Abstract
Fibrinogen binding by integrin αIIbβ3 is promoted by platelet agonists that increase the affinity and avidity of αIIbβ3 for fibrinogen through a process called “inside-out” signaling. Having previously demonstrated that inside-out activation of αIIbβ3 is defective in murine megakaryocytes that lack the transcription factor NF-E2, we screened for NF-E2–regulated genes that affect αIIbβ3 activation. Caspase-12 is the most down-regulated gene we identified in NF-E2–/– megakaryocytes. Therefore, the role of this protein in αIIbβ3 activation was determined using platelets from caspase-12–/– mice. Despite wild-type levels of αIIbβ3, caspase-12–/– platelets exhibit reduced fibrinogen binding to αIIbβ3 following stimulation by adenosine diphosphate (ADP) or protease-activated receptor 4 (PAR4) receptor-activating peptide. The defect in αIIbβ3 activation is associated with decreased cytosolic free calcium and inositol triphosphate levels, and with reduced aggregation, despite wild-type phospholipase Cβ expression levels. In contrast, agonist-induced surface expression of P-selectin, suppression of cAMP levels following ADP stimulation, and spreading on immobilized fibrinogen are unimpaired. Moreover, although caspase-12 is highly expressed in mature megakaryocytes, it is undetectable in platelets. Taken together, these studies establish that caspase-12 expression in murine megakaryocytes is regulated, directly or indirectly, by NF-E2, and suggest that caspase-12 participates in the development of fully functional signaling pathways linking some G-protein–coupled receptors to αIIbβ3 activation.
Introduction
Platelets are anucleate blood cells produced by megakaryocytes. They play a critical role in hemostasis by first binding at the site of vascular injury and then aggregating to form a platelet plug (for review see Ruggeri1 ). Their hemostatic function is intimately linked to the interaction between adhesive ligands present at the site of vascular injury and their cognate receptors on the platelet surface. The primary platelet fibrinogen receptor, integrin αIIbβ3, does not effectively bind fibrinogen until the platelet is stimulated by an agonist such as thrombin, adenosine diphosphate (ADP), or collagen.2,3 Agonist stimulation triggers an intracellular process called “inside-out” signaling that increases the affinity and avidity of αIIbβ3 for fibrinogen or von Willebrand factor, a response necessary for platelet aggregation.2
Platelet studies have demonstrated that agonist stimulation typically leads to elevations in intracellular calcium levels, activation of protein kinase C and phosphatidylinositol 3-kinase, and involves cytoskeletal proteins such as talin.2,4,5 Mice genetically deficient in specific agonist receptors6-13 or heterotrimeric G-proteins14-19 have furthered our understanding of αIIbβ3 signaling, as has the use of cell lines with heterologously expressed αIIbβ3.18,19 Furthermore, recent reports have begun to provide details of the structural changes that occur when β3 goes from a resting to an active state.2,20-22 Nonetheless, inside-out signaling remains an incompletely understood process, and the identification of additional molecular regulators or participants in this pathway could provide novel targets for new classes of antiplatelet agents.
Platelets and cell lines engineered to express αIIbβ3 have proven invaluable in gaining our current understanding of inside-out signaling, but each of these systems has its limitations. For example, the lack of a nucleus precludes in vitro genetic manipulations of platelets. Moreover, the cell lines most commonly used are immortalized and not of megakaryocytic, or even hematopoietic, origin. To overcome these limitations, we established methods for studying αIIbβ3 signaling in primary murine bone marrow–derived megakaryocytes, the immediate precursor of circulating platelets.23
We previously reported that megakaryocytes from mice lacking the hematopoietic-restricted transcription factor NF-E2 (nuclear factor–erythroid2) fail to undergo agonist-induced inside-out activation of αIIbβ3 despite normal levels of αIIbβ3 on the cell surface.23 Reasoning that one or more NF-E2–regulated genes may therefore be involved in αIIbβ3 activation, we performed expression array analysis using RNA from NF-E2+/+ and NF-E2–/– megakaryocytes to identify such gene products. One such identified gene product, the Rap1 guanine nucleotide exchange factor CalDAG-GEF1, enhances agonist-induced activation of αIIbβ3.24 Another gene, caspase-12, is the most down-regulated gene we identified in the NF-E2–/– megakaryocytes. Herein we present the characterization of a defect in αIIbβ3 activation in caspase-12–/– platelets.
Materials and methods
Reagents and animals
All chemicals were from Sigma Aldrich (St Louis, MO) unless otherwise noted. Iscove modified Dulbecco medium (IMDM) was from Gibco Life Technologies (Carlsbad, CA), murine interleukin 6 (IL-6) and IL-11 were from PeproTech (Rocky Hill, NJ), and recombinant human thrombopoietin (TPO) was a generous gift from Genentech (South San Francisco, CA). The NF-E2–/– mice25 and the caspase-12–/– mice26 were previously described. All animals were housed and handled in accordance with institutional guidelines.
Megakaryocyte isolation
Bone marrow was harvested from 6- to 8-week-old NF-E2+/+ and NF-E2–/– mice as described previously.23 Briefly, both tibias and fibulas were flushed with CATCH buffer (calcium- and magnesium-free phosphate-buffered saline [PBS-CMF] containing 2% bovine serum albumin [BSA] and 0.38% trisodium citrate). Following gentle shear force to generate a single-cell suspension, low-density marrow cells were separated by layering the cell suspension over Isoprep (specific gravity 1.077; Robbins Scientific, Sunnyvale, CA) followed by room temperature centrifugation at 405g for 30 minutes. Low-density cells removed from the interface were cultured for 5 to 6 days at a starting concentration of 1 × 106 cells/mL in IMDM supplemented with 0.5% BSA, 4 μg/mL low-density lipoprotein (LDL), 200 μg/mL saturated human transferrin, 10 μg/mL human insulin, 50 μM β-mercaptoethanol, 20 μM each of nucleotide triphosphate (NTP) and deoxynucleotide triphosphate (dNTP), 1× l-glutamine (Gibco), 1× pen/strep (Gibco), TPO (50 ng/mL), IL-6 (10 ng/mL), and IL-11 (10 ng/mL). Mature, polyploid megakaryocytes were enriched by gravity sedimentation for 60 minutes at 37° C in BSA-coated (1%) 50-mL conical tubes. The settled cells were then applied to a 2-step (1.5%/3.0%) BSA gradient in PBS-CMF. After 30 minutes at room temperature, pelleted cells were resuspended in 5 mL PBS-CMF and applied to a second identical 2-step BSA gradient and allowed to settle for 30 minutes. Sedimented cells were resuspended, and cytospins of 400 to 500 cells were Wright-Giemsa stained to determine cell morphology and purity. Preparations with greater then 95% purity were used for protein and RNA isolation.
Platelet isolation
Whole blood was collected from the inferior vena cava of ketamine-xylazine–anesthetized mice using a 23-gauge needle and a 1-cc syringe that contained 150 μLACD (75 mM trisodium citrate, 124 mM dextrose, and 38 mM citric acid monohydrate). The anticoagulated blood was transferred to a 1.5-mL eppendorf tube, 500 μL PIPES buffer (0.15 mM NaCl, 20 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] pH 6.5) was gently added, and the sample was centrifuged at 83g for 10 minutes in a Sorvall RC5 (Kendro, Newton, CT) to generate platelet-rich plasma (PRP). To generate washed platelets, the PRP was transferred to a 15-mL conical tube, apyrase (grade IV; 1 U/mL final) was added, the platelets were centrifuged at 630g for 10 minutes, the supernatant was removed, and the platelets were gently resuspended in calcium-free HEPES-Tyrode buffer (137 mM NaCl, 2.9 mM KCl, 12 mM NaHCO3, 0.1 mM MgCl2, 0.1% BSA, 0.1% glucose, 5 mM HEPES, pH 7.4).
Soluble fibrinogen binding assay
Human fibrinogen (60 μL of 30 mg/mL; Enzyme Research Labs, Lafayette, IN) was mixed with 100 μL of 1 M NaHCO3 and 150μL fluorescein isothiocyanate (FITC)–cellite (28 mg/mL in dH20; Calbiochem, San Diego, CA), rotated for 90 minutes at room temperature, and FITC-fibrinogen was isolated by separation over a PD-10 column (Amersham, Piscataway, NJ). Agonist-induced soluble fibrinogen binding was assayed in 50 μL HEPES-Tyrode buffer (0.1 mM Ca++) containing 5 × 105 platelets and 200 μg/mL FITC-fibrinogen. Following agonist addition, the platelets were incubated undisturbed for 30 minutes at room temperature in the dark. Samples were then diluted with 0.5 mL HEPES-Tyrode buffer and analyzed on a FACScan (Becton Dickinson). Soluble fibrinogen binding was measured in the FL1 channel.
Platelet aggregation
Platelet aggregation was monitored by measuring light transmission using a lumi-aggregometer (Chronolog, Havertown, PA). All reactions were performed in a 300 μL volume in siliconized glass vials at 37° C with constant stirring (1000 rpm). Washed platelets at 2 × 108/mL in calcium-free HEPES-Tyrode buffer were added to the siliconized glass vial. CaCl2 (1.8 mM) and fibrinogen (1 mg/mL) were added 30 seconds prior to the addition of 3 μL agonist or carrier control. Changes in light transmission were recorded against a blank of HEPES-Tyrode buffer (100% platelet aggregation). Concentrations for PAR4 receptor activating peptide (AYPGFK; Anaspec, San Jose, CA), ADP, U46619 (Cayman Chemicals, Ann Arbor, MI), type I collagen (Chronolog), convulxin (Pentapham, Norwalk, CT), thrombin, and A23187 varied and are indicated in the text and figure legends. The pan-caspase inhibitor Z-VAD (Biomol, Plymouth, CA) was incubated with washed platelets for 30 minutes at room temperature prior to addition of agonist.
P-selectin (CD62-P) surface expression
Washed platelets (5 × 105) in 100 μL HEPES-Tyrode buffer were activated by incubation with PAR4 receptor activating peptide (AYPGFK) for 30 minutes in the dark at room temperature. Fifteen minutes into the incubation, FITC-conjugated anti–CD62-P antibody (Pharmingen, Palo Alto, CA) was added. The amount of surface CD62-P expression was determined by flow cytometry. Resting CD62-P expression levels were measured in the same manner, using carrier only as the platelet agonist.
Cyclic AMP generation
Washed platelets (1 × 107) in 50 μL HEPES-Tyrode buffer were assayed in the presence of 1.8 mM CaCl in a siliconized glass tube (Chronolog). After incubating with 1 μM prostaglandin E1 (PGE1) for 10 minutes, ADP (20-0.2 μM) was added to the stirred platelet suspension, which was incubated at room temperature for an additional 5 minutes. Platelets were lysed with 5 volumes of 0.1 M HCl for 10 minutes at room temperature, centrifuged for 10 minutes at 600g, and the supernatant containing the free cyclic AMP (cAMP) was removed. The cAMP generated was determined using an immunoassay per the manufacturer's recommendations (R&D Systems, Minneapolis, MN).
Western blotting
Washed platelets or washed BSA-purified megakaryocytes were lysed using 2× RIPA buffer or 2× sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing a 1× protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitors (sodium fluoride, sodium orthovanadate, and calyculin A). Cleared lysates were separated by SDS-PAGE, transferred to nitrocellulose, blocked with BSA or milk in tris-buffered saline Tween (TBST; 0.5%) for 60 minutes and incubated with primary antibody against caspase-12 (rat monoclonal; generous gift of Junying Yuan, Harvard Medical School, and Sigma-Aldrich), Gαq (Calbiochem), phospholipase Cγ2 (PLCγ2), PLCβ1, PLCβ2, PLCβ3, ERK2 (Santa Cruz Biotechnology, Santa Cruz, CA), phospho-PLCβ3(ser1105) or phospho-PLCβ3(ser537) (Cell Signaling Technology, Beverly MA). Protein bands were detected using species-specific, horseradish peroxidase–conjugated secondary antibody (Amersham, Piscataway, NJ) and chemiluminescence (Pierce, Rockford, IL). The von Willebrand factor–positive (VWF+), CD41+ early megakaryocyte lineage cells were generated as described.27
Calcium mobilization
Free intraplatelet calcium levels were determined using FURA-2AM–loaded platelets. In brief, 1 U/mL apyrase and 4 μg/mL FURA-2AM (Molecular Probes, Eugene, OR) were added to 2 × 108 platelets in 1 mL calcium-free HEPES-Tyrode buffer and incubated for 45 minutes at 37° C. Platelets were then washed once (630g for 10 minutes) and resuspended in calcium-free HEPES-Tyrode buffer. CaCl2 (1.8 mM final) was added immediately prior to stimulation with agonist in a stirred cuvette in a dark chamber of the fluorimeter. Cytoplasmic free calcium levels were determined by monitoring the fluorescence intensity emitted at 510 nm following excitation at 340 nm and 380 nm using a Luminometer (Photon Technology International, London, ON, Canada). The data are presented as the 340:380 fluorescence ratio.
Inositol 1,4,5 triphosphate (IP3) measurement
Washed platelets (100 μL containing 2 × 108) were stimulated with thrombin (3 U/mL) in the presence of 1.8 mM CaCl2. After 3 seconds and 6 seconds the reaction was terminated by the addition of 20 μL ice-cold 20% perchloric acid. Inositol 1,4,5 triphosphate (IP3) measurement was performed using a radioimmuno assay according to the manufacturer's (Amersham) recommendations.
Differential interference contrast microscopy
Glass coverslips were coated with 0.1% poly-l-lysine overnight at room temperature, washed with PBS, and then coated with either 1% BSA or 20 μg/mL fibrinogen (Enzyme Research Labs) for 2 hours at 37° C in a humidity chamber and then blocked with 1% BSA. Washed wild-type and caspase-12–/– platelets (200 μL containing 5 × 107/mL in calcium-free HEPES-Tyrode buffer) were activated with PAR4 receptor-activating peptide (250 μM final) and layered onto the slides for 45 minutes at 37° Cin a humidity chamber. The slides were mounted and immediately viewed using differential interference contrast microscopy (Zeiss laser scanning confocal microscope with LSM META S10 software; 63×/1.4 objective).
Statistics
Statistics were performed using InStat Statistical (GraphPad Software, San Diego, CA) and Excel (Microsoft, Seattle, WA) software. Data shown are the means plus or minus standard error of the mean (SEM) and comparisons between mean values were performed using the Student paired t test.
Results
Megakaryocyte caspase-12 expression is markedly reduced in NF-E2–/– mice
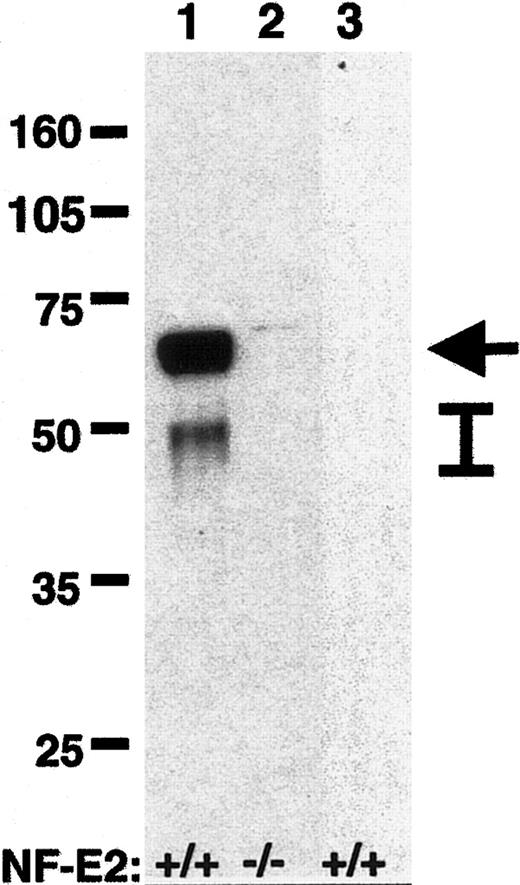
We previously demonstrated that megakaryocytes lacking the hematopoietic transcription factor NF-E2 have markedly decreased agonist-induced fibrinogen binding despite wild-type levels of integrin αIIbβ3 on the cell surface.23 Reasoning that one or more NF-E2–regulated genes may therefore influence or regulate αIIbβ3 inside-out signaling, we performed screening cDNA expression array analysis using RNA from mature polyploid NF-E2–/– and NF-E2+/+ megakaryocytes in an attempt to identify such genes. We found caspase-12 to be the most down-regulated gene identified in the NF-E2–/– megakaryocytes, quantified at more than 200-fold reduced by real-time TaqMan reverse trancriptase (RT)–PCR. Western blots of megakaryocyte protein lysates from NF-E2–/– and NF-E2+/+ megakaryocytes confirmed the marked deficiency of caspase-12 protein in the NF-E2–/– megakaryocytes (Figure 1). Consistent with the previous observation that NF-E2 functions late in megakaryocytopoiesis,25 we were unable to detect caspase-12 in early megakaryocyte-lineage cells from wild-type mice (Figure 1). Megakaryocyte caspase-12 expression is therefore directly or indirectly regulated by the transcription factor NF-E2.
Western blot for caspase-12 in megakaryocytes. Protein lysates (20 μg) from large polyploid NF-E2+/+ (lane 1) and NF-E2–/– (lane 2) megakaryocytes were separated by SDS-PAGE (10%), and caspase-12 was detected by Western blot after transfer of proteins to nitrocellulose membrane. Lane 3 contains protein lysate (20 μg) from CD41+, VWF+ early megakaryocyte-lineage cells from wild-type mice.27 The NF-E2 genotype is indicated below each lane, molecular weight markers (kDa) are indicated on the left, and caspase-12 (arrow) and caspase-12 cleavage products (bar) are indicated on the right.
Western blot for caspase-12 in megakaryocytes. Protein lysates (20 μg) from large polyploid NF-E2+/+ (lane 1) and NF-E2–/– (lane 2) megakaryocytes were separated by SDS-PAGE (10%), and caspase-12 was detected by Western blot after transfer of proteins to nitrocellulose membrane. Lane 3 contains protein lysate (20 μg) from CD41+, VWF+ early megakaryocyte-lineage cells from wild-type mice.27 The NF-E2 genotype is indicated below each lane, molecular weight markers (kDa) are indicated on the left, and caspase-12 (arrow) and caspase-12 cleavage products (bar) are indicated on the right.
Caspase-12–/– platelets have a defect in αIIbβ3 inside-out signaling
Caspase-12–/– mice are viable and have a normal life span.26 NF-E2–/– mice have a markedly decreased platelet count secondary to abnormal platelet formation and release during the late stage of megakaryocytopoiesis,25 which is due in part to decreased expression of β1 tubulin.28 Platelet counts were therefore obtained to determine if caspase-12 contributes to the thrombocytopenia seen in NF-E2–/– mice. In contrast with the approximate 60% reduction in platelet count reported for β1 tubulin–/– mice,28 we found no significant difference (P = .22) between the platelet counts of wild-type (mean ± SEM: 898 ± 170 × 109/L [898 000 ± 170 000/μL]; n = 8) and caspase-12–/– (mean ± SEM: 949 ± 96 × 109/L [949 000 ± 96 000/μL]; n = 8) mice, demonstrating that caspase-12 does not independently contribute to the low resting platelet count in NF-E2–/– mice. Transmission electron microscopy did not reveal any apparent difference between wild-type and caspase-12–/– platelets, including no difference in the number of α-granules per platelet.
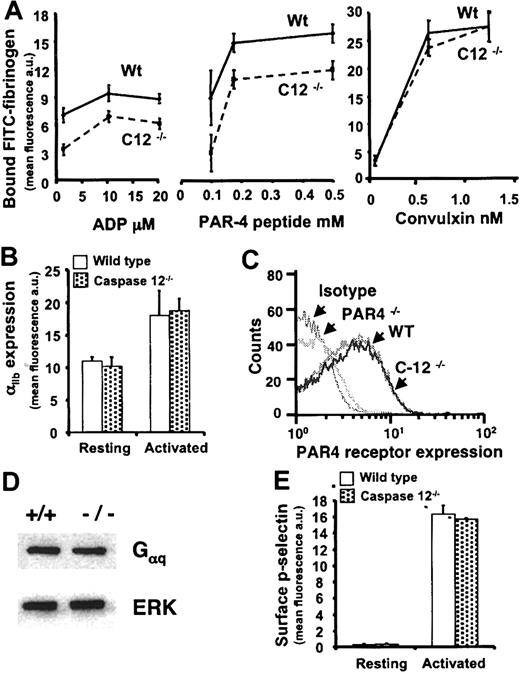
To determine if caspase-12 contributes to the αIIbβ3 signaling defect in NF-E2–/– megakaryocytes,23 wild-type and caspase-12–/– platelets were assayed for agonist-induced soluble fibrinogen binding. The caspase-12–/– platelets bind significantly less fibrinogen than do wild-type platelets when stimulated by ADP or the PAR4 receptor-activating peptide (AYPGFK) over a range of agonist concentrations. However, no defect was found when platelets were activated by the GPVI collagen receptor agonist convulxin (Figure 2A). The diminished soluble fibrinogen binding by caspase-12–/– platelets is not due to reduced αIIbβ3 expression (Figure 2B). These data demonstrate that caspase-12–/– platelets have a defect in integrin αIIbβ3 inside-out signaling that is manifest by a subset of platelet agonists.
Soluble fibrinogen binding to activated platelets. (A) Washed platelets incubated with soluble FITC-fibrinogen were activated with ADP, PAR4 receptor-activating peptide (AYPGFK), or convulxin, and bound fibrinogen was detected by FACS analysis. Agonist-induced FITC-fibrinogen bound to the platelets is indicated on the y-axis in fluorescence units. Agonist concentration is provided on the x-axis. Data are the means ± SEM for 3 or 4 independent experiments. Caspase-12–/– platelets bound significantly less soluble fibrinogen following stimulation with ADP (P values range from .02 to .003 for the 3 concentrations shown) and PAR4 receptor-activating peptide (P values range from .01 to .005 for the 3 concentrations shown). There was no significant difference in soluble fibrinogen binding at any of the convulxin concentrations. (B) The expression level of surface integrin αIIb (CD41) as measured by FACS analysis and FITC-conjugated anti-CD41 antibody is indicated on the y-axis in fluorescence units for wild-type (white bars) and caspase-12–/– (dotted bars) platelets at rest and following agonist stimulation. (C) Histograms demonstrate the relative PAR4 receptor expression levels as determined by FACS analysis using FITC-conjugated anti-PAR4 receptor antibody. Wild-type (WT), caspase-12–/– (C12–/–), and PAR4–/– platelets were analyzed. The isotype control is indistinguishable from the PAR4–/– platelets, and the wild-type platelets do not differ from the caspase-12–/– platelets. (D) A Western blot shows no difference in Gαq expression between wild-type (+/+) and caspase-12–/– (–/–) platelets. The same membrane was probed for ERK as a loading control. (E) Surface P-selectin (CD62-P) expression was determined using FACS analysis and FITC-conjugated anti–CD62-P antibody. The expression level is indicated on the y-axis in fluorescence units for wild-type (white bars) and caspase-12–/– (dotted bars) platelets at rest and following agonist stimulation. Data in panels B and E are the means ± SEM for 3 independent experiments, and there is no statistical difference between wild-type and caspase-12–/– platelets in either panel.
Soluble fibrinogen binding to activated platelets. (A) Washed platelets incubated with soluble FITC-fibrinogen were activated with ADP, PAR4 receptor-activating peptide (AYPGFK), or convulxin, and bound fibrinogen was detected by FACS analysis. Agonist-induced FITC-fibrinogen bound to the platelets is indicated on the y-axis in fluorescence units. Agonist concentration is provided on the x-axis. Data are the means ± SEM for 3 or 4 independent experiments. Caspase-12–/– platelets bound significantly less soluble fibrinogen following stimulation with ADP (P values range from .02 to .003 for the 3 concentrations shown) and PAR4 receptor-activating peptide (P values range from .01 to .005 for the 3 concentrations shown). There was no significant difference in soluble fibrinogen binding at any of the convulxin concentrations. (B) The expression level of surface integrin αIIb (CD41) as measured by FACS analysis and FITC-conjugated anti-CD41 antibody is indicated on the y-axis in fluorescence units for wild-type (white bars) and caspase-12–/– (dotted bars) platelets at rest and following agonist stimulation. (C) Histograms demonstrate the relative PAR4 receptor expression levels as determined by FACS analysis using FITC-conjugated anti-PAR4 receptor antibody. Wild-type (WT), caspase-12–/– (C12–/–), and PAR4–/– platelets were analyzed. The isotype control is indistinguishable from the PAR4–/– platelets, and the wild-type platelets do not differ from the caspase-12–/– platelets. (D) A Western blot shows no difference in Gαq expression between wild-type (+/+) and caspase-12–/– (–/–) platelets. The same membrane was probed for ERK as a loading control. (E) Surface P-selectin (CD62-P) expression was determined using FACS analysis and FITC-conjugated anti–CD62-P antibody. The expression level is indicated on the y-axis in fluorescence units for wild-type (white bars) and caspase-12–/– (dotted bars) platelets at rest and following agonist stimulation. Data in panels B and E are the means ± SEM for 3 independent experiments, and there is no statistical difference between wild-type and caspase-12–/– platelets in either panel.
The agonist-restricted defect in αIIbβ3 signaling suggested a possible receptor-specific abnormality. To begin to address this, PAR4 receptor expression levels on the surface of wild-type and caspase-12–/– platelets were determined by fluorescence-activated cell sorting (FACS) analysis. PAR4 receptor expression did not differ between the wild-type and caspase-12–/– platelets (Figure 2C). Knowing that the ADP and the PAR4 receptor are each G-protein–coupled, that both use the Gαq subunit, and that convulxin does not signal through a G-protein–coupled receptor, Western blots were performed to determine Gαq protein levels. Again no difference was found between wild-type and caspase-12–/– platelets (Figure 2D). The defect in αIIbβ3 activation in caspase-12–/– platelets is therefore not a function of diminished agonist receptor expression on the platelet surface or a reduced level of platelet Gαq.
Agonist-induced platelet activation triggers a variety of platelet changes in addition to activation of integrin αIIbβ3. One such event is the redistribution of P-selectin (CD62-P) from its intracellular residence in α-granule membranes to a position on the outer surface of the platelet. There was no difference in CD62-P levels between wild-type and caspase-12–/– platelets at rest or following PAR4 receptor-activating peptide stimulation (Figure 2E). The defect in caspase-12–/– platelet activation is therefore restricted in terms of both the agonist and the nature of the activation event.
Caspase-12–/– platelets are defective in platelet aggregation but not in platelet spreading
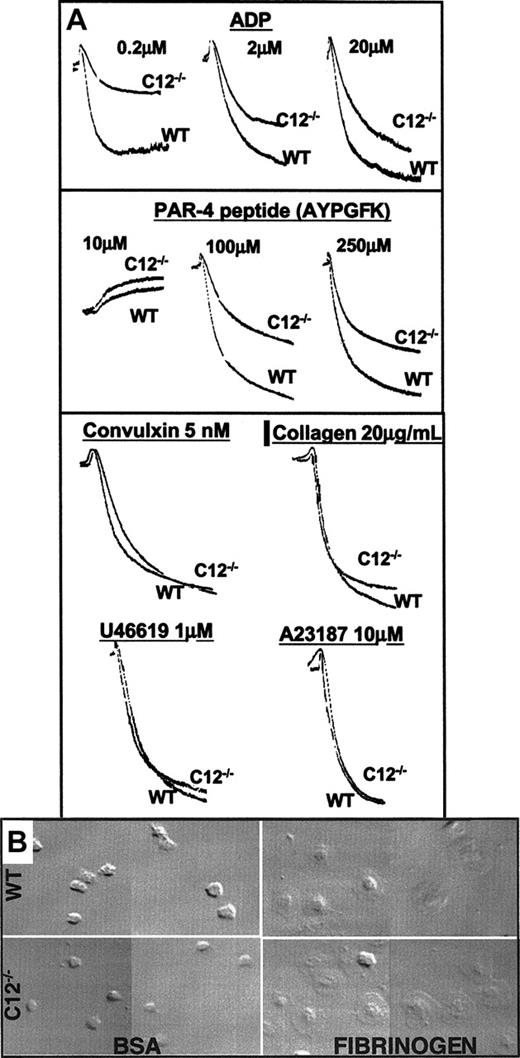
Platelet aggregation studies were performed to further characterize integrin αIIbβ3 signaling in caspase-12–/– platelets. Similar to the defect in agonist-induced soluble-fibrinogen binding, caspase-12–/– platelets also have impaired aggregation over a range of concentrations of ADP and PAR4 receptor-activating peptide (Figure 3A). A similar defect was seen following stimulation by thrombin (data not shown). However, no aggregation defect was seen when caspase-12–/– platelets were stimulated by convulxin (1-10 nM), collagen (0.2-20 μg/mL), the thromboxane mimetic U46619 (0.1-10 μM), or the calcium ionophore A23187 (1-10 μM) (Figure 3A). Of note, the initial shape change was consistently the same between the wild-type and caspase-12–/– platelets for each agonist tested. To determine if the aggregation defect in caspase-12–/– platelets reflects an outside-in signaling abnormality in addition to the αIIbβ3 inside-out signaling defect, platelet spreading experiments were performed using fibrinogen-coated glass coverslips. No difference was observed between wild-type and caspase-12–/– platelets (Figure 3B), arguing against a significant outside-in αIIbβ3 signaling defect in the caspase-12–/– platelets.
Platelet aggregation and spreading. (A) Platelet aggregation results are presented with light transmission increasing from top to bottom along the y-axis and time along the x-axis. The agonists and concentrations used are indicated. A single agonist concentration aggregation tracing is provided for the 4 agonists that showed no difference between wild-type and caspase-12–/– platelets. However, the lack of a difference between wild-type and caspase-12–/– platelets was seen over a range of concentrations for convulxin (1-50 nM), collagen (0.2-20 μg/mL), U46619 (0.1-10 μM), and A23187 (1-10 μM). (B) Washed wild-type (top) and caspase-12–/– (bottom) platelets were allowed to spread on BSA-coated (left) or fibrinogen-coated (right) glass coverslips. Images were obtained using differential interference contrast microscopy. Images are representative fields taken from 1 of 3 independent experiments that all yielded similar results.
Platelet aggregation and spreading. (A) Platelet aggregation results are presented with light transmission increasing from top to bottom along the y-axis and time along the x-axis. The agonists and concentrations used are indicated. A single agonist concentration aggregation tracing is provided for the 4 agonists that showed no difference between wild-type and caspase-12–/– platelets. However, the lack of a difference between wild-type and caspase-12–/– platelets was seen over a range of concentrations for convulxin (1-50 nM), collagen (0.2-20 μg/mL), U46619 (0.1-10 μM), and A23187 (1-10 μM). (B) Washed wild-type (top) and caspase-12–/– (bottom) platelets were allowed to spread on BSA-coated (left) or fibrinogen-coated (right) glass coverslips. Images were obtained using differential interference contrast microscopy. Images are representative fields taken from 1 of 3 independent experiments that all yielded similar results.
Caspase-12–/– platelets have normal agonist-induced cAMP suppression
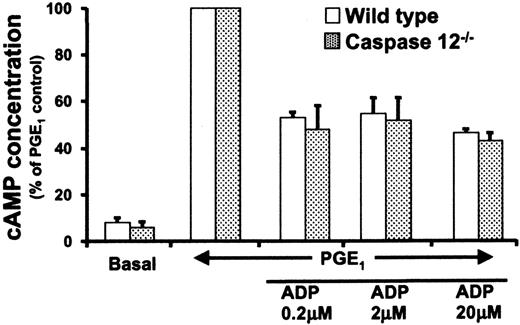
Platelets have 2 ADP receptors, P2Y1 involved in shape change and calcium flux,10,11 and P2Y12 involved in the inhibition of cAMP generation.12 cAMP is a potent inhibitor of platelet activation, and impaired P2Y12 signaling can contribute to impaired platelet activation due to a failure to suppress platelet cAMP levels. Therefore, the possibility that a P2Y12 signaling abnormality could contribute to the defect in soluble fibrinogen binding and aggregation by caspase-12–/– platelets was evaluated. Wild-type and caspase-12–/– platelets were pretreated with PGE1, stimulated with ADP, and the amount of intracellular cAMP was determined. We found no difference in cAMP levels between wild-type and caspase-12–/– platelets (Figure 4). Moreover, the absolute basal and PGE1-stimulated cAMP concentrations were not significantly different for the 2 genotypes. These results suggest that the activation defect in caspase-12–/– platelets is not due to an abnormality in resting cAMP levels or to abnormal cAMP levels secondary to defective P2Y12 signaling.
cAMP concentrations in ADP-stimulated platelets. Washed wild-type (white bars) and caspase-12–/– (dotted bars) platelets were incubated with PGE1 followed by ADP stimulation at the indicated concentrations. The y-axis shows the cAMP concentration, with PGE1-stimulated levels given a value of 100%. Data presented are the means ± SEM from 3 independent experiments. Platelet cAMP levels prior to PGE1 stimulation (basal) are less than 10% of that seen following PGE1 stimulation.
cAMP concentrations in ADP-stimulated platelets. Washed wild-type (white bars) and caspase-12–/– (dotted bars) platelets were incubated with PGE1 followed by ADP stimulation at the indicated concentrations. The y-axis shows the cAMP concentration, with PGE1-stimulated levels given a value of 100%. Data presented are the means ± SEM from 3 independent experiments. Platelet cAMP levels prior to PGE1 stimulation (basal) are less than 10% of that seen following PGE1 stimulation.
Caspase-12–/– platelets have abnormal agonist-induced intracellular free calcium levels
Cytoplasmic free calcium levels typically rise following agonist-induced platelet activation.29 To determine if the defective soluble fibrinogen binding by caspase-12–/– platelets is associated with abnormal cytoplasmic calcium levels, FURA-2am–loaded platelets were stimulated and relative intracellular free calcium levels were measured. Abnormally low cytoplasmic free calcium levels were observed for caspase-12–/– platelets following ADP and PAR4 receptor-activating peptide stimulation (Figure 5A), independent of the presence or absence of added extracellular calcium. This defect mirrored that seen for soluble fibrinogen binding (Figure 2A) and aggregation (Figure 3A) with these same agonists. In contrast, the thromboxane mimetic U46619 produces wild-type levels of cytoplasmic free calcium, as does convulxin (Figure 5A), consistent with the wild-type aggregation seen in response to each of these 2 agonists (Figure 3A). To better understand the mechanism underlying the blunted rise in cytoplasmic free calcium, platelet IP3 levels were determined following agonist stimulation. Relative to wild-type platelets, caspase-12–/– platelets had significantly diminished IP3 levels at 3 (P = .002) and 6 (P = .006) seconds following agonist stimulation (Figure 5B).
Cytoplasmic calcium and 1,4,5-inositol triphosphate (IP3) levels following agonist stimulation. (A) FURA-2am–loaded washed platelets were stimulated with the indicated agonists (ADP, 20 μM; PAR4 activating peptide [AYPGFK], 250 μM; U46619, 100 μM; convulxin, 1 nM) and cytoplasmic-free calcium was determined by measuring the fluorescence emission spectra following excitation by 340-nm and 380-nm wavelength UV light. Data are representative of 3 independent experiments and are presented as a 340:380 ratio. (B) Intracellular IP3 levels are presented for wild-type (white bars) and caspase-12–/– (dotted bars) platelets at 3 and 6 seconds following stimulation with thrombin. Data presented are the means ± SEM from 3 independent experiments. Wild-type platelets are assigned a value of 100% for each experiment. (C) Western blots for expression of PLCβ1, β2, and β3, isoforms, for PLCβ3 phosphoserine 537 and 1105 before and following activation with PAR4 receptor-activating peptide, and for total PLCγ2 are presented. All show similar expression levels in wild-type (WT) and caspase-12–/– (C12–/–) platelets. Each membrane was reprobed for the indicated loading control.
Cytoplasmic calcium and 1,4,5-inositol triphosphate (IP3) levels following agonist stimulation. (A) FURA-2am–loaded washed platelets were stimulated with the indicated agonists (ADP, 20 μM; PAR4 activating peptide [AYPGFK], 250 μM; U46619, 100 μM; convulxin, 1 nM) and cytoplasmic-free calcium was determined by measuring the fluorescence emission spectra following excitation by 340-nm and 380-nm wavelength UV light. Data are representative of 3 independent experiments and are presented as a 340:380 ratio. (B) Intracellular IP3 levels are presented for wild-type (white bars) and caspase-12–/– (dotted bars) platelets at 3 and 6 seconds following stimulation with thrombin. Data presented are the means ± SEM from 3 independent experiments. Wild-type platelets are assigned a value of 100% for each experiment. (C) Western blots for expression of PLCβ1, β2, and β3, isoforms, for PLCβ3 phosphoserine 537 and 1105 before and following activation with PAR4 receptor-activating peptide, and for total PLCγ2 are presented. All show similar expression levels in wild-type (WT) and caspase-12–/– (C12–/–) platelets. Each membrane was reprobed for the indicated loading control.
PLC catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-triphosphate (IP3) and diacylglycerol, and there are 4 PLC subclasses: β, γ, δ, and ϵ.30 G-protein–coupled receptors, the receptor type used by ADP and the PAR4 activating peptide, signal specifically through PLCβ isoforms.30 Of the 4 known PLCβ isoforms, only β1, β2, and β3 are expressed outside of the nervous system. Diminished expression of one of these PLCβ isoforms would be the most direct explanation of the signaling defect in caspase-12–/– platelets, but no differences were found for any of the PLCβ isoforms between wild-type and caspase-12–/– platelets (Figure 5C). PLCβ3 serine 1105 phosphorylation has been associated with inhibition of PLC activity.31,32 Western blot analysis for serine 1105 phosphorylation before and after platelet activation with PAR4 receptor–activating peptide demonstrated no consistent difference between wild-type and caspase-12–/– platelets (Figure 5C). PLCβ3 serine 537 phosphorylation may also affect PLC activity,33 but again there was no difference between wild-type or caspase-12–/– platelets at rest or following activation with PAR4 receptor–activating peptide (Figure 5C). Since PLCγ2 is the most highly expressed PLC isoform in human platelets,34 we also assessed its expression. Again, there was no difference between wild-type and caspase-12–/– platelets (Figure 5C). Therefore, whereas caspase-12–/– platelets have a clear defect in PLC activity as manifest by diminished IP3 levels, it is not related to the above-described PLC expression or phosphorylation.
Caspase-12 acts during thrombopoiesis to affect platelet function
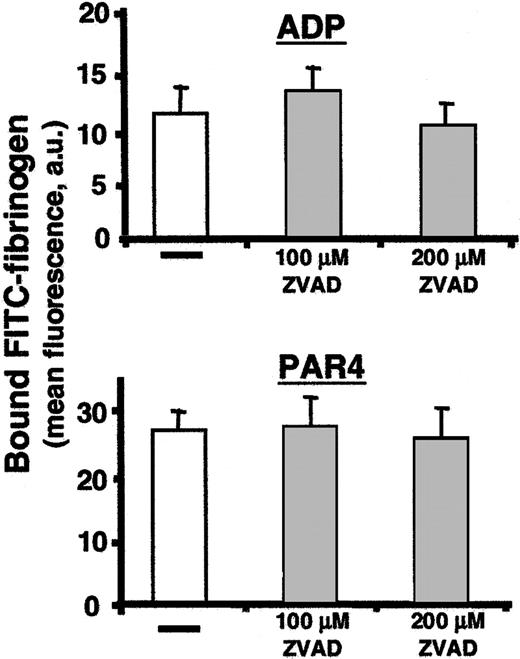
Given the above abnormalities in caspase-12–/– platelets, we predicted that agonist stimulation of wild-type platelets would induce caspase-12 activation by cleavage of the inactive proenzyme form to its active cleavage product. However, despite high levels of pro-caspase-12 expression in mature megakaryocytes (Figure 1), we were surprisingly unable to detect pro-caspase-12 or its activated cleavage products in wild-type platelets before or after agonist stimulation (data not shown). This unexpected finding suggests that caspase-12 most likely functions within the megakaryocyte during thrombogenesis to produce a fully functional platelet. Such a model would predict that a caspase-12 inhibitor should be unable to reproduce the caspase-12–/– platelet phenotype in wild-type platelets. Although there are no caspase-12–specific inhibitors, treating wild-type platelets with the pan-caspase inhibitor Z-VAD-FMK failed to impair soluble fibrinogen binding following stimulation with ADP or PAR4 receptor–activating peptide (Figure 6).
Soluble fibrinogen binding following treatment with protease inhibitors. Soluble FITC-fibrinogen binding was determined following agonist stimulation of platelets pretreated with the caspase inhibitor ZVAD. Control platelets for each inhibitor, treated with carrier buffer only, are indicated by a horizontal bar. The amount of bound fibrinogen was determined by the mean fluorescence in the FL1 channel. Data presented are means ± SEM for 3 independent experiments. There is no significant difference between untreated and treated platelets at either ZVAD concentration tested.
Soluble fibrinogen binding following treatment with protease inhibitors. Soluble FITC-fibrinogen binding was determined following agonist stimulation of platelets pretreated with the caspase inhibitor ZVAD. Control platelets for each inhibitor, treated with carrier buffer only, are indicated by a horizontal bar. The amount of bound fibrinogen was determined by the mean fluorescence in the FL1 channel. Data presented are means ± SEM for 3 independent experiments. There is no significant difference between untreated and treated platelets at either ZVAD concentration tested.
Discussion
To search for essential signaling molecules involved in agonist-induced activation of the integrin αIIbβ3, we performed comparative expression array analysis using mRNA from NF-E2+/+ and NF-E2–/– megakaryocytes. Caspase-12 was found to be the most down-regulated gene product in the absence of NF-E2. Using platelets from caspase-12–/– mice, we have identified a role for caspase-12 in G-protein–coupled receptor activation of integrin αIIbβ3. More specifically, the absence of caspase-12 leads to a defect in αIIbβ3 activation following stimulation of the PAR4 thrombin receptor or the P2Y1 ADP receptor despite normal levels of platelet αIIbβ3. The activation defect is associated with a blunted rise in agonist-induced cytosolic free calcium and IP3 levels, whereas agonist-induced P-selectin surface expression is not altered. Normal αIIbβ3 activation and aggregation following convulxin stimulation of caspase-12–/– platelets indicates that the defect is agonist restricted, and normal cytosolic free calcium and aggregation following U46619 stimulation imply that not all G-protein–coupled receptors are equally affected. Furthermore, normal ADP suppression of cAMP in PGE1-stimulated caspase-12–/– platelets suggests that the defect does not involve the P2Y12 ADP receptor. In addition, normal spreading of caspase-12–/– platelets on fibrinogen-coated surfaces argues against a role for caspase-12 in αIIbβ3 outside-in signaling. Interestingly, caspase-12 is not detectable in platelets despite high levels within mature megakaryocytes, and a pan-caspase inhibitor fails to recapitulate the caspase-12–/– platelet phenotype. Taken together, these data suggest that caspase-12 performs a developmental function during late megakaryocytopoiesis that helps endow platelets with a complete complement of fully active pathways for agonist-induced αIIbβ3 activation.
It is well established that agonist stimulation of platelets via the PAR4 thrombin receptor or the P2Y1 ADP receptor leads to the activation of PLC, which in turn hydrolyzes membrane-bound phosphoinositol 4,5-diphosphate to yield IP3 and diacylglycerol.29,30 IP3 then binds to its receptor, a calcium channel on the platelet-dense tubular system, which results in release of stored calcium and a rise in cytosolic free calcium, and which in turn impacts a number of platelet functions including αIIbβ3 activation. The impaired αIIbβ3 activation in caspase-12–/– platelets, combined with diminished cytosolic free Ca++ and IP3, suggest a defect in the signaling pathway downstream of agonist-receptor engagement and upstream of the generation of IP3, strongly implicating a defect in PLC activity. However, within the limits of Western blot anlaysis, none of the G-protein–coupled PLCβ isoforms, β1, β2, or β3, are abnormally expressed. Also, wild-type and caspase-12–/– platelets show no difference in phosphorylation at serine 537 or 1105 on PLCβ3 (Figure 5C), 2 phosphorylations thought to influence PLC activity.32,33 Together, the data suggest that the impaired PLC activity in caspase-12–/– platelets is not due to diminished PLC expression, but to abnormal development during platelet biogenesis that prevents fully functioning PLC activity in the context of specific physiologic signaling events involved in αIIbβ3 activation pathways. Interestingly, a family with a bleeding diathesis secondary to impaired PLCβ2 expression, and presumably activity, has been reported, and their platelets show a blunted rise in cytoplasmic free calcium and IP3 levels following thrombin receptor stimulation.34,35
Platelet agonist receptors can be grouped into those that are G-protein–coupled and those that are not. Receptors involved in the initiation of platelet plug formation, such as GPVI, are not G-protein–coupled. On the other hand, the PAR4 thrombin receptor and the P2Y1 ADP receptor are G-protein–coupled, and both are thought to signal through Gαq. Therefore, one simple unifying explanation for the caspase-12–/– phenotype is a defect in Gαq signaling. However, this mechanism is not supported by the normal caspase-12–/– platelet aggregation induced by the thromboxane mimetic U46619, which also signals through a Gαq-coupled receptor.16 Moreover, Gαq expression level is not altered in caspase-12–/– platelets (Figure 2D). The agonist-restricted defect in αIIbβ3 activation in caspase-12–/– platelets could alternatively be explained by a reduced number of agonist receptors on the platelet surface. However, caspase-12–/– platelets have wild-type levels of PAR4 receptor on their surface (Figure 2C).
Mammalian caspases constitute a family of cysteine proteases that can be sorted into 2 subgroups based on their amino acid sequence similarity to the mammalian interleukin-1β–converting enzyme (ICE). Caspase-12 belongs to the ICE-like subgroup, which includes caspases 1, 4, 5, 11, and 13. The non–ICE-like caspases, most notably caspases 2, 3, 6, 7, 8, 9, and 10, are classically associated with programmed cell death.36,37 The ICE-like caspases are more commonly linked to processes other than cell death. For example, mouse caspases 1 and 11 and human caspases 1, 4, and 5 are associated with immunologic functions through their role in the maturation of cytokines IL-1β and IL-18. More recently, caspase-8 has been implicated in T-cell proliferation and cell-cycle progression, and T-cell activation.38-40 Our findings for caspase-12 and platelet function provide another example of caspase function that is not directly related to programmed cell death, and it is the first link between a caspase and integrin signaling.
Murine caspase-12 is expressed to varying degrees in most mouse tissues.41,42 It is largely localized to the cytoplasmic surface of the endoplasmic reticulum (ER) where it is thought to play a role in ER stress–induced apoptosis.26 ER stress–induced caspase-12 activation can activate cytoplasmic caspase-9, which in turn can activate caspase-3, a known executioner of cell death,43 suggesting that caspase-12 may be a critical link between ER stress and cell death.44 Moreover, m-calpain in the S-100 fraction of mouse cortical tissue appears to cleave purified caspase-12 in a manner similar to that seen during oxygen deprivation of mouse glial cells, suggesting that m-calpain may be a physiologic activator of caspase-12 during ER stress.41 It remains to be seen whether these in vitro findings using purified substrates will hold true in megakaryocytes. Given the reported platelet aggregation defect in μ-calpain–/– mice,45 it will be interesting to determine the relationship between calpain family members and caspase-12 in primary megakaryocytes.
Reports using cell lines and primary marrow megakaryocytes in combination with caspase inhibitors suggest that platelet formation is linked to compartmentalized caspase activation in the megakaryocyte.46,47 Interestingly, despite high levels of caspase-9 in megakaryocytes, these investigators could not detect caspase-9 in platelets.46 Caspase-12 is therefore the second caspase to be highly expressed in mature megakaryocytes yet undetectable in platelets. Given the proposed link between caspase-12 and caspase-9,43 it will be important to determine the spatial and enzymatic relationship between caspase-12 and caspase-9 within megakaryocytes.
In this report we demonstrate that caspase-12 expression in murine megakaryocytes is directly or indirectly regulated by the transcription factor NF-E2, and we describe a novel role for caspase-12 in the development of a complete complement of fully functional pathways involved in agonist-induced αIIbβ3 activation. As with many caspases, the physiologic substrates for megakaryocyte caspase-12 remain unknown, but their identification should further our understanding of the role of caspase-12 in megakaryocytopoiesis and in integrin signaling. Caspase-12 is the second αIIbβ3 signaling effector molecule that we have identified whose expression is down-regulated in NF-E2–/– megakaryocytes,24 supporting the idea that additional NF-E2–regulated genes may play a role in αIIbβ3 signaling.
Prepublished online as Blood First Edition Paper, April 1, 2004; DOI 10.1182/blood-2003-10-3633.
Supported by National Institutes of Health (NIH) grants HL54476 (Transfusion Medicine SCORE) and HL65198.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank J. Kang for excellent animal care and handling, R. Shivdasani and S. Orkin (Harvard Medical School) for the NF-E2–/– mice, J. Yuan (Harvard Medical School) for the caspase-12–/– mice, S. Coughlin (University of California, San Francisco) for the PAR4–/– mice, Genentech (South San Francisco, CA) for recombinant TPO, and I. Hsieh and D. Bainton (University of California, San Francisco) for help with electron microscopy.

![Figure 5. Cytoplasmic calcium and 1,4,5-inositol triphosphate (IP3) levels following agonist stimulation. (A) FURA-2am–loaded washed platelets were stimulated with the indicated agonists (ADP, 20 μM; PAR4 activating peptide [AYPGFK], 250 μM; U46619, 100 μM; convulxin, 1 nM) and cytoplasmic-free calcium was determined by measuring the fluorescence emission spectra following excitation by 340-nm and 380-nm wavelength UV light. Data are representative of 3 independent experiments and are presented as a 340:380 ratio. (B) Intracellular IP3 levels are presented for wild-type (white bars) and caspase-12–/– (dotted bars) platelets at 3 and 6 seconds following stimulation with thrombin. Data presented are the means ± SEM from 3 independent experiments. Wild-type platelets are assigned a value of 100% for each experiment. (C) Western blots for expression of PLCβ1, β2, and β3, isoforms, for PLCβ3 phosphoserine 537 and 1105 before and following activation with PAR4 receptor-activating peptide, and for total PLCγ2 are presented. All show similar expression levels in wild-type (WT) and caspase-12–/– (C12–/–) platelets. Each membrane was reprobed for the indicated loading control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/5/10.1182_blood-2003-10-3633/5/m_zh80170465960005.jpeg?Expires=1765895909&Signature=qIqUn22QEYI9i7rLZTyoIwqr9r~A7SVtQ9cwmhTGwDAyGhe-afOpRZEHJZAIhaS47xfQhc7TwOQvaupsJHGTjN-kds15v68ll7jYpdtJMMqe6zjL9TM5z3AgbI7RMFsJlp2SNOOWM0sa1XJJ8VRO-FFI9CuKDYpCR18WHuLUfenmjNB9T1ZkC3My7C70ETX6MoZJPBnckm2jfiNphAcYabcZQ7c3x~2qwxKvuiF7W1v-2D8srhCdFr1V1yWsz4Wvb0gybkX2Eq7OJDYarYrgRBV4ohYqyiTRa0w4adNvse8LgrLyXMzJeeGactbMuouwXPkZvFPKmsC00ebKlq9Gtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal