologue SLP-76. These results indicate that LAT/SLP-76 can rescue PLCγ2 activation in the absence of SLP-65.43 However, in this model the capacity of LAT/SLP-76 to replace SLP-65 function would be dependent on the presence of Btk as an adaptor molecule. Although SLP-65 or LAT/SLP-76, together with Btk, may serve as a scaffold to bring other kinase proteins in close proximity to PLCγ2, phosphorylation of 2 of 4 regulatory tyrosines in PLCγ2 appears to be entirely dependent upon Btk/Tec-family kinases, whereas Syk/zeta-associated protein 70 (ZAP-70) fails to modify these sites.44 Further experiments are required to investigate if Btk can interact with LAT or SLP-76.
In summary, our results show that Btk not only activates PLCγ2 by phosphorylation in a complex with SLP-65 but also has a scaffolding function in an SLP-65-independent pathway in pre-B cells. This kinase-independent Btk function provides the molecular basis of the observed cooperation of Btk with SLP-65 as a tumor suppressor in pre-B cells. Based on recent findings in mature B cells,15 we hypothesize that Btk tumor suppressor function involves its capacity to recruit PIP5K to the membrane, which allows Btk to provide substrate for PLCγ2.
Prepublished online as Blood First Edition Paper, August 26, 2004; DOI 10.1182/blood-2004-07-2708.
Supported in part by the Netherlands Organization for Scientific Research (R.W.H.; grant no. 901-07-209).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank K. Dahlenborg, M. van Zelm, E. de Haas (Dept Immunology), and H. Diepstraten (Erasmus MC Animal Facility) for their assistance at various stages of the project.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal