Abstract
The role of the Gi-coupled platelet P2Y12 receptor in platelet function has been well established. However, the functional effector or effectors contributing directly to αIIbβ3 activation in human platelets has not been delineated. As the P2Y12 receptor has been shown to activate G protein–gated, inwardly rectifying potassium (GIRK) channels, we investigated whether GIRK channels mediate any of the functional responses of the platelet P2Y12 receptor. Western blot analysis revealed that platelets express GIRK1, GIRK2, and GIRK4. In aspirin-treated and washed human platelets, 2 structurally distinct GIRK inhibitors, SCH23390 (R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride) and U50488H (trans-(±)-3,4-dichloro-N-methyl-N-[2-(pyrrolidinyl)cyclohexyl] benzeneacetamide methanesulfonate), inhibited adenosine diphosphate (ADP)–, 2-methylthioADP (2-MeSADP)–, U46619-, and low-dose thrombin–mediated platelet aggregation. However, the GIRK channel inhibitors did not affect platelet aggregation induced by high concentrations of thrombin, AYPGKF, or convulxin. Furthermore, the GIRK channel inhibitors reversed SFLLRN-induced platelet aggregation, inhibited the P2Y12-mediated potentiation of dense granule secretion and Akt phosphorylation, and did not affect the agonist-induced Gq-mediated platelet shape change and intracellular calcium mobilization. Unlike AR-C 69931MX, a P2Y12 receptor–selective antagonist, the GIRK channel blockers did not affect the ADP-induced adenlylyl cyclase inhibition, indicating that they do not directly antagonize the P2Y12 receptor. We conclude that GIRK channels are important functional effectors of the P2Y12 receptor in human platelets.
Introduction
Platelets play an important role in normal hemostasis and abnormal activation of platelets leads to thrombosis. During vascular injury or conditions of high shear, exposure of the collagen-rich subendothelium activates the platelets and results in the formation of a stable thrombus due to the combined action of adenosine diphosphate (ADP) secreted from platelet-dense granules and generated thrombin.1 Any perturbations in this system can have pathologic cardiovascular implications such as intravascular thrombus formation and vascular occlusion.
ADP is an important component of the platelet-dense granules and also an important platelet agonist that stimulates platelets by acting on the Gq-coupled P2Y1 receptor and Gi-coupled P2Y12 receptor.2,3 Once released, it amplifies the primary responses of other agonists such as collagen and thrombin, leading to enhanced platelet aggregation and stability of the thrombus.4,5
It is now well established that concomitant signaling through Gq-coupled P2Y1 and Gi-coupled P2Y12 is necessary and sufficient for the integrin GPIIb/IIIa activation.3 Furthermore, selective Gi activation, when coupled with selective G12/13 stimulation, also results in platelet aggregation.6,7 Notably, the P2Y12-coupled Gi signaling is also crucial for potentiating dense granule secretion, thromboxane A2 (TXA2) generation, and irreversible aggregation.8-11 The P2Y12 receptor deficiency leads to dysfunctional platelets and results in a bleeding diathesis.12-14 Furthermore, platelets obtained from P2Y12–/– mice also show prolonged bleeding times and decreased aggregation in response to ADP.15 The P2Y12 receptor is the target of antithrombotic drugs like clopidogrel and ticlopidine. Clopidogrel irreversibly blocks the ADP binding to platelet P2Y12 receptor16,17 and it has been suggested that it interacts with C17 and C270 in the extracellular domains of the receptor.18
The P2Y1 receptor stimulation results in the generation of inositol 3,4,5-trisphosphate (IP3) and diacylglycerol (DAG) following phospholipase C (PLC) activation.19 IP3 mobilizes calcium from internal stores which results in platelet filopodial extension and shape change whereas elevation of DAG levels activates protein kinase C (PKC).20 The P2Y12 receptor stimulation results in Gαi-mediated inhibition of stimulated adenylyl cyclase and Gβγ-mediated activation of phophatidylinositide (PI) 3-kinase γ21,22 and Rap 1b.23,24 We have recently shown that ADP causes Akt phosphorylation through the P2Y12 receptor.25 However, the signaling events occurring downstream of the P2Y12 receptor mediating the fibrinogen receptor activation still remain obscure.8 We and others have demonstrated that adenylyl cyclase inhibition associated with Gαi activation is not a direct mediator of GPIIb/IIIa activation.8,26-28
The activation of G protein–gated, inwardly rectifying potassium (GIRK; Kir3.0) channels was used in an expression cloning approach to identify the P2Y12 receptor.29 Subsequently, the rat P2Y12 receptor was shown to activate GIRK channels in sympathetic neurons.30 GIRK channels are expressed in the brain and heart, where they modulate resting membrane potential and cellular excitability.31-33 GIRK channels are tetrameric complexes formed by the variable assembly of 4 subunits (GIRK1-4).34-37 While GIRK2, GIRK3, and GIRK4 can form functional homomultimeric channels,34-36,38,39 GIRK1 requires assembly with either GIRK2, GIRK3, and/or GIRK4 to achieve surface membrane expression.38,40 The Gβγ subunits of pertussis toxin–sensitive (Gi/o) G proteins directly bind and activate GIRK channels.35,41-44 Recently, 2 structurally unrelated compounds, SCH23390 (R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride) and U50488H (trans-(±)-3,4-dichloro-N-methyl-N-[2-(pyrrolidinyl)cyclohexyl] benzeneacetamide methanesulfonate), were shown to directly block GIRK channels with different potencies.45,46
In this study, we used a pharmacologic approach to investigate whether GIRK channels mediate any of the downstream effects of P2Y12 receptor activation in platelets, culminating in fibrinogen receptor activation. We demonstrate that 2 structurally distinct GIRK channel blockers inhibit P2Y12 receptor–mediated functional responses in platelets, while sparing functional responses associated with Gq signaling.
Materials and methods
Materials
SCH23390, U50488H, SKF38393 ((±)-1-phenyl-2,3,4,5-tetrahydro-(1H)-3-benzazepine-7,8-diol hydrochloride), 2-methylthioADP (2MeSADP), apyrase grade VII, human fibrinogen, thrombin, and acetylsalicylic acid were obtained from Sigma (St Louis, MO). ADP was purchased from Chrono-Log (Havertown, PA). Antibodies against GIRK1 and GIRK2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies against GIRK1-3 were obtained from Alomone Labs (Jerusalem, Israel). The antibody against the GIRK4 isoform was described previously.47 Fura-2am was purchased from Molecular Probes (Eugene, OR). [2,8-3H] adenine was purchased from Perkin Elmer Life Sciences (Boston, MA). AR-C69931MX was a generous gift from Astra-Zeneca Research Laboratories (Charnwood, Loughborough, United Kingdom). The stable thromboxane/prostaglandin endoperoxide analog 9, 11-dideoxy-9, 11-epoxymethanoprostaglandin F2 (U46619), prostaglandin (PGE1), and Ro 31-8220 (bisindolylmaleimide IX) were from Biomol (Plymouth Meeting, PA). Hexapeptide SFLLRN was custom synthesized at Research Genetics (Huntsville, AL). Luciferin-luciferase reagent was purchased from Chrono-Log. SC57101A was a gift from Searle Research and Development (Skokie, IL). All other reagents were of reagent grade, and deionized water was used throughout.
Preparation of washed human platelets
Whole blood was drawn from healthy, consenting human volunteers selected from the students, staff, and workers at Temple University. Donated blood was collected in tubes containing one-sixth volume of ACD (2.5 g sodium citrate, 1.5 g citric acid, and 2 g glucose in 100 mL deionized water). Citrated blood was centrifuged (Eppendorf 5810R centrifuge, Hamburg, Germany) at 230g for 20 minutes at room temperature (RT) to obtain platelet-rich plasma (PRP). PRP was incubated with 1 mM acetylsalicylic acid (aspirin) for 30 minutes at 37° C, and then allowed to remain at RT for 15 minutes. The PRP was then centrifuged for 10 minutes at 980g at RT. Platelet pellet was resuspended in calcium-free Tyrode buffer (138 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 3 mM NaH2PO4, 5 mM glucose, 10 mM Hepes adjusted to pH 7.4) containing 0.01 U/mL apyrase. Cells were counted using the Z1 Coulter Particle Counter (Beckman Coulter, Miami, FL) and adjusted to 2 × 108 platelets/mL.
cAMP assay
The PRP was incubated with 1 mM aspirin and 2 μL/mL (3-H) adenine48 for 1 hour at 37° C and washed platelets were prepared as described above. Forskolin (20 μM) was added at zero seconds to stimulate the adenylyl cyclase. All inhibitors (AR-C69931MX, SCH23390, and U50488H) were added 30 seconds after forskolin stimulation. ADP (10 μM) was added to inhibit the cAMP level in forskolin-stimulated platelets 60 seconds after forskolin addition. The reaction was stopped at 210 seconds by adding an equal volume of the stopping solution comprised of 2 mM cAMP, 2 mM adenosine triphosphate (ATP), and 10% trichloroacetic acid. The cAMP levels in the prepared samples were determined as described earlier.49 All experiments were repeated at least 3 times using blood obtained from 3 different donors.
Intracellular calcium mobilization
Calcium mobilization was measured in platelets that were loaded with 2 mM Fura-2am along with 1 mM aspirin in PRP for 45 minutes at 37° C, and washed platelets were prepared as described in “Preparation of washed human platelets.” Platelet samples (0.5 mL) were placed in a quartz cuvette with a magnetic stir bar in a temperature-controlled chamber. An Aminco Bowman Series 2 flourescence spectrometer (Thermospectronics, Rochester, NY) was used for measurement of intracellular calcium mobilization. Two wavelengths (340 nm and 380 nm) were used for excitation, and the emitted light was measured at 510 nm. Samples were stimulated with the agonist after 30 seconds of incubation at 37° C, and SCH23390 and U50488H were added prior to agonist addition, at zero seconds. Minimum fluorescence (Fmin) was obtained by addition of 20 mM Tris and 4 mM EGTA (ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid), and maximum fluorescence (Fmax) was determined by adding 0.25% Triton X-100 and saturating levels of CaCl2. Calculation of the calcium mobilization was performed as outlined previously.50 Experiments were repeated at least 3 times using blood obtained from 3 different donors.
Aggregometry
Aggregation responses of 0.5 mL platelets in response to various agonists and inhibitors were determined using the PICA lumi-aggregometer (Chrono-Log) by measuring the light transmission under stirring conditions (900 rpm) at 37° C. The baseline was set using Tyrode buffer as blank. GIRK channel inhibitors were added prior to the addition of agonist. Preincubation at 37° C for 15 minutes was needed for 10 μM Ro31-8220 to block platelet secretion. The chart recorder (Kipp and Zonen, Bohemia, NY) was set for 0.2 mm/s. Platelets were stimulated with 10 μM ADP and 100 nM 2-MeSADP in the presence of exogenously added human fibrinogen (1 mg/mL). For studying platelet shape change response, SC57101A (10 μM), a fibrinogen receptor antagonist, was added prior to agonist addition. Experiments were repeated at least 3 times using blood obtained from 3 different donors.
Analysis of PAC-1 mAb binding
GPIIb/IIIa activation in aspirin-treated, washed human platelets was carried out by measuring PAC-1 mAb (Becton Dickinson, San Jose, CA) binding and analyzed by flow cytometry. Platelets were prepared as previously mentioned and were brought to a final concentration of 3 × 107 platelets/mL. The agonist (5 μL) and AR-C, SCH23390, or U50488H were added into each of the tubes. PAC-1 mAb (5 μL) was also added into all the labeled tubes except the one needed to set the baseline, marked “unstained.” Tyrode solution was used to normalize the volume, where the final volume after the addition of agonist/inhibitors and PAC-1 mAb was less than 20 μL. Addition of an aliquot of 50 μL of platelets to the 20 μL of agonist/inhibitor/mAb resulted in a final concentration of 3 × 106 platelets/mL. The addition of platelets to each tube was done at 30-second increments to begin stimulation. The samples were stimulated for a period of 10 minutes in the dark, and then diluted with 450 μL Tyrode buffer. Each sample (450 μL) was transferred to a 12 × 75 mm cuvette (Fisher Scientific, Pittsburgh, PA) and analyzed by flow cytometry, using FACSCAN (BD Biosciences), to measure an increase in fluorescence that indicates an increase in GPIIb/IIIa receptor activation. The experiment was performed 3 times, and data are presented as the mean plus or minus the standard error of the mean (SEM).
Preparation of washed mouse platelets
Blood was collected from anesthetized mice by cardiac puncture into syringes containing 3.8% sodium citrate as anticoagulant. The whole blood was centrifuged (IEC Micromax centrifuge; International Equipment Company, Needham Heights, MA) at 100g for 10 minutes to isolate the PRP. PGE1 (1 μM) was added to PRP. The platelets were centrifuged at 400g for 10 minutes and the pellet was resuspended in Tyrode buffer containing 0.01 U/mL apyrase.
Western blotting
Washed human or mouse platelets (0.1 mL) were lysed using sample loading buffer and boiled for 10 minutes. The platelet lysates were loaded on to a 10% Tris-glycine gel, subjected to SDS-PAGE, and transferred to polyvinylidene difluoride (PVDF) membrane. The membrane was then treated with blocking buffer (0.5% nonfat dry milk, 3% bovine serum albumin [BSA], 20 mM Tris, 140 mM NaCl) for 30 minutes at RT under rocking conditions. Following blocking, the membrane was incubated with primary antibody (1:200 dilution for anti-GIRK1/2, 1:200 dilution for anti-GIRK3, and 1:333 dilution for anti-GIRK4, 1:1000 for phospho-Akt) in Tris buffered saline Tween (TBST; 20 mM Tris, 140 mM NaCl, and 0.1% [vol/vol] Tween 20) with 2% BSA overnight at 4° C with gentle rocking. Wherever required, 5-fold higher amounts of blocking peptides (against GIRK1 and GIRK2) were first incubated with the primary antibody in 500 μL antibody-dilution buffer, at RT for 2 hours. After electrophoresis, transfer and blocking was done, the blocking peptide-primary antibody solution was diluted with antibody dilution buffer to achieve a final concentration of 1:200 for the primary antibody. After three 5-minute washes with TBST, the membrane was probed with alkaline phosphatase-labeled secondary antibody (1:5000 dilution in TBST with 2% BSA) for 1 hour at RT. The membrane was washed 3 times for 5 minutes each time using the 1× TBST, once with deionized water and last with 1× tropix buffer (Tropix, Bedford, MA). Membrane was then incubated with CDP-Star chemiluminescent substrate (Tropix) for 10 minutes at RT and immunoreactivity was detected using Fujifilm Luminescent Image Analyzer (Fujifilm Medical Systems, Stamford, CT). The lane loading control was established with anti–PKC-δ-antibodies (Santa Cruz Biotechnology) by the procedures published earlier.51
Results
Detection of GIRK subunits in platelets by Western blotting
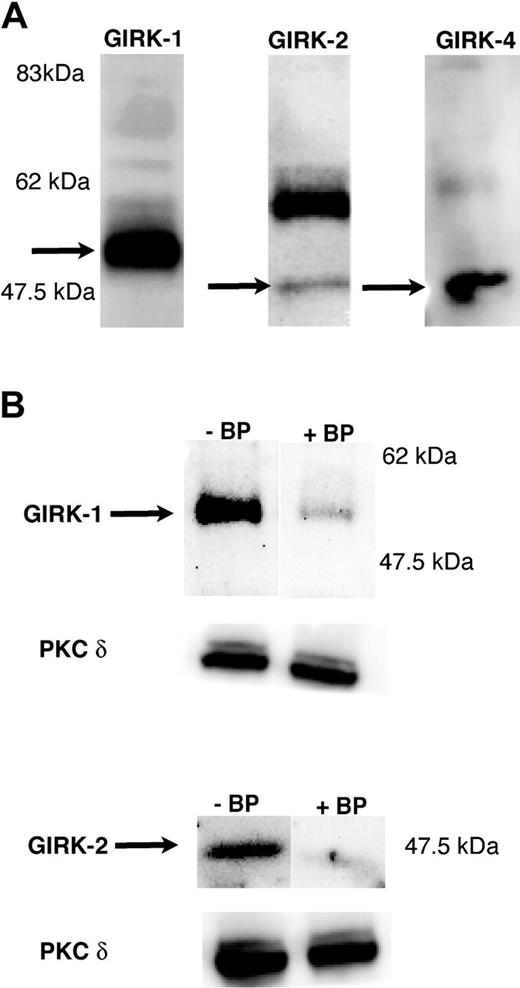
As a first step toward investigating a potential role of GIRK channels in platelet function, Western blot analysis was carried out to determine whether any of the 4 GIRK channel subunits were expressed in human platelet samples. As shown in Figure 1, GIRK1, GIRK2, and GIRK4 were found in human platelets at the expected molecular sizes.52-54 GIRK3 was not detected in either human or mouse platelets (not shown). These results were confirmed using GIRK1 and GIRK2 antibodies from 2 different sources and by using blocking peptides (Figure 1).
Detection of GIRK subunits in platelets by Western blotting. Washed and aspirin-treated human platelets were lysed with (3×) sample buffer, and 10 μLof protein lysate was loaded onto a 10% Tris-glycine gel. Western blotting was performed using specific antibodies against GIRK1, GIRK2, and GIRK4 (A) and in the presence of blocking peptides as described in “Materials and methods” (B). The molecular weights are indicated in each of the blots. The predominant GIRK1 band was noticed at approximately 55 kDa. GIRK2 and GIRK4 both were detected at 47.5 kDa molecular weight range. The blot was probed with antibodies against PKC-δ to ensure equal loading of lanes with protein.
Detection of GIRK subunits in platelets by Western blotting. Washed and aspirin-treated human platelets were lysed with (3×) sample buffer, and 10 μLof protein lysate was loaded onto a 10% Tris-glycine gel. Western blotting was performed using specific antibodies against GIRK1, GIRK2, and GIRK4 (A) and in the presence of blocking peptides as described in “Materials and methods” (B). The molecular weights are indicated in each of the blots. The predominant GIRK1 band was noticed at approximately 55 kDa. GIRK2 and GIRK4 both were detected at 47.5 kDa molecular weight range. The blot was probed with antibodies against PKC-δ to ensure equal loading of lanes with protein.
Effect of GIRK inhibitors on agonist-induced platelet aggregation
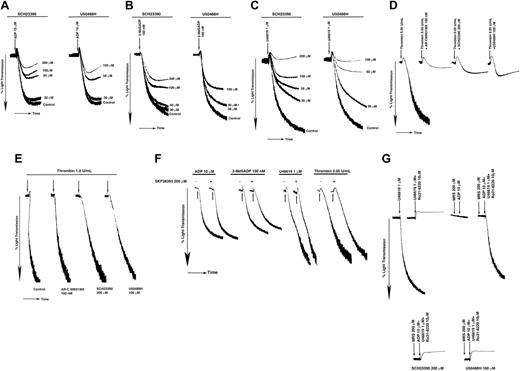
P2Y12 receptor plays an important role in the platelet aggregation induced by ADP and thromboxane A2.3,55,56 We investigated the effect of 2 structurally distinct GIRK channel inhibitors, SCH23390 and U50488H, to evaluate the role of GIRK channels in P2Y12-dependent platelet aggregation in aspirin-treated and washed human platelets. As shown in Figure 2, these inhibitors blocked the aggregation induced by ADP (Figure 2A), 2-MeSADP (Figure 2B), and U46619 (Figure 2C) in a concentration-dependent manner, with dramatic inhibition seen at 200 μM SCH23390 and 100 μM U50488H. At this concentration, neither SCH23390 nor U50488H alone produced any aggregation response (not shown). It is known that aggregation induced by a low dose of thrombin (0.05 U/mL) depends on ADP secreted from platelet-dense granules to provide the Gi signaling.5 Hence if GIRK channel activation is a functional effector in P2Y12 receptor–coupled Gi signaling pathway, blocking GIRKs should impair low-dose thrombin–induced aggregation. Indeed, both SCH23390 (200 μM) and U50488H (100 μM) inhibited the platelet aggregation induced by a low dose of thrombin, similar to the effect observed with AR-C69931MX (100 nM) treatment (Figure 2D). Importantly, the GIRK channel inhibitors did not affect platelet aggregation induced by high concentrations of thrombin (1 U/mL; Figure 2E), AYPGKF (not shown), or convulxin (100 ng/mL), a glycoprotein VI agonist (data not shown), which is known to occur independently of P2Y12 receptor activation.5,57
Effect of the GIRK channel blockers on agonist-induced platelet aggregation. Aspirinated, washed human platelets were stimulated with (A) 10 μM ADP, (B) 100 nM 2-MeSADP, (C) 1 μM U46619, (D) 0.05 U/mL thrombin, and (E) 1.0 U/mL thrombin, in the presence of 200 μM SKF38393 (F) at 37° C in a lumi-aggregometer and percent light transmission was recorded. Panel G shows the ability of Gi stimulation to rescue platelet aggregation when stimulated with U46619 in the presence of Ro 31-8220 and the effect of GIRK inhibitors. SCH23390 (200 μM), U50488H (100 μM), or AR-C69931MX (100 nM) were added prior to the addition of the agonist. Fibrinogen (2 mg/mL) was exogenously added to ADP-, 2-MeSADP–, or U46619-stimulated platelets. Arrow indicates the addition of reagents. Data are representative of at least 3 independent experiments performed using platelets from 3 different donors.
Effect of the GIRK channel blockers on agonist-induced platelet aggregation. Aspirinated, washed human platelets were stimulated with (A) 10 μM ADP, (B) 100 nM 2-MeSADP, (C) 1 μM U46619, (D) 0.05 U/mL thrombin, and (E) 1.0 U/mL thrombin, in the presence of 200 μM SKF38393 (F) at 37° C in a lumi-aggregometer and percent light transmission was recorded. Panel G shows the ability of Gi stimulation to rescue platelet aggregation when stimulated with U46619 in the presence of Ro 31-8220 and the effect of GIRK inhibitors. SCH23390 (200 μM), U50488H (100 μM), or AR-C69931MX (100 nM) were added prior to the addition of the agonist. Fibrinogen (2 mg/mL) was exogenously added to ADP-, 2-MeSADP–, or U46619-stimulated platelets. Arrow indicates the addition of reagents. Data are representative of at least 3 independent experiments performed using platelets from 3 different donors.
To investigate the specificity of SCH23390 toward blocking GIRK channels we used SKF38393, which is structurally related to SCH23390 but inactive at blocking GIRK channels.45 We evaluated the effect of SKF38393 (200 μM) on ADP-, 2-MeSADP–, U46619-, and low-dose thrombin–induced aggregation. We noticed that SKF38393 did not affect platelet aggregation induced by any of the above agonists (Figure 2F). These results suggest that inhibition of platelet functional responses by SCH23390 are restricted to its GIRK channel-blocking ability.
U46619, a stable thromboxane A2 analog, acts via the Gq-coupled TP receptor, activates PKC, and causes the secretion of ADP from the dense granules. Secreted ADP stimulates the P2Y12 receptor and provides the Gi component of the signaling pathway.56 When aspirin-treated and washed human platelets were stimulated with U46619 (1 μM) in the presence of Ro31-8220 (a PKC inhibitor), only shape change was seen due to lack of Gi stimulation by secreted ADP. The aggregation under this condition could be rescued by the selective activation of the P2Y12 receptor using ADP (10 μM) in the presence of the P2Y1 antagonist, MRS-2179 (200 μM). Both SCH23390 (200 μM) and U50488H (100 μM) dramatically blocked the Gi rescue of aggregation (Figure 2G). These results suggest that inhibition of GIRK channels impairs aggregation dependent on P2Y12 stimulation.
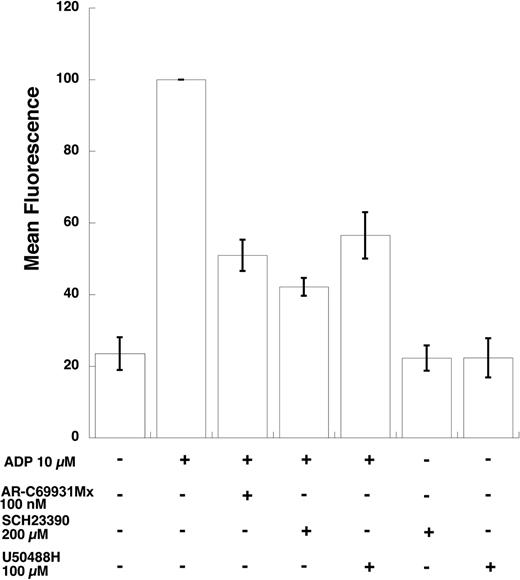
We then investigated whether the effect of GIRK channel inhibitors is on fibrinogen receptor activation or by other indirect means. We used PAC-1, a monoclonal antibody that binds only to the activated form of the fibrinogen receptor, to evaluate the effect of the GIRK channel inhibitors on ADP-induced fibrinogen activation. As shown in Figure 3, the 2 GIRK inhibitors blocked ADP-induced fibrinogen receptor activation to a similar extent as AR-C 69931MX, a P2Y12 receptor–selective antagonist. Thus, we ruled out the possibility that these inhibitors are blocking fibrinogen binding to its receptor and thereby inhibiting aggregation by directly measuring GPIIb/IIIa activation. These data indicate that the GIRK channel inhibitors block inside-out signaling leading to fibrinogen receptor activation.
Effect of GIRK channel blockers on ADP-induced PAC-1 binding. PAC-1 binding in aspirin-treated, washed human platelets on ADP stimulation was measured in the presence of various inhibitors as noted. After stimulation of platelets in the dark for 10 minutes, they were diluted with Tyrode buffer and immediately analyzed on a FACSCAN flow cytometer for increases in fluorescence that correlate with GPIIb/IIIa activation. Data were calculated as median fluorescence by multiplying the median point of the cell population with the percentage of the cell population in the marker. Each bar is the average of 3 experiments ± SEM from 3 donors. *P < .05.
Effect of GIRK channel blockers on ADP-induced PAC-1 binding. PAC-1 binding in aspirin-treated, washed human platelets on ADP stimulation was measured in the presence of various inhibitors as noted. After stimulation of platelets in the dark for 10 minutes, they were diluted with Tyrode buffer and immediately analyzed on a FACSCAN flow cytometer for increases in fluorescence that correlate with GPIIb/IIIa activation. Data were calculated as median fluorescence by multiplying the median point of the cell population with the percentage of the cell population in the marker. Each bar is the average of 3 experiments ± SEM from 3 donors. *P < .05.
Effect of GIRK channel inhibitors on Gq-mediated platelet functional responses
Thus far, these experiments demonstrate that GIRK channel activation is essential for P2Y12 receptor–mediated platelet aggregation. However, it is possible that GIRK channel blockade might affect the Gq-mediated functional responses as well. To ensure that SCH23390 and U50488H are selective in blocking the P2Y12 receptor effects, we tested their effect on the typical Gq functional responses (platelet shape change and mobilization of calcium). The platelet shape change was measured in the presence of SC57101A (10 μM), a competitive fibrinogen receptor antagonist.58 Even after SCH23390 (200 μM) was added, the platelet shape change in response to ADP (10 μM), 2-MeSADP (100 nM), or U46619 (1 μM) was intact. We repeated the same set of experiments with U50488H (100 μM) and noticed no difference in the shape-change response (Figure 4A). These data indicate that neither SCH23390 nor U50488H affect Gq-mediated platelet shape change.
Effect of GIRK channel blockers on G protein–mediated platelet functional responses. (A) Effect of GIRK channel blockers on platelet shape change. Aspirin-treated and washed human platelets were treated with fibrinogen receptor antagonist SC57101A (10 μM) to block aggregation prior to addition of agonist. ADP (10 μM) and 2-MeSADP (100 nM) were added under stirring conditions to observe the shape-change response. SCH23390 (200 μM) and U50488H (100 μM) were added prior to agonist stimulation. (B) Effect of SCH23390 on ADP-mediated platelet intracellular calcium release. Fura-2am–loaded human platelets were activated with 10 μM ADP and 1 μM U46619 in the presence of SC57101A (10 μM) to measure calcium mobilization. (C) Effect of GIRK channel blockers on P2Y12 receptor–mediated inhibition of adenylyl cyclase. Washed, aspirinated, and [3H]-adenine treated and (20 μM) forskolin-stimulated platelets were stimulated with ADP (10 μM) in the presence of AR-C69931MX (100 nM), SCH23390 (200 μM), or U50488H (100 μM) as noted and inhibition of adenylyl cyclase was studied. Data are expressed as percent of total [3H]-adenine nucleotides. Data are representative of 3 independent experiments done using platelets from 3 donors, and error bars indicate SEM.
Effect of GIRK channel blockers on G protein–mediated platelet functional responses. (A) Effect of GIRK channel blockers on platelet shape change. Aspirin-treated and washed human platelets were treated with fibrinogen receptor antagonist SC57101A (10 μM) to block aggregation prior to addition of agonist. ADP (10 μM) and 2-MeSADP (100 nM) were added under stirring conditions to observe the shape-change response. SCH23390 (200 μM) and U50488H (100 μM) were added prior to agonist stimulation. (B) Effect of SCH23390 on ADP-mediated platelet intracellular calcium release. Fura-2am–loaded human platelets were activated with 10 μM ADP and 1 μM U46619 in the presence of SC57101A (10 μM) to measure calcium mobilization. (C) Effect of GIRK channel blockers on P2Y12 receptor–mediated inhibition of adenylyl cyclase. Washed, aspirinated, and [3H]-adenine treated and (20 μM) forskolin-stimulated platelets were stimulated with ADP (10 μM) in the presence of AR-C69931MX (100 nM), SCH23390 (200 μM), or U50488H (100 μM) as noted and inhibition of adenylyl cyclase was studied. Data are expressed as percent of total [3H]-adenine nucleotides. Data are representative of 3 independent experiments done using platelets from 3 donors, and error bars indicate SEM.
ADP and U46619 elevate intracellular calcium levels by acting via their Gq-coupled P2Y1 and TP receptors, respectively. Aspirin-treated, Fura-2am–loaded, and washed human platelets were stimulated with ADP (10 μM) or U46619 (1 μM) in the absence and presence of SCH23390 (200 μM) or U50488H (100 μM). Neither compound significantly affected the ADP- or U46619-induced increases in intracellular calcium levels (Figure 4B), further emphasizing that the GIRK channel inhibitors do not influence Gq-mediated platelet functional responses.
GIRK channel blockers do not antagonize the P2Y12 receptor
Prior experiments show that SCH23390 and U50488H are selective in blocking the P2Y12 receptor–mediated responses. A plausible explanation for their effects is that they are antagonists of the P2Y12 receptor. We addressed this concern by measuring the cAMP levels in forskolin-stimulated platelets following the stimulation with ADP in the absence and presence of SCH23390 or U50488H. ADP inhibits adenylyl cyclase and reduces the forskolin-stimulated cAMP levels, which are reversed by AR-C69931MX.5 As shown in Figure 4C, SCH23390 and U50488H failed to relieve the ADP-induced inhibition of adenylyl cyclase in forskolin-stimulated platelets. Furthermore, these inhibitors, by themselves, do not cause increases in cAMP levels in platelets (Figure 4C), which could affect platelet functional responses. These data indicate that SCH23390 and U50488H block P2Y12-induced platelet functional responses through a mechanism independent of receptor antagonism.
Effect of GIRK inhibitors on SFLLRN-induced irreversible aggregation
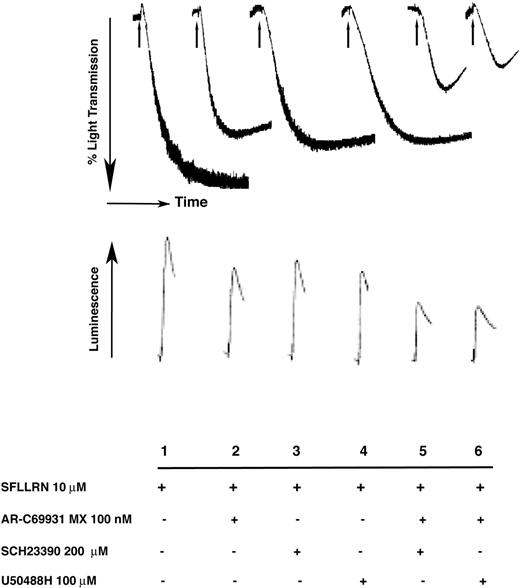
The ability of the PAR-1 activating peptide, SFLLRN, to cause irreversible aggregation depends solely on secreted ADP, which acts via the P2Y12 receptor and stimulates the Gi signaling cascade.5,10 If GIRK channels were the functional effectors downstream of the P2Y12 receptor, then one would predict that their blockade should render the SFLLRN-induced aggregation reversible. As shown in Figure 5, in the presence of SCH23390 and U50488H, 10 μM SFLLRN-induced irreversible aggregation was reversed in a manner similar to that obtained with the P2Y12 receptor antagonist, AR-C69931MX. A combination of AR-C69931MX and GIRK inhibitors caused more inhibition of SFLLRN-induced aggregation. These results further suggest that GIRK channels are important effectors downstream of P2Y12 receptor activation in platelets.
Effect of GIRK channel blockers on thrombin receptor activating peptide (TRAP)–induced aggregation and dense granule secretion. Aspirin-treated, washed platelets were activated with 10 μM SFLLRN and the corresponding aggregation or the amount of ATP secreted from dense granules (luciferin-luciferase assay) was measured as mentioned in “Materials and methods.” Arrow indicates addition of the agonist. Data are representative of 3 independent experiments done using platelets from 3 donors.
Effect of GIRK channel blockers on thrombin receptor activating peptide (TRAP)–induced aggregation and dense granule secretion. Aspirin-treated, washed platelets were activated with 10 μM SFLLRN and the corresponding aggregation or the amount of ATP secreted from dense granules (luciferin-luciferase assay) was measured as mentioned in “Materials and methods.” Arrow indicates addition of the agonist. Data are representative of 3 independent experiments done using platelets from 3 donors.
Effect of GIRK channel inhibitors on SFLLRN-induced dense granule secretion
Stimulation of Gi signaling leads to potentiation of dense granule secretion in platelets.8 Agonists like thrombin, AYPGKF, SFLLRN, and U46619 cause primary dense granule secretion by acting via their respective Gq-coupled receptors. Secreted ADP further potentiates the primary secretion by acting on the P2Y12 receptor.8 We investigated whether GIRK channels play any role in the P2Y12-mediated potentiation of dense granule release. Aspirin-treated and washed human platelets were stimulated with 10 μM SFLLRN in the absence and presence of GIRK channel blockers. A luciferinluciferase assay was used to measure the amount of ATP secreted from the dense granules. Figure 5 shows that SCH23390 and U50488H inhibited the SFLLRN-induced secretion to an extent similar to that caused by the AR-C69931MX. A combination of AR-C69931MX and GIRK inhibitors caused further inhibition of dense granule release. These results indicate that GIRK channels are important for mediating the functions downstream of the P2Y12 receptor.
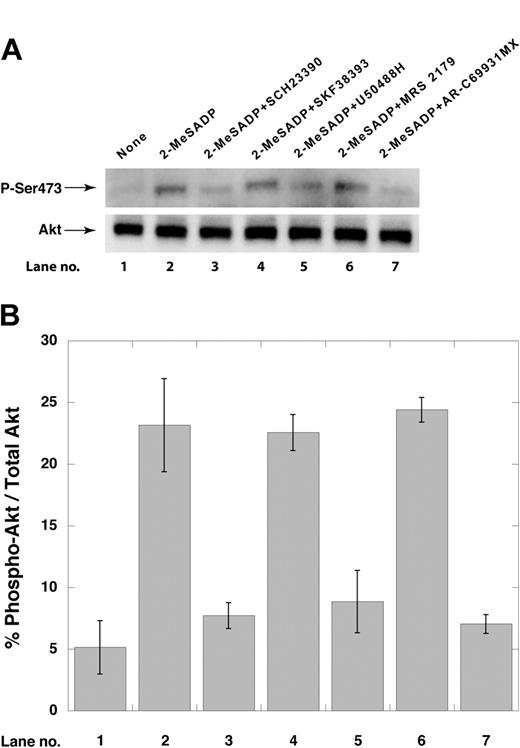
Effect of GIRK channel blockade on Gi-mediated Akt phosphorylation
We have demonstrated that Akt is activated in a PI3-kinase–dependent mechanism upon P2Y12 receptor stimulation by ADP.25 We hence evaluated the effect of GIRK inhibitors on ADP-induced PI3-kinase activation using Akt phosphorylation as the readout. As shown in Figure 6, AR-C69931MX, SCH23390 (200 μM), and U50488H (100 μM) blocked the Akt-phosphorylation. SKF38393 (200 μM), however, did not affect the Akt phosphorylation, providing additional evidence about the specificity of SCH23390 (Figure 6).
Effect of GIRK inhibitors on ADP-induced Akt phosphorylation. (A) Aspirin-treated, washed human platelets were stimulated with ADP in the presence or absence of reagents as indicated. Platelet lysates were then electrophoresed and probed with phospho-specific Ser473 anti-Akt antibodies as described in “Materials and methods.” (B) Densitometric analysis of the immunoblots from panel A was performed using the ImageGauge Program (Fujifilm Luminescent Image Analyzer). Lane numbers correspond to those in panel A. Data are representative of 3 independent experiments done using platelets from 3 donors, and error bars indicate SEM.
Effect of GIRK inhibitors on ADP-induced Akt phosphorylation. (A) Aspirin-treated, washed human platelets were stimulated with ADP in the presence or absence of reagents as indicated. Platelet lysates were then electrophoresed and probed with phospho-specific Ser473 anti-Akt antibodies as described in “Materials and methods.” (B) Densitometric analysis of the immunoblots from panel A was performed using the ImageGauge Program (Fujifilm Luminescent Image Analyzer). Lane numbers correspond to those in panel A. Data are representative of 3 independent experiments done using platelets from 3 donors, and error bars indicate SEM.
Discussion
Although the P2Y12 receptor has been shown to play an important role in platelet activation, the functional effectors mediating its effects have not been identified. Because GIRK channels are activated by Gi signaling upon P2Y12 receptor activation in other systems,29,30 we investigated whether GIRK channels are functional effectors of the P2Y12 receptor–mediated Gi signaling in platelets.
Two structurally distinct GIRK channel blockers, SCH23390 and U50488H, blocked P2Y12-mediated platelet aggregation, potentiation of dense granule release, SFLLRN-mediated irreversible aggregation, and ADP-induced PI3-kinase activation. However, platelet aggregation induced by high concentrations of thrombin, AYPGKF, and convulxin was unaffected by these inhibitors. Furthermore, the inhibitors did not affect Gq-mediated functional responses such as mobilization of calcium ions from intracellular stores or platelet shape change. We ruled out the antagonistic effect of these compounds on the P2Y12 receptor as well as their stimulatory effect on adenylyl cyclase, both of which could affect platelet functional responses. Based on all of our observations, we conclude that the GIRK channel blockers affect platelet responses only in cases of stimulation by agonists depending on the P2Y12-mediated Gi signaling pathway. A combination of P2Y12 receptor antagonism and GIRK channel inhibition caused more inhibition of SFLLRN-induced aggregation and dense granule release reaction. The plausible reason for this enhanced inhibition is that the other secreted components could cause Gi stimulation,56,59 independently of the P2Y12 receptor activation, that contributes to the potentiation of SFLLRN-mediated effects. The GIRK inhibitors could be blocking these pathways in addition to the P2Y12 receptor pathways, thereby causing further inhibition of SFLLRN-induced aggregation and secretion.
SCH23390 is also known to antagonize dopamine D1 receptors.60 As there is no dopamine source present in our experiments, we conclude that SCH23390 is not mediating its effects through the dopamine D1 receptor. Toward ascertaining the specificity of SCH23390, we used SKF38393, a structurally similar compound that lacks the GIRK channel-blocking ability,45 in our study. SKF38393 did not have any effect on agonist-induced platelet aggregation, implying that effects seen with SCH23390 are mainly due to GIRK channel blockade.
Similarly, though U50488H is a known κ-opioid receptor agonist that causes inhibition of stimulated adenylyl cyclase,61 there is no evidence for κ-opioid receptor expression on platelets to date.62,63 Even if κ-opioid receptors were present on platelets, one would predict that U50488H would potentiate platelet activation due to stimulation of Gi pathways. On the contrary, we observed inhibition of Gi-mediated platelet responses by U50488H. Hence, we conclude that the effects of U50488H are not through stimulation of κ-opioid receptors.
In CHO cells, the IC50 of SCH23390 for blocking the GIRK1/2 heteromultimeric channel and GIRK2 homomultimeric channels were 7.8 μM and 83 μM, respectively. For U50488H, the inhibitory concentration (IC50) for inhibition of the GIRK1/2 heteromultimeric channel was 70.28 ± 3.68 μM.45 However, lack of complete inhibition of the platelet functional responses by 200 μM SCH23390 and 100 μM U50488H in our experiments argues against a homogeneous population of GIRK1/GIRK2 heteromultimeric channels and could be due to the presence of multiple combinations of the different GIRK subunits on platelets, differing potency of GIRK inhibitors with the varying subunit assembly, or tissue-specific GIRK channel splice variants in platelets.64
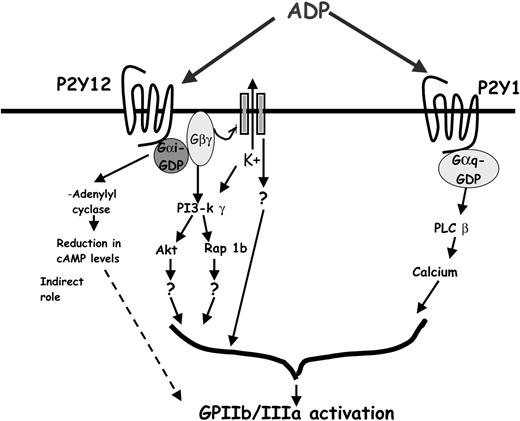
Previous studies have implicated Rap 1b and PI3-kinase γ as possible signaling molecules downstream of the P2Y12 receptor activation. Although PI3-kinase γ knockout mice have protection from thromboembolism, ADP-induced platelet aggregation is unaffected when higher concentrations of the agonist are used,65 suggesting that other effectors might be contributing to the functional responses downstream of the P2Y12 receptor activation. Rap 1b is a small GTPase that is activated in a PI3-kinase γ–dependent mechanism following Gαi/Gαz-coupled receptor stimulation.24,66 It has been shown to play an important role in fibrinogen receptor activation in megakaryocytes.23 Recent studies have also shown that platelets from Rap 1b knockout mice have a lesser extent of aggregation induced by ADP, compared with the wild-type mouse platelets.67 Thus, Rap 1b appears to be one of the functional effectors downstream of the P2Y12 receptor. Platelets also express 2 isoforms of Akt (protein kinase B).68 Platelets from mice deficient in either isoform of Akt do not show significant abnormalities in agonist-induced platelet aggregation.68 Lack of both isoforms is embryonically lethal, suggesting their role in the early development. However, platelets from mice with 3 of the 4 alleles that were knocked out show considerable deficiencies in aggregation.68 As Rap 1b and Akt are downstream of PI3-kinase γ, these 2 effectors might be in the same functional effector pathway as PI3-kinase γ (Figure 7).
Schematic of possible effectors downstream of the P2Y12 receptor. The figure depicts the signaling events that occur on ADP-induced P2Y12 receptor stimulation. PI3-kinase γ, Akt, and Rap 1b are known effectors, which contribute to P2Y12 receptor–mediated GPIIb/IIIa activation. The βγ subunit of the Gi released on receptor stimulation binds to and activates GIRK channels. GIRK channel activation results in the efflux of K+ and in the generation of an intracellular signaling cascade leading to integrin activation. Functional transducers mediating this signal are yet to be identified.
Schematic of possible effectors downstream of the P2Y12 receptor. The figure depicts the signaling events that occur on ADP-induced P2Y12 receptor stimulation. PI3-kinase γ, Akt, and Rap 1b are known effectors, which contribute to P2Y12 receptor–mediated GPIIb/IIIa activation. The βγ subunit of the Gi released on receptor stimulation binds to and activates GIRK channels. GIRK channel activation results in the efflux of K+ and in the generation of an intracellular signaling cascade leading to integrin activation. Functional transducers mediating this signal are yet to be identified.
In the sinoatrial cells of the heart, acetylcholine-mediated GIRK channel activation results in the hyperpolarization and subsequent slowing of the heart rate, whereas in the brain, GIRK channel activation is essential for the postsynaptic inhibitory effects of many neurotransmitters. The presence of GIRK channels in platelets has not been reported to date. Neuronal cells and platelets, however, may share some common signal transduction mechanisms. It is known that stem cells can be differentiated into glial cells when treated with hematopoietic growth factors and cytokines.69 In addition, brain cells have been shown to differentiate into hematopoietic cells.70 It should also be noted that the process of exocytosis is highly homologous both in platelets and neuronal cells and they both have revealed the presence of similar secretory machinery.71 Hence, the presence of GIRK channels in platelets is at least conceivable.
Whether membrane potential is an initiator or mediator of platelet activation mechanism is controversial.72,73 Previous studies have shown that ADP and thrombin cause depolarization of platelet membrane potential and sodium influx possibly via the sodiumproton exchanger.74-76 Thus, it is not unreasonable to speculate that GIRK channel–induced hyperpolarization may serve as the driving force for the sodium influx and resulting depolarization of membrane potential, and increase in intracellular pH. It is also possible that certain proteins may associate with GIRK channels by interacting with their intracellular domains. GIRK activation might activate some associated intracellular proteins that might initiate the inside-out signaling cascade, resulting in integrin activation independent of the transmembrane potential change. Thus, GIRK channels might be involved in events responsible for platelet aggregation and also might contribute to the events associated with the platelet activation process.
In conclusion, we have provided evidence using pharmacologic inhibitors that GIRK channels are functional effectors downstream of P2Y12 receptor activation. We propose that GIRK channel activation contributes to GPIIb/IIIa activation, potentiation of dense granule release, irreversible aggregation by SFLLRN, and PI3-kinase activation. The exact mechanism by which the GIRK channels contribute to these platelet functional responses and the composition of the GIRK isoforms in the functional channel remains to be studied.
Prepublished online as Blood First Edition Paper, May 13, 2004; DOI 10.1182/blood-2004-01-0069.
Supported by research grants HL60683 and HL64943 from the National Institutes of Health (S.P.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Robert T. Dorsam and Todd M. Quinton, Sol Sherry Thrombosis Research Center, for critically reviewing the manuscript.

![Figure 4. Effect of GIRK channel blockers on G protein–mediated platelet functional responses. (A) Effect of GIRK channel blockers on platelet shape change. Aspirin-treated and washed human platelets were treated with fibrinogen receptor antagonist SC57101A (10 μM) to block aggregation prior to addition of agonist. ADP (10 μM) and 2-MeSADP (100 nM) were added under stirring conditions to observe the shape-change response. SCH23390 (200 μM) and U50488H (100 μM) were added prior to agonist stimulation. (B) Effect of SCH23390 on ADP-mediated platelet intracellular calcium release. Fura-2am–loaded human platelets were activated with 10 μM ADP and 1 μM U46619 in the presence of SC57101A (10 μM) to measure calcium mobilization. (C) Effect of GIRK channel blockers on P2Y12 receptor–mediated inhibition of adenylyl cyclase. Washed, aspirinated, and [3H]-adenine treated and (20 μM) forskolin-stimulated platelets were stimulated with ADP (10 μM) in the presence of AR-C69931MX (100 nM), SCH23390 (200 μM), or U50488H (100 μM) as noted and inhibition of adenylyl cyclase was studied. Data are expressed as percent of total [3H]-adenine nucleotides. Data are representative of 3 independent experiments done using platelets from 3 donors, and error bars indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/5/10.1182_blood-2004-01-0069/5/m_zh80170466130004.jpeg?Expires=1765893855&Signature=LiUjafHcDDZMRRahHAKXIkptV6c~dHCa3~EDbBt148btbkJiOB~9IkP27S~cNDB0qy7NoN-K2hJ72qidIngKZUHHYcVVyRJgVRZ3WTK71viOX31aunhvOO4LhAvoMTfwrWFcxdRQdAXbkxBTNMYCKj5K~E-Fv6laRm2cOVF5Mi871ExnUaQ7KJxkIgUGMOlmoqAXJuB7eKpZoyYIMKh8ZfbehmLmzzYC0V4ZbyNcyHG6vQeR3skf0fDrNpVbzsi7GXBmS5C1PCdqgEdvl-9uP~DlO7d64zLU8WGLVio7cLkNwevB-yWGJrWuX88c5wc73TkFiMHslXiLCp~zbKoE9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal