Abstract
We have generated transgenic mice expressing the leech anticoagulant hirudin and human tissue factor pathway inhibitor tethered to the cell surface by fusion with fragments of human CD4 and P-selectin. Expression of the transgenes is under the control of the CD31 (platelet endothelial cell adhesion molecule [PECAM]) promoter, limiting expression to endothelial cells, monocytes, and platelets. In addition, the P-selectin sequence directs expression to secretory granules. Functional cell surface expression only occurs when the cells are activated. In a mouse model of systemic lipopolysaccharide (LPS)–induced endotoxemia, we show that expression of either anticoagulant on activated endothelium inhibits the widespread intravascular thrombosis, thrombocytopenia, and consumptive coagulopathy associated with endotoxemia. Importantly, non– LPS-treated transgenic mice had normal baseline bleeding times. We speculate that targeted delivery of anticoagulants to the endothelium may be a strategy worth pursuing in clinical sepsis to improve efficacy of systemic anticoagulation while minimizing potential hemorrhagic side effects.
Introduction
Severe sepsis with associated organ failure is a serious clinical syndrome that is estimated to cause as many deaths annually in the United States as acute myocardial infarction.1 The condition has been studied in a variety of different animal models, one of which involves injection of the lipopolysaccharide (LPS) component of gram-negative bacterial cell walls to produce systemic endotoxemia. This model is characterized by fever, shock, coagulopathy, disseminated intravascular coagulation, multiple organ failure and, ultimately, death. Although the relevance of experimental endotoxemia to clinical sepsis has been questioned,2 one strength is that its pathophysiologic basis is relatively well understood. A critical early event is widespread intravascular activation of coagulation by tissue factor (TF). Accordingly, inhibition of the initiation phase of clotting3-9 or preventing thrombin generation10 using suitable agents has a significant impact on disease morbidity and mortality in experimental models.
The dramatic effect of anticoagulants in animal studies led to several recent large-scale phase 3 clinical trials of anticoagulants in human sepsis, including recombinant tissue factor pathway inhibitor (TFPI),11 antithrombin III,12 and activated protein C.13 Although the use of activated protein C was associated with a significant reduction in 20-day mortality in treated patients, neither antithrombin III nor TFPI demonstrated a benefit when compared with controls, and the use of all 3 novel reagents was associated with an increased risk of severe bleeding. However, it is significant that in all 3 trials the nonrandomized administration of heparin was associated with reduced mortality in the control groups and appeared to reduce the impact of the study agent. This has led to significant confusion about the importance of clotting factors in septicemia and the therapeutic potential of anticoagulation.14
We have been developing a strategy to direct the expression of novel, tethered anticoagulant fusion proteins to activated endothelial cells (ECs). We have previously shown that TFPI and hirudin tethered to the cell surface are functionally active anticoagulants.15,16 We also have shown that the addition of a P-selectin cytoplasmic sequence directs expression to the secretory granules of appropriate cells, such that functional cell surface expression only occurs when these cells become activated.17,18 We now describe the generation and characterization of transgenic mice expressing transgenes encoding these novel fusion proteins under a CD31 promoter to drive expression on ECs, platelets, and monocytes.
We hypothesized that expression of these anticoagulants on activated endothelium would efficiently inhibit the widespread intravascular activation of clotting seen after LPS. Using bone marrow reconstitution experiments to generate mice with isolated expression on ECs, we confirm that this is the case; inhibition of clotting on EC membranes is effective at preventing the widespread fibrin deposition, profound thrombocytopenia, and consumptive coagulopathy that is characteristic of severe endotoxemia.
Materials and methods
Constructs for microinjection
Constructs encoding human TFPI–CD4–P-selectin and hirudin–CD4–P-selectin and in vitro characterization after expression on ECs have been described.15-19 In preliminary experiments using a chromogenic assay with a source of mouse TF and factor VII (FVII), soluble human TFPI inhibited the conversion of murine factor X (FX) to FXa (data not shown), indicating that human TFPI is capable of inhibiting the initiation of coagulation in mice. To generate transgenic mice, a 0.446-kb human CD31 promoter subfragment was generated by polymerase chain reaction (PCR) from genomic DNA prepared from fresh human peripheral blood mononuclear cells (PBMCs) and cloned into vector pGEM-T (Promega, Southampton, United Kingdom) for sequencing. The promoter fragment was subcloned as a SacI-SacII fragment into pEGFP-1 (BD Biosciences Clontech, Oxford, United Kingdom). A 0.24-kb SacI-SalI fragment from the chicken β-globin insulator, derived from pSL 1180 (a PPL Therapeutics in-house vector), was directionally subcloned into the vector upstream of the CD31 promoter. TFPI–CD4–P-selectin and hirudin–CD4–P-selectin were separately subcloned into the vector to replace the enhanced green fluorescent protein (EGFP) sequence. All junctions were sequenced to confirm integrity.
Generation and breeding of transgenic mice
Transgenic founder mice were generated by microinjection. Animals carrying the transgenes were identified by Southern blot analysis of tail-tip DNA using a DIG Southern starter Kit (Roche Diagnostics, Mannhein, Germany) and copy number determined by slot-blot analyses. Four lines were established from each strain, but only 1 from each, derived from a founder with high copy number (more than 30), was used to establish a breeding colony for backcrossing onto a C57BL/6 (B6) background. All experiments were carried out on mice that had been backcrossed for at least 5 generations.
Immunohistochemistry
Organs were embedded in optimal cutting temperature (OCT) (VWR International, Dorset, United Kingdom) by freezing with dry ice, sectioned, and fixed in methanol at –20° C. Frozen sections were immersed in 1% bovine serum albumin–phosphate-buffered saline (BSA-PBS) and 10% goat serum (Sigma, Dorset, United Kingdom) for 30 minutes and then incubated overnight at 4° C with one of the following antibodies: rabbit antihuman TFPI immunoglobulin G (IgG), sheep antihirudin (both from Enzyme Research Laboratories, Swansea, United Kingdom), rabbit antimouse TF (generated in-house by J.H.M.), fluorescein isothiocyanate (FITC)–conjugated rabbit antihuman fibrinogen that also binds fibrin (DAKO, Glostrup, Denmark), rat antimouse CD31 (Pharmingen, San Diego, CA), or mouse antihuman α-actin (Sigma). Second-layer staining was with goat antirabbit IgG-FITC, donkey antisheep IgG-FITC, sheep antimouse IgG-FITC (all from Sigma), or rabbit antirat IgG-FITC (DAKO). Sections were examined on an immunofluorescence microscope (Axiovert S100 TV; Zeiss, Welwyn Garden City, United Kingdom) with Plan-NEOFLUAR objectives using a KTL/CCD-1300/Y/HS camera from Princeton Instruments (Trenton, NJ). Images were analyzed using the MetaMorph imaging system (Universal Imaging, Downingtown, PA). Hematoxylin and eosin (H&E) staining was performed according to standard protocols.
Purification of mouse microvascular ECs
Organs were collected, rinsed in Dulbecco modified Eagle medium (DMEM), minced, and digested with collagenase (3 mg/mL; Roche Diagnostics) for 30 minutes at 37° C. Digested tissue was passed through a cell strainer and the collected cells washed twice in 0.5% fetal calf serum (FCS)–DMEM before incubation for 30 minutes at 4° C with rat antimouse CD31 and CD105 (both from Pharmingen). After 2 washes, cells were resuspended in 0.5% FCS-DMEM containing goat antirat microbeads (Miltenyi Biotec, Auburn, CA) and incubated at 4° C for 15 minutes. After washing, cells were passed through a magnetic column (Miltenyi Biotec). Retained beads were washed and released from the magnet before culture in DMEM with 20% FCS medium. All ECs were passage 1. In some experiments, ECs were activated by phorbol myristate acetate (PMA) (1 μM; Sigma) for 30 minutes at 37° C prior to analysis.
Leukocyte isolation
Mouse blood, collected by tail-tip resection, was diluted 1:20 in ACK buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA [ethylenediaminetetraacetic acid]) to lyse erythrocytes. After 20 minutes at room temperature, cells were washed and resuspended in 3% FCS-DMEM medium. For analysis, monocyte phenotype was confirmed by flow cytometric analysis with an antimouse CD14 monoclonal antibody (mAb) (Pharmingen). Splenocytes were isolated from homogenized mouse spleen, suspended in 10 mL ACK buffer, incubated for 5 minutes at room temperature, and resuspended in RPMI containing 10% FCS. Cells were activated with concanavalin A at the final concentration of 5 μg/mL for 48 hours at 37° C.
Purification of platelets
Platelet-rich plasma was obtained from blood by centrifugation at 80g for 10 minutes, followed by dilution 1:20 with 1% ammonium oxalate and 2.5 mM Gly-Pro-Arg-Pro peptide (Sigma). Samples were placed in a counting chamber in a moist Petri dish, allowed to settle, and the platelets in 1 mm2 counted (= N). The number of platelets per liter of blood equals 2N × 109. Platelet phenotype was confirmed by flow cytometric analysis with an anti-CD41 mAb (Pharmingen). In some experiments, platelets were activated by 1 U/mL thrombin (Enzyme Research Laboratories) for 30 minutes at 37° C prior to analysis.
Flow cytometry analysis
A total of 1 × 105 cells or platelets were incubated with primary antibodies for 30 minutes on ice, followed by secondary antibodies for 30 minutes. Antibodies used were as described above. Stained cells were analyzed on a cytofluorometer (Becton Dickinson, Franklin Lakes, NJ).
In vitro clotting assay
Purified microvascular ECs were included in a recalcified mouse plasma clotting assay similar to that previously described.17 Plasma was collected from B6 mice. ECs were seeded at a density of 105/mL in a 6-well plate and grown to near confluence before addition of interleukin-1α (IL-1α; 10 ng/mL) (Sigma) for 12 hours and PMA for the final 30 minutes. After washing 3 times with serum-free DMEM, cells were detached with EDTA. A total of 5 × 106 ECs were added to 200 μL recalcified plasma and the time to clot measured. Similar assays were performed with porcine EC transfectants and human plasma.
Endotoxic shock
All mice weighed 25 ± 1 g. Experiments were performed under terminal anesthesia and conformed to United Kingdom national and institutional guidelines. Mice were anesthetized with sodium pentobarbitone (Sagatal; 60 ng/g) (Rhone Merieux, Harlow, United Kingdom) and given a single injection of LPS (Escherichia coli serotype 0127:B8, Sigma) 2 μg/g or saline (control) intraperitoneally. Nitro-l-arginine methyl ester (l-NAME) (Alexis, Nottingham, United Kingdom) (50 μg/g) was administered intraperitoneally 30 minutes before LPS and again 0, 2, and 4 hours after LPS. In some experiments, recombinant soluble hirudin, antihirudin antibodies, or anti-TFPI antibodies were administered at variable doses with LPS. Mice were killed 4.5 hours after LPS or saline administration, and blood samples were withdrawn by puncture of the heart into 0.1 vol of 3.8% sodium citrate (Sigma).
Bleeding time assay
Anesthetized mice were maintained in a restrainer (Becton Dickinson), and a distal 2-mm segment of tail was severed with a razor blade. The tail was immediately immersed in 0.9% saline at 37° C. Bleeding time was defined as the time required for the bleeding to stop.20
Fibrinogen assay
Fibrinogen levels were measured using the Clauss method with bovine thrombin (Sigma). Assays were terminated at 20 minutes if no clot was observed. Results are expressed as percentage fibrinogen relative to control B6 mice. Serial dilutions of pooled B6 mouse plasma (n = 5) in 0.05 M imidazole per 0.1 M sodium chloride (pH 7.3) were used to determine the relationship between clotting times and normal mouse plasma fibrinogen levels.
Bone marrow (BM) reconstitution
Bone marrow (BM) was flushed from the excised ends of long bones from humanely killed mice and resuspended at 5 × 107 cells per milliliter in fresh RPMI. Recipient mice were irradiated with 12 Gy (1200 rad) before injection of 1 × 107 BM cells into a tail vein. Mice were isolated for 4 weeks before further experimentation.
Detection of free TFPI and hirudin fusion protein in mouse plasma
Plasma was processed by centrifuging freshly citrated blood at 2000g at 4° C for 15 minutes and stored at –80° C before analysis. Saturating amounts of anti-TFPI or antihirudin mAb were added and incubated at room temperature for 30 minutes. The resulting solution was added to 105 stable pig EC transfectants,17 incubated for 15 minutes at 4° C, and then analyzed by flow cytometry using appropriate FITC-conjugated second layers.
Results
Generation and characterization of transgenic mice
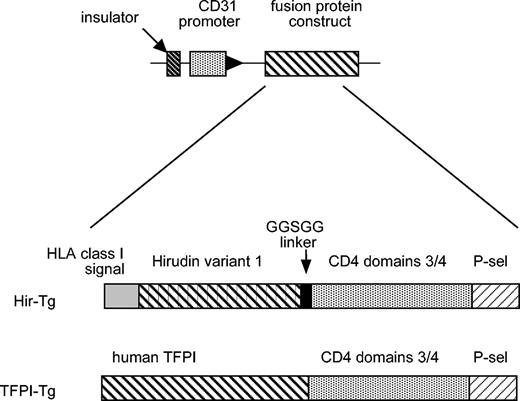
A diagrammatic representation of the transgene used to generate the transgenic strains is shown in Figure 1. The assembly of the fusion protein constructs has been described in detail elsewhere.15,16 Transgenic mice (hereafter called TFPI-Tg or Hir-Tg mice) were viable, had normal litters, and transmitted the transgenes at expected frequencies. There were no significant differences in the breeding or growth characteristics between the 2 strains. Northern analysis revealed expression of transgene mRNA in all major tissues (data not shown). All further analysis has been performed on heterozygous animals, although homozygous animals are similarly viable and breed normally (data not shown).
Diagrammatic representation of the constructs used to generate the Hir-Tg and TFPI-Tg mice.
Diagrammatic representation of the constructs used to generate the Hir-Tg and TFPI-Tg mice.
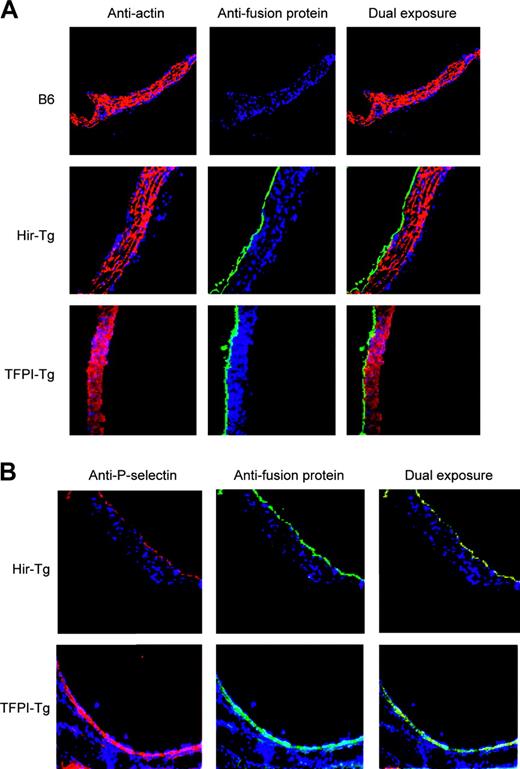
The CD31 promoter fragment was expected to drive expression in ECs, platelets, and leukocytes. Immunohistology of aortas confirmed fusion protein expression limited to the intima (Figure 2A) and complete colocalization with P-selectin, confirming EC expression (Figure 2B). Expression in the ECs of large, medium, and small arterioles and venules was also documented in tissue sections of heart, kidney, lung, liver, and spleen (data not shown).
Immunohistology of mouse aortas using antifusion protein mAb (antihirudin for Hir-Tg and antihuman TFPI for TFPI-Tg). All sections stained with DAPI (4,6 diamidino-2-phenylindole) nuclear stain (blue). (A) Expression of the fusion proteins is restricted to the intima. Antifusion protein mAb (green) contrasting with anti–α-actin mAb (red) used to stain media (original magnification × 100). (B) Colocalization of fusion proteins (green) with P-selectin (red) in ECs of the transgenic mice (original magnification × 100).
Immunohistology of mouse aortas using antifusion protein mAb (antihirudin for Hir-Tg and antihuman TFPI for TFPI-Tg). All sections stained with DAPI (4,6 diamidino-2-phenylindole) nuclear stain (blue). (A) Expression of the fusion proteins is restricted to the intima. Antifusion protein mAb (green) contrasting with anti–α-actin mAb (red) used to stain media (original magnification × 100). (B) Colocalization of fusion proteins (green) with P-selectin (red) in ECs of the transgenic mice (original magnification × 100).
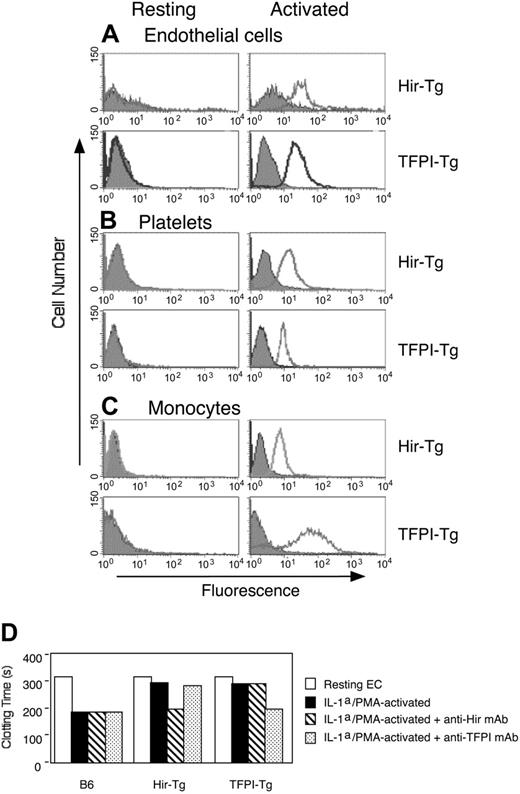
Microvascular ECs were purified from heart, kidneys, and lung and examined by flow cytometry using human TFPI- or hirudin-specific mAb. As illustrated for cardiac ECs in Figure 3A, no fusion protein was detected on resting ECs, but positive staining appeared within several minutes after PMA activation. Fusion protein expression was also demonstrated on platelets and monocytes from the 2 transgenic strains (Figure 3B-C) but only following activation with thrombin and LPS, respectively. Splenocytes, activated by concanavalin A, were negative for either fusion protein (data not shown), highlighting that expression was not seen in CD31– lineages. In clotting assays with mouse plasma, cytokine-activated microvascular ECs from both types of mice inhibited clot formation (Figure 3D), demonstrating that the fusion proteins were biologically active.
Examination of microvascular ECs. (A-C) Flow cytometric analysis of fusion protein expression in microvascular cardiac ECs (A), platelets (B), or monocytes (C) from the 2 transgenic strains. Open profiles indicate antihirudin or anti-TFPI mAb; gray shaded profiles, isotype-matched control mAb; ordinate, cell number; abscissa, intensity of fluorescence. (A) EC phenotype confirmed by expression of CD31 and anti-CD105. Activation induced in vitro by incubation with 1 μM PMA for 30 minutes. (B) Platelet phenotype confirmed by expression of CD41. Activation induced in vitro by incubation with 1 U/mL thrombin for 30 minutes. (C) Monocyte phenotype confirmed by expression of CD14. Resting monocytes purified from saline-treated mice. Activated monocytes purified from LPS/l-NAME–treated mice. (D) Clotting of recalcified mouse plasma in the presence of ECs from B6, Hir-Tg, or TFPI-Tg mice. Comparing results using resting ECs (open bar) from B6 with those using resting ECs from either transgenic strain, P = NS. Comparing results using IL-1α/PMA-activated ECs (filled bars) from B6 with those using similar ECs from either of the transgenic strains, P < .0001. Error bars have been included but are too small to see.
Examination of microvascular ECs. (A-C) Flow cytometric analysis of fusion protein expression in microvascular cardiac ECs (A), platelets (B), or monocytes (C) from the 2 transgenic strains. Open profiles indicate antihirudin or anti-TFPI mAb; gray shaded profiles, isotype-matched control mAb; ordinate, cell number; abscissa, intensity of fluorescence. (A) EC phenotype confirmed by expression of CD31 and anti-CD105. Activation induced in vitro by incubation with 1 μM PMA for 30 minutes. (B) Platelet phenotype confirmed by expression of CD41. Activation induced in vitro by incubation with 1 U/mL thrombin for 30 minutes. (C) Monocyte phenotype confirmed by expression of CD14. Resting monocytes purified from saline-treated mice. Activated monocytes purified from LPS/l-NAME–treated mice. (D) Clotting of recalcified mouse plasma in the presence of ECs from B6, Hir-Tg, or TFPI-Tg mice. Comparing results using resting ECs (open bar) from B6 with those using resting ECs from either transgenic strain, P = NS. Comparing results using IL-1α/PMA-activated ECs (filled bars) from B6 with those using similar ECs from either of the transgenic strains, P < .0001. Error bars have been included but are too small to see.
Histology and coagulopathy in systemic endotoxemia
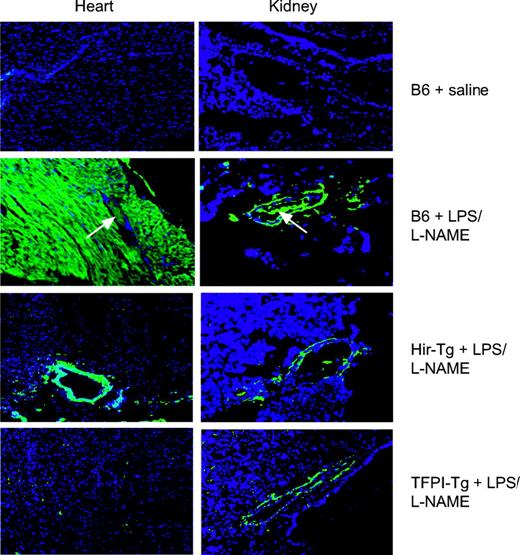
For these studies a protocol to maximize the thrombotic manifestations of endotoxin, as previously described by Jaimes et al, was used.21 Mice were coinjected with LPS and the nitric oxide synthase inhibitor l-NAME to induce systemic endotoxemia and monitored continuously. Following humane killing after 4.5 hours, organs were examined by histology. Representative cardiac and renal sections are shown in Figure 4. By H&E staining, B6 control mice given LPS/l-NAME displayed widespread intravascular thrombosis in subepicardial vessels whereas the vessels in transgenic hearts were fully patent (data not shown). By immunohistology (Figure 4), B6 mice displayed widespread intravascular fibrin deposition as well as fibrin deposits in extravascular sites. In the heart, these were extensive and found throughout the myocardium around cardiac myocytes, whereas in the kidneys extravascular deposits were located mainly in perivascular areas. In contrast, no intravascular fibrin deposition was seen in either of the transgenic mice, and extravascular deposition was largely abrogated, though some residual perivascular fibrin deposition was noted in the heart and kidney.
Immunohistology of heart and kidney from saline- or LPS/l-NAME–treated control and Tg mice. All sections were stained with an antifibrinogen antibody that also detects fibrin (green) and with the nuclear stain DAPI (blue) (original magnification × 100). White arrows indicate vessels with intraluminal clot. No staining was detected in organs from saline-treated Hir-Tg or TFPI-Tg mice (data not shown).
Immunohistology of heart and kidney from saline- or LPS/l-NAME–treated control and Tg mice. All sections were stained with an antifibrinogen antibody that also detects fibrin (green) and with the nuclear stain DAPI (blue) (original magnification × 100). White arrows indicate vessels with intraluminal clot. No staining was detected in organs from saline-treated Hir-Tg or TFPI-Tg mice (data not shown).
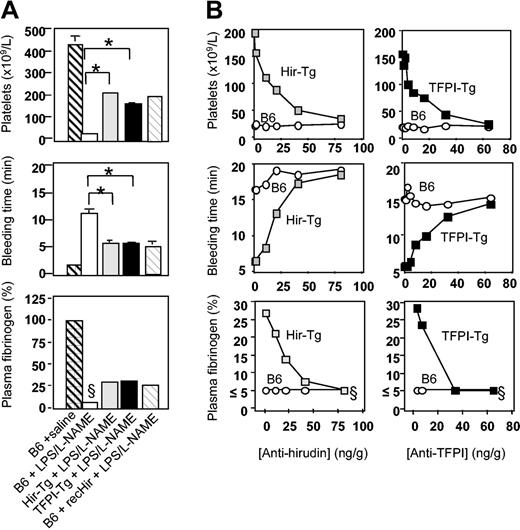
Further analysis was performed to characterize the coagulation abnormalities that had developed 4.5 hours after LPS/l-NAME treatment. Saline-injected TFPI-Tg, Hir-Tg, and fusion protein–negative littermate control mice had platelet counts, bleeding times, and fibrinogen levels that were indistinguishable from those seen in B6 control mice (Figure 5; data not shown for Tg mice or negative littermate controls). After LPS/l-NAME treatment, B6 control mice developed a severe thrombocytopenia, prolonged bleeding times, and profound fibrinogen depletion (Figure 5A). Experiments with B6 control mice confirmed that injection of LPS/l-NAME caused greater derangements of all measured parameters compared with LPS alone (data not shown). Fusion protein–negative littermates of both transgenic strains had responses to LPS/l-NAME that were indistinguishable from B6 (data not shown). In contrast, TFPI-Tg and Hir-Tg mice displayed a markedly modified response to LPS/l-NAME due entirely to the expression of the fusion protein, because coinjection of specific anti-TFPI or antihirudin mAb returned the phenotype to that of B6 controls (Figure 5B). The phenotype of hirudin-Tg mice resembled closely that of B6 control mice given 225 ng/g soluble recombinant hirudin (Figure 5A), further indicating that the phenotype of these mice was due to expression of hirudin fusion protein (because of a shortage of recombinant TFPI, similar studies with this compound have not been possible). No evidence of circulating free TFPI or hirudin was detected in any of the LPS/l-NAME–treated transgenic mice using a modified flow cytometric technique (data not shown).
Coagulation abnormalities after LPS/l-NAME treatment. (A) Consumptive coagulopathy 4.5 hours after administration of LPS and l-NAME. Recombinant hirudin (recHir) was administered to B6 mice at a dose of 225 ng/g at the same time as LPS. Data are derived from 3 to 6 mice per group (error bars = SEM). (B) Inhibition of phenotype in transgenic mice by titration with antifusion protein mAb. Inhibitory mAbs were injected at the same time as LPS. Each data point is from a single mouse. §Clauss fibrinogen assay was stopped at 20 minutes (5% or less normal mouse plasma fibrinogen) if no clot was observed; therefore, this value is minimum fibrinogen concentration detectable by this assay. *Comparing B6 plus LPS/l-NAME with either of the transgenic strains, P < .0001
Coagulation abnormalities after LPS/l-NAME treatment. (A) Consumptive coagulopathy 4.5 hours after administration of LPS and l-NAME. Recombinant hirudin (recHir) was administered to B6 mice at a dose of 225 ng/g at the same time as LPS. Data are derived from 3 to 6 mice per group (error bars = SEM). (B) Inhibition of phenotype in transgenic mice by titration with antifusion protein mAb. Inhibitory mAbs were injected at the same time as LPS. Each data point is from a single mouse. §Clauss fibrinogen assay was stopped at 20 minutes (5% or less normal mouse plasma fibrinogen) if no clot was observed; therefore, this value is minimum fibrinogen concentration detectable by this assay. *Comparing B6 plus LPS/l-NAME with either of the transgenic strains, P < .0001
BM reconstitution experiments
To assess the functional impact of expression of the fusion proteins on ECs alone compared, alternatively, with expression on platelets and monocytes, BM from TFPI-Tg or Hir-Tg mice was infused into irradiated B6 recipients (generating mice termed “BMTFPI → B6” and “BMHir → B6,” respectively) and vice versa (generating mice termed “BMB6 → TFPI” or “BMB6 → Hir,” respectively). All analyses were performed 4 to 5 weeks after reconstitution, at which time average platelet and whole leukocyte counts were 87% (± 2%) and 89% (± 2%), respectively, of unmanipulated B6 control mice.
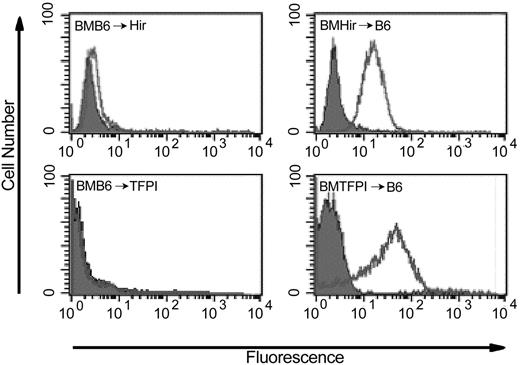
As expected, when analyzed by flow cytometry, expression of the fusion proteins was not evident on the platelets or leukocytes of LPS-treated BMB6 → Hir or BMB6 → TFPI mice (Figure 6) but was present on the platelets of BMHir → B6 and BMTFPI → B6 mice. In these, the proportion of platelets expressing the fusion protein was always more than 95% (Figure 6).
Flow cytometric analysis of hirudin or TFPI fusion protein on platelets from bone marrow–reconstituted mice after administration of LPS and l-NAME. Open profiles indicate antihirudin mAb for BMB6 → Hir and BMHir → B6 and antihuman TFPI mAb for BMB6 → TFPI and BMTFPI → B6; gray shaded profiles, isotype-matched control mAb.
Flow cytometric analysis of hirudin or TFPI fusion protein on platelets from bone marrow–reconstituted mice after administration of LPS and l-NAME. Open profiles indicate antihirudin mAb for BMB6 → Hir and BMHir → B6 and antihuman TFPI mAb for BMB6 → TFPI and BMTFPI → B6; gray shaded profiles, isotype-matched control mAb.
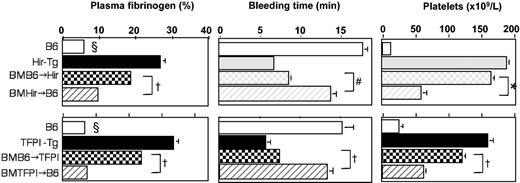
Unmanipulated and BM-reconstituted mice were given LPS/l-NAME and parameters of coagulopathy measured (Figure 7). Compared with B6 controls, all animals showed some alleviation of the coagulopathy, indicating that expression of the fusion protein on either ECs or on platelets and monocytes had an effect and, as anticipated, the unmanipulated transgenic animals (with expression on all 3 cell types) showed the greatest alleviation. When BM-reconstituted animals were compared, the transgenic animals reconstituted with B6 marrow were significantly better protected from the coagulopathy, by all parameters, than B6 animals reconstituted with transgenic marrow. These experiments indicated that greater inhibition of consumptive coagulopathy was achieved when fusion protein expression was limited to ECs in comparison to expression limited to platelets and leukocytes. A small number of B6 and transgenic mice were reconstituted with autologous BM, and after LPS/l-NAME treatment these had phenotypes identical to unmanipulated mice (data not shown).
Consumptive coagulopathy 4.5 hours after administration of LPS and l-NAME in bone marrow–reconstituted mice. Data from unmanipulated Hir-Tg and TFPI-Tg mice are also presented in Figure 4. All data are derived from 3 to 6 mice per group (error bars = SEM). §Clauss fibrinogen assay was stopped after 20 minutes (5% or less normal mouse plasma fibrinogen) if no clot was observed; therefore, this value is minimum fibrinogen value detectable by this assay. Comparing the BM-reconstituted mice. *P = .007, †P < .0001, #P = .0015.
Consumptive coagulopathy 4.5 hours after administration of LPS and l-NAME in bone marrow–reconstituted mice. Data from unmanipulated Hir-Tg and TFPI-Tg mice are also presented in Figure 4. All data are derived from 3 to 6 mice per group (error bars = SEM). §Clauss fibrinogen assay was stopped after 20 minutes (5% or less normal mouse plasma fibrinogen) if no clot was observed; therefore, this value is minimum fibrinogen value detectable by this assay. Comparing the BM-reconstituted mice. *P = .007, †P < .0001, #P = .0015.
Discussion
In this paper, we have described the generation of 2 strains of mice expressing transgenic anticoagulant fusion proteins on activated ECs, platelets, and monocytes. The fusion proteins consisted of human TFPI or hirudin, each linked to the membrane proximal domains of human CD4, under the control of the CD31 promoter. Cell membrane surface expression was regulated by inclusion of a P-selectin cytoplasmic sequence, which directed resting fusion protein expression to secretory granules of CD31+ cells. On tissue sections, expression was restricted to endothelium of visible arteries, arterioles, veins, and venules, whereas flow cytometry of isolated cell populations revealed expression on activated but not resting microvascular ECs, platelets, and monocytes.
Clotting is initiated by the expression of TF.22 During this phase, only a fraction of the circulating factor IX (FIX) and FX is converted to their active forms, FIXa and FXa.23 In the absence of the activated cofactor factor Va (FVa), FXa generates barely detectable levels of thrombin. Although insufficient to initiate significant fibrin polymerisation alone, the trace amounts of thrombin formed are able to back-activate intrinsic pathway cofactors FV and factor VIII, ultimately generating much larger amounts of thrombin, which are sufficient to generate a clot. This “propagation” phase of coagulation occurs on plasma membranes and is dependent on the exposure of procoagulant phospholipids.24
Both strains of transgenic mice showed resistance to the thrombocytopenia, consumptive coagulopathy, and widespread intravascular thrombosis that occurred in wild-type B6 controls after injection of LPS, although neither was completely protected. By this analysis, no significant difference between either strain was observed, suggesting that in both strains the propagation phase was aborted.
BM reconstitution experiments suggested that isolated expression of the tethered fusion proteins on ECs was effective at preventing LPS-induced coagulopathy, because the alleviation of coagulopathy by EC expression was almost as great as that in the unmanipulated transgenic animals. In contrast, isolated expression on platelets and monocytes was not as effective. However, from these data alone, it is difficult to compare the relative efficiency of the anticoagulants on platelets/monocytes with that on ECs, because we have not addressed the likelihood of quantitative differences in the total amount of fusion protein expressed on the pool of platelets and monocytes compared with that expressed on ECs.
We suggest that targeted expression of anticoagulants to the EC surface may be an effective strategy for influencing the pathophysiology of sepsis. As illustrated by the normal baseline bleeding times in these transgenic mice, such a strategy may avoid the potentially dangerous systemic side effects of soluble anticoagulants. In considering how to achieve targeted expression on activated ECs in nontransgenic organisms, 2 broad approaches, tested in other models of thrombosis, may be relevant. The first used anticoagulants covalently linked to antibodies or Fab fragments specific for fibrin25 or activated EC-specific molecules such as EC selectin,26 while the second involved delivery to the endothelium of genetic constructs encoding anticoagulants for local expression.27 We suggest that both be tested in endotoxemia or other sepsis models to determine if the results illustrated here can be replicated using an approach that may have potential for clinical application.
Prepublished online as Blood First Edition Paper, May 4, 2004; DOI 10.1182/blood-2003-12-4365.
Supported by PPL Therapeutics and the Medical Research Council, United Kingdom.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal