Abstract
There appear to be 2 pathways involved in the early pathogenesis of premalignant monoclonal gammopathy of undetermined significance (MGUS) and malignant multiple myeloma (MM) tumors. Nearly half of these tumors are nonhyperdiploid and mostly have immunoglobulin H (IgH) translocations that involve 5 recurrent chromosomal loci, including 11q13 (cyclin D1), 6p21 (cyclin D3), 4p16 (fibroblast growth factor receptor 3 [FGFR3] and multiple myeloma SET domain [MMSET]), 16q23 (c-maf), and 20q11 (mafB). The remaining tumors are hyperdiploid and contain multiple trisomies involving chromosomes 3, 5, 7, 9, 11, 15, 19, and 21, but infrequently have IgH translocations involving the 5 recurrent loci. Dysregulated expression of cyclin D1, D2, or D3 appears to occur as an early event in virtually all of these tumors. This may render the cells more susceptible to proliferative stimuli, resulting in selective expansion as a result of interaction with bone marrow stromal cells that produce interleukin-6 (IL-6) and other cytokines. There are 5 proposed tumor groups, defined by IgH translocations and/or cyclin D expression, that appear to have differences in biologic properties, including interaction with stromal cells, prognosis, and response to specific therapies. Delineation of the mechanisms mediating MM cell proliferation, survival, and migration in the bone marrow (BM) microenvironment may both enhance understanding of pathogenesis and provide the framework for identification and validation of novel molecular targets.
Introduction
On January 1, 2000, there were estimated to be 47 000 patients with multiple myeloma (MM) in the United States, reflecting a yearly incidence of nearly 14 000 and a median survival of about 3 years.1 Currently it remains an incurable malignancy that often is preceded by an exceptionally common (3.4% of the population over the age of 502 ) premalignant tumor, monoclonal gammopathy of undetermined significance (MGUS). Monoclonal gammopathies are usually asymptomatic, but they may sometimes cause primary amyloidosis as a result of pathologic, and ultimately lethal, deposits of monoclonal immunoglobulin (Ig) in critical tissues.3 Although MGUS is stable, it stochastically progresses to frankly malignant MM at a rate of 0.6% to 3% per year, depending on the level of monoclonal Ig.4 For both MGUS and MM, the incidence is markedly age dependent, about 2-fold higher in American blacks than whites, and significantly higher in males.5 Although for many years the incidence of MM appeared to be increasing, since 1992 the incidence appears to have become stable. The roles of genetic background and environment are poorly defined, although there may be clustering within families.6
MM is a plasmablast/plasma cell tumor characterized by frequent Ig translocations
Germinal center B cells uniquely modify the DNA of Ig genes through sequential rounds of somatic hypermutation and antigen selection, and also by IgH switch recombination. Postgerminal center B cells can generate plasmablasts (PBs) that have successfully completed somatic hypermutation and IgH switching before migrating to the bone marrow (BM), where stromal cells enable terminal differentiation into long-lived plasma cells (PCs).7,8 MGUS and MM are characterized by the accumulation of transformed PBs/PCs at multiple sites in the BM. Importantly, although MM is more proliferative than MGUS, both tumors have an extremely low rate of proliferation, typically with less than 1% of cells synthesizing DNA until late stages of MM. The combination of karyotypic complexity, an inability to efficiently perform conventional cytogenetics on low proliferative tumors, and the telomeric location of some translocation partners delayed the identification of Ig translocations in MGUS and MM. An important initial step in solving this problem was the identification and cloning of IgH switch region translocation breakpoints in human myeloma cell lines (HMCLs).9 Interphase fluorescence in situ hybridization (FISH) using probes flanking the cloned breakpoints identifies karyotypic abnormalities even in nondividing cells and enabled the analysis of primary MGUS and MM tumors.10,11 Several studies have shown that a majority of MM tumors have an IgH translocation that nonrandomly involves one of many potential chromosomal partners.9,12-14 The prevalence of IgH translocations varies with the stage of disease: 46% to 48% in MGUS or smoldering MM (SMM), 55% to 73% in intramedullary MM, 85% in primary plasma cell leukemia, and more than 90% in HMCLs.8,14
Recurrent chromosomal partners for Ig translocations
There are 5 well-defined recurrent chromosomal partners (oncogenes) that are involved in IgH translocations in MGUS and MM: 11q13 (cyclin D1), 6p21 (cyclin D3), 4p16 (fibroblast growth factor receptor 3 [FGFR3] and multiple myeloma SET domain [MMSET]), 16q23 (c-maf), and 20q11 (mafB).9 Together the combined prevalence of these 5 IgH translocation partners is about 40%, with approximately 15% 11q13, 3% 6p21, 15% 4p16, 5% 16q23, and 2% 20q11.8,12,15-18 The t(4;14) translocation is unusual in that it appears to dysregulate 2 potential oncogenes, MMSET on der(4) and FGFR3 on der(14), although FGFR3 on der(14) is lost or not expressed in about 20% of MM tumors that have a t(4;14) translocation.19-23 The apparently lower incidence of 4p16 and/or 16q23 in MGUS/SMM compared with MM may be due to these translocations resulting in de novo MM without preceding MGUS, or a more rapid progression of MGUS to MM, a hypothesis supported by the fact that patients with translocations involving 4p16 or 16q23 have an extremely poor prognosis.12,13,22,24
Primary versus secondary translocations in MM
Primary translocations occur as early and perhaps initiating events during tumor pathogenesis, whereas secondary translocations occur as progression events. Most translocations involving the 5 recurrent translocation partners appear to be primary translocations that occurred from errors in IgH switch recombination during B-cell development in germinal centers. In contrast, translocations of c-myc appear to be very late secondary events that do not involve B-cell–specific recombination mechanisms, are often complex, and sometimes do not involve Ig loci. By FISH analysis, rearrangements of c-myc are reported in only 15% of MM tumors (with frequent heterogeneity within a tumor), but in nearly 40% of advanced MM tumors and 90% of HMCLs.25,26 Regarding the approximately 20% of IgH translocations not involving the 6 recurrent partners, little is known about the multitude of partners and oncogenes, the mechanisms that mediate these translocations, or the time(s) at which these translocations occur.
Dysregulation of cyclin D1, D2, or D3: a unifying oncogenic event in MM
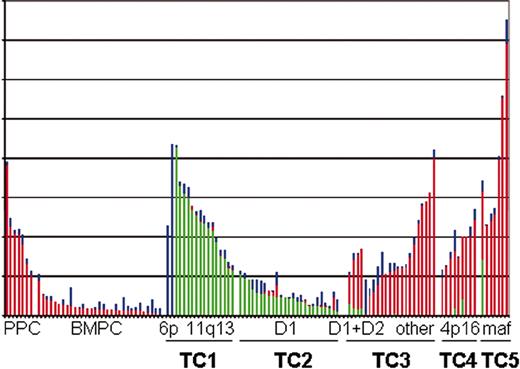
MGUS and MM appear closer to normal, nonproliferating PCs than to normal, but highly proliferating PBs, for which 30% or more of the cells can be in S phase. It is surprising therefore that analysis by Bergsagel and Kuehl27 of combined gene expression profiling data published from 2 laboratories28,29 shows that the expression level of cyclin D1, cyclin D2, or cyclin D3 mRNA in MM and MGUS is distinctly higher than in normal PCs, comparable with the levels of cyclin D2 mRNA expressed in normal proliferating PBs (Figure 1). Normal hematopoietic cells, including normal B lymphocytes, PCs, and PBs, express cyclin D2 and/or D3, but little or no cyclin D1.30 Given the lack of cyclin D1 expression in normal lymphocytes, the occurrence of Ig translocations that dysregulate cyclin D1 or cyclin D3 in about 20% of MM tumors, the expression of cyclin D1 in nearly 40% of tumors lacking a t(11;14) translocation, and the increased expression levels of cyclin D2 in most remaining tumors, it seems apparent that almost all MM tumors dysregulate at least one of the cyclin D genes.
Cyclin D expression in normal and malignant plasma cells. The raw scores for each of the 3 D-cyclins (D1, green bars; D2, red bars; D3, blue bars) from the Affymetrix HuFL dataset published by Tarte et al28 and Zhan et al29 are plotted one above the other. The samples are divided into 9 groups, and arranged by the level of expression of the predominant cyclin D within each group. The samples are CD138+ selected cells from 6 peripheral blood generated plasmablasts and 1 reactive plasmacytosis (PPC), 31 bone marrow PCs (BMPC) from healthy volunteers, and 78 samples from patients with newly diagnosed MM and 3 with plasma cell leukemia. Among these there are 2 with high CCND3 (6p21) and 15 with high CCND1 (11q13) (TC1); 25 with lower levels of CCND1 without t(11;14) (TC2); 4 with lower levels of D1 and elevated CCND2 (D1 + D2), 17 remaining patients with elevated CCND2 (other), and 2 patients without an elevated cyclin D (TC3); 9 with elevated FGFR3 (4p16) (TC4); and 7 with elevated CX3CR1 and β7 integrin, a marker of maf dysregulation (maf, 16q23 and 20q11) (TC5).
Cyclin D expression in normal and malignant plasma cells. The raw scores for each of the 3 D-cyclins (D1, green bars; D2, red bars; D3, blue bars) from the Affymetrix HuFL dataset published by Tarte et al28 and Zhan et al29 are plotted one above the other. The samples are divided into 9 groups, and arranged by the level of expression of the predominant cyclin D within each group. The samples are CD138+ selected cells from 6 peripheral blood generated plasmablasts and 1 reactive plasmacytosis (PPC), 31 bone marrow PCs (BMPC) from healthy volunteers, and 78 samples from patients with newly diagnosed MM and 3 with plasma cell leukemia. Among these there are 2 with high CCND3 (6p21) and 15 with high CCND1 (11q13) (TC1); 25 with lower levels of CCND1 without t(11;14) (TC2); 4 with lower levels of D1 and elevated CCND2 (D1 + D2), 17 remaining patients with elevated CCND2 (other), and 2 patients without an elevated cyclin D (TC3); 9 with elevated FGFR3 (4p16) (TC4); and 7 with elevated CX3CR1 and β7 integrin, a marker of maf dysregulation (maf, 16q23 and 20q11) (TC5).
A model for the molecular pathogenesis of multiple myeloma
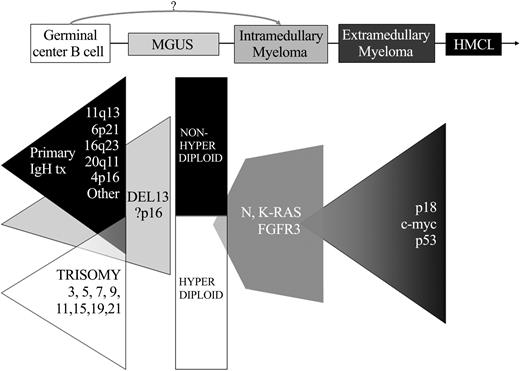
Based on the results summarized, a model for the molecular pathogenesis of MM has been proposed.8 Chromosome content appears to identify 2 different—but perhaps overlapping— pathways of pathogenesis: nonhyperdiploid tumors with a very high incidence of IgH translocations involving the 5 recurrent partners and a relatively high incidence of chromosome 13/13q14 loss; and hyperdiploid tumors associated with multiple trisomies involving chromosomes 3, 5, 7, 9, 11, 15, 19, and 21, but a low incidence of both chromosome 13/13q14 loss and IgH translocations involving the 5 recurrent partners31-33 (Figure 2). In about half of tumors, a primary chromosome translocation results in the dysregulated expression of an oncogene. This may lead directly (11q13-cyclin D1 and 6p21-cyclin D3) or indirectly (4p16, 16q23, other cyclin D2) to cyclin D dysregulation. Alternatively, the remaining tumors are mostly hyperdiploid, and cyclin D1 (or less often cyclin D2) usually is dysregulated by an undefined mechanism.27 The dysregulation of 1 of 3 cyclin D genes may render the cells more susceptible to proliferative stimuli, resulting in selective expansion as a result of interaction with bone marrow stromal cells (BMSCs) that produce interleukin-6 (IL-6) and other cytokines. Karyotypic abnormalities, most notably IgH translocations, trisomies of chromosomes 3, 5, 7, 9, 11, 15, 19, and 21, and monosomy of chromosome 13 or 13q14 deletion, often are present in premalignant MGUS, the earliest identified stage of tumorigenesis.12,14 Even though dysregulation of a cyclin D gene appears to be a nearly universal event in early pathogenesis, there is evidence that the retinoblastoma (Rb) pathway is further disrupted by p16INK4a methylation and inactivation in a substantial fraction of MGUS and MM tumors.34,35 Tumor progression is associated with secondary chromosome translocations, of which c-myc provides a paradigm.8,26 Mutually exclusive activating mutations of K- or N-Ras (or FGFR3 when there is a t(4;14) translocation) are rare or absent in MGUS, whereas Ras mutations are present in 30% to 40% of early MM, and FGFR3 mutations occur more frequently in advanced MM.21,36-38 Mutations and/or monoallelic deletion of p53 occur frequently but only late in the course of the disease.39 Further disruption of the Rb pathway by inactivation of Rb or p18INK4c can also occur at a low frequency, most likely as a late progression event.40,41 The frequency and timing of other events, such as inactivation of phosphatase and tensin homolog (PTEN), remain to be determined.42,43
Two pathways for progression to plasma cell neoplasia. Defined stages of pathogenesis are depicted, with shaded triangles indicating the possible timing and frequency of known oncogenic events. The earliest changes include 2 partially overlapping pathways, indicated by primary IgH translocations (tx) and multiple trisomies. Deletion 13 (most often in nonhyperdiploid tumors) and p16 methylation might be included among the earliest changes, but might sometimes be involved in progression. Activating mutations of N- and K-RAS appear to mark, if not cause, the MGUS to MM transition in some tumors, but can also occur as later progression events. Late oncogenic changes include inactivation of p18 and p53, and also translocations that dysregulate c-myc. Inactivation of Rb, PTEN, and secondary translocations not involving c-myc are not depicted.
Two pathways for progression to plasma cell neoplasia. Defined stages of pathogenesis are depicted, with shaded triangles indicating the possible timing and frequency of known oncogenic events. The earliest changes include 2 partially overlapping pathways, indicated by primary IgH translocations (tx) and multiple trisomies. Deletion 13 (most often in nonhyperdiploid tumors) and p16 methylation might be included among the earliest changes, but might sometimes be involved in progression. Activating mutations of N- and K-RAS appear to mark, if not cause, the MGUS to MM transition in some tumors, but can also occur as later progression events. Late oncogenic changes include inactivation of p18 and p53, and also translocations that dysregulate c-myc. Inactivation of Rb, PTEN, and secondary translocations not involving c-myc are not depicted.
Proposal for 5 MM subtypes defined by translocations and cyclin D expression (TC classification)
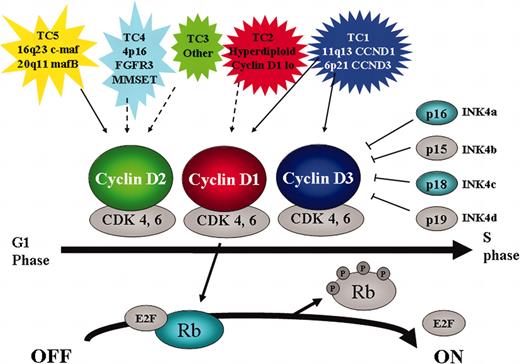
In addition to determining the expression level of cyclin D1, D2, and D3, gene expression profiling can effectively identify MM tumors that overexpress the oncogenes dysregulated by the 5 recurrent IgH translocations: 11q13 (cyclin D1); 6p21 (cyclin D3); 4p16 (MMSET and usually FGFR3); 16q23 (c-maf); and 20q11 (mafB).27,29 We propose 5 translocation and cyclin D (TC) groups (Table 1) that can be distinguished based on the 5 recurrent Ig translocations and cyclin D expression: TC1 tumors (18%) express high levels of either cyclin D1 or cyclin D3 as a result of an Ig translocation; TC2 tumors (37%) ectopically express low to moderate levels of cyclin D1 despite the absence of a t(11;14) translocation; TC3 tumors (22%) are a mixture of tumors that do not fall into one of the other groups, with most expressing cyclin D2, but a few also expressing low levels of cyclin D1 or cyclin D3; TC4 tumors (16%) express high levels of cyclin D2, and also MMSET (and in most cases FGFR3) as a result of a t(4;14) translocation; and TC5 tumors (7%) express the highest levels of cyclin D2, and also high levels of either c-maf or mafB, consistent with evidence that both maf transcription factors up-regulate the expression of cyclin D2.44
Translocation and cyclin D (TC) molecular classification of multiple myeloma
Group . | Primary translocation . | Gene(s) at breakpoint . | D-Cyclin . | Ploidy . | Frequency of each TC group in newly diagnosed MM, % . |
|---|---|---|---|---|---|
| TC1 | |||||
| 11q13 | CCND1 | D1 | NH | 15 | |
| 6p21 | CCND3 | D3 | NH | 3 | |
| TC2 | None | None | D2 | H | 37 |
| TC3 | None | None | D2 | H = NH | 22 |
| TC4 | 4p16 | FGFR3/MMSET | D2 | NH > H | 16 |
| TC5 | |||||
| 16q23 | c-maf | D2 | NH | 5 | |
| 20q11 | mafB | D2 | NH | 2 |
Group . | Primary translocation . | Gene(s) at breakpoint . | D-Cyclin . | Ploidy . | Frequency of each TC group in newly diagnosed MM, % . |
|---|---|---|---|---|---|
| TC1 | |||||
| 11q13 | CCND1 | D1 | NH | 15 | |
| 6p21 | CCND3 | D3 | NH | 3 | |
| TC2 | None | None | D2 | H | 37 |
| TC3 | None | None | D2 | H = NH | 22 |
| TC4 | 4p16 | FGFR3/MMSET | D2 | NH > H | 16 |
| TC5 | |||||
| 16q23 | c-maf | D2 | NH | 5 | |
| 20q11 | mafB | D2 | NH | 2 |
Bergsagel and Kuehl27 ; and P.L.B., W.M.K., and J. Shaughnessy, unpublished data, January 2004.
H indicates hyperdiploid; NH, nonhyperdiploid.
The TC molecular classification predicts prognosis and response to therapies
In addition to tumor mass and secondary features that represent a host response to MM (anemia, thrombocytopenia, bone disease, immunodeficiency, etc), intrinsic properties of the tumor cell are also informative in predicting prognosis and response to existing therapies. For example, it has been well documented that an unfavorable outcome is associated with each of the following: increased plasma cell labeling index, the generation of tumor cells with an abnormal karyotype (perhaps a surrogate for increased proliferation), hypodiploidy compared with hyperdiploidy, monosomy of chromosome 13/13q, monosomy of chromosome 17/deletion of p53, and lack of cyclin D1 expression.7,13,31-33,45,46 It also has been reported and independently confirmed that activating mutations of K-Ras (but not N-Ras) represent an adverse prognostic factor.37,38 More recently, it has become clear that specific IgH translocations also have a profound prognostic significance13,24 (Table 1). In particular, patients with tumors that have a t(4;14) translocation (TC4) have a substantially shortened survival either with standard or high-dose therapy (median overall survival [OS], 26 months and 33 months, respectively), and patients with a t(14;16) (TC5) have a similarly poor if not worse prognosis (median OS, 16 months with conventional therapy). By contrast, patients with tumors that have a t(11;14) translocation (TC1) appear to have a marginally better survival following conventional chemotherapy (median OS, 50 months), but apparently a remarkably better survival following intense therapy (predicted 88% OS at 80 months). These results suggest that the TC classification, which appears to be based on the earliest events in pathogenesis, may be a clinically useful way to classify patients into groups that have distinct subtypes of MM (and MGUS) tumors.13,47-49 The TC classification identifies clinically important molecular subtypes of MM with different prognoses and with unique responses to different treatments (eg, high dose therapy [HDT] and TC1, microenvironment-directed therapy and TC2, FGFR3 inhibitor and TC4, maf dominant-negative and TC5).
Identification of novel therapeutic strategies targeting genetic abnormalities
The critical role of cyclin D dysregulation in the pathogenesis of MM highlights the importance of the cyclin D/Rb pathway and suggests that there may be a therapeutic window in targeting this pathway50 for all molecular subtypes of MM (Figure 3). For example, epigenetic silencing of cyclin-dependent kinase (CDK) inhibitor mRNA expression might be reversed by histone deacetylase (HDAC) inhibitors (suberoylanilide hydroxamic acid [SAHA], depsipeptide) or inhibitors of DNA methyl transferase (5 aza-2′deoxy-cytidine).51 To target cyclin D, per se, there are a number of possible strategies including modulation of mRNA translation (eg, desferroxamine, eicosapentaenoic acid),52,53 posttranslational modifications (ubiquitination and proteasomal degradation),54,55 enzyme function (selective CDK kinase inhibitors),50,56 and perhaps even inhibition of expression of cyclin D mRNA (the TC2 group may be particularly dependent on interaction with BM stromal cells for the ectopic expression of cyclin D1). Additional specificity may be achieved by targeting the genes directly dysregulated by translocations. This seems to be especially true in the case of the t(4;14) where 2 enzymes are overexpressed: FGFR3, a tyrosine kinase receptor, and MMSET, which has homology to histone methyltransferases. As a surface receptor, FGFR3 may be targeted by monoclonal antibodies, and as a tyrosine kinase, by selective tyrosine kinase inhibitors. Preclinical studies have validated FGFR3 as a therapeutic target in t(4;14) MM,57 inhibitors of histone methyltransferases are being developed, and studies are under way to validate MMSET as a target in t(4;14) MM.
Critical role for cyclin D/Rb pathway in multiple myeloma. The 5 TC molecular subtypes of myeloma are characterized by either direct (solid arrow) or indirect (dashed arrow) dysregulation of a D cyclin. Cyclin D together with CDK4 and 6 is involved in G1-S cell cycle progression by phosphorylating and inactivating Rb. This reaction is inhibited by the CDK inhibitors INK4a-d. In addition to cyclin D, other members that are targeted by genetic mutation in MM are highlighted (p16 by methylation, p18 by small homozygous deletions, and Rb by monoallelelic deletion).
Critical role for cyclin D/Rb pathway in multiple myeloma. The 5 TC molecular subtypes of myeloma are characterized by either direct (solid arrow) or indirect (dashed arrow) dysregulation of a D cyclin. Cyclin D together with CDK4 and 6 is involved in G1-S cell cycle progression by phosphorylating and inactivating Rb. This reaction is inhibited by the CDK inhibitors INK4a-d. In addition to cyclin D, other members that are targeted by genetic mutation in MM are highlighted (p16 by methylation, p18 by small homozygous deletions, and Rb by monoallelelic deletion).
Critical but variable role for the bone marrow (BM) microenvironment
Similar to their normal BM plasma cell (BMPC) counterpart, MGUS and MM tumors are dependent on mutual interactions with cells and extracellular components of the BM for survival and growth. Exceptions to this include primary plasma cell leukemia (PCL) and terminal phases of MM, which sometimes extend to extramedullary sites. Significantly, virtually all HMCLs are derived from PCL or extramedullary tumor. Although not yet well understood, there is increasing evidence that some of the earliest oncogenic events differentially affect the interaction of tumor cells with BM components. First, tumors in the TC1 and TC2 groups are more strongly associated with lytic bone lesions than tumors in the TC4 and TC5 groups (P.L.B., unpublished observations, January 2004).58 Second, the maf transcription factor–stimulated expression of β7 integrin and other surface receptors or cytokines seems likely to influence the interactions of the TC5 tumor group in the BM.44 Third, in contrast to tumors in the other TC groups, TC2 tumors (hyperdiploid with multiple trisomies and cyclin D1 expression without a t(11;14)) are greatly underrepresented or absent in primary PCL59 and HMCLs.27 Thus TC2 tumors may be uniquely dependent on the BM environment, with the possibility that the ectopic/increased expression of cyclin D1 is dependent on the BM microenvironment. For example, IL-6 secreted by BMSCs triggers phosphorylation of Akt and downstream glycogen synthase kinase 3β (GSK-3β).60 GSK-3β in turn induces phosphorylation of cyclin D1 followed by degradation through the ubiquitin-proteasome pathway, thereby promoting the transition from G1 to S phase.61 Tumor necrosis factor α (TNFα) in the BM milieu activates nuclear factor κB (NF-κB), thereby modulating expression of adhesion molecules on both MM cells and BMSCs, as well as inducing IL-6 transcription and secretion in BMSCs. Activated NF-κB also binds to the promoter of cyclin D1, thereby regulating its expression.62,63
Growth and survival of MM in the bone marrow (BM) milieu
MM cells home to the BM and adhere to extracellular matrix (ECM) proteins and to BM stromal cells (BMSCs), which not only localizes tumor cells in the BM milieu but also has important functional sequelae.64 Specifically, adhesion of MM cells to ECM proteins confers cell adhesion–mediated drug resistance (CAM-DR),65-67 and binding of MM cells to BMSCs triggers transcription and secretion of cytokines (ie, IL-6, insulin-like growth factor 1 [IGF-1], or vascular endothelial growth factor [VEGF]) from BMSCs, which not only promotes growth, survival, and migration of MM cells but also further confers resistance to conventional chemotherapy.60,68-72 Delineation of mechanisms mediating MM cell growth, survival, and drug resistance in the BM milieu provides the framework to develop and validate novel anti-MM agents to overcome drug resistance and improve patient outcome.73
Role of adhesion molecules
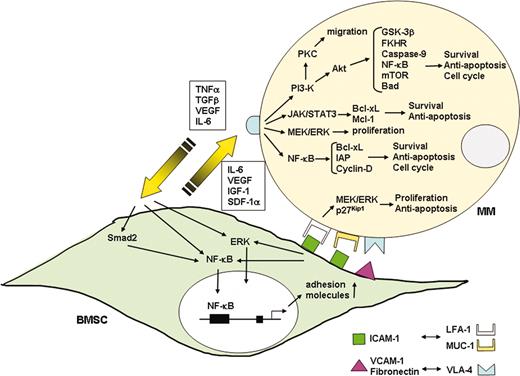
Adhesion molecules mediate both homotypic and heterotypic adhesion of MM cells to either ECM proteins or BMSCs.64 Adhesion molecules CD44, very late antigen 4 (VLA-4), VLA-5, leukocyte function-associated antigen-1 (LFA-1, CD11a), CD56 (neural cell adhesion molecule), CD54 (intercellular adhesion molecule-1 [ICAM-1]), syndecan-1 (CD138), and MPC-1 mediate homing of MM cells to the BM. Subsequently, tumor cells binds to ECM proteins (ie, via syndecan-1 and VLA-4 on MM cells to type I collagen and fibronectin, respectively) and to BMSCs (ie, via VLA-4 on MM cells to vascular cell adhesion molecule 1 [VCAM-1, CD106] on BMSCs; Figure 4). Binding not only localizes tumor cells in the BM microenvironment, but also has important functional and clinical sequelae. Syndecan-1 regulates tumor cell growth and survival, and elevated serum levels correlate with increased tumor cell mass, decreased metalloproteinase-9 activity, and poor prognosis.74,75 Furthermore, adhesion of MM cells via syndecan-1 to collagen induces matrix metalloproteinase-1, thereby promoting bone resorption and tumor invasion. Importantly, binding via VLA-4 on MM cells to the ECM protein fibronectin triggers up-regulation of p27Kip1 and other genetic changes that confer CAM-DR.65-67 Adhesion of MM cells to BMSCs triggers nuclear factor κB (NF-κB)–dependent transcription and secretion of IL-6,76 whereas inhibition of NF-κB activity abrogates this response.77,78 Moreover, MM cells localized in the BM milieu secrete cytokines such as TNFα,79 transforming growth factor β (TGF-β),80 and VEGF,81 which further up-regulate IL-6 secretion from BMSCs. Most importantly, within the BM microenvironment these cytokines mediate growth (IL-6, IGF-1, VEGF), survival (IL-6, IGF-1), drug resistance (IL-6, IGF-1, VEGF), and migration (IGF-1, VEGF, SDF-1α) of MM cells, and also trigger angiogenesis (VEGF) (Figure 4). Novel agents that can overcome CAM-DR and the growth advantage conferred in the BM in vitro, including thalidomide, immunomodulatory drugs (IMiDs), and proteasome inhibitors, hold great promise to overcome conventional drug resistance and improve patient outcome.73
Interaction of MM cells and their BM milieu. Binding of MM cells to BMSCs triggers both adhesion- and cytokine-mediated MM cell growth, survival, drug resistance, and migration. MM cell binding to BMSCs up-regulates cytokine (IL-6, IGF-1, VEGF, SDF-1α) secretion from both BMSCs and MM cells. These cytokines subsequently activate 3 major signaling pathways (ERK, JAK/STAT3, and/or PI3-K/Akt), and their downstream targets including cytokines (IL-6, IGF-1, VEGF) and antiapoptotic proteins (Bcl-xL, IAPs, Mcl-1) in MM cells. Adhesion-mediated activation of NF-κB up-regulates adhesion molecules (ICAM-1, VCAM-1) on both MM cells and BMSCs, further enhancing adhesion of MM cells to BMSCs.
Interaction of MM cells and their BM milieu. Binding of MM cells to BMSCs triggers both adhesion- and cytokine-mediated MM cell growth, survival, drug resistance, and migration. MM cell binding to BMSCs up-regulates cytokine (IL-6, IGF-1, VEGF, SDF-1α) secretion from both BMSCs and MM cells. These cytokines subsequently activate 3 major signaling pathways (ERK, JAK/STAT3, and/or PI3-K/Akt), and their downstream targets including cytokines (IL-6, IGF-1, VEGF) and antiapoptotic proteins (Bcl-xL, IAPs, Mcl-1) in MM cells. Adhesion-mediated activation of NF-κB up-regulates adhesion molecules (ICAM-1, VCAM-1) on both MM cells and BMSCs, further enhancing adhesion of MM cells to BMSCs.
Since adhesion molecules play a central role in the pathogenesis of MM, therapeutic strategies targeting these molecules and the sequelae of adhesion have been developed and tested in animal models (ie, anti–ICAM-1 antibodies inhibit tumor development in severe combined immunodeficient [SCID] mice). In addition, SCID mice bearing human fetal bone grafts (SCID-hu mice) have been used to study in vivo homing and binding of human MM cells to human ECM proteins and BMSCs; related induction of human cytokines; associated tumor cell growth, survival, drug resistance, and migration; as well as evaluation of novel therapeutics.82-84 This model allows for direct evaluation of the impact of the human BM microenvironment and cytokines on tumor cell pathogenesis. Most recently, we have used green fluorescent protein (GFP)–labeled MM cells within a similar SCID mouse model to demonstrate that binding of MM cells in BM induces genes in tumor cells conferring growth, survival, drug resistance, and migration as well as genes in BMSCs modulating cytokines.85 This model allows for whole body imaging to assess tumor migration and localization in vivo. Moreover, evaluation of novel therapies in this model has identified agents (ie, IGF-1 and heat shock protein [Hsp] 90 inhibitors)86,87 with great promise to overcome drug resistance and improve patient outcome. One drawback of this model is the study of human MM cells in murine BM, given the lack of cross-reactivity of some murine cytokines with human tumor cells.
Role of cytokines in MM
IL-6
Although some MM cells secrete IL-6 and grow in an autocrine fashion,88 it is primarily produced in BMSCs89,90 and mediates paracrine MM cell growth. Its secretion is up-regulated in MM cells by CD40 activation91 and in BMSCs by either adhesion of MM cells or cytokines (TNFα, VEGF, IL-1β).79,81,92 Binding of MM cells to BMSCs induces NF-κB activation and related up-regulation of IL-6 transcription and secretion in BMSCs; conversely, specific inhibition of NF-κB by IκB kinase (IKK) inhibitor down-regulates both constitutive and induced IL-6 secretion.78 CD45+ MM cells have recently been identified as those MM cells responsive to IL-6.93,94
IL-6 binds to gp80 (CD80, IL-6R) expressed on most MM cell lines and patient tumor cells, thereby inducing phosphorylation and homodimerization of gp130.95-98 Phosphorylation of gp130 in turn activates Ras/Raf/mitogen-activated protein kinase (MAPK) kinase (MEK)/extracellular signal-related kinase (ERK), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), and phosphatidylinositol-3 kinase (PI3-K)/Akt (PKB) downstream signaling pathways in MM cells (Figure 4) mediating MM cell growth, survival, and drug resistance, respectively.60,71,99-104 Targeted inhibition of Ras/Raf/MEK/ERK signaling using farnesyl-transferase inhibitor (R115777),102,103 ERK antisense oligonucleotide, and MEK1 inhibitor PD98059,100 or MEK1/2 inhibitor U0126,104 abrogates tumor cell growth. Survival and drug resistance of MM cells are mediated via activation of both JAK2/STAT3 and PI3-K/Akt signaling cascades. STAT3 regulates downstream protein expression of Bcl-2 family members Bcl-xL101 and Mcl-1,105-107 and expression of these antiapoptotic proteins can be selectively down-regulated by dominant/negative STAT3 or JAK inhibitor101,108,109 and antisense Mcl-1.110 Moreover, cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in MM cells, at least in part, by down-regulating Mcl-1.111 IL-6 confers resistance to dexamethasone (Dex),68,69,112 a common conventional therapy, via PI3-K/Akt signaling.60 Dex-mediated apoptosis in MM cells is associated with downstream release of second mitochondria activator of caspase (Smac), but not of cytochrome-c (cyto-c),113 from mitochondria114 ; cytosolic Smac disrupts X-linked inhibitor of apoptosis protein (XIAP)/caspase-9 complexes, thereby allowing activation of caspase-9, caspase-3 cleavage, and apoptosis. Since caspase-9 is a downstream target of Akt,115,116 IL-6 protects against Dex-induced apoptosis via inactivation of caspase-960 ; conversely, PI3-K inhibitors block IL-6–mediated protection against Dex-induced apoptosis.60,71 Recent studies show that X-box binding protein 1 (XBP-1), a transcription factor that induces differentiation of normal B cells to plasma cells, is induced by IL-6 in patient MM cells117-119 ; proteasome inhibitors act against MM cells, at least in part, by targeting XBP-1 and the unfolded protein response.120 Finally, it has shown that IL-6 induces caveolin-1 phosphorylation on the MM cell surface, whereas inhibition of caveolin-1 function blocks IL-6–mediated signaling and growth in MM cells.121
Clinically, serum IL-6 and IL-6 receptors (IL-6Rs) are prognostic factors in MM reflective of the proliferative fraction of tumor cells.122-124 Treatment strategies targeting IL-6 to date include antibodies to IL-6 and IL-6 receptor, as well as IL-6 superantagonists that compete for IL-6R binding but do not activate downstream signaling125-129 ; to date, however, only transient responses have been observed.
IGF-1
IGF-1 induces proliferation, survival, and drug resistance in MM cells via MEK/ERK and PI3-K/Akt signaling cascades. Although IGF-1 does not induce JAK2/STAT3 signaling in MM cells, it is a more potent inducer of Akt signaling than IL-6 and confers protection against Dex.71,130-132 IGF-1 triggers phosphorylation of FKHR (forkhead transcription factor); up-regulates intracellular antiapoptotic proteins including FLICE-inhibitory protein, survivin, cellular inhibitor of apoptosis protein 2, A1/Bfl-1, and XIAP132 ; and increases telomerase activity via induction of PI3-K/Akt/NF-κB signaling133 (Figure 4). Inhibitors of IGF-1 receptor demonstrate promising anti-MM activity in preclinical studies72 ; PI3-K or IKK inhibitors also block IGF-1–induced telomerase activity.133,134
VEGF
VEGF is produced in MM cells and BMSCs and accounts, at least in part, for increased angiogenesis in MM patient BM.135,136 Furthermore, both adhesion of MM cells to BMSCs and exogenous IL-6 up-regulate VEGF secretion, as does CD40 activation of human MM cells81,92,137 (Figure 4). VEGF triggers Flt-1 phosphorylation and activation of MEK/ERK and PI-3K/PKCα signaling cascades in MM cell lines and patient cells, thereby promoting modest proliferation and pronounced migration, respectively.138,139 These direct effects of VEGF on MM cells and BM angiogenesis suggest potential use of novel therapies targeting VEGF; already VEGF receptor tyrosine kinase inhibitor PTK787 has demonstrated promise in preclinical studies.140,141
TNFα and CD40 ligand
Both patient MM cells and BM mononuclear cells (BMMCs) express TNFα mRNA and protein,142-144 and TNFα secretion is significantly higher in those MM patients with bone disease.145 It induces tumor cell apoptosis via Fas-associated death domain/caspase-8 signaling,146,147 as well as survival via NF-κB activation and up-regulation of antiapoptotic proteins (ie, Bcl-xL, XIAP, inhibitor of apoptosis protein [IAP])148-151 (Figure 4). Combining TNFα inhibition with NF-κB blockade using IKK inhibitors enhances cytotoxicity.78 Although TNFα secreted by MM cells does not induce growth, survival, or drug resistance in tumor cells, it binds to a TNFα response element in the IL-6 promoter in BMSCs, and more potently triggers paracrine IL-6 transcription and secretion than does either VEGF or TGF-β.79 Moreover, TNFα secreted by MM cells induces NF-κB–dependent up-regulation of adhesion molecules on both MM and BMSCs (CD49d, CD54),79 thereby increasing the binding of MM to BMSCs with associated CAM-DR and induction of cytokine (IL-6, IGF-1, VEGF) secretion in BMSCs.76,78,79 Novel agents targeting TNFα, including thalidomide and the IMiDs,152 act against MM, at least in part, by inhibiting sequelae of these induced cytokines.
CD40 ligand, a TNFα family member, modulates MM cell growth both directly and indirectly via its actions in the BM milieu.91,153,154 Specifically, activation of MM cells via CD40 directly triggers p53-dependent growth versus apoptosis, as well as PI3/Akt NF-κB–dependent MM cell migration.155 CD40 ligation also induces VEGF secretion in BMSCs, which mediates MM cell homing and migration as well as angiogenesis in the BM milieu.137 Finally, CD40 activation up-regulates both HLA and costimulatory molecules on MM cells, thereby augmenting their antigenpresenting capacity.
Other cytokines
Stromal cell–derived factor 1 α (SDF-1α), the ligand for chemokine receptor CXCR4, is present in supernatants from MM patient BMSCs, whereas CXCR4 is expressed on MM cells.156 SDF-1α transiently up-regulates VLA-4–mediated MM cell adhesion in the BM milieu,157 as well as inducing modest proliferation, migration, and protection against Dex-induced apoptosis via activation of ERK, PI3-K/Akt, and NF-κB signaling cascades, respectively.156 SDF-1α also induces secretion of IL-6 and VEGF in BMSCs, thereby further promoting MM cell growth, survival, drug resistance, and migration (Figure 4). Transforming growth factor beta (TGF-β) secreted by MM cells triggers paracrine IL-6 secretion in BMSCs91 ; conversely, TGF-β receptor inhibitors down-regulate IL-6 secretion in BMSCs and associated paracrine MM cell growth.158 IL-21 induces DNA synthesis in patient MM cells via phosphorylation of JAK1, STAT3, and ERK.159 B-cell stimulation factor 3 (BSF-3) triggers ERK and STAT3 phosphorylation, thereby stimulating growth and conferring Dex resistance in MM cells.160 IL-1, IL-6, receptor activator of NF-κB ligand (RANKL), parathyroid hormone-related protein (PTHrP), and macrophage inflammatory protein 1-α (MIP-1α) mediate bone destruction via activation of osteoclasts. RANKL triggers osteoclastogenesis and bone resorption as well as enhancing induction of osteoclasts by MIP-1α and/or IL-6.161-164 Most recently, increased Dickkopf 1 (DKK1) expression has been reported in MM cells, implicating Wnt signaling in suppression of osteoblasts and lytic lesions of bone in MM.58
Death signaling in MM
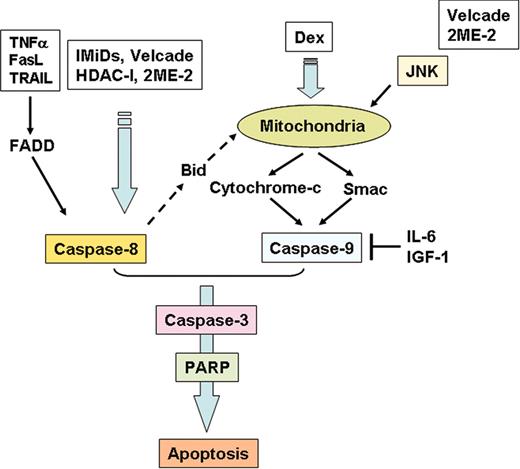
Both conventional and novel chemotherapeutic agents target specific kinases mediating MM cell growth, survival, and apoptosis.73 Most agents trigger apoptosis, which can be distinguished from necrosis by the lack of an associated inflammatory response.165 Cytosolic aspartate–specific proteases (CASPases), which disassemble cells into apoptotic bodies, are present as inactive proenzymes and commonly activated by proteolytic cleavage.166 In MM cells, caspase-8 is activated in response to extracellular apoptosis-inducing ligand (ie, TNFα, TRAIL), by cytoplasmic death domain (ie, Fas-associated death domain [FADD]),167 and by novel agents such as the immunomodulatory drug CC-5013 (lenalidomide, also known as Revimid or Revlimid)168 (Figure 5). On the other hand, caspase-9 is activated in response to agents that trigger release of cyto-c from mitochondria to the cytosol. Importantly, Dex-induced apoptosis in MM cells is associated with caspase-9 activation and release of second mitochondria-derived activator of caspases (Smac), but not cyto-c.114 Activation of either caspase-8 or caspase-9 is followed by downstream activation of caspase-3, poly adenosine diphosphate–ribose polymerase (PARP), and DNA fragmentation factor (DFF). Cross-talk of apoptotic signaling from caspase-8 to caspase-9 can occur (ie, via Bid).169,170 Furthermore, novel proteasome inhibitor bortezomib (Velcade), 2-methoxyestradiol (2ME2), and lysophosphatidic acid acyltransferase-β (LPAAT-β) inhibitors activate c-Jun NH2-terminal kinase (JNK), which translocates from cytosol to mitochondria, thereby facilitating release of mitochondrial cyto-c/Smac to the cytosol and sequential activation of caspases-9 and -3; conversely, blocking JNK using either specific JNK inhibitor SP600125 or dominant-negative JNK abrogates both stress-induced release of cyto-c/Smac and induction of apoptosis171-173 (Figure 5).
Apoptotic signaling pathways triggered by conventional and novel therapies. Fas/FasL, TRAIL, Thal/IMiDs, and HDAC inhibitors trigger activation of caspase-8, whereas Dex activates caspase-9. Bortezomib (Velcade) and 2ME-2 induce both caspase-8 and -9 activation. Both IL-6 and IGF-1 inhibit caspase-9/caspase-3 apoptotic signaling via activation of Akt signaling.
Apoptotic signaling pathways triggered by conventional and novel therapies. Fas/FasL, TRAIL, Thal/IMiDs, and HDAC inhibitors trigger activation of caspase-8, whereas Dex activates caspase-9. Bortezomib (Velcade) and 2ME-2 induce both caspase-8 and -9 activation. Both IL-6 and IGF-1 inhibit caspase-9/caspase-3 apoptotic signaling via activation of Akt signaling.
Apoptosis can also be triggered or enhanced by down-regulation of inhibitor of apoptosis protein (IAP) activity (ie, inhibition of NF-κB activity down-regulates IAPs and enhances MM sensitivity to various drugs).168 Bcl-2 family proteins regulate the release of cyto-c from mitochondria to cytosol; inhibition of these proteins (ie, Bcl-2, Bcl-xL, Mcl-1) therefore also enhances sensitivity of MM cells to apoptotic stimuli.110,174 Importantly, these preclinical studies delineating apoptotic signaling induced by conventional and novel agents provide the framework for clinical treatment protocols. For example, both TRAIL and Revimid trigger caspase-8–mediated MM cell death168 and enhanced killing via intrinsic signaling in vitro, suggesting their potential combined clinical use (Figure 5). On the other hand, bortezomib induces predominantly activation of caspase-8, whereas Dex triggers activation of capase-9, providing the preclinical framework for combining agents in clinical protocols to induce dual apoptotic signaling. To date, thalidomide plus dexamethasone treatment has demonstrated significant antitumor activity against newly diagnosed175 as well as relapsed refractory MM.176 Bortezomib inhibits DNA repair by cleaving DNA-PKCs and ataxiatelangiectasia protein (ATM),171 thereby enhancing cytotoxicity or overcoming resistance to DNA damaging agents in vitro.177 Importantly, recent studies show that bortezomib can also overcome clinical resistance to doxorubicin and melphalan.178,179
Targeting the MM cell in its BM microenvironment
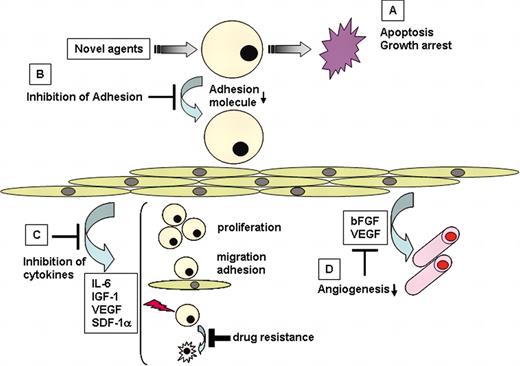
Both in vitro systems and in vivo animal models to characterize mechanisms of MM cell homing to BM have been developed, including the factors (MM cell–BMSCs interactions, cytokines, angiogenesis) promoting MM cell growth, survival, drug resistance, and migration in the BM milieu73 (Figure 6). These model systems have allowed for the development of several promising biologically based therapies that can target directly or indirectly both the MM cell and BM microenvironment including thalidomide/CC-5013 (Revimid),168,180-183 PS-341 (bortezomib),77,171,184-186 VEGF receptor kinase inhibitor PTK787,140 HDAC inhibitors SAHA187 and LAQ-824,188 2-ME2,118,189 arsenic trioxide,190 and LPAAT-β inhibitor173 ; those that target MM cells including telomestatin,191 Hsp-90 inhibitor 17-AAG,87 TRAIL,168,192 statins,193 IGF-1R inhibitor72 ; and those that target only the BM microenvironment including IκB kinase inhibitors78 and p38MAPK inhibitors194 (Table 2). It is our hypothesis that drugs in these classes will need to be combined to achieve complete eradication of MM cells, and we are presently studying their mechanisms of action at a gene and protein level in order to provide the framework for rational combination clinical trials to overcome drug resistance and improve patient outcome.118
Novel biologically based therapies targeting MM cells and the BM microenvironment. Novel agents (A) induce G1 growth arrest and/or apoptosis in MM cell lines and patient cells resistant to conventional chemotherapy; (B) inhibit MM cell adhesion to BMSCs; (C) decrease cytokine production and sequelae in the BM microenvironment; and (D) decrease angiogenesis.
Novel biologically based therapies targeting MM cells and the BM microenvironment. Novel agents (A) induce G1 growth arrest and/or apoptosis in MM cell lines and patient cells resistant to conventional chemotherapy; (B) inhibit MM cell adhesion to BMSCs; (C) decrease cytokine production and sequelae in the BM microenvironment; and (D) decrease angiogenesis.
Novel agents for myeloma
Actions . | Agents . |
|---|---|
| Targeting both MM cells and interaction of MM cells with the bone marrow microenvironment | Thalidomide and its analogs (Revlimid) |
| Proteasome inhibitor (bortezomib) | |
| Arsenic trioxide | |
| 2-Methoxyestradiol (2-ME2) | |
| Lysophosphatidic acid acyltransferase-β inhibitor | |
| Triterpenoid 2-cyano-3, 12-dioxoolean-1, 9-dien-28-oic acid (CDDO) | |
| N-N-diethl-8,8-dipropyl-2-azaspiro [4.5] decane-2-propanamine (Atiprimod) | |
| Targeting circuits mediating MM cell growth and survival | VEGF receptor tyrosine kinase inhibitor (PTK787/ZK222584, GW654652) |
| Farnesyltransferase inhibitor | |
| Histone deacetylase inhibitor (SAHA, LAQ824) | |
| Heat shock protein-90 inhibitor (Geldanamycin, 17-AAG) | |
| Telomerase inhibitor (Telomestatin) | |
| bcl-2 antisense oligonucleotide (Genasense) | |
| Inosine monophosphate dehydrogenase (VX-944) | |
| Rapamycin | |
| Targeting the bone marrow microenvironment | IκB kinase (IKK) inhibitor (PS-1145) |
| p38 MAPK inhibitor (VX-745, SCIO-469) | |
| TFG-β receptor inhibitor (SD-208) | |
| Targeting cell surface receptors | TNF-related apoptosis-inducing ligand (TRAIL)/Apo2 ligand |
| IGF-1 receptor inhibitor (ADW) | |
| HMG-CoA reductase inhibitor (Statins) | |
| Anti-CD20 antibody (Rituximab) |
Actions . | Agents . |
|---|---|
| Targeting both MM cells and interaction of MM cells with the bone marrow microenvironment | Thalidomide and its analogs (Revlimid) |
| Proteasome inhibitor (bortezomib) | |
| Arsenic trioxide | |
| 2-Methoxyestradiol (2-ME2) | |
| Lysophosphatidic acid acyltransferase-β inhibitor | |
| Triterpenoid 2-cyano-3, 12-dioxoolean-1, 9-dien-28-oic acid (CDDO) | |
| N-N-diethl-8,8-dipropyl-2-azaspiro [4.5] decane-2-propanamine (Atiprimod) | |
| Targeting circuits mediating MM cell growth and survival | VEGF receptor tyrosine kinase inhibitor (PTK787/ZK222584, GW654652) |
| Farnesyltransferase inhibitor | |
| Histone deacetylase inhibitor (SAHA, LAQ824) | |
| Heat shock protein-90 inhibitor (Geldanamycin, 17-AAG) | |
| Telomerase inhibitor (Telomestatin) | |
| bcl-2 antisense oligonucleotide (Genasense) | |
| Inosine monophosphate dehydrogenase (VX-944) | |
| Rapamycin | |
| Targeting the bone marrow microenvironment | IκB kinase (IKK) inhibitor (PS-1145) |
| p38 MAPK inhibitor (VX-745, SCIO-469) | |
| TFG-β receptor inhibitor (SD-208) | |
| Targeting cell surface receptors | TNF-related apoptosis-inducing ligand (TRAIL)/Apo2 ligand |
| IGF-1 receptor inhibitor (ADW) | |
| HMG-CoA reductase inhibitor (Statins) | |
| Anti-CD20 antibody (Rituximab) |
Having demonstrated preclinical promise of these novel agents, we have rapidly translated our laboratory studies to phase 1, 2, and 3 clinical trials to evaluate their clinical use and toxicity, and to move them rapidly from the bench to the bedside. Most excitingly, bortezomib195 and Revlimid196 have already demonstrated marked clinical anti-MM activity even in patients with refractory relapsed MM, confirming the use of our preclinical models to identify and validate novel therapeutics. Importantly, gene array and proteomic studies have helped to identify in vivo mechanisms of action and drug resistance, as well as aiding in their clinical application. For example, gene microarray profiling of bortezomib-treated MM cells reveals induction of heat shock protein 90 stress response,171,184 providing the rationale for the combined clinical use of bortezomib and 17-AAG to enhance anti-MM activity. The study of proteomics also forms the basis for clinical application. For example, protein profiling of bortezomib-treated MM cells demonstrated cleavage of DNA repair enzymes, providing the rationale for combining bortezomib with DNA damaging agents to enhance sensitivity or overcome resistance to these conventional therapies.177 These studies have provided the framework for a new treatment paradigm targeting MM cell-host BMSC interactions and their sequelae in the BM milieu to overcome drug resistance and improve patient outcome in MM.
Future directions
Recent studies have enabled the definition of molecular subtypes of MM and also the role of the BM milieu in pathogenesis. These studies have provided the framework for identification and validation of novel targeted therapies to overcome drug resistance and improve patient outcome. In the future, gene and protein profiling will enable patient-specific selection of targeted therapies197 and will also provide the framework for development of more potent and less toxic targeted therapies.
Prepublished online as Blood First Edition Paper, April 15, 2004; DOI 10.1182/blood-2004-01-0037.
Supported by National Institutes of Health Grant Specialized Programs of Research Excellence (SPORE) IP50 CA10070-01, PO-1 78378, and RO-1 CA 50947; the Doris Duke Distinguished Clinical Research Scientist Award; the Multiple Myeloma Research Foundation; and the Cure for Myeloma Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal