Abstract
Transforming growth factor-β1 (TGF-β1), an immunosuppressive cytokine, inhibits cytotoxic T cell (CTL) immune responses. In contrast, 4-1BB (CD137), a costimulatory molecule in the tumor necrosis factor (TNF) receptor family, amplifies CTL-mediated antitumor immune responses. We investigated whether TGF-β1 responses could be reversed by 4-1BB costimulation during in vitro differentiation of naive CD8+ T cells into effector CTL cells. TGF-β1 potently suppressed CTL differentiation of human cord blood naive CD8+ T cells as determined by reduced induction of characteristic phenotypes of effector cells and cytotoxic activity. TGF-β1-mediated suppression of CTL differentiation was abrogated by 4-1BB costimulation but not by CD28 or another member in the TNF receptor family, CD30. 4-1BB costimulation suppressed Smad2 phosphorylation induced by TGF-β1, suggesting that 4-1BB effects were at the level of TGF-β1 signaling. 4-1BB effects on the TGF-β1-mediated suppression were enhanced by interleukin 12 (IL-12) but counteracted by IL-4; 4-1BB expression was up- or down-regulated, respectively, by IL-12 and IL-4. IL-4 was more dominant than IL-12 when both cytokines were present during 4-1BB costimulation in the presence of TGF-β1. This indicates critical roles for IL-4 and IL-12 in regulating 4-1BB effects on TGF-β1-mediated suppression. (Blood. 2005;105:274-281)
Introduction
Differentiation of naive CD8+ T cells into effector or memory cytotoxic T cells (CTLs) is initiated by T-cell receptor (TCR) activation through their encountered peptide antigens presented by major histocompatibility complex (MHC) class I at the surface of antigen-presenting cells (APCs). This event alone does not necessarily establish productive CTL differentiation. Most T cells activated in the absence of appropriate costimulatory events appear to cause peripheral tolerance or die prematurely.1 Current evidence indicates that the CTL-mediated cytotoxic process requires not only triggering of the TCR but also costimulation signals provided by APCs.1 Thus, adaptive CTL immune responses are mounted primarily by mature dendritic cells (DCs), the most potent APCs with highly enriched costimulatory molecules.2
Tumor-T-cell interactions might not provide sufficient costimulatory signals for full T-cell activation and establishment of the cytotoxic activity of tumor-specific CTLs.3,4 Consistent with this possibility, TCR signaling of tumor-specific CTLs is often defective.5,6 As a result, tumor-infiltrated CTLs show skewed maturation toward a preterminally differentiated state with reduced expression of perforin and granzyme B, key proteins for CTL activity.3 Despite the presence of antitumor antigen-specific CTLs in tumor infiltrates, signs of tumor destruction are usually not evident.3,7
Another reason for defective antitumor CTLs may reflect an array of immunosuppressive mediators produced by tumors. Tumors secret large amounts of transforming growth factor-β (TGF-β), which limits CTL differentiation.8-10 The detrimental effect of TGF-β1 on CTL cytotoxic activity is consistent with enhanced tumor eradication in mice with T cells lacking TGF-β signaling.8-10 Therefore, secretion of TGF-β may be 1 mechanism by which tumors can evade host CTL immune responses. Tumor-derived TGF-β also reduces the effectiveness of DC-based immunotherapy.11
Efforts have been devoted to enhance antitumor CTL response by introducing exogenous costimulatory signals.12 CD28 is a primary T-cell costimulatory molecule. However, in humans, effector activity is detected primarily in the CD28- subpopulation of CD8+ T cells.13,14 CD28- effector cells do not usually proliferate and are prone to apoptosis.15 Survival of the effector cells, which is critical for further differentiation into memory type cells, requires inducible costimulatory molecules other than CD28.1,16 4-1BB, a member of the tumor necrosis factor (TNF) receptor family that includes Fas, CD27, CD30, and RANK (receptor activator of nuclear factor-κB), is induced on activated CD4+ and CD8+ T cells17 and has the ability to prevent activation-induced apoptosis of a CD28- subpopulation of CD8+ T cells.18 In particular, 4-1BB ligation enhances long-term effector and memory CTL responses1,19,20 and boosts antitumor CTL immune responses against weakly immunogenic tumors21-23 and viruses.24,25 4-1BB costimulation also contributes to exacerbating graft-versus-host disease (GVHD)26 and allograft rejection.24
To date, there is little evidence of a function for 4-1BB in antagonizing the suppressive effects of TGF-β1. In this study, we investigated whether TGF-β1, 4-1BB, interleukin 12 (IL-12), and IL-4 functionally interact for differentiation of CTLs from immunologically immature human cord blood (CB) CD8+ T cells. CD8+ T cells isolated from CB were used because they comprise an exclusively naive cell population and express little cytotoxic activity. This reflects their absence of detectable levels of CD28- effector and CD45RO memory cells.18 IL-15 is a critical cytokine for survival and differentiation of effector and memory CD8+ T cells.27-29 We previously demonstrated that naive CB CD8+ T cells could be induced to differentiate into highly cytotoxic CTLs by stimulation with 5-day IL-15 and IL-12 and subsequent 4-1BB stimulation.18
We report that TGF-β1 strongly suppresses CB CTL effector differentiation induced by IL-15, and that the suppressive effect of TGF-β1 can be blocked by 4-1BB costimulation. The effects of 4-1BB were at the level of TGF-β1 signaling. 4-1BB activity in reversing the suppressive effect of TGF-β1 was significantly amplified in the presence of IL-12 but abrogated by IL-4 even in the presence of IL-12.
Materials and methods
Reagents
Monoclonal antibody to 4-1BB (mouse immunoglobulin G1 [IgG1]) was purified with rec-Protein G-Sepharose 4B (Zymed, South San Francisco, CA) from ascites collected from Balb/C mice injected intraperitoneally with the hybridoma, 4B4.18 Isotype control IgG1 (MOPC 21) was purchased from Sigma (St Louis, MO). Purified anti-4-1BB was labeled with N-hydroxysuccinimide (NHS)-fluorescein (Pierce, Rockford, IL) according to the manufacturer's manual. Human IL-12 was purchased from R&D Systems (Minneapolis, MN). Anti-4-1BB-phycoerythrin (PE), granzyme B-PE, anti-interferon-γ (INF-γ)-fluorescein isothiocyanate (FITC), and anti-CD28 (CD28.2) were purchased from BD Biosciences (San Diego, CA). Anti-CD62L-FITC, anti-CD8-Tri-Color, isotype control mouse IgG1-FITC, and mouse IgG2a-Tri-Color were purchased from Caltag (Burlingame, CA). Human TGF-β1, IL-15, IL-4, IL-10, and IL-13 were purchased from PeproTech (Rocky Hill, NJ). Anti-human CD3 (OKT3) anti-CD30 (M44) were kind gifts from Ortho Biotech (Raritan, NJ) and Amgen (Seattle, WA), respectively.
Cells
Human umbilical CB was collected in 50-mL tubes containing 1 mL heparin sodium (1000 USP U/mL; American Pharmaceutical Partners, Schaumburg, IL) with proper consent from mothers in the Wishard Hospital, Indianapolis, IN. CB mixed with an equal volume of phosphate-buffered saline (PBS) was layered over Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden), and interface cells were collected. T cells were purified by negative selection using a combination of monoclonal antibodies specific for red blood cells, granulocytes, monocytes, and B lymphocytes and complement (Lympho-Kwik; One Lambda, Canoga Park, CA). The purity of T cells was greater than 90% as determined by flow analysis for the expression of CD3 and the αβ subunits of TCR using anti-CD3-FITC (BD Biosciences) and anti-αβ TCR-PE (BD Biosciences). Enriched T cells were incubated in complete RPMI-1640 medium containing 10% fetal bovine serum, 2 mg/mL l-glutamine, 50 μg/mL gentamicin (Sigma, St Louis MO), and 50 μM β-mercaptoethanol with IL-15 (10 ng/mL) for 5 days with greater than 95% viability as determined by trypan blue exclusion and further purified for CD8+ T cells by fluorescence-activated cell sorting (FACS) resulting in greater than 99% purity.
Cell activation
IL-15-stimulated CD8+ T cells were placed in 24-well plates (Costar, Corning, NY) precoated with anti-CD3 (1 μg/mL) and either anti-4-1BB, anti-CD28, anti-CD30, or isotype control IgG at 10 μg/mL or graded anti-4-1BB concentrations (0.1, 1.0, 0.5, and 10 μg/mL). Antibody precoating was accomplished by incubation of plates with antibodies in PBS at 4°C overnight. To determine effects of TGF-β1 and anti-4-1BB costimulation, IL-15-stimulated cells were activated in complete RPMI-1640 medium in 5% CO2 at 37°C with or without TGF-β1 (10 ng/mL) in combination with IL-12 (2 or 10 ng/mL) or IL-4 (10 ng/mL). IL-15 (10 ng/mL) was included in all the activation cultures. After 3 days, cells were harvested and analyzed for the induction of cytotoxic activity and intracellular levels of granzyme B and IFN-γ and for surface expression of 4-1BB.
Anti-CD3 redirected cytotoxicity assay
Cytotoxicity was determined in a standard 4-hour 51Cr release assay using an Epstein-Barr virus (EBV)-transformed B-cell line, kindly provided by Dr Karen Pollok (Indiana University, Indianapolis), as target cells. The EBV-transformed B-cell line was derived from human primary B cells by infection with EBV and subsequent incubation in a humidified 37°C, 5% CO2 incubator for 3 weeks to select immortalized cells. Cells were then maintained in complete RPMI-1640 medium. Target cells (2 × 106 cells) were incubated in 0.5 mL RPMI-1640 culture medium containing 250 μCi (9.25 MBq) 51Na2 CrO4. Target cells were washed 3 times with PBS and plated in 96-well round-bottom plates at 5000 cells per well. CD8+ T cells were mixed with target cells at designated effector-to-target cell ratios. The plates were briefly centrifuged at 200g and incubated at 37°C in the presence of anti-CD3 (1 μg/mL). After 4 hours, supernatants were counted in a gamma counter. Percentage of specific lysis was calculated by the following formula: % specific lysis = 100 × (experimental release - spontaneous release)/(maximum release - spontaneous release). Maximum release was determined by counting supernatants of wells containing target cells plus 2% Triton X-100, and minimum release was determined by counting supernatants of wells containing target cells plus medium.
Flow cytometry analysis
For multiple-color staining, 5 to 10 × 105 cells were incubated with anti-CD8-Tri-Color plus anti-CD62L-FITC and anti-4-1BB-PE in 50 μL staining buffer (PBS containing 1% bovine serum albumin) for 30 minutes at 4°C. The cells were washed 2 times with staining buffer and analyzed in a FACSCalibur cytofluorograph (Becton Dickinson, Mountain View, CA). Two-color staining was performed to detect 4-1BB, intracellular granzyme B, or IFN-γ in CD8+ T cells, using anti-4-1BB-FITC (in some cases, anti-4-1BB-PE) and anti-CD8-Tri-Color following T-cell activation. For detecting intracellular granzyme B or IFN-γ in response to anti-4-1BB costimulation, cells were stained with anti-CD8-Tri-Color and subsequently permeabilized for 15 minutes at room temperature with a cell permeabilization kit (FIX & PERM; Caltag Laboratories, Burlingame, CA) before staining with anti-granzyme B-PE or anti-IFN-γ-FITC. For detecting IFN-γ, Brefeldin A (GolgiPlug; BD Biosciences, San Diego, CA) was added at 10 μg/mL for 4 hours to block secretion of IFN-γ from cells before harvesting cells. Ten thousand events were acquired from the viable cells gated based on forward scatter (FSC) and side scatter (SSC) and analyzed for the expression of various marker proteins using relevant antibodies.
Western blot analysis
IL-15-stimulated CD8+ T cells were activated with plate-coated anti-CD3 (1 μg/mL) plus anti-CD28 (10 μg/mL) or anti-4-1BB (10 μg/mL) for 3 days. Cells were then treated with TGF-β1 for 0, 10, and 60 minutes and boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, and DNA was sheared with a 18-gauge needle. Protein concentrations were measured to normalize the loading samples. Induction of phospho-Smad2 in response to TGF-β1 was analyzed by Western blot using anti-phospho-Smad2 rabbit polyclonal antibody (Cell Signaling Technology, Beverly, MA) and horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (BioRad, Hercules, CA). For total Smad2, the blot was stripped and reprobed with anti-Smad 2 mouse IgG (BD Biosciences) and HRP-conjugated anti-mouse IgG (BioRad). Blots were scanned with densitometer, and band intensities were subtracted by background obtained from the cells not treated with TGF-β1. Phospho-Smad2 intensities induced by TGF-β1 were normalized by dividing by the respective total Smad2. For the relative phospho-Smad2 intensities, we defined phospho-Smad2 intensity of 10-minute TGF-β1-treated anti-CD28-costimulated cells as 1.
Statistical analysis
At least, 3 to 4 CB units were used for each experiment. For cytotoxicity assays, averaged percentages of specific lysis calculated from triplicate assay data were further processed to obtain the mean ± standard deviation (SD) from 4 independent experiments. Mean ± SD from multiple flow cytometry experiments were also calculated to assure reproducibility. P values were calculated by Student t test to determine the significance of anti-4-1BB and IL-4 effects on reversing TGF-β1 inhibition under various conditions.
Results
TGF-β1 inhibits CTL cytotoxic activity and 4-1BB costimulation reverses TGF-β-mediated suppression
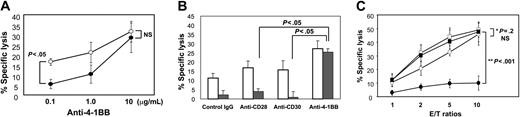
The immunosuppressive potential of TGF-β1 is an important promoter of malignant cell growth. This is partly caused by TGF-β1-induced interference with the generation of tumor-specific cytotoxic T lymphocytes.9,10 We examined whether TGF-β1 inhibited development of cytotoxic activity from naive CB CD8+ T cells using in vitro killing assays with an EBV-transformed B-cell line as target cells. CB T cells negatively selected by complement were incubated with IL-15 for 5 days, and FACS-purified CD8+ T cells were subsequently activated by plate-coated anti-CD3 plus increasing amounts of anti-4-1BB in the absence or presence of TGF-β1. The cells developed significant degrees of cytotoxic activity, but the presence of TGF-β1 during anti-CD3 activation markedly inhibited their cytotoxic activity (P < .05), and inhibition by TGF-β1 was effectively blocked by anti-4-1BB costimulation in a dose-dependent fashion (Figure 1A). To evaluate specificity of anti-4-1BB effects against TGF-β1, we compared effects of anti-4-1BB with other known costimulatory antibodies against CD28 (clone CD28.2) and CD30 (clone M44) at maximal concentrations of 10 μg/mL. CD30, a member of the TNF receptor superfamily, was included as a control because it is structurally related to 4-1BB. Agonistic antibodies for either CD28 or CD30 failed to reverse TGF-β1-mediated inhibition of cytotoxic activity (P < .05) (Figure 1B). We confirmed this distinct anti-4-1BB effect in comparison to anti-CD28 throughout different effector-to-target cell ratios. However, the anti-4-1BB effects appeared significant in the presence (P < .001) but not absence of TGF-β1. This suggests that 4-1BB is unique among the other costimulatory molecules tested for reversing the inhibitory effect of TGF-β1 on development of CTLs from naive CB CD8+ T cells.
Anti-4-1BB but not anti-CD28 or anti-CD30 costimulation reverses TGF-β1-mediated inhibition of cytotoxic activity. (A) T cells isolated from CB were stimulated with IL-15 for 5 days and further purified for CD8+ T cells by FACS. These cells were activated with plate-coated anti-CD3 (1 μg/mL) plus increasing concentrations of anti-4-1BB at 0.1, 1.0, and 10 μg/mL in the presence (filled circles) or absence (open circles) of TGF-β1 (10 ng/mL) for 3 days. Cells were analyzed for cytotoxic activity by a standard 4-hour 51Cr release assay using an EBV-transformed B-cell line as target cells. The effector-to-target cell ratio was 2:1. Percentage of specific lysis was calculated as described in “Materials and methods.” Means ± SD of percentage of specific lysis obtained from data of 4 separate experiments each performed in triplicate are plotted. P values between cells treated with and without TGF-β1 in the presence of 0.1 and 10 μg/mL anti-4-1BB are shown. NS indicates not significant. (B) Similarly, IL-15-stimulated CD8+ T cells were activated with plate-coated anti-CD3 (1 μg/mL) plus control IgG, anti-CD28, anti-CD30, or anti-4-1BB at 10 μg/mL in the presence (filled bars) or absence (open bars) of TGF-β1 (10 ng/mL) for 3 days. Cells were analyzed for cytotoxic activity as reflected by percentage of specific lysis. Mean ± SD obtained from data of 4 separate experiments each performed in triplicate are plotted, with P values calculated between mean percentage of specific lysis obtained with either anti-4-1BB and anti-CD28 or anti-CD30. (C) Cells were activated as in panel B with anti-CD3 (1 μg/mL) and anti-4-1BB (10 μg/mL; squares), or anti-CD28 (10 μg/mL; circles) in the presence (filled) or absence (open) of TGF-β1 (10 ng/mL) for 3 days at different effector-to-target cell ratios. Cells were analyzed for cytotoxic activity. Mean ± SD percentage of specific lysis from 4 independent experiments each performed in triplicate are plotted. P values between effects of anti-4-1BB and anti-CD28 in the presence (**P < .001) and absence of TGF-β1 (*P = .2, NS) are shown.
Anti-4-1BB but not anti-CD28 or anti-CD30 costimulation reverses TGF-β1-mediated inhibition of cytotoxic activity. (A) T cells isolated from CB were stimulated with IL-15 for 5 days and further purified for CD8+ T cells by FACS. These cells were activated with plate-coated anti-CD3 (1 μg/mL) plus increasing concentrations of anti-4-1BB at 0.1, 1.0, and 10 μg/mL in the presence (filled circles) or absence (open circles) of TGF-β1 (10 ng/mL) for 3 days. Cells were analyzed for cytotoxic activity by a standard 4-hour 51Cr release assay using an EBV-transformed B-cell line as target cells. The effector-to-target cell ratio was 2:1. Percentage of specific lysis was calculated as described in “Materials and methods.” Means ± SD of percentage of specific lysis obtained from data of 4 separate experiments each performed in triplicate are plotted. P values between cells treated with and without TGF-β1 in the presence of 0.1 and 10 μg/mL anti-4-1BB are shown. NS indicates not significant. (B) Similarly, IL-15-stimulated CD8+ T cells were activated with plate-coated anti-CD3 (1 μg/mL) plus control IgG, anti-CD28, anti-CD30, or anti-4-1BB at 10 μg/mL in the presence (filled bars) or absence (open bars) of TGF-β1 (10 ng/mL) for 3 days. Cells were analyzed for cytotoxic activity as reflected by percentage of specific lysis. Mean ± SD obtained from data of 4 separate experiments each performed in triplicate are plotted, with P values calculated between mean percentage of specific lysis obtained with either anti-4-1BB and anti-CD28 or anti-CD30. (C) Cells were activated as in panel B with anti-CD3 (1 μg/mL) and anti-4-1BB (10 μg/mL; squares), or anti-CD28 (10 μg/mL; circles) in the presence (filled) or absence (open) of TGF-β1 (10 ng/mL) for 3 days at different effector-to-target cell ratios. Cells were analyzed for cytotoxic activity. Mean ± SD percentage of specific lysis from 4 independent experiments each performed in triplicate are plotted. P values between effects of anti-4-1BB and anti-CD28 in the presence (**P < .001) and absence of TGF-β1 (*P = .2, NS) are shown.
4-1BB counteracts TGF-β1 effect on development of effector phenotypes possibly by inhibiting TGF-β1 signaling
Expression of granzyme B and IFN-γ is an essential element for CTL effector function. We determined whether development of effector phenotypes reflected altered cytotoxic activities by TGF-β1 and anti-4-1BB. IL-15-stimulated CB CD8+ T cells were subsequently activated with anti-CD3 plus anti-4-1BB or anti-CD28 for 3 days in the absence or presence of TGF-β1. A large portion of the cells expressed granzyme B following anti-CD3 activation regardless of whether costimulation was with anti-CD28 or anti-4-1BB, although anti-4-1BB costimulation enhanced levels of granzyme B+ cells compared with anti-CD28 (66.7% versus 88.2%; Figure 2A). Differences between the 2 costimulatory effects were dramatically apparent when the activation cultures included TGF-β1. TGF-β1 during anti-CD3 activation dramatically reduced granzyme B+ cells and IFN-γ+ cells in the presence of anti-CD28 (P < .05) (Figure 2A-B). In contrast, the suppressive effects of TGF-β1 were significantly blocked by anti-4-1BB. IFN-γ expression correlated with enlarged cell size as indicated by increased FSC. We noticed that consistent with low IFN-γ expression, cells treated with TGF-β1 had a relatively low FSC even after anti-CD3 activation. Anti-4-1BB increased the cell size of TGF-β1-treated cells (Figure 2B).
Anti-4-1BB costimulation restores TGF-β1-suppressed expression of granzyme B and IFN-γ. IL-15-stimulated CD8+ T cells were activated with plate-coated anti-CD3 (1 μg/mL) plus anti-CD28 (10 μg/mL) or anti-4-1BB (10 μg/mL) in the presence (filled bars) or absence (open bars) of TGF-β1 (10 ng/mL) for 3 days. Cells were analyzed for intracellular expression of granzyme B (A) and IFN-γ (B) by 2-color immunostaining. Granzyme B and IFN-γ levels were plotted against CD8 and FSC, respectively. Means ± SD of percentage of granzyme B+ and IFN-γ+ cells from 3 separate experiments are shown in the histograms. P values are shown between cells treated with and without TGF-β1 in the presence of anti-CD28 or anti-4-1BB. (C) IL-15-stimulated CD8+ T cells were costimulated with anti-CD28 or anti-4-1BB for 3 days as in panels A and B and then treated with TGF-β1 (10 ng/mL) for 0, 10, and 60 minutes. Phosphorylation of Smad2 induced by TGF-β1 was detected by Western blot using phospho-Smad2-specific antibody. Equivalent total amounts of Smad2 levels in each sample were demonstrated by reprobing the stripped same blot with the Smad2-specific antibody. One representative result of 2 experiments with similar results is shown. Relative phospho-Smad2 intensities are shown as relative numbers normalized to 0 and 1 for anti-CD28 costimulated cells treated with TGF-β1 for 0 and 10 minutes, respectively, as described in “Materials and methods.”
Anti-4-1BB costimulation restores TGF-β1-suppressed expression of granzyme B and IFN-γ. IL-15-stimulated CD8+ T cells were activated with plate-coated anti-CD3 (1 μg/mL) plus anti-CD28 (10 μg/mL) or anti-4-1BB (10 μg/mL) in the presence (filled bars) or absence (open bars) of TGF-β1 (10 ng/mL) for 3 days. Cells were analyzed for intracellular expression of granzyme B (A) and IFN-γ (B) by 2-color immunostaining. Granzyme B and IFN-γ levels were plotted against CD8 and FSC, respectively. Means ± SD of percentage of granzyme B+ and IFN-γ+ cells from 3 separate experiments are shown in the histograms. P values are shown between cells treated with and without TGF-β1 in the presence of anti-CD28 or anti-4-1BB. (C) IL-15-stimulated CD8+ T cells were costimulated with anti-CD28 or anti-4-1BB for 3 days as in panels A and B and then treated with TGF-β1 (10 ng/mL) for 0, 10, and 60 minutes. Phosphorylation of Smad2 induced by TGF-β1 was detected by Western blot using phospho-Smad2-specific antibody. Equivalent total amounts of Smad2 levels in each sample were demonstrated by reprobing the stripped same blot with the Smad2-specific antibody. One representative result of 2 experiments with similar results is shown. Relative phospho-Smad2 intensities are shown as relative numbers normalized to 0 and 1 for anti-CD28 costimulated cells treated with TGF-β1 for 0 and 10 minutes, respectively, as described in “Materials and methods.”
TGF-β receptor ligation results in phosphorylation of Smad2.10 To determine whether counteracting 4-1BB effects on TGF-β1 inhibition were at the level of TGF-β1 signaling, we assessed the degree of Smad activation by measuring phosphorylation of Smad2 in response to TGF-β1 for 10 and 60 minutes for the CD8+ T cells costimulated with anti-CD28 or anti-4-1BB. Western blot analysis results show that the cells costimulated with anti-4-1BB were significantly weaker in responding to TGF-β1 compared with those costimulated with anti-CD28 as evidenced by reduced phospho-Smad2 levels (P = .03) (Figure 2C). No noticeable changes in TGF-β type II receptor expression levels were detected on the cell surface after anti-4-1BB costimulation (data not shown). Thus, our results suggest that the blockade of inhibitory TGF-β1 responses by anti-4-1BB costimulation as seen in Figure 2A-B may be mediated at the level of TGF-β1 signaling.
TGF-β1 down-regulates expression of 4-1BB
4-1BB induction is highly dependent on anti-CD3 activation. Because TGF-β1 prevented cells from forming blasts, a typical morphology of activated cells (data not shown, but implicated from Figure 2B), in response to anti-CD3, we postulated that TGF-β1 might inhibit 4-1BB expression induced by anti-CD3. IL-15-stimulated CD8+ T cells were placed in the plates precoated with anti-CD3 (1 μg/mL) plus either control IgG, anti-CD28, or anti-4-1BB at 10 μg/mL and levels of 4-1BB expression on the surface of CD8+ T cells were determined by flow cytometry (Figure 3). A large portion of the cells expressed 4-1BB in response to anti-CD3, resulting in 64.4%, 74.2%, or 87.8% of the cells with 4-1BB expression when respectively costimulated with IgG, anti-CD28, or anti-4-1BB. Presence of TGF-β1 greatly reduced 4-1BB expression to 8.6% and 8.2% for the cells which were activated with anti-CD3 in the plates precoated with control IgG or anti-CD28 (P < .05). In contrast, no significant TGF-β1 effect was detected for the cells costimulated with anti-4-1BB (P = .4, NS). The TGF-β1-mediated inhibition of 4-1BB expression was almost completely restored by anti-4-1BB to 85.2%.
TGF-β1 inhibits 4-1BB induction and the TGF-β1 effect can be reversed by anti-4-1BB costimulation. IL-15-stimulated CD8+ T cells were activated with plate-coated anti-CD3 plus either control IgG, anti-CD28, or anti-4-1BB in the absence (upper panels) or presence (lower panels) of TGF-β1 for 3 days. Cells were analyzed for expression of 4-1BB on CD8+ T cells. (A) Means ± SD of the percentage of 4-1BB+ cell populations in CD8+ T cells from 4 experiments are shown in the histograms. (B) P values between cells treated with and without TGF-β1 in the presence of control IgG, anti-CD28, or anti-4-1BB are shown.
TGF-β1 inhibits 4-1BB induction and the TGF-β1 effect can be reversed by anti-4-1BB costimulation. IL-15-stimulated CD8+ T cells were activated with plate-coated anti-CD3 plus either control IgG, anti-CD28, or anti-4-1BB in the absence (upper panels) or presence (lower panels) of TGF-β1 for 3 days. Cells were analyzed for expression of 4-1BB on CD8+ T cells. (A) Means ± SD of the percentage of 4-1BB+ cell populations in CD8+ T cells from 4 experiments are shown in the histograms. (B) P values between cells treated with and without TGF-β1 in the presence of control IgG, anti-CD28, or anti-4-1BB are shown.
IL-12 enhances anti-4-1BB effects
IL-12 can induce cytotoxic effector cells,30-32 although the mechanism for this effect is not well defined. TGF-β1 inhibits IL-12 signaling.33 In light of these characteristics, we hypothesized that IL-12 might also counteract TGF-β1 suppression of 4-1BB expression. To this end, we evaluated anti-CD3-induced 4-1BB expression with or without IL-12 and TGF-β1 using IL-15-stimulated CB CD8+ T cells (Figure 4A). TGF-β1 greatly suppressed 4-1BB induction from 72.5% to 2.7% in the absence of IL-12. IL-12 partially restored 4-1BB expression inhibited by TGF-β1, increasing the percentage of 4-1BB+ cells from 2.7 to 41.6 (left lower panels of Figure 4A). This positive IL-12 effect on 4-1BB induction became more significant in the presence of TGF-β1 (P < .05) (right panels of Figure 4A). To test whether IL-12 enhanced anti-4-1BB effects, we measured the expression levels of 4-1BB in the presence of different suboptimal concentrations of anti-4-1BB with or without IL-12. The effect of suboptimal concentrations of anti-4-1BB on reversing TGF-β1 inhibition was considerably amplified by IL-12 (Figure 4B). For example, IL-12 allowed concentrations of anti-4-1BB as low as 1 μg/mL to reverse the 4-1BB expression inhibited by TGF-β1 to levels almost equivalent to those achieved by 10 μg/mL anti-4-1BB without IL-12.
IL-12 amplifies anti-4-1BB blocking effects on TGF-β1 inhibition of 4-1BB expression. (A) IL-15-stimulated CD8+ T cells were activated with plate-coated anti-CD3 (1 μg/mL) for 3 days in the absence or presence of IL-12 (2 ng/mL) or TGF-β1 (10 ng/mL). Cells were analyzed for the expression of 4-1BB+CD8+ T cells as shown by percentages in the dot plots. Means ± SD of the percentage of 4-1BB+CD8+ T cells from 4 experiments are shown in the histograms. P values between effects of absence (open bars) or presence (filled bars) of TGF-β1 in the presence and absence of IL-12 are shown. (B) IL-15-stimulated CD8+ T cells were activated as in panel A with different concentrations of anti-4-1BB in the presence or absence of IL-12 (2 ng/mL). All activation was undertaken with TGF-β1 (10 ng/mL). Percentages of 4-1BB+ cells produced with (filled squares) or without IL-12 (open squares) from 4 independent experiments, each performed in triplicate, were plotted as mean ± SD against the concentrations of anti-4-1BB.
IL-12 amplifies anti-4-1BB blocking effects on TGF-β1 inhibition of 4-1BB expression. (A) IL-15-stimulated CD8+ T cells were activated with plate-coated anti-CD3 (1 μg/mL) for 3 days in the absence or presence of IL-12 (2 ng/mL) or TGF-β1 (10 ng/mL). Cells were analyzed for the expression of 4-1BB+CD8+ T cells as shown by percentages in the dot plots. Means ± SD of the percentage of 4-1BB+CD8+ T cells from 4 experiments are shown in the histograms. P values between effects of absence (open bars) or presence (filled bars) of TGF-β1 in the presence and absence of IL-12 are shown. (B) IL-15-stimulated CD8+ T cells were activated as in panel A with different concentrations of anti-4-1BB in the presence or absence of IL-12 (2 ng/mL). All activation was undertaken with TGF-β1 (10 ng/mL). Percentages of 4-1BB+ cells produced with (filled squares) or without IL-12 (open squares) from 4 independent experiments, each performed in triplicate, were plotted as mean ± SD against the concentrations of anti-4-1BB.
IL-4 inhibits anti-4-1BB-induced granzyme B expression
IL-4 can antagonize IL-12 activity.34 Because IL-12 amplified anti-4-1BB responses, we evaluated the possibility that IL-4 might inhibit anti-4-1BB responses. To this end, we measured anti-4-1BB-induced granzyme B expression in response to IL-4 or IL-12 (Figure 5A). In the absence of TGF-β1 (left panel of Figure 5A), our culture conditions (5-day IL-15 stimulation followed by anti-CD3 activation) produced high levels of granzyme B expression, which ranged from 61% to 79% granzyme B+ cells in the presence of IL-4 or IL-12. In the presence of TGF-β1 (right panel of Figure 5A), cells maintained low granzyme B expression levels, which ranged from 6.4% to 14.2% with anti-CD28 costimulation (as shown by open bars). In contrast, cells costimulated with anti-4-1BB costimulation (as shown by filled bars) demonstrated restoration of granzyme B expression inhibited by TGF-β1 to levels seen with the cells not treated with TGF-β1. The reversal of anti-4-1BB effects against TGF-β1-mediated inhibition was noted for control (43.4% granzyme B+ cells) (P < .05) and IL-12-treated cells (59.2% granzyme B+ cells) (P < .05) but not for IL-4-treated cells (19.8% granzyme B+ cells) (P = .1, NS). These results indicate that anti-4-1BB reversed TGF-β1-mediated inhibition, but IL-4 blocked the anti-4-1BB effects. The inhibitory IL-4 effect was detected only when both anti-4-1BB and TGF-β1 were present. This suggests that IL-4 may interfere with 4-1BB effects on reversing the TGF-β1 inhibition.
IL-4 allows TGF-β1 to inhibit granzyme B expression by abrogating effect of anti-4-1BB. (A) IL-15-stimulated CD8+ T cells were activated with anti-CD3 and anti-CD28 (open bars) or anti-4-1BB (filled bars) for 3 days in the absence or presence of IL-4 (10 ng/mL) or IL-12 (2 ng/mL). All activation cultures were done with (right graph) or without (left graph) TGF-β1 (10 ng/mL). Cells were analyzed for intracellular levels of granzyme B. Mean ± SD of the percentage of granzyme B+ cells from 4 experiments are shown in the histograms. P values between effects of anti-CD28 and anti-4-1BB in the presence and absence of IL-4 or IL-12 were compared for the cells treated with or without TGF-β1. (B) IL-15-stimulated CD8+ T cells were activated with anti-CD3 and anti-4-1BB for 3 days as in panel A in the presence of 0, 2, 5, and 10 ng/mL IL-4 with or without 10 ng/mL IL-12. All the activations were undertaken with TGF-β1 (10 ng//mL). Cells were analyzed for the expression of granzyme B, and percentages of granzyme B+ cells were plotted against the concentrations of IL-4 and IL-12. Data are shown as mean ± SD of 4 independent experiments, each performed in triplicate. P values between cells treated with and without IL-4 in the presence (**) and absence (*) of IL-12 are shown.
IL-4 allows TGF-β1 to inhibit granzyme B expression by abrogating effect of anti-4-1BB. (A) IL-15-stimulated CD8+ T cells were activated with anti-CD3 and anti-CD28 (open bars) or anti-4-1BB (filled bars) for 3 days in the absence or presence of IL-4 (10 ng/mL) or IL-12 (2 ng/mL). All activation cultures were done with (right graph) or without (left graph) TGF-β1 (10 ng/mL). Cells were analyzed for intracellular levels of granzyme B. Mean ± SD of the percentage of granzyme B+ cells from 4 experiments are shown in the histograms. P values between effects of anti-CD28 and anti-4-1BB in the presence and absence of IL-4 or IL-12 were compared for the cells treated with or without TGF-β1. (B) IL-15-stimulated CD8+ T cells were activated with anti-CD3 and anti-4-1BB for 3 days as in panel A in the presence of 0, 2, 5, and 10 ng/mL IL-4 with or without 10 ng/mL IL-12. All the activations were undertaken with TGF-β1 (10 ng//mL). Cells were analyzed for the expression of granzyme B, and percentages of granzyme B+ cells were plotted against the concentrations of IL-4 and IL-12. Data are shown as mean ± SD of 4 independent experiments, each performed in triplicate. P values between cells treated with and without IL-4 in the presence (**) and absence (*) of IL-12 are shown.
As IL-4 and IL-12 showed contrasting effects in granzyme B expression, we investigated whether one was more dominant than the other. To this end, we compared the effects of a combination of IL-4 and IL-12 with that of individual cytokines on granzyme expression for the CD8+ T cells costimulated with anti-4-1BB in the presence of TGF-β1 (Figure 5B). As seen by inhibitory IL-4 effects without IL-12 (*P < .05), IL-4 significantly reduced granzyme B expression induced by anti-4-1BB in a dose-dependent manner even in the presence of a maximal concentration of IL-12 (10 ng/mL) (**P < .05), indicating dominant IL-4 activity over IL-12.
IL-4 inhibits CTL activity induced by anti-4-1BB
Since IL-4 inhibited expression of granzyme B induced by anti-4-1BB, we assessed whether IL-4 could inhibit the cytotoxic activity induced by anti-4-1BB (Figure 6A-B). We used cells maximally costimulated with anti-4-1BB (10 μg/mL) to detect a possible negative IL-4 effect on cytotoxic activities. IL-15-stimulated cells were activated with anti-CD3 under 6 different conditions. TGF-β1-mediated inhibition of cytotoxic activities was not apparent because cells were fully costimulated with anti-4-1BB (Figure 6A). However, cells treated with IL-4 manifested exceptionally low levels of cytotoxic activity in the presence of TGF-β1 and anti-4-1BB (P < .01). IL-12 showed nonsignificant effects on cytotoxic activity, probably because anti-4-1BB costimulation was at a maximal level. Consistent with the IL-4 effect on inhibiting granzyme B expression as shown in Figure 5, IL-4 down-regulated cytotoxic activity. Although IL-12 enhanced cytotoxic activity, the inhibitory IL-4 effect was not overcome by IL-12 even at a maximal concentration (10 ng/mL) (*P < .01) (Figure 6B).
Inhibitory IL-4 effect on cytotoxic activity in the presence of TGF-β1 results from inhibition of 4-1BB expression. (A) IL-15-stimulated CD8+ T cells were activated with plate-coated anti-CD3 (1 μg/mL) plus anti-4-1BB (10 μg/mL) for 3 days in the absence or presence of IL-4 (10 ng/mL) or IL-12 (2 ng/mL). The activation was undertaken with (filled bars) or without (open bars) TGF-β1 (10 ng/mL). Cells were analyzed for cytotoxic activity to an EBV-transformed B-cell line at a 2:1 effector-to-target cell ratio using a standard 51Cr release assay. Cells activated with TGF-β1 in the absence of anti-4-1BB costimulation demonstrated low (below 5%) specific lysis. Mean ± SD of the percentage of specific lysis from 4 separate experiments each performed in triplicate are plotted. (B) Similarly, cells activated in the presence and absence of IL-4 (10 ng/mL) with or without IL-12 (10 ng/mL each) were assayed for cytotoxic activity as in panel A at different effector-to-target cell ratios. The activation was undertaken with TGF-β1 (10 ng/mL). Mean ± SD of the percentage of specific lysis from 4 separate experiments each performed in triplicate are plotted against E/T ratios. P values for the inhibitory IL-4 effects in the presence (*P < .01) and absence of IL-12 (**P < .01) are shown. (Ci) IL-15-stimulated T cells were activated with plate-coated anti-CD3 (1 μg/mL) plus anti-4-1BB (10 μg/mL) for 3 days in the absence or presence of IL-4. The activation was undertaken with TGF-β1 (10 ng/mL). Cells prior to and after anti-CD3 activation were analyzed for the expression of 4-1BB and CD62L on CD8+ T cells as shown by percentages in the dot plots. Mean ± SD of the percentage of CD62L+4-1BB- (□), CD62L+4-1BB+ (▨), and CD62L-4-1BB+ (▪) cell types from 4 experiments before (Cii) and after (Ciii) activation with or without IL-4 are plotted in the histograms. P values for IL-4 effects on production of CD62L+4-1BB- and CD62L-4-1BB+ cell types are shown.
Inhibitory IL-4 effect on cytotoxic activity in the presence of TGF-β1 results from inhibition of 4-1BB expression. (A) IL-15-stimulated CD8+ T cells were activated with plate-coated anti-CD3 (1 μg/mL) plus anti-4-1BB (10 μg/mL) for 3 days in the absence or presence of IL-4 (10 ng/mL) or IL-12 (2 ng/mL). The activation was undertaken with (filled bars) or without (open bars) TGF-β1 (10 ng/mL). Cells were analyzed for cytotoxic activity to an EBV-transformed B-cell line at a 2:1 effector-to-target cell ratio using a standard 51Cr release assay. Cells activated with TGF-β1 in the absence of anti-4-1BB costimulation demonstrated low (below 5%) specific lysis. Mean ± SD of the percentage of specific lysis from 4 separate experiments each performed in triplicate are plotted. (B) Similarly, cells activated in the presence and absence of IL-4 (10 ng/mL) with or without IL-12 (10 ng/mL each) were assayed for cytotoxic activity as in panel A at different effector-to-target cell ratios. The activation was undertaken with TGF-β1 (10 ng/mL). Mean ± SD of the percentage of specific lysis from 4 separate experiments each performed in triplicate are plotted against E/T ratios. P values for the inhibitory IL-4 effects in the presence (*P < .01) and absence of IL-12 (**P < .01) are shown. (Ci) IL-15-stimulated T cells were activated with plate-coated anti-CD3 (1 μg/mL) plus anti-4-1BB (10 μg/mL) for 3 days in the absence or presence of IL-4. The activation was undertaken with TGF-β1 (10 ng/mL). Cells prior to and after anti-CD3 activation were analyzed for the expression of 4-1BB and CD62L on CD8+ T cells as shown by percentages in the dot plots. Mean ± SD of the percentage of CD62L+4-1BB- (□), CD62L+4-1BB+ (▨), and CD62L-4-1BB+ (▪) cell types from 4 experiments before (Cii) and after (Ciii) activation with or without IL-4 are plotted in the histograms. P values for IL-4 effects on production of CD62L+4-1BB- and CD62L-4-1BB+ cell types are shown.
To delineate the mechanism by which IL-4 prevented the blocking effect of anti-4-1BB against TGF-β1, we postulated that IL-4 might prevent induction of 4-1BB expression on CD8+ T cells. We compared 4-1BB expression levels of cells treated with or without IL-4 during anti-CD3 activation. Prior to anti-CD3 activation, most cells did not express 4-1BB and were positive for CD62L, a marker for naive cells (left panel of Figure 6Ci and 6Cii). To detect an IL-4 effect as seen in Figure 6A, we used cells that were activated with anti-CD3 in the presence of both anti-4-BB and TGF-β1. CD8+ T cells treated with IL-4 expressed significantly lower levels of 4-1BB as compared with cells not treated with IL-4 (35.9% versus 75.9%, P < .05) (right panels in Figure 6Ci and 6Ciii). In contrast to the cells not treated with IL-4, the majority of cells treated with IL-4 remained as a CD62L+4-1BB- naive phenotype (P < .05) (Figure 6Ciii). These results suggest that the inhibitory IL-4 effects on granzyme B expression, cytotoxic activity, and effector differentiation may result from inhibition of 4-1BB expression.
Discussion
TGF-β1 is a potent immune suppressor cytokine, driving immune tolerance in peripheral tissues through inhibitory effects on induction of inflammatory responses.9,35,36 TGF-β is secreted by various tumors.11 As a result, tumor-infiltrating CD8+ T cells fail to differentiate into effector or effector memory cells, leading to premature cell death.35 This is consistent with markedly reduced expression of perforin and granzyme B among tumor-infiltrating CD8+ T cells.3 TGF-β is also found in the fetoplacental unit at high levels.37 Anti-inflammatory effects of TGF-β1 as well as IL-4 may play important roles in accommodating the maternal immune response against paternal alloantigens of the fetus during pregnancy and preventing potentially harmful effects of proinflammatory mediators in the fetoplacental unit.37-39 Thus, functionally impaired CD8+ T cells in CB may be due to preexposure of these cells to such anti-inflammatory cytokines. CB-derived monocytes and dendritic cells, in comparison with adult peripheral blood (PB)-derived cells, are defective in producing IL-12 and IL-15 at an mRNA and protein level on lipopolysaccharide (LPS) stimulation.40-42 Conditions at tumor sites may be analogous to those present in CB that contains CD8+ T cells defective in cytotoxic activity.18,43 It is conceivable that the mechanisms by which tumors evade the host immune systems may have been adopted from natural evolution for fetal-maternal immune tolerance. In light of these possibilities, we believed that study of the regulation of CB CTL differentiation might provide insight into defective effector differentiation of tumor-infiltrating CD8+ T cells.
We have previously demonstrated that, unlike adult peripheral CD8+ T cells, IL-15 prestimulation appeared to be important for CB immature CD8+ T-cell response to anti-CD3 and induced cytotoxic activity as well as granzyme B expression.18 In this study, using immature noncytotoxic CD8+ T cells, we demonstrated that TGF-β1 acts as a powerful suppressor for CTL differentiation, and 4-1BB costimulation reverses the inhibitory activity of TGF-β1 during CTL differentiation induced by anti-CD3 activation following IL-15 stimulation. Other costimulatory molecules, anti-CD28 and anti-CD30,44 did not mimic the effect of anti-4-1BB, suggesting that 4-1BB may be a unique costimulatory molecule with the ability to block the TGF-β1-mediated inhibition of CTL effector differentiation. The counteracting 4-1BB effects may be at the level of TGF-β1 signaling.
4-1BB is barely, if at all, detected on CD8+ T cells freshly isolated from CB as well as from adult PB.18 4-1BB can be preferentially induced on CD28- subpopulations of CB CD8+ T cells during long-term in vitro IL-15 stimulation.18 Enhanced 4-1BB expression may be critical, especially in the presence of TGF-β1, which might otherwise down-regulate 4-1BB expression below threshold levels. 4-1BB signals mediate a positive feedback loop for further increased 4-1BB expression, which may better protect the cells from the suppressive effects of TGF-β1. The CD28- subpopulation, which is less able to undergo extensive expansion and which is low in mitogenic potential,15,45 may be the most vulnerable to TGF-β1 suppression. 4-1BB is a critical costimulatory molecule contributing to survival of the CD28- subpopulation and development of effector memory phenotypes, mainly by preventing the suppressive effects of TGF-β1. Consistent with these results, 4-1BB ligand-deficient mice develop markedly fewer antigen-reactive effector as well as memory CD8+ T cells, mainly due to apoptosis.25,46 4-1BB is likely involved in the selection process from CD28- effector CTLs susceptible to death to the generation of more functionally active CD28+ effector memory cells.18,19
IL-15 is important for survival of the effector and memory cells as well.28 However, experiments in IL-15-deficient mice indicate that IL-15 is not required for the generation of tumor-specific immune responses.47 IL-15 did not prevent inhibitory TGF-β1 activity (data not shown). Our data reflect the notion reported by others27,47 that IL-15 may not be essential for the generation of effector or memory CD8 T cells, although it is required for homeostatic proliferation to maintain memory populations in vivo. IL-15 does not appear to be able to substitute for 4-1BB costimulation to resist the inhibitory effect of TGF-β1 on CTL differentiation.
Like 4-1BB, 4-1BB ligand is not constitutively expressed by resting or immature DCs but is inducible over several days of activation by molecular triggers for DC activation.1 The factors that govern 4-1BB ligand expression by DCs may support the induction of powerful CTL responses perhaps by promoting long-term CTL survival.19,20 These include ligands for Toll-like receptor (TLR) such as LPS (TLR4 ligand) or CD40.47 Expression of 4-1BB ligand approximately coincides with the peak expression level of 4-1BB on T cells.1 Therefore, timely interaction of DC-expressed 4-1BB ligand with its receptor 4-1BB on CTL may be important for full expansion of effector CTLs and maintenance of persistent antitumor responses, especially at a point when TGF-β1 is present.
IL-12 has long been known to promote antitumor CTL responses.30-32 Our data indicated that IL-12 promoted 4-1BB expression on the surface of CD8+ T cells inhibited by TGF-β1 (Figure 4A). Furthermore, IL-12 amplified anti-4-1BB effects for reversing TGF-β1 inhibition, thereby further increasing 4-1BB expression (Figure 4B). These results are in agreement with the synergism between IL-12 and 4-1BB in antitumor T-cell immune responses.30,48,49 TLR signals are potent stimulators of both IL-12 and 4-1BB ligand expression by DCs.23,50 We previously observed that IL-12 production from myeloid DCs was enhanced by 4-1BB ligand stimulation.51 Therefore, danger signals through TLR may be important at the time of 4-1BB costimulation to protect effector or effector memory CD8+ T cells from TGF-β1, as observed with OX40 costimulation for memory CD4+ T cells.52 These are consistent with enhanced development of memory CD8+ T cells in vivo by products of infectious agents such as LPS that trigger TLR on DCs.53 CD8+ T-cell differentiation into memory cells in humans appears to be complex, presenting heterogeneous memory subsets which include central and effector memory cells, based on functional characteristics.45,54 The CTLs we generated by anti-4-1BB costimulation may include an effector memory population.
IL-4 prevented anti-4-1BB from blocking TGF-β1-mediated inhibition of cytotoxic activity. IL-4-mediated inhibition was not detected without TGF-β1, suggesting that the inhibitory IL-4 effect was mainly derived from the failure of anti-4-1BB to prevent TGF-β1-mediated inhibition. These results are in agreement with our data that demonstrated a marked IL-4-mediated inhibition of granzyme B expression only in the presence of both anti-4-1BB and TGF-β1 (as shown in Figure 5A). Thus, in contrast to IL-12, IL-4 down-regulates 4-1BB costimulation, thereby preventing 4-1BB blockade of TGF-β1-mediated inhibition of CTL effector differentiation. Furthermore, IL-4 has a dominant effect over IL-12, thereby preventing 4-1BB costimulation from reversing TGF-β1 inhibition of cytotoxic activity even in the presence of IL-12.
IL-4, a type 2 cytokine, down-regulates CTL functions with low expression of granzyme B.55-57 Long-term coculture of tumor targets and tumor-specific type CTLs indicated that type 1 CTL (Tc1) cells proliferated and killed tumor cells, whereas IL-4-treated Tc1 cells failed to proliferate and hence were unable to curtail the proliferation of tumor cells.58 These results suggest that IL-4 in vivo would compromise immunity by reducing the long-term functional capability of CTLs. IL-4 overrides IL-12 effects when both cytokines are present during 4-1BB costimulation in the presence of TGF-β1. Such an IL-4-mediated immune deviation might be due in part to down-regulation of 4-1BB expression. In this context, T helper 1 and T helper 2 (Th1/Th2) polarization may play a pivotal role for shifting the subtle balance between TGF-β1 and 4-1BB dominancy in functional activation of effector CTLs. We did not detect the relevant IL-4 effects from other type 2 cytokines, such as IL-10 and IL-13, suggesting that IL-4 is a unique cytokine, among the type 2 cytokines tested, with this inhibitory effect on 4-1BB expression.
TCR signaling is a major driving factor for CTL effector and memory differentiation programs.59,60 TGF-β1 may interfere with TCR signaling.61 This notion is consistent with observations that T cells from TGF-β1-deficient mice display increased expression of the activation markers lymphocyte function-associated antigen-1 (LFA-1), CD69, and CD122.62 TGF-β1-mediated inhibition of 4-1BB expression may be primarily due to TGF-β1-mediated inhibition of TCR activation. This TGF-β1 effect is opposite that of costimulatory molecules, which lower the TCR activation threshold. Thus, TGF-β1 may act as a “counter-costimulatory” factor reducing TCR signaling strength. In contrast to TGF-β1, cells that receive 4-1BB costimulation may differentiate into effector or effector memory cells and survive for long periods by establishing high TCR signaling strength even in the presence of TGF-β1. Thus, we favor the idea that the magnitude of CTL effector responses may reflect the outcome of a competition between TGF-β1 and 4-1BB signals. Control of this balance may be essential for generating antitumor CTL immune responses.
Most human tumors do not regress and often continue to grow in spite of the presence of tumor-specific CTLs.7 A major goal of therapeutic antitumor vaccination is to strongly activate antigen-specific effector memory CD8+ T cells such that they kill malignant cells.23 For this, naive T-cell precursors have to be activated, undergo clonal expansion, and differentiate to effector cells requiring a series of cascade activation steps. TGF-β produced by tumors significantly reduces the potency of DC/tumor fusion vaccines.11 TGF-β1 influences IL-12 responsiveness by inhibiting early signaling events essential to gene induction in IL-12-activated T cells.33 TGF-β also drives the shift in the Th1/Th2 balance toward Th2.63 Boosting T-cell-mediated immune response to tumor antigens, through vaccines, may be achieved by antagonizing anti-inflammatory cytokines such as TGF-β1 and IL-4.64 Knowledge of the mechanisms by which TGF-β1 interferes with the development of an antitumor response and strategies to counteract these immunosuppressive activities by 4-1BB and IL-12 may be of relevance to improving cancer immunotherapeutic approaches.
Prepublished online as Blood First Edition Paper, September 7, 2004; DOI 10.1182/blood-2003-12-4343.
Supported by US Public Health Service grants (RO1 HL56416, RO1 HL67384, and RO1 DK53674) (H.E.B.) from the National Institute of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to Ortho Biotech and Amgen for providing anti-CD3 and anti-CD30, respectively.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal