Abstract
The study of hematopoiesis has been greatly facilitated by transplantation of blood cell populations into recipient animals. Efficient engraftment of donor cells generally requires ablation of the host hematopoietic system. The zebrafish has recently emerged as a developmental and genetic system to study hematopoiesis. To enable the study of hematopoietic stem cell (HSC) biology, immune cell function, and leukemogenesis in zebrafish, we have developed hematopoietic cell transplantation (HCT) into adult recipient animals conditioned by γ irradiation. Dose-response experiments showed that the minimum lethal dose (MLD) of 40 Gy led to the specific ablation of hematolymphoid cells and death by 14 days after irradiation. Sublethal irradiation doses of 20 Gy predominantly ablated lymphocytes and permitted transplantation of a lethal T-cell leukemia. Finally, transplantation of hematopoietic cells carrying transgenes yielding red fluorescent erythrocytes and green fluorescent leukocytes showed that HCT is sufficient to rescue the MLD, that recipient hematolymphoid tissues were repopulated by donor-derived cells, and that donor blood cell lineages can be independently visualized in living recipients. Together, these results establish transplantation assays to test for HSC function and oncogenic transformation in zebrafish.
Introduction
The study of the biologic effects of γ irradiation began over a century ago,1 but it was not until deployment of the atomic bomb in 1945 that the clinical manifestations of lethal total body irradiation (TBI) were fully realized. Acute irradiation injury was replicated using animal models, and seminal experiments by Jacobsen et al2,3 and Lorenz et al4 showed that shielding or transplantation of hematopoietic tissues was sufficient for radioprotection of otherwise lethal doses. Although humoral factors produced from these tissues were initially proposed to be the radioprotective elements,2 subsequent studies showed that survival correlated with the level of hematopoietic repopulation by donor-derived cells.5-8 Pioneering studies by Till and McCulloch and colleagues showed that donor bone marrow contained rare cells that generated clonal, macroscopic spleen colonies,9,10 a fraction of which regenerated spleen colonies11 or repopulated multilineage hematopoiesis12 in hosts receiving serial transplants. These experiments led to the concept of the hematopoietic stem cell (HSC).
TBI has since been used to condition hosts prior to transplantation of donor bone marrow, both therapeutically in humans and as a means of testing murine blood cell subsets for the presence of HSCs. The development of monoclonal antibodies and multiparameter flow cytometry, combined with hematopoietic cell transplantation (HCT), has led to the prospective isolation of murine HSCs,13-16 committed lymphoid17 and myeloerythroid18 progenitors, and their downstream progeny by cell-surface phenotypes. These powerful techniques have allowed individual components of the immune system to be tested functionally, which has led to the dissection of the hematopoietic hierarchy and the resolution of effector cell function. HCT also provides the means to study the effects of genetic mutations in isolated cell types when introduced into a normal cellular environment.
The zebrafish has recently emerged as a unique vertebrate model in which forward genetic screens can uncover novel genes involved in blood cell development and function.19 Many zebrafish blood mutants identified in these screens are embryonic lethal, and we have recently described embryonic HCT to address issues of cell autonomous mutant gene function.20 Similar transplantation of whole kidney marrow (WKM) cells into unconditioned adult recipients resulted in the disappearance of donor-derived cells within several weeks of transplantation. This suggests that donor-derived cells are either rejected by the host immune system or that engraftment of donor cells requires the creation of niche space in host hematopoietic tissues.
Here we present the first study of the biologic response to ionizing irradiation in adult zebrafish. We determine that the minimum lethal dose (MLD) specifically ablates the zebrafish blood-forming system, and show that transplantation of WKM is sufficient for radioprotection. We also demonstrate that sublethal irradiation doses transiently ablate lymphocytes, which is both necessary and sufficient for the transfer of leukemia from rag2-EGFP-mMyc transgenic zebrafish. Similar to mammals, these studies show that the zebrafish hematopoietic system is the first to fail following increasing doses of γ irradiation. Transplantation into conditioned adults now provides the means to test for oncogenic transformation, HSC enrichment strategies, and immune cell function in a genetically tractable vertebrate organism.
Materials and methods
Zebrafish
Zebrafish were mated, staged, and raised as described21 and maintained in accordance with Children's Hospital animal research guidelines. Kaplan-Meier survival experiments were performed on age- and sex-matched adult zebrafish purchased from EkkWill Aquatic suppliers (Gibsonton, FL). Transgenic β-actineGFP and GATA-1dsRED zebrafish were generated as previously described.20
Irradiation
Adult zebrafish were anesthetized with 0.02% tricaine then placed 5 at a time into 60-mm by 15-mm Petri dishes (Falcon, Bedford, MA) containing fish water. Each dish was wrapped in parafilm then placed atop a 3-inch spacer to achieve a dose rate of 978 cGy/min in a 137Cesium source irradiator. Calibration of the machine (Gammacell 1000; MDS Nordion, Ottawa, ON, Canada) showed this dose rate to be uniform among calibration chips placed within euthanized animals under water, under water alone, or in air alone, verifying that the tissue dosage via TBI was accurate.
Cell collection
Wild-type adult zebrafish were anesthetized with 0.02% tricaine prior to blood, kidney, and spleen collection. The spleen and kidney were dissected following a ventral, midline incision and placed into ice-cold 0.9X phosphate-buffered saline (PBS) containing 5% fetal calf serum. Single-cell suspensions were generated by aspiration followed by gentle teasing of each organ atop a 40-μm nylon mesh filter using a plunger from a 1-cc syringe.
Histology and cytology
Adult animals were killed and placed in 4% paraformaldehyde at 4° C for 4 days and then transferred to 0.25 M EDTA [ethylenediaminetetraacetic acid], pH 8.0, for no less than 2 days.22 Fish were then dehydrated in alcohol, cleared in xylene, and infiltrated with paraffin. Tissue sections (4 μm) from paraffin-embedded tissue blocks were placed on charged slides, deparaffinized in xylene, rehydrated through graded alcohol solutions, and stained with hematoxylin and eosin. For detection of green fluorescent protein (GFP), slides were deparaffinized and pretreated with 10 mM citrate, pH 6.0 (Zymed, South San Francisco, CA), in a steam pressure cooker (Decloaking Chamber, BioCare Medical, Walnut Creek, CA) as per the manufacturer's instructions, followed by washing in distilled water. All additional steps were performed at room temperature in a hydrated chamber. Slides were pretreated with Peroxidase Block (Dako, Carpinteria, CA) for 5 minutes to quench endogenous peroxidase activity, followed by a 1:5 dilution of goat serum in 50 mM Tris-Cl, pH 7.4, for 20 minutes to block nonspecific binding sites. Primary murine anti-GFP antibody (clone JL-8; Clontech, Palo Alto, CA) was applied at a 1:1000 dilution in 50 mM Tris-Cl, pH 7.4, with 3% goat serum for one hour. After washing in 50 mM Tris-Cl, pH 7.4, goat antimouse horseradish peroxidase–conjugated antibody (Envision detection kit; Dako) was applied for 30 minutes. Immunoperoxidase staining was developed using a diaminobenzidine (DAB)–positive chromogen kit (Dako) per manufacturer's instructions and counterstained with hematoxylin. Transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining was performed using an Apoptag kit according to the manufacturer's instructions (Serologicals, Norcross, GA). Cytospin preparations were performed using 1 × 105 to 2 × 105 kidney cells or splenocytes cytocentrifuged at 300 rpm for 3 minutes onto glass slides (Shandon, Waltham, MA). Cytospin preparations were processed through May-Grünwald and Giemsa stains (Sigma-Aldrich-Fluka, St Louis, MO) for morphologic analyses. Total cell counts were performed using a Brightline Hemocytometer (Fisher Scientific, Hampton, NH) and Türks stain (Stem Cell Technologies, Vancouver, BC, Canada). Visible light imaging was performed on an Olympus BX-51 microscope (Olympus, Melville, NY) using 40 × and 100 × oil objectives and a QImaging micropublisher digital camera and software (Burnaby, BC, Canada).
Flow cytometry
Hematopoietic cells isolated from wild-type zebrafish were processed as above, washed, and resuspended in ice-cold 0.9x PBS plus 5% fetal bovine serum (FBS) and passed through a 40-μm filter. Propidium iodide (PI; Sigma, St Louis, MO) was added to 1 μg/mL to exclude dead cells and debris. FACS analysis and sorting was performed based on PI exclusion, forward scatter, and side scatter using a FACS Vantage flow cytometer (Becton Dickinson, Hialeah, FL).
Leukemia transplantation
Leukemic cell suspensions were prepared from moribund transgenic zRag2-GFP fish as previously described.23 Two days after irradiation with 20 Gy, 1 million or 5 million cells were injected intraperitoneally into wild-type recipients using a 10-μL Hamilton (Reno, NV) syringe following anesthesia with tricaine.
Hematopoietic cell transplantation
Whole kidney marrow cells were isolated from wild-type or transgenic adult donors as described in “Cell collection” and filtered and washed 3 times before resuspending at a final concentration of 2.0 × 105 cells per microliter in 0.9X PBS containing 5% fetal calf serum (FCS). DnaseI (1 unit; Gibco, Carlsbad, CA) and heparin (3 units; Sigma) were added to lessen aggregation. Transplant recipients were anesthetized in tricaine and injected intracardially with approximately 1 × 106 WKM cells using borosilicate glass capillary needles (1-mm outer diameter [OD], no filament; World Precision Instruments, Sarasota, FL) made using a Flaming/Brown micropipette puller (Sutter Instruments, Novato, CA). Cell suspensions were backloaded into each needle and injected into circulation by forced air using a Narishige injection station and hand-held needle holder. Animals that received transplants of transgenic WKM were visualized weekly under an inverted fluorescent microscope (Leica DM-IRE2; Leica, Heidelberg, Germany) to monitor GFP+ and dsRED+ cells over the first 30 days after transplantation. Imaging of transplant recipients was performed using a Hamamatsu Orca-ER digital camera (Hamamatsu, Hamamatsu City, Japan) and Openlab software (Improvision, Lexington, MA).
Results
Survival of adult zebrafish following total body irradiation
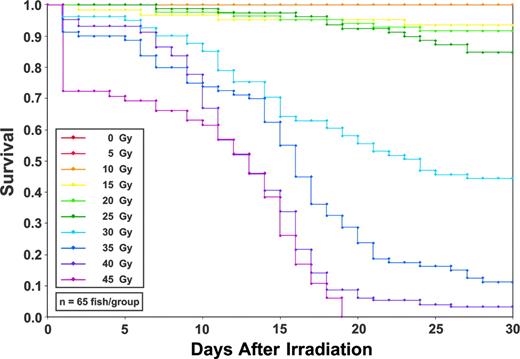
In order to determine the approximate MLD of γ irradiation, we administered graded doses of radiation to groups of 10 adult zebrafish in 5-Gy increments from 0 to 50 Gy. Animals receiving between 0 Gy and 25 Gy showed more than 80% survival for up to 4 months, whereas doses of more than 45 Gy showed high mortality on day one after irradiation (not shown). Based on these results, large-scale Kaplan-Meier survival curves were generated by irradiating 10 groups of 65 fish each at 5-Gy intervals between 0 Gy and 45 Gy (Figure 1). The MLD, which we initially defined as less than 10% survival over 30 days, was 40 Gy. Compared with the steep drop in survival between 12 and 14 days following the MLD in mice, zebrafish survival showed a gradual and consistent decline over 21 days (Figure 1). Approximately 5% of the cohort receiving a 40-Gy dose survived past this time point. These outliers are likely due to the genetic variability present in the outbred animals used in this large-scale experiment, since subsequent experiments using standard laboratory strains that are less polymorphic showed more uniform death rates at identical doses.
Kaplan-Meier survival curves following graded doses of total body irradiation. Groups of 65 adult zebrafish were irradiated at doses from 0 Gy to 45 Gy in 5-Gy increments. The minimum lethal dose is 40 Gy, as defined as the dose resulting in less than 10% survival over 30 days after irradiation.
Kaplan-Meier survival curves following graded doses of total body irradiation. Groups of 65 adult zebrafish were irradiated at doses from 0 Gy to 45 Gy in 5-Gy increments. The minimum lethal dose is 40 Gy, as defined as the dose resulting in less than 10% survival over 30 days after irradiation.
Histologic analyses following irradiation
To assess the effects of irradiation, parallel studies using other groups of animals were performed. Animals were killed on days 1 to 4, 8, 12, 16, and 20 after irradiation for histologic analyses. Morphologic analysis of whole animal sections stained with hematoxylin and eosin over 0 Gy to 45 Gy showed an apparent threshold dose of 20 Gy that specifically ablated the majority of cells within hematopoietic tissues. Compared with unirradiated tissue sections, sections from animals receiving 20 Gy showed a time-dependent decrease in cellularity in the kidney, the bone marrow equivalent in teleosts, and the thymus (Figure 2A). By 8 days after irradiation, cellularity in each tissue reached a nadir but then recovered to preirradiation levels by day 20 (Figure 2A). No significant alterations in cellularity or tissue architecture were observed in other organ systems, with the exception of the testis (not shown).
Histologic analyses following irradiation. (A) Histology of hematolymphoid tissue over time following sublethal irradiation. Compared with unirradiated controls (top left panel), kidney interrenal cells decrease daily following a dose of 20 Gy until reaching a nadir on day 8. Thymic sections (bottom panels) show similar decreases in thymocytes over time. By day 8, most thymi are involuted and difficult to detect. After a dose of 20 Gy, both the kidney and thymus recover cellularity by day 20 (right panels). T indicates renal tubule. (B) Histology of hematolymphoid tissue following lethal irradiation. Compared with unirradiated controls (left panels), kidney and thymic hematopoietic cells are largely depleted by day 12 (right panels). Unlike the sublethal time course, hematopoietic cells do not recover after a nadir on day 8 following a dose of 40 Gy. Tips of red chevrons point to erythrocytes, and green chevrons point to leukocytes. (C) TUNEL staining of kidney and thymus sections at 10 hours after irradiation. Both tissues showed a dose-dependent increase in the number of dying cells. (D) Effect of 40 Gy on nonhematopoietic tissues. Compared with unirradiated controls (top panels), only the testis exhibited gross alterations, with spermatogonial progenitor cells (arrowheads) disappearing by day 12 (bottom left panel). Other tissues receiving a 40-Gy dose, including the intestine, gill, liver, and pancreas, did not show gross abnormalities (bottom panels).
Histologic analyses following irradiation. (A) Histology of hematolymphoid tissue over time following sublethal irradiation. Compared with unirradiated controls (top left panel), kidney interrenal cells decrease daily following a dose of 20 Gy until reaching a nadir on day 8. Thymic sections (bottom panels) show similar decreases in thymocytes over time. By day 8, most thymi are involuted and difficult to detect. After a dose of 20 Gy, both the kidney and thymus recover cellularity by day 20 (right panels). T indicates renal tubule. (B) Histology of hematolymphoid tissue following lethal irradiation. Compared with unirradiated controls (left panels), kidney and thymic hematopoietic cells are largely depleted by day 12 (right panels). Unlike the sublethal time course, hematopoietic cells do not recover after a nadir on day 8 following a dose of 40 Gy. Tips of red chevrons point to erythrocytes, and green chevrons point to leukocytes. (C) TUNEL staining of kidney and thymus sections at 10 hours after irradiation. Both tissues showed a dose-dependent increase in the number of dying cells. (D) Effect of 40 Gy on nonhematopoietic tissues. Compared with unirradiated controls (top panels), only the testis exhibited gross alterations, with spermatogonial progenitor cells (arrowheads) disappearing by day 12 (bottom left panel). Other tissues receiving a 40-Gy dose, including the intestine, gill, liver, and pancreas, did not show gross abnormalities (bottom panels).
Morphologic analyses of animals receiving the MLD of 40 Gy showed similar but more pronounced effects on hematolymphoid tissues. Hematopoietic cells within the kidney and thymus showed a similar time-dependent decrease in cellularity and were almost completely absent by day 12 (Figure 2B). Unlike animals receiving 20 Gy, however, cell numbers did not recover after this time point. We performed TUNEL staining to determine whether hematolymphoid cells died by apoptosis following irradiation doses of 20 Gy or 40 Gy. As shown in Figure 2C, unirradiated kidney sections showed little to no TUNEL+ cells, whereas kidney sections from irradiated animals showed a dose-dependent increase in dying cells at 10 hours after irradiation. Thymus sections showed limited cell death in unirradiated animals, whereas 20-Gy doses led to most cells becoming TUNEL+ and 40-Gy doses led to nearly all cells exhibiting TUNEL positivity by 10 hours (Figure 2C). Histologic analyses of other organ systems showed no abnormalities indicative of organ failure (Figure 2D). As also observed at 20 Gy, the testis showed a specific loss of spermatogonia following 40-Gy doses (Figure 2D). These spermatocyte precursors deceased over time and were largely absent by day 8 following doses as low as 10 Gy. Following a dose of 20 Gy, spermatogonia regenerated to unirradiated levels by day 20, resulting in restoration of fertility by day 30 (not shown).
Kinetic analyses of hematopoietic cells following sublethal irradiation
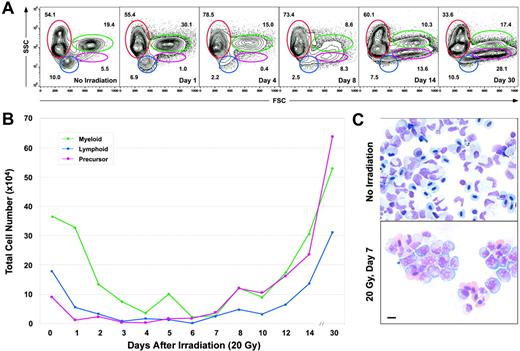
We have recently reported that the major blood lineages in the zebrafish kidney can be resolved using light scatter characteristics by flow cytometry (FACS).20 To enumerate changes in each blood cell lineage within kidney marrow, we examined a cohort of animals by FACS over one month following irradiation at 20 Gy. Relative changes to each of the erythroid, myeloid, lymphoid, and precursor populations are presented in Figure 3A, and mean total cell numbers for each lineage (total kidney count × percentage of each lineage) are presented in Figure 3B. One day after irradiation, the percentages of lymphoid and precursor cells decreased, resulting in relative increases in the erythroid and myeloid fractions. By day 4 after irradiation, large decreases in each of the nonerythroid lineages were apparent. As shown in Figure 3B, total cell counts for each of these lineages showed approximate 10-fold, 11-fold, and 35-fold decreases by day 4 for myeloid, lymphoid, and precursor cells, respectively. Total numbers of erythroid cells were omitted from our analyses because variable numbers are recovered during dissection of the kidney due to its intimate association with the dorsal aorta.
Kinetics of disappearance of kidney blood cell lineages following sublethal irradiation. (A) Light scatter profiles of whole kidney marrow cells showing the effects of 20 Gy on the relative percentages of erythrocytes (red gate), lymphocytes (blue gate), precursors (pink gate), and myeloid cells (green gate) over time. At each time point, 5 animals were killed; the mean values for each lineage are presented in the FACS plots. Representative plots were chosen from each time point based on these mean values. (B) Absolute cell counts of each leukocyte subset over time. The numbers of each population decreased to their low points by days 4 to 6. Cell counts gradually increased after this point until reaching numbers much higher than pre-irradiation levels by day 30. (C) Cytospin preparation of unirradiated (top) versus irradiated (bottom) WKM on day 7, showing that most cells within irradiated WKM display immature morphologies. Scale bar equals 10 μm.
Kinetics of disappearance of kidney blood cell lineages following sublethal irradiation. (A) Light scatter profiles of whole kidney marrow cells showing the effects of 20 Gy on the relative percentages of erythrocytes (red gate), lymphocytes (blue gate), precursors (pink gate), and myeloid cells (green gate) over time. At each time point, 5 animals were killed; the mean values for each lineage are presented in the FACS plots. Representative plots were chosen from each time point based on these mean values. (B) Absolute cell counts of each leukocyte subset over time. The numbers of each population decreased to their low points by days 4 to 6. Cell counts gradually increased after this point until reaching numbers much higher than pre-irradiation levels by day 30. (C) Cytospin preparation of unirradiated (top) versus irradiated (bottom) WKM on day 7, showing that most cells within irradiated WKM display immature morphologies. Scale bar equals 10 μm.
A large relative increase in the precursor fraction, where all hematopoietic progenitors appear to reside,20 was observed by day 8, and cytospin preparations of WKM at this time showed the majority of cells to have the immature morphology of hematopoietic progenitors (Figure 3C). The precursor population continued to increase over time, and by day 30 reached more than a 6-fold increase in both relative and absolute cell numbers (Figure 3A-B) compared with unirradiated controls. Accordingly, the absolute numbers of myeloid and lymphoid cells also increased over their pre-irradiation levels.
Sublethal irradiation is necessary and sufficient for transfer of lethal T-cell leukemia
The benchmark for oncogenic transformation is transfer of disease to animals receiving transplants. Zebrafish carrying a rag2-EGFP-mMyc fusion transgene develop a lethal T-cell leukemia that can be transferred to irradiated adult recipients and visualized by fluorescent microscopy.23 In this report, we wished to test whether irradiation is necessary to transfer disease to wild-type hosts. Since a sublethal dose of 20 Gy both ablates the majority of lymphocytes and creates niche space in all hematolymphoid tissues, we chose this dose for all leukemia transplant experiments. Animals were either irradiated or mock-irradiated then received transplants of graded numbers of leukemic lymphoblasts 2 days later. Transplant recipients were monitored daily for survival. As shown in Figure 4A, only irradiated animals died after transplantation. Increasing cell dose led to a more rapid death, and GFP+ tumor cells could be seen colonizing the thymus and subsequently spreading throughout the body (Figure 4B). At 25 days after transplantation of 1 × 106 leukemic cells, several moribund animals were killed and analyzed by flow cytometry. Compared with unirradiated transplant recipients with no sign of disease (Figure 4C left), WKM from irradiated transplant recipients was largely comprised of leukemic lymphoblasts (Figure 4C middle and right), and displayed FACS profiles similar to those of primary leukemic animals. Doses as low as 5 × 103 cells were found to transfer leukemia to irradiated recipients (not shown) whereas unirradiated animals never developed disease, even at doses as high as 5 × 106 cells. Transplantation of aberrant cell types can thus be performed following sublethal irradiation to test oncogenic potential in zebrafish.
Transplantation of lethal T-cell leukemia requires irradiation. (A) Kaplan-Meier survival curves following transplantation of leukemic T cells into recipient animals receiving either no irradiation or 20 Gy of TBI. Performing transplantation into irradiated animals led to a dose-dependent decrease in survival over 30 days, whereas unirradiated controls remained healthy. (B) GFP+ donor cells colonize recipient thymi only after irradiation. (C) Examination of moribund animals 25 days after irradiation and transplantation showed the kidney to be almost completely replaced by leukemic lymphoblasts by FACS (middle) and WKM cytospins (right).
Transplantation of lethal T-cell leukemia requires irradiation. (A) Kaplan-Meier survival curves following transplantation of leukemic T cells into recipient animals receiving either no irradiation or 20 Gy of TBI. Performing transplantation into irradiated animals led to a dose-dependent decrease in survival over 30 days, whereas unirradiated controls remained healthy. (B) GFP+ donor cells colonize recipient thymi only after irradiation. (C) Examination of moribund animals 25 days after irradiation and transplantation showed the kidney to be almost completely replaced by leukemic lymphoblasts by FACS (middle) and WKM cytospins (right).
Kinetic analyses of hematopoietic cells following lethal irradiation
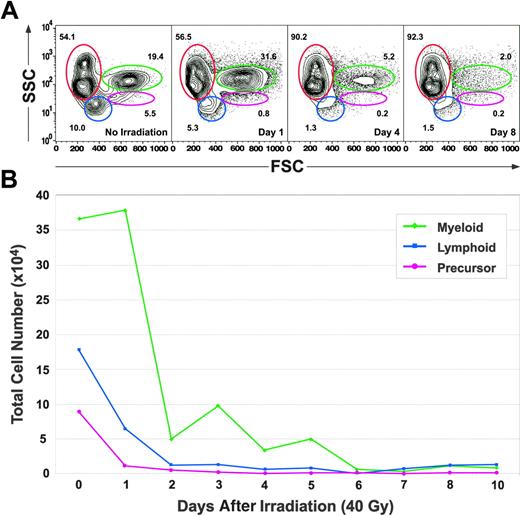
We next analyzed the kidneys of animals receiving lethal doses of 40 Gy. One day after irradiation, significant reductions were seen in the lymphoid and precursor populations, and the relative ratios of each blood cell lineage appeared similar to those reported in Figure 3 at the 20-Gy dose. By 4 days, the vast majority of nonerythroid populations were depleted from the kidney (Figure 5A). Absolute cell counts confirmed this depletion, with myeloid cells showing an 11-fold decrease, lymphoid cells a 29-fold decrease, and precursor cells a 115-fold decrease. Unlike sublethally irradiated animals, the nadir of absolute cell counts on day 6 did not recover (Figure 5B), but remained near zero until day 10, the last time point at which the animals were analyzed before death.
Kinetics of disappearance of kidney blood cell lineages following lethal irradiation. (A) Light scatter profiles of whole kidney marrow showing the effects of 40 Gy on the relative percentages of erythrocytes (red gate), lymphocytes (blue gate), precursors (pink gate), and myeloid cells (green gate) over time. (B) Absolute cell counts of each leukocyte subset over time. The numbers of each population decreased to their low points by day 6 and did not subsequently recover before death.
Kinetics of disappearance of kidney blood cell lineages following lethal irradiation. (A) Light scatter profiles of whole kidney marrow showing the effects of 40 Gy on the relative percentages of erythrocytes (red gate), lymphocytes (blue gate), precursors (pink gate), and myeloid cells (green gate) over time. (B) Absolute cell counts of each leukocyte subset over time. The numbers of each population decreased to their low points by day 6 and did not subsequently recover before death.
Transplantation of whole kidney marrow is sufficient for radioprotection
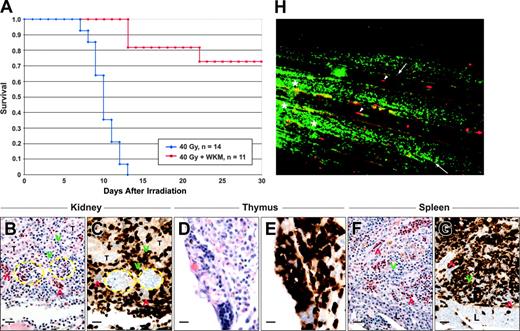
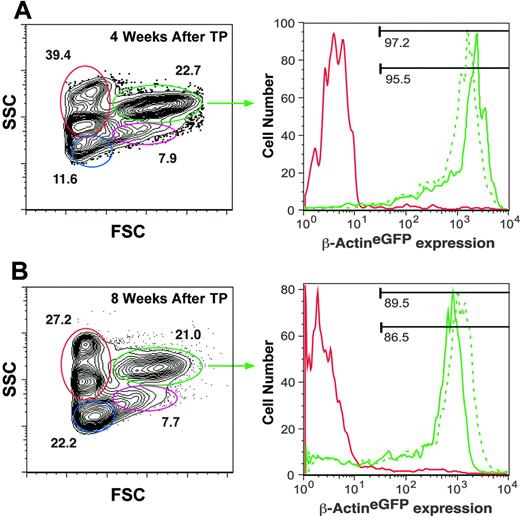
That the MLD specifically ablates cells within blood-forming tissues, and that nearly all leukocyte subsets are depleted from the kidney within a week before death suggests that irradiation-induced mortality is due to hematopoietic failure. If hematopoietic failure is the only lethal consequence of a 40-Gy dose, then HCT should rescue survival of recipient animals. Our previous results demonstrated that the adult zebrafish kidney contains long-term HSCs, as well as their downstream progenitor cells.20 We thus performed transplantation with one kidney equivalent into lethally irradiated animals 2 days after irradiation. Compared to control animals that did not receive transplants, which all died by 14 days after irradiation, animals receiving WKM transplants showed over 70% survival over 30 days (Figure 6A). This radioprotection was associated with repopulation of the kidney marrow, thymus, and spleen. Transplantation of WKM from β-actineGFP transgenic animals showed that repopulation of each hematopoietic tissue derived from donor cells (Figure 6B-G). Transplantation of WKM from β-actineGFP × GATA-1dsRED double-transgenic animals permitted independent visualization of donor erythrocytes and donor leukocytes by fluorescent videomicroscopy (Figure 6H). In this way, multilineage transplant engraftment efficiency can be monitored over time in living recipient animals. Transplantation of double-transgenic WKM cells also permits the relative contribution of donor cells to steady-state hematopoiesis to be measured by FACS. In β-actineGFP animals, over 95% of cells within the kidney myeloid scatter fraction express GFP (not shown). This finding, in combination with the relatively short lifespans of myelomonocytic cells, allows donor contribution to be accurately quantitated in a transplantation setting. As shown in Figure 7, analysis of individual animals that received transplants of double-transgenic WKM following the MLD showed that over 95% of kidney myeloid cells were donor-derived at 4 weeks after transplantation and more than 86% were donor-derived at 8 weeks after transplantation. These results confirm the findings presented in Figure 6B-G that the vast majority of leukocytes present in the hematolymphoid tissues of rescued recipients are of donor derivation.
Hematopoietic cell transplantation rescues survival of the minimum lethal dose. (A) Kaplan-Meier survival curves of adult zebrafish receiving a dose of 40 Gy with (red line) or without (blue line) transplantation of 1 × 106 WKM. All irradiated animals not receiving transplants died by 14 days. By contrast, more than 70% of animals that received a transplant survived for at least 30 days. (B-G) Animals receiving transplants of transgenic β-actineGFP WKM cells that survived 30 days showed robust reconstitution of hematolymphoid tissues by donor-derived cells, including the kidney (B-C), thymus (D-E), and spleen (F-G). Sections stained for GFP are serial sections following those stained with hematoxylin and eosin and are separated by 4 to 12 μm. Dashed yellow lines demarcate glomeruli; red chevrons, erythrocytes; and green chevrons, leukocytes. T indicates renal tubules; and L, liver. Scale bar equals 20 μm. (H) Transplantation of double-transgenic β-actineGFP × GATA-1dsRED WKM allows visualization of multilineage hematopoietic reconstitution in living recipients. Still image from the Supplemental Video (see the Supplemental Video link at the top of the online article on the Blood website) shows a closeup (× 100) view of the capillary network in the tail of an HCT recipient at 30 days after transplantation. Arrows denote donor-derived leukocytes and arrowheads show donor-derived erythrocytes. Asterisk denotes regions of skin autofluorescence.
Hematopoietic cell transplantation rescues survival of the minimum lethal dose. (A) Kaplan-Meier survival curves of adult zebrafish receiving a dose of 40 Gy with (red line) or without (blue line) transplantation of 1 × 106 WKM. All irradiated animals not receiving transplants died by 14 days. By contrast, more than 70% of animals that received a transplant survived for at least 30 days. (B-G) Animals receiving transplants of transgenic β-actineGFP WKM cells that survived 30 days showed robust reconstitution of hematolymphoid tissues by donor-derived cells, including the kidney (B-C), thymus (D-E), and spleen (F-G). Sections stained for GFP are serial sections following those stained with hematoxylin and eosin and are separated by 4 to 12 μm. Dashed yellow lines demarcate glomeruli; red chevrons, erythrocytes; and green chevrons, leukocytes. T indicates renal tubules; and L, liver. Scale bar equals 20 μm. (H) Transplantation of double-transgenic β-actineGFP × GATA-1dsRED WKM allows visualization of multilineage hematopoietic reconstitution in living recipients. Still image from the Supplemental Video (see the Supplemental Video link at the top of the online article on the Blood website) shows a closeup (× 100) view of the capillary network in the tail of an HCT recipient at 30 days after transplantation. Arrows denote donor-derived leukocytes and arrowheads show donor-derived erythrocytes. Asterisk denotes regions of skin autofluorescence.
FACS analysis of irradiated recipients rescued by transplantation of WKM cells carrying a β-actineGFP transgene. (A) Visualization of kidney scatter populations (left) shows that 97% of myeloid cells (green gate) in recipient 1 (dotted green histogram, right panel) and 95% of myeloid cells in recipient 2 (solid green histogram, right panel) are donor-derived at 4 weeks after transplantation based on expression of the GFP transgene. (B) Similar analysis of 2 transplant recipients at 8 weeks after transplantation shows 89% and 86% donor-derived myeloid cells. Erythroid cells (red gate, left panels) do not express the β-actineGFP transgene and were used as internal negative controls for GFP expression (solid red histograms, right panels).
FACS analysis of irradiated recipients rescued by transplantation of WKM cells carrying a β-actineGFP transgene. (A) Visualization of kidney scatter populations (left) shows that 97% of myeloid cells (green gate) in recipient 1 (dotted green histogram, right panel) and 95% of myeloid cells in recipient 2 (solid green histogram, right panel) are donor-derived at 4 weeks after transplantation based on expression of the GFP transgene. (B) Similar analysis of 2 transplant recipients at 8 weeks after transplantation shows 89% and 86% donor-derived myeloid cells. Erythroid cells (red gate, left panels) do not express the β-actineGFP transgene and were used as internal negative controls for GFP expression (solid red histograms, right panels).
Discussion
The sensitivity of mammalian cells to γ radiation has led to many important discoveries, both in clinical medicine and in basic research. The clinical application of x-rays to cancer treatment in fact began only 1 year after their discovery by Roentgen in 1895, and this first experiment suggested that X irradiation could lead to palliative effects against breast tumors.1 Radiation therapy is now commonly used against many tumor types, and when followed by bone marrow transplantation (BMT) can be administered at supralethal doses against particularly aggressive hematologic malignancies.24,25 In animal models of cancer, irradiation has been important in the ablation of the immune response, which permits the transplantation of aberrant populations to test oncogenic potential. Transplantation to test transformation has become the gold standard in mouse cancer models, and we now present evidence that a similar approach can be used in the zebrafish.
In our dose-response experiments, we determined that at a threshold of approximately 20 Gy, cells within hematolymphoid tissues were largely ablated within one week. This effect was transient, however, and total numbers of all hematopoietic populations recovered within one month. Of particular relevance for cancer studies, lymphoid cells were almost completely depleted within 3 days. Previous studies showed that Myc-induced leukemias could be transplanted into irradiated recipients.23 We were uncertain, however, whether irradiation was required for transfer of disease. Together, our current results show that irradiation is both necessary and sufficient for propagation of lethal leukemia in animals that received a transplant. When transplanted into irradiated animals, doses as low as 5 × 103 leukemic cells could confer lethal disease, demonstrating frank cellular transformation and that this model of acute leukemia is particularly aggressive. That transplant doses as high as 5 × 106 cells could not transfer leukemia in unconditioned recipients suggests that leukemic cells are recognized as foreign and rejected by the host immune system. It will now be possible to test whether this sublethal irradiation regimen is sufficient for the transplantation of other hematopoietic malignancies as well as solid tumors from zebrafish harboring cancer-predisposing mutations.
The seminal murine studies showing that acute irradiation syndrome could be rescued by BMT demonstrated that the MLD specifically causes death by hematopoietic failure.4,26 In this report we demonstrate that, despite nearly 450 million years of evolutionary divergence from mammals, the major consequences following graded doses of ionizing irradiation are quite similar in zebrafish. Our studies show that the MLD is approximately 40 Gy, and that this dose is lethal due to hematopoietic failure. The kinetics of hematopoietic cell depletion over the first 2 days after irradiation are quite similar to those initially reported in mouse bone marrow and spleen following the MLD of 9 Gy.26 Daily sections performed following 40-Gy doses in zebrafish showed that the vast majority of hematopoietic cells were depleted in the kidney and thymus. Compared with unirradiated controls, mean cell counts of each of the kidney myeloid, lymphoid, and precursor populations showed over 7-, 14-, and 16-fold decreases in absolute cell numbers, with the total kidney leukocyte number dropping from an average of 6.3 × 105 to 6.8 × 103 by day 2. All cell lineages continued to decline over time until death. Continually decreasing cellularity was observed only in hematopoietic tissues, suggesting that a dose of 40 Gy leads specifically to hematopoietic failure.
Also similar to results in early rodent models, reconstitution of irradiated zebrafish by HCT showed radioprotective effects and correlated with donor cell repopulation of host hematolymphoid tissues over time. The first experiments in which lethally irradiated mice underwent BMTs showed approximately 75% survival over 28 days.4 Our results are very similar, and like the 25% of mice that did not survive over one month, we found that zebrafish killed when appearing moribund generally showed a failure of hematopoietic engraftment. In addition to the engraftment failure observed in the mouse, bacteremia was usually seen in these animals between 5 and 12 days after transplantation.4 By contrast, we never observed bacteremia in irradiated zebrafish, even in the absence of HCT. Rather, we occasionally observed the apparent outgrowth of Pseudoloma neurophila (microsporidia), an opportunistic pathogen that we rarely found in untreated animals. Large cysts of microsporidia were often observed throughout the tissues of irradiated animals that either did not receive a transplant or failed to engraft transplanted donor cells. We did not observe appreciable outgrowth in those recipients showing robust blood cell repopulation, suggesting that propagation of these pathogens occurred predominantly in the absence of hematopoietic reconstitution. Future studies should address whether outgrowth of microsporidia can be prevented by drug treatment.
Following sublethal irradiation, hematopoiesis recovers despite the transient ablation of nearly all hematopoietic progenitor cells. This suggests that HSCs are spared, and that sublethal irradiation may be useful for enriching stem cell subsets akin to the use of 5-fluorouracil treatment in mice.27,28 The response to sublethal irradiation may also be useful for genetic screens designed to uncover functional defects in the repopulation of blood cells by HSCs. Since spermatogonial progenitors are also transiently ablated in the testis, abnormal repopulation of this organ could simultaneously be assessed to find stem cell mutations in the male germ line. It may also be possible to identify mutants that are either significantly radiosensitive or radioresistant. Our survival results using outbred zebrafish were much more variable when compared with laboratory strains, suggesting that the response to irradiation may be affected by genetic determinants.
One major difference between the results published in this study and the results of studies done with mammals is the relatively large dose of irradiation required for the MLD. The lethal dose for mice is generally 9 Gy to 10 Gy, whereas we have found that zebrafish require approximately 40 Gy. One contributing difference may be genome size. The zebrafish genome is approximately 1.7 × 109 bp, which is roughly half the size of the mouse genome. Since a smaller genome requires less DNA repair, the irradiation dose necessary to elicit the same biologic response observed in mice would therefore be larger, assuming the efficiency of DNA repair is equivalent between species. If the relationship of dose response to genome size is linear, however, the approximate 4-fold difference in the MLD between zebrafish and mice is likely more complex than genome size alone would dictate. Perhaps the most important difference in terms of the biologic response to irradiation between teleosts and mammals is temperature. Whereas mammals maintain a constant temperature relative to their environments, teleosts are poikilothermic and thus are dependent on ambient conditions. In general, cold-blooded animals have been shown to be more radioresistant than warm-blooded animals.29 Previous studies in other teleosts such as goldfish (Carassius auratus) and medaka (Oryzias latipes) have shown MLDs of approximately 30 Gy to 50 Gy,30-32 similar to the MLD reported here for zebrafish. Reports on the effects of temperature on the MLD in many organisms, including teleosts, have shown a strong negative correlation between temperature and radioresistance.29 In mice, for example, the temporary reduction in ambient temperature to 0° C to 0.5° C causes the lethal dose at which half of the animals die over 30 days (LD 50/30) to increase from 6.2 Gy to 18 Gy if the animals are rewarmed 30 minutes after exposure.33 Shifting frogs from 25° Cto 5° C, and tench from 18° Cto10° C similarly increased the LD 50/30 from 10 Gy to 100 Gy,34 and from 12 Gy to 550 Gy,35 respectively. The mechanisms for the radioprotective effects of decreasing temperature are not entirely clear. In both mammals and teleosts, histologic studies have shown that radiation damage appears less severe at lower temperatures,36,37 perhaps due to slowed metabolism or lessened generation of free radicals in the relatively hypoxic environments. The life span of erythrocytes has also been shown to increase with decreasing temperature.38 It has recently been demonstrated in mouse studies that transplantation of megakaryocyte/erythrocyte-restricted progenitors is sufficient for radioprotection following the MLD.39 Hematopoietic failure is thus due to the specific loss of erythrocytes and/or platelets over the critical window of 30 days after irradiation, after which surviving host-derived HSCs repopulate all blood cell lineages. Taken together, the temperature effects on radiosensitivity are likely due both to metabolic alterations and to the ability of irradiated erythrocytes to persist until host hematopoiesis recovers.
The zebrafish has proven utility as a genetically tractable vertebrate organism, but has lacked methodologies to test function at the cellular level. By characterizing the effects of TBI in zebrafish, we have shown that the biologic response to γ irradiation is very similar to that in mammals. This now permits the study of cell autonomy of mutant gene function, and allows prospective isolation strategies to test immune cell function and HSC enrichment strategies. Determination of a sublethal dose that transiently ablates lymphocytes should also permit transplantation of a wide array of cell types by lessening immune surveillance and rejection. The use of transgenic donor cell populations also serves as a useful tool to independently observe the kinetics of multilineage repopulation over time in living recipients.
Prepublished online as Blood First Edition Paper, May 13, 2004; DOI 10.1182/blood-2004-01-0100.
Supported by the Irvington Institute for Immunological Research (D.T.); National Institutes of Health (NIH) grants 5K08-DK061849 (H.M.S.), CA-06516 (J.L.K.), and CA-68484 (A.T.L.); a National Science Foundation (NSF) predoctoral fellowship (D.M.L.); and the Howard Hughes Medical Institute, the Grousbeck family, and Legal Sea Foods (L.I.Z.).
D.T. and A.W. contributed equally to this study.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank A. Kiger and J. Amatruda for critical evaluation of the manuscript, C. Belair and B. Barut for animal and laboratory management, and A. Flint and M. Handley for assistance with flow cytometry.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal