Abstract
Acquisition of CCR7 expression is an important phenotype change during dendritic cell (DC) maturation that endows these cells with the capability to migrate to lymph nodes. We have analyzed the possible role of CCR7 on the regulation of the survival of DCs. Stimulation with CCR7 ligands CCL19 and CCL21 inhibits apoptotic hallmarks of serum-deprived DCs, including membrane phosphatidylserine exposure, loss of mitochondria membrane potential, increased membrane blebs, and nuclear changes. Both chemokines induced a rapid activation of phosphatidylinositol 3′-kinase/Akt1 (PI3K/Akt1), with a prolonged and persistent activation of Akt1. Interference with PI3K, Gi, or G protein βγ subunits abrogated the effects of the chemokines on Akt1 activation and on survival. In contrast, inhibition of extracellular signal-related kinase 1/2 (Erk1/2), p38, or c-Jun N-terminal kinase (JNK) was ineffective. Nuclear factor–κB (NFκB) was involved in the antiapoptotic effects of chemokines because inhibition of NFκB blunted the effects of CCL19 and CCL21 on survival. Furthermore, chemokines induced down-regulation of the NFκB inhibitor IκB, an increase of NFκB DNA-binding capability, and translocation of the NFκB subunit p65 to the nucleus. In summary, in addition to its well-established role in chemotaxis, we show that CCR7 also induces antiapoptotic signaling in mature DCs.
Introduction
Apoptosis, or programmed cell death, is a physiologic process involved in the normal development and maintenance of tissue homeostasis.1 The final stage of this process that leads to the demise of the cell is executed by proteases that degrade vital molecular components of the cell.1 Hallmarks of cells undergoing apoptosis include disruption of mitochondria transmembrane potential, apparition of numerous blebs on the membrane, increased nuclear condensation, and increased appearance of phosphatidylserine (PS) in the outer leaflet of the cell membrane.
Apoptosis is a programmed process that is regulated through a complex mechanism that involves multiple molecular intermediates. Surface receptors may inhibit apoptosis by relaying intracellular signals that either repress proapoptotic molecules and/or stimulate antiapoptotic ones.1 Multiple pathways that inhibit apoptosis use as a common signaling intermediate phosphatidylinositol 3′-kinase (PI3K) and its downstream effector Akt1.1-3 Akt1 phosphorylates and inhibits a variety of proapoptotic regulators and also regulates proteins that promote cell survival.1-3 In this regard, it has been shown that Akt1 may activate IκB kinase, which induces phosphorylation and subsequent degradation of IκB, a molecule that binds and retains transcription factor nuclear factor–κB (NFκB) in the cytoplasm.1-3 Upon IκB degradation, NFκB translocates to the nucleus and stimulates transcription from a variety of antiapoptotic genes.2,4 Apart from PI3K/Akt1, in some cell settings, mitogen-activated protein kinase (MAPK) family members have also been shown to play an important role as regulators of apoptosis.5-7
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) that play a fundamental role in the initiation of the immune response.8,9 In the developmental stage called “immature DCs,” these cells are mainly in tissues and display low APC ability. Following encounter with foreign antigens, they rapidly undergo a process called maturation. Maturation involves an increase in the antigen-presenting ability and in the migration of the cells to secondary lymphoid organs, where they present antigens to naive T cells and induce specific immune responses. During maturation DCs up-regulate surface expression of chemokine receptor CCR7. Ligands for CCR7, chemokines CCL19 and CCL21, which are constitutively expressed at high levels in lymph nodes (LNs), are powerful attractants that direct DCs to these tissues.10-14
Signaling pathways that emanate from CCR7 in DCs and other leukocytes are just starting to be characterized.15-18 CCR7 and other serpentine-type receptors relay intracellular signals using G protein family members. G proteins are heterodimers, formed by α and the βγ subunits, which are classified in 4 families.19 Commonly, chemokine receptors relay signals that regulate chemotaxis through Gi protein family members.19 Recently, it has been demonstrated that, in addition to chemotaxis, CCR7 also regulates DC cytoarchitecture and endocytic ability, suggesting that this receptor can regulate several signaling pathways in DCs.15,16 Herein, we show that CCR7 induces intracellular signals that inhibit apoptosis of DCs, including strong and persistent activation of Akt1. Transmission of antiapoptotic signals could be an important mechanism whereby CCR7 may contribute to improve the adaptive immune response.
Materials and methods
Reagents and materials
Granulocyte-macrophage colony-stimulating factor (GM-CSF) (Leucomax) was purchased from Schering-Plough (Kenilworth, NJ). DePsipher, interleukin-4 (IL-4), CCL21, and CXCL12 were obtained from R&D Systems (Minneapolis, MN). CCL19 was from PeproTech (Rocky Hill, NJ). Tumor necrosis factor-α (TNF-α) was from Alexis Biochemicals (San Diego, CA). N-acetyl-l-cysteine, bovine serum albumin (BSA), poly-l-lysine, LY294002, wortmannin, carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), pertussis toxin, Hoechst 33342, l-α-phosphatidylinositol (PtdIns), and anti–β-actin antibody were from Sigma (St Louis, MO). SN50 was from Biomol (Plymouth Meeting, PA). UO126, SB203580, and SP600125 were from Calbiochem (Nottingham, United Kingdom). The blocking antihuman CCR7 (3D12) monoclonal antibody (mAb),20 annexin V–fluorescein isothiocyanate (FITC), 7-amino-actinomycin D (7-AAD), the anti-CCR7–phycoerythrin (PE)–conjugated monoclonal antibody, and the antitotal Akt1 and the antiphospho-Akt1 (P-Ser473) polyclonal antibodies were from BD Pharmingen (San Diego, CA). The rabbit polyclonal anti-PI3K antibody (which recognizes the p85 subunit) was from Upstate Biotechnology (Lake Placid, NY). The anti–Erk-2 (C14), anti-IκB (C-21), and the anti-βARK (H222) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit antiphospho–extracellular signal-regulated kinase (antiphospho-ERK1/2), antiphospho-p38, and antiphospho–c-Jun N-terminal kinase (antiphospho-JNK) polyclonal antibodies that recognize the active form of the corresponding kinases were from Cell Signaling Technology (Beverly, MA). Thin-layer chromatography (TLC) plates were from MERCK (Darmstadt, Germany). 32P–γ–adenosine triphosphate (32P-γ-ATP) (3000 Ci/mmol [111000 GBq/mmol]) was obtained from Hartmann Analytic (Braunschweig, Germany).
Cells and culture conditions
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats from healthy donors over a Lymphoprep (Nycomed, Norway), and the monocytes were induced to differentiate to DCs by adding GM-CSF.21-24 Briefly, monocytes (purity of CD14 more than 95%) were resuspended at 0.5 × 106/mL to 1 × 106/mL and cultured in complete medium (RPMI 1640) (Gibco Life Technologies, Paisley, Scotland) containing 10% heat-inactivated fetal calf serum (FCS), HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (25 mM), glutamine (2 mM), penicillin (100 U/mL) and streptomycin (100 μg/mL), GM-CSF (1000 U/mL), and IL-4 (1000 U/mL). Cells were cultured for 6 to 7 days, with cytokine addition every second day to obtain a population of immature DCs. To induce maturation of the cells, GM-CSF, IL-4, and 50 ng/mL TNF-α were added for a further 72-hour period. Phenotypic analysis of the cells revealed that purity of CD80+, CD86+, CD83+, and HLA-DR+ cells was more than 85%. Less than 1% of the cells were CD3+, CD14+, CD16+, or CD19+.
Assays of apoptotic damage
DCs were incubated in 0.1% BSA in RPMI plus 20 mM HEPES for 6 hours in the presence or absence of CCL19 or CCL21 and then harvested. At the indicated time points, DCs were stained with FITC-conjugated annexin V and 7-AAD, according to the manufacturer's instructions, and staining assessed by flow cytometry. In these experiments the cells that were apoptotic were those that are annexin V–positive/7-AAD–negative. To analyze mitochondria membrane potential disruption, DCs were incubated with DePsipher and immediately analyzed by flow cytometry or immunofluorescence, according to manufacturer protocol. Apoptotic nuclear morphology was assessed using Hoechst 33342 staining.
Scanning electron microscopy and immunofluorescence
For scanning electron microscopy, cells were washed in phosphate-buffered saline (PBS) and prefixed in 2.5% glutaraldehyde and then rinsed in 0.1 M cacodylate buffer. After postfixation in 1% osmium tetroxide, tissues were stained in 2% uranyl acetate and then dehydrated in graded ethanols. Scanning electron microscopy (SEM) sections were critical point dried using liquid carbon dioxide and coated with gold. The resulting sections were examined with a scanning electron microscope (Stereoscan 250 MK III; Leica, Deerfield, IL). Immunofluorescence and confocal microscopy analysis were performed as described with slight changes.25,26 Briefly, DCs (50 × 103 cells) suspended in complete medium were incubated at 37° C onto coverslips coated with poly-l-lysine (20 μg/mL). Cells were fixed in 4% paraformaldehyde in PBS (10 minutes at room temperature) and permeabilized with 0.2% Triton X-100 (10 minutes at room temperature). Before processing for immunofluorescence, the cells were treated with 1% BSA (15 minutes) to block unspecific binding. Then, samples were extensively washed with PBS and distilled water. Coverslips were mounted in fluorescent mounting medium (Dako, Carpinteria, CA), and representative fields of cells were photographed through an oil immersion lens (63×). Confocal microscopy was performed using an MRC-1000 Confocal Laser Scanning System (Bio-Rad, Hercules, CA) connected to a Nikon Diaphot 200 inverted microscope (Nikon, Tokyo, Japan). Images of 20 serial vertical cellular sections were acquired every 0.4 μm with the Bio-Rad COMOS graphical user interface and software (Biorad, Hercules, CA). Image analysis was performed using Adobe Photoshop 5.0 (Adobe System) and Image software. Green fluorescent protein (GFP)–expressing cells were analyzed using the FITC channel.
Immunoprecipitation of PI3K and kinase assays
Lysis of the cells and subsequent immunoprecipitation was performed as described.25,26 After precipitating PI3K with the anti-p85 subunit antibody, PI3K assays were carried out as described.27 Briefly, DCs (500 × 103 cells) were solubilized in lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane]–HCl [pH 7.4], 140 mM NaCl, 1% Triton X-100, 10% glycerol, 1 mM sodium orthovanadate, and a protease inhibition cocktail) and immunoprecipitated with anti-PI3K antibody (anti-p85). Kinase reactions were performed in kinase buffer (20 mM Tris-HCl [pH 7.5], 75 mM NaCl, 20 mM HEPES, 10 mM MgCl2, 200 μM adenosine) in the presence of 10 μgofthe substrate l-α-phosphatidylinositol (PtdIns) and 10 μCi (0.37 MBq) 32P-γ-ATP. The product of the reaction PtdIns-3-phosphate (PIP) was analyzed through thin-layer chromatography (TLC) and autorradiography.
Cell lysis and Western blot analysis
DCs (300 × 103 cells) were stimulated or not with the corresponding chemokines for the indicated period. The stimulation was terminated by solubilizing the cells in 50 μL ice-cold lysis Akt buffer (20 mM Tris-HCl [pH 7.5], 120 mM NaCl, 1% Nonidet P-40 [NP-40], 10% glycerol, 1 mM sodium pyrophosphate, 20 mM NaF, 1 mM sodium orthovanadate, plus a protease inhibitor cocktail [Sigma]). Supernatants were mixed with 5 × sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (500 mM Tris-HCl [pH 6.8], 0.25 mM sodium orthovanadate, 2.5 mM EDTA [ethylenediaminetetraacetic acid], 15% SDS, 5 mM EDTA, 10% 2-mercaptoethanol, 25% glycerol), boiled, and then fractionated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking with 5% nonfat milk protein in triethanolamine-buffered saline (TBS), pH 7.5, filters were incubated with the indicated antibodies in 1 × TBST (TBS plus 0.1% Tween 20) and visualized with the appropriate horseradish peroxidase (HRP)–conjugated secondary antibodies (Bio-Rad) and an enhanced chemiluminescence (ECL) substrate (Pierce, Rockford, IL) detection system.
Expression constructs and transfections
Electrophoretic mobility shift assay (EMSA)
This assay was performed essentially as described.30,31 For competition experiments, unlabeled oligonucleotides (100-fold molar excess) were preincubated with cell extracts at 4° C for 30 minutes before the probe was added. Oligonucleotide probe for NFκB was 5′-AGTTGAGGGGACTTTCCCAGGC-3′, which contains consensus-binding site for NFκB.
Results
Stimulation of dendritic cells with CCL19 and CCL21 inhibits apoptosis of mature DCs
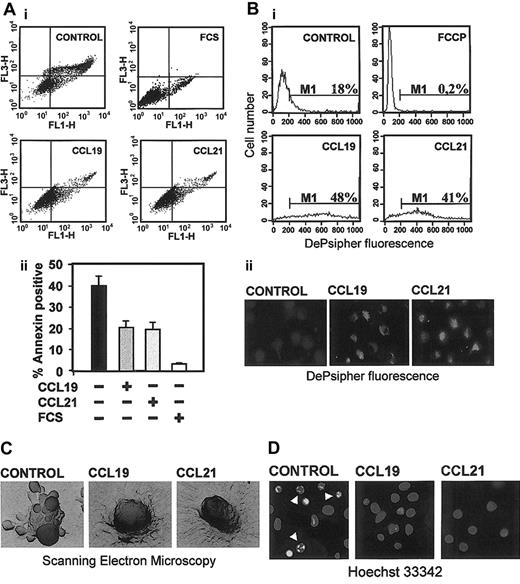
To determine whether CCL19 or CCL21 exerted any effect on the viability of DCs, cells were maintained for 6 hours in RPMI plus 0.1%BSA (see “Materials and methods”) in the presence or the absence of chemokines. After this time, we assessed the percentage of apoptotic cells estimating DCs that were annexin V–positive/7-AAD–negative. As shown in Figure 1A, after 6 hours almost 40% of control cells were apoptotic. This percentage was consistently reduced to 18% to 20% when the cells were cultured in the presence of 200 ng/mL CCL19 or CCL21 (Figure 1Aii), implying that chemokines reduced between 50% and 60% the percentage of apoptotic cells. The protection conferred by both chemokines was concentration dependent, with a maximal effect reached at 200 ng/mL and no further increase at higher concentrations until 1000 ng/mL. The protective effects of CCL19 or CCL21 were abrogated when the DCs were pretreated with a neutralizing anti-CCR7 monoclonal antibody (mAb 3D12) but not with an isotype control (not shown). The effect of both chemokines was less potent than that elicited by fetal calf serum (Figure 1A). In this case, after 6 hours in complete medium (RPMI including 10% fetal calf serum) less than 5% of the DCs were apoptotic (Figure 1A). Because CXCR4, a homeostatic chemokine receptor that is expressed in mature DCs, may transmit antiapoptotic signals,32,33 we also treated the cells with the CXCR4 ligand CXCL12 (100 ng/mL). Treatment of the DCs with CXCL12 reduced the number of apoptotic cells almost 37%, implying that the protective effect of this chemokine was less potent than that of CCL19 and CCL21, which reduced the number of apoptotic cells from 50% to 60% (Figure 1A and not shown).
Stimulation with CCL19 and CCL21 reduces the percentage of apoptotic DCs. (A) Cells were washed and then incubated for 6 hours in 0.1% BSA in RPMI in the absence (control) or in the presence of 200 ng/mL CCL19, CCL21, or 10% fetal calf serum (FCS). (i) DCs were analyzed for annexin V (FL1-H) and 7-amino actinomycin (7-AAD) (FL3-H) by flow cytometry. To exclude necrotic cells (7-AAD–positive), only annexin V–positive/7-AAD–negative cells were considered apoptotic. (ii) Quantification of the percentage of annexin V–positive/7-AAD–negative cells. Results represent the mean ± SEM (n = 8). (B) Apoptotic cells were also stained with DePsipher to detect loss of mitochondria potential and then analyzed by flow cytometry (i). In this experiment we used as positive control cells treated with carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), a protonophore that dissipates the H+ gradient across the inner membrane of mitochondria and induces apoptosis. Figure viewed with the Nikon Diaphot microscope. (ii) DePsipher immunofluorescence staining. Healthy cells that were stained with DePsipher give an intense red labeling (observed as a bright labeling on this black-and-white figure) under the fluorescent microscope. Figure viewed with the Nikon Diaphot microscope. (C) Scanning electron microcopy of a representative apoptotic cell presenting numerous apoptotic blebs (control) and a CCL19-treated and CCL21-treated cell. This figure was viewed with the Stereoscan 250 microscope. The number of cells presenting blebs was reduced by half in the chemokine-treated cells (not shown). (D) Photographs taken from cells stained with Hoechst 33342. Arrowheads point to condensed or fragmented nuclei.
Stimulation with CCL19 and CCL21 reduces the percentage of apoptotic DCs. (A) Cells were washed and then incubated for 6 hours in 0.1% BSA in RPMI in the absence (control) or in the presence of 200 ng/mL CCL19, CCL21, or 10% fetal calf serum (FCS). (i) DCs were analyzed for annexin V (FL1-H) and 7-amino actinomycin (7-AAD) (FL3-H) by flow cytometry. To exclude necrotic cells (7-AAD–positive), only annexin V–positive/7-AAD–negative cells were considered apoptotic. (ii) Quantification of the percentage of annexin V–positive/7-AAD–negative cells. Results represent the mean ± SEM (n = 8). (B) Apoptotic cells were also stained with DePsipher to detect loss of mitochondria potential and then analyzed by flow cytometry (i). In this experiment we used as positive control cells treated with carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), a protonophore that dissipates the H+ gradient across the inner membrane of mitochondria and induces apoptosis. Figure viewed with the Nikon Diaphot microscope. (ii) DePsipher immunofluorescence staining. Healthy cells that were stained with DePsipher give an intense red labeling (observed as a bright labeling on this black-and-white figure) under the fluorescent microscope. Figure viewed with the Nikon Diaphot microscope. (C) Scanning electron microcopy of a representative apoptotic cell presenting numerous apoptotic blebs (control) and a CCL19-treated and CCL21-treated cell. This figure was viewed with the Stereoscan 250 microscope. The number of cells presenting blebs was reduced by half in the chemokine-treated cells (not shown). (D) Photographs taken from cells stained with Hoechst 33342. Arrowheads point to condensed or fragmented nuclei.
We substantiate the results obtained using annexin V, analyzing how CCL19 and CCL21 affected the apparition of other well-known phenotypic changes typical of apoptotic cells—namely, loss of mitochondria membrane potential (Δψm) (Figure 1B), the presence of apoptotic blebs on the membrane of the cells (Figure 1C), and increased nuclear condensation (Figure 1D). As shown, induction of the apoptotic changes was inhibited in the CCL19- or CCL21-treated DCs compared with control, untreated cells. In all cases, there was a reduction of almost 50% in the number of apoptotic cells. Taken together the data clearly demonstrate that stimulation with CCL19 and CCL21 inhibits apoptosis of DCs.
Stimulation of DCs with CCL19- or CCL21-induced activation of PI3K and Akt1
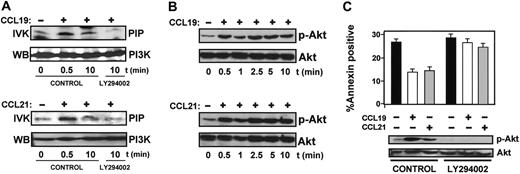
Because PI3K regulates survival of many cell types through its downstream effector Akt1,2 we analyzed whether CCL19 and CCL21 induced activation of PI3K and Akt.1 To analyze PI3K activity, DCs were treated with chemokines for various times and lysed. The lysates were incubated with the anti-PI3K antibody, and the resulting immune complexes were subjected to in vitro kinase assays using l-α-phosphatidylinositol as a substrate. As shown in Figure 2A, both chemokines induced a rapid increase in the kinase activity of PI3K. The maximum increase was observed after 0.5 minutes, decreasing after 10 minutes (Figure 2A and not shown). Chemokine treatment resulted in a 7 ± 0.3-fold increase (n = 3) in PI3K activity. We also examined the effect of chemokines on Akt1 using a phosphospecific antibody that specifically recognizes phosphorylated/active Akt.1 As shown in Figure 2B, CCL19 or CCL21 induced an increase in the phosphorylation of Akt1 that also took place at 0.5 minutes, but in contrast to PI3K, Akt1 persisted phosphorylated/active after 10 minutes (Figure 2B). Densitometric scanning showed that CCL19 and CCL21 induced an 8 ± 0.2-fold (n = 4) increase in the phosphorylation of Akt1 as early as 30 seconds after the addition of chemokines to intact cells and remained at this high level for an additional 120 minutes (Figure 2B and not shown). Phosphorylation of Akt1 in CCL19- and CCL21-stimulated DCs was also observed by immunofluorescence using the antiphospho-Akt1 antibody (not shown).
PI3K and Akt are activated in DCs stimulated with CCL19 and CC21 and regulate apoptosis. (A) DCs suspended in 0.1% BSA in RPMI that were untreated (control) or pretreated with LY294002 (100 μM) for 60 minutes (LY294002) were left unstimulated (–) or were stimulated (+) with CCL19 or CCL21 for the indicated times. Cells were lysed, PI3K was precipitated, and in vitro kinase (IVK) performed as described in “Materials and methods.” PIP indicates PtdIns-3-phosphate. In parallel experiments, immunoprecipitates were also analyzed by Western blotting (WB) to show equal levels of PI3K. A representative experiment out of 4 performed is shown. (B) Whole-cell lysates of cells stimulated with CCL19 or CCL21 for the indicated times were separated on SDS-PAGE and transferred to PVDF membranes for subsequent Western blotting. Activated Akt was detected with an antibody reacting with phosphorylated P-Ser 473 (p-Akt). To confirm equal loading, blots were reprobed with an antibody reacting with total Akt1. (C) DCs suspended in 0.1% BSA in RPMI were left untreated (control) or pretreated with LY294002 (100 μM) for 60 minutes. Then, DCs were either left unstimulated (–) or stimulated (+) with CCL19 or CCL21. (Bottom) Western blots. After 2.5 minutes of stimulation with chemokines, aliquots of DCs were taken to analyze the level of phosphorylated/active Akt1 (p-Akt) and total Akt1 by Western blotting. A representative experiment out of 3 performed is shown. (Top) Bar diagrams. Remaining DCs were left for an additional 6 hours, and then the percentage of annexin V–positive/7-AAD–negative cells was quantified. The results represent the mean ± SEM of 3 independent experiments.
PI3K and Akt are activated in DCs stimulated with CCL19 and CC21 and regulate apoptosis. (A) DCs suspended in 0.1% BSA in RPMI that were untreated (control) or pretreated with LY294002 (100 μM) for 60 minutes (LY294002) were left unstimulated (–) or were stimulated (+) with CCL19 or CCL21 for the indicated times. Cells were lysed, PI3K was precipitated, and in vitro kinase (IVK) performed as described in “Materials and methods.” PIP indicates PtdIns-3-phosphate. In parallel experiments, immunoprecipitates were also analyzed by Western blotting (WB) to show equal levels of PI3K. A representative experiment out of 4 performed is shown. (B) Whole-cell lysates of cells stimulated with CCL19 or CCL21 for the indicated times were separated on SDS-PAGE and transferred to PVDF membranes for subsequent Western blotting. Activated Akt was detected with an antibody reacting with phosphorylated P-Ser 473 (p-Akt). To confirm equal loading, blots were reprobed with an antibody reacting with total Akt1. (C) DCs suspended in 0.1% BSA in RPMI were left untreated (control) or pretreated with LY294002 (100 μM) for 60 minutes. Then, DCs were either left unstimulated (–) or stimulated (+) with CCL19 or CCL21. (Bottom) Western blots. After 2.5 minutes of stimulation with chemokines, aliquots of DCs were taken to analyze the level of phosphorylated/active Akt1 (p-Akt) and total Akt1 by Western blotting. A representative experiment out of 3 performed is shown. (Top) Bar diagrams. Remaining DCs were left for an additional 6 hours, and then the percentage of annexin V–positive/7-AAD–negative cells was quantified. The results represent the mean ± SEM of 3 independent experiments.
We used LY294002, a potent inhibitor of PI3K, to study if inhibition of this kinase affected the survival of DCs. Pretreatment of DCs with the inhibitor abrogated completely PI3K activity (Figure 2A), the phoshorylation of Akt1 (Figure 2C), and the effects of CCL19 and CCL21 on the survival of the cells (Figure 2C). Similar results were obtained when wortmannin, another selective PI3K inhibitor, was used (not shown). Taken together, the data indicate that stimulation of CCR7 induces activation of PI3K/Akt1, with a prolonged activation of Akt1, and that these molecules regulate the survival of the DCs.
CCR7-dependent survival and activation of Akt1 is pertussis toxin sensitive and involves βγ subunits of G proteins
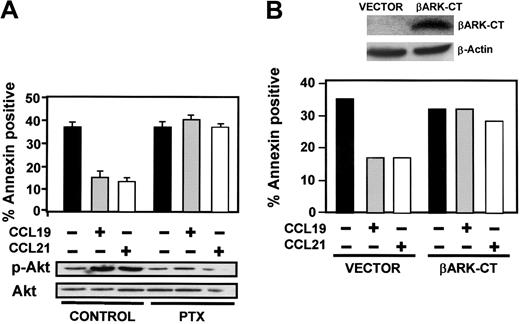
Chemokine receptors may couple to several G protein family members.19 To analyze the involvement of Gi in the prosurvival signals induced by CCL19 and CCL21, DCs were pretreated with the Gi-selective inhibitor pertussis toxin (PTX). This treatment abrogated the effects of the CCL19 and CCL21 on the survival of DCs and the activation of PI3K (not shown) and Akt1 (Figure 3A), indicating the involvement of a Gi protein in the CCR7-mediated effects. To determine which subunits of trimeric Gi proteins relay the signals from CCR7, we transfected DCs with β adrenergic receptor kinase carboxyl terminus (βARK-CT), a plasmid encoding a peptide inhibitor of Gβγ signaling,28 and analyzed if such overexpression modulates the protection of apoptosis conferred by chemokines. As shown in Figure 3B, overexpression of βARK-CT abrogated completely the prosurvival effects of chemokines, suggesting that βγ subunits of Gi regulate the signaling downstream of CCR7 that controls survival of DCs.
Gi and βγ subunits regulate apoptosis and activation of Akt. (A) DCs were left untreated (control) or pretreated with PTX (100 ng/mL) for 120 minutes. Subsequently, DCs were left unstimulated (–) or stimulated (+) with CCL19 or CCL21. (Bottom) After 2.5 minutes of stimulation with chemokines, aliquots were taken to analyze the level of phosphorylated/active Akt1 (p-Akt) and total Akt1 by Western blotting. A representative experiment out of 3 performed is shown. (Top) Remaining DCs were left for an additional 6 hours, and then the percentage of annexin V–positive/7-AAD–negative cells was quantified. Fluorescence-activated cell sorter (FACS) analysis performed in parallel showed that PTX treatment did not affect the levels of CCR7 (not shown). The results represent the mean and SEM of 3 independent experiments. (B) DCs were transfected either with vector or with βARK-CT. (Top) Eighteen hours after transfection, aliquots of vector- and βARK-CT–transfected DCs were taken to analyze βARK-CT levels by Western blotting using an anti-βARK antibody.28 β-actin levels show equal loading of the gels. (Bottom) Vector- and βARK-CT–expressing DCs were washed and subsequently incubated for 6 hours in 0.1% BSA in RPMI without (–) or with (+) CCL19 or CCL21. The percentage of annexin V–positive/7-AAD–negative cells was determined. A representative experiment out of 3 performed is shown.
Gi and βγ subunits regulate apoptosis and activation of Akt. (A) DCs were left untreated (control) or pretreated with PTX (100 ng/mL) for 120 minutes. Subsequently, DCs were left unstimulated (–) or stimulated (+) with CCL19 or CCL21. (Bottom) After 2.5 minutes of stimulation with chemokines, aliquots were taken to analyze the level of phosphorylated/active Akt1 (p-Akt) and total Akt1 by Western blotting. A representative experiment out of 3 performed is shown. (Top) Remaining DCs were left for an additional 6 hours, and then the percentage of annexin V–positive/7-AAD–negative cells was quantified. Fluorescence-activated cell sorter (FACS) analysis performed in parallel showed that PTX treatment did not affect the levels of CCR7 (not shown). The results represent the mean and SEM of 3 independent experiments. (B) DCs were transfected either with vector or with βARK-CT. (Top) Eighteen hours after transfection, aliquots of vector- and βARK-CT–transfected DCs were taken to analyze βARK-CT levels by Western blotting using an anti-βARK antibody.28 β-actin levels show equal loading of the gels. (Bottom) Vector- and βARK-CT–expressing DCs were washed and subsequently incubated for 6 hours in 0.1% BSA in RPMI without (–) or with (+) CCL19 or CCL21. The percentage of annexin V–positive/7-AAD–negative cells was determined. A representative experiment out of 3 performed is shown.
Inhibition of MAPK family members Erk1/2, p38, and JNK did not abrogate the effect of CCL19 and CCL21 on the survival of DCs
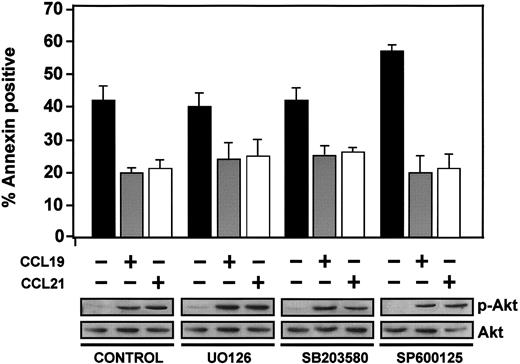
MAPK family members have been implicated in the regulation of apoptosis in different systems.7,34 To investigate if these kinases could mediate the CCR7-dependent effects on survival, we treated DCs with UO126, SB203580, or SP600125, which are selective inhibitors of Erk1/2, p38, or JNK, respectively. Despite the strong inhibition of the activity of the latter kinases (not shown), MAPK inhibitors caused only a slight increase in the basal death of the cells. Furthermore, in these cells, stimulation with CCL19 or CCL21 still caused a reduction in the percentage of apoptosis that was similar to that of control cells (Figure 4). Also, inhibition of different MAPK family members did not abrogate the stimulation of phosphorylation of Akt1 induced by CCL19 or CCL21 (Figure 4). In sum, the results indicate that MAPK family members are not regulating CCR7-mediated survival of DCs under our conditions.
MAPK family members do not mediate CCR7-dependent inhibition of apoptosis. DCs were left untreated (control) or pretreated with UO126 (5 μM), SB203580 (13 μM), or SP600125 (50 μM) for 60 minutes to inhibit, respectively, Erk1/2, p38, and JNK. Parallel control experiments demonstrated that these enzymes were completely blocked by the respective inhibitors (not shown). Subsequently, DCs were left unstimulated (–) or stimulated (+) with CCL19 or CCL21. (Bottom) After 2.5 minutes of stimulation with chemokines, aliquots were taken to analyze the level of active Akt1 (p-Akt) and total Akt1 by Western blotting. A representative experiment out of 3 performed is shown. (Top) Remaining DCs were left for an additional 6 hours, and then the percentage of annexin-positive/7-AAD–negative cells was quantified. The results represent the mean ± SEM of 3 independent experiments.
MAPK family members do not mediate CCR7-dependent inhibition of apoptosis. DCs were left untreated (control) or pretreated with UO126 (5 μM), SB203580 (13 μM), or SP600125 (50 μM) for 60 minutes to inhibit, respectively, Erk1/2, p38, and JNK. Parallel control experiments demonstrated that these enzymes were completely blocked by the respective inhibitors (not shown). Subsequently, DCs were left unstimulated (–) or stimulated (+) with CCL19 or CCL21. (Bottom) After 2.5 minutes of stimulation with chemokines, aliquots were taken to analyze the level of active Akt1 (p-Akt) and total Akt1 by Western blotting. A representative experiment out of 3 performed is shown. (Top) Remaining DCs were left for an additional 6 hours, and then the percentage of annexin-positive/7-AAD–negative cells was quantified. The results represent the mean ± SEM of 3 independent experiments.
Involvement of NFκB in the CCR7-induced survival of DCs
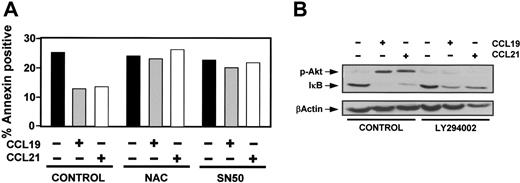
NFκB regulates a variety of antiapoptotic genes and is involved in regulating survival of DCs2,4,35 ; therefore, we analyzed if this transcription factor was involved in the effects elicited by CCL19 and CCL21. As shown in Figure 5A, treatment of DCs with the NFκB inhibitors N-acetyl-l-cysteine (NAC)36 and SN50 37 prevented the effects of chemokines on survival, suggesting the involvement of this transcriptional factor in signaling from CCR7. In most cells NFκB is kept in the cytoplasm as a latent, inactive form, by forming a complex with the inhibitor IκB. IκB kinase (IKK)–mediated phosphorylation of IκB targets this molecule for ubiquitylation and degradation, resulting in NFκB activation and translocation to the nucleus. Because the levels of IκB are inversely correlated to the activity of NFκB, we analyzed the levels of IκBin control and chemokine-treated DCs as an index of the activity of NFκB. As shown in Figure 5B, CCL19 and CCL21 induced an important down-regulation of IκB, suggesting that these chemokines induce activation of NFκB. To analyze if PI3K/Akt1 could be regulating the levels of IκB, we pretreated DCs with the PI3K inhibitor LY294002 and examined in control and chemokine-treated cells the levels of IκB. As shown in Figure 5B, inhibition of Akt1 phosphorylation led to a partial inhibition in the reduction of the levels of IκB induced by chemokines, suggesting that Akt1 contributes but is not the only mechanism leading to CCR7-dependent IκB degradation.
NFκB is involved in the antiapoptotic signaling induced from CCR7. (A) DCs were untreated (control) or pretreated with 20 mM n-acetyl-L-cysteine (NAC) or 20 μM SN50 for 60 minutes. Then, DCs were left unstimulated (–) or stimulated (+) with CCL19 or CCL21 for 6 hours. The percentage of annexin-positive/7-AAD–negative DCs was quantified. The data presented are representative of 2 independent experiments. (B) DCs untreated (control) or pretreated with LY294002 (100 μM) for 60 minutes were either left unstimulated (–) or stimulated (+) with CCL19 or CCL21 for an additional 30 minutes. (Top) DCs were taken to analyze by Western blotting the level of phosphorylated Akt1 (p-Akt) and subsequently, in the same blot, the levels of IκB. (Bottom) Blots were stripped and equal loading shown with an anti–β-actin antibody. A representative experiment out of 3 performed is shown.
NFκB is involved in the antiapoptotic signaling induced from CCR7. (A) DCs were untreated (control) or pretreated with 20 mM n-acetyl-L-cysteine (NAC) or 20 μM SN50 for 60 minutes. Then, DCs were left unstimulated (–) or stimulated (+) with CCL19 or CCL21 for 6 hours. The percentage of annexin-positive/7-AAD–negative DCs was quantified. The data presented are representative of 2 independent experiments. (B) DCs untreated (control) or pretreated with LY294002 (100 μM) for 60 minutes were either left unstimulated (–) or stimulated (+) with CCL19 or CCL21 for an additional 30 minutes. (Top) DCs were taken to analyze by Western blotting the level of phosphorylated Akt1 (p-Akt) and subsequently, in the same blot, the levels of IκB. (Bottom) Blots were stripped and equal loading shown with an anti–β-actin antibody. A representative experiment out of 3 performed is shown.
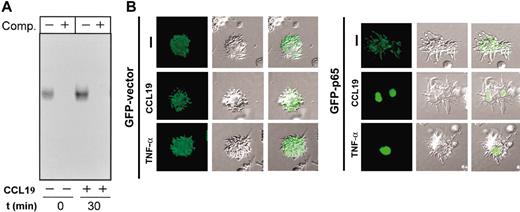
To analyze directly if chemokines induced activation of NFκB, we performed electrophoretic mobility shift assays (EMSAs). As shown in Figure 6A, stimulation of the cells with CCL19 caused an increase in the DNA-binding activity of NFκB, indicating that NFκB was activated by stimulating DCs with this chemokine. Because activated NFκB translocates to the nucleus, we analyzed if stimulation with chemokines induced such translocation of NFκB.2,4 RelA/p65 is a subunit of the NFκB family of transcription factors that is expressed in DCs38 ; therefore, we transfected DCs with a construct that encodes p65-GFP and then stimulated the DCs with CCL19 (Figure 6B) or CCL21 (not shown).29 Between 30% and 40% of the transfected cells expressed p65-GFP; however, following stimulation with chemokines almost 70% of these tranfected DCs showed a clear nuclear p65-GFP localization (Figure 6B). In contrast, in DCs transfected with GFP vector, the staining remained in the cytoplasm despite the stimulation with chemokines or TNF-α (Figure 6B). Taken together, the results clearly indicate that stimulation of DCs with CCL19 or CCL21 induces activation of NFκB.
Stimulation of DCs with CCL19 induces activation and translocation of NFκB to the nucleus. (A) Determination of NFκB DNA-binding activity in total cell extracts obtained from DCs that were left unstimulated (–) or stimulated (+) with CCL19 for 30 minutes. Extracts were used to perform EMSA in the absence (–) or presence (+) of a 100-fold molar excess of unlabeled competitor (Comp) oligonucleotides. A representative experiment out of 2 performed is shown. (B) Stimulation of DCs with CCL19 induces translocation of NFκB to the nucleus. DCs were transfected with either pEGFP-C1 vector (GFP-vector) or pEGFP-p65 (GFP-p65). Eighteen hours after transfection, DCs were washed with RPMI and then either left unstimulated (–) or stimulated for 45 minutes with CCL19 or TNF-α used as positive control. The cells were then plated onto polylysine-coated coverslips, fixed, permeabilized, and the nucleus stained with Hoechst 33342 (not shown). The figure show Nomarski optics and GFP protein staining analyzed using the FITC fluorescence channel. Hoechst 33342 staining marked the position of the nucleus in all the cases (not shown). The figure was viewed with a Nikon Diaphot microscope. A representative experiment out of 3 performed is shown.
Stimulation of DCs with CCL19 induces activation and translocation of NFκB to the nucleus. (A) Determination of NFκB DNA-binding activity in total cell extracts obtained from DCs that were left unstimulated (–) or stimulated (+) with CCL19 for 30 minutes. Extracts were used to perform EMSA in the absence (–) or presence (+) of a 100-fold molar excess of unlabeled competitor (Comp) oligonucleotides. A representative experiment out of 2 performed is shown. (B) Stimulation of DCs with CCL19 induces translocation of NFκB to the nucleus. DCs were transfected with either pEGFP-C1 vector (GFP-vector) or pEGFP-p65 (GFP-p65). Eighteen hours after transfection, DCs were washed with RPMI and then either left unstimulated (–) or stimulated for 45 minutes with CCL19 or TNF-α used as positive control. The cells were then plated onto polylysine-coated coverslips, fixed, permeabilized, and the nucleus stained with Hoechst 33342 (not shown). The figure show Nomarski optics and GFP protein staining analyzed using the FITC fluorescence channel. Hoechst 33342 staining marked the position of the nucleus in all the cases (not shown). The figure was viewed with a Nikon Diaphot microscope. A representative experiment out of 3 performed is shown.
Discussion
CCR7 plays a fundamental role directing mature DCs to the lymph nodes.10-14 Apart from this well-established function, it is emerging that this receptor plays additional roles, which are important for the physiology of DCs, including regulation of DC cytoarchitecture and endocytosis.15,16 Herein we show that stimulation of DCs with CCR7 ligands CCL19 and CCL21 blocks well-known apoptotic hallmarks of serum-starved DCs, including increased phosphatidyl exposure and membrane blebs on the membrane, nuclear changes, and loss of mitochondria potential (Figure 1). We show that PI3K/Akt1, a signaling axis that regulates apoptosis in a variety of cells settings,1-3 plays an important role in the protective effects elicited by CCR7. First, we observed a potent activation of PI3K/Akt1 that, strikingly, in the case of Akt1 was persistent and prolonged for at least 120 minutes (Figure 2A-B and not shown). Interestingly, the unusual and persistent activation of Akt that we observe has been also observed in lymphocytes for CXCR4, another member of the family of homeostatic chemokine receptors, like CCR7.33 Second, we demonstrated the important role of PI3K/Akt in the CCR7-mediated stimulation of the survival of DCs by showing that interference with these kinases using pharmacologic inhibitors (LY294002 or wortmannin) abrogated the effects of the chemokines (Figure 2C). Because it was shown before that in naive T cells CCL19 induced only transient activation of Akt1,33 the prolonged activation observed in DCs indicates the context dependence of the activation of this kinase. Akt1 phosphorylates/inhibits in the cytoplasm a variety of proapoptotic molecules including caspase 9 and bcl-2 members and also regulates the activation of prosurvival transcriptional factor NFκB.2,3 Furthermore, Akt1 can also translocate to the nucleus, where it inhibits proapoptotic transcriptional factors.2,3,39 Therefore, given the number of antiapoptotic targets, the persistent activation of Akt1 induced from CCR7 may have a strong antiapoptotic effect in DCs. In this regard, in other leukocytes it has been shown that Akt can maintain cell survival even in the absence of extrinsic signals.40
We also show that downstream from CCR7, Gi proteins are important to regulate the signaling that converges in PI3K/Akt and survival because pretreatment with pertussis toxin blocked completely the effect of the chemokines (Figure 3A). Furthermore, by transfecting cells with a construct encoding a peptide inhibitor of Gβγ,28 we show that βγ subunits, instead of αi subunits, regulate the signaling from CCR7 that leads to inhibition of apoptosis (Figure 3B). Interestingly, we found that despite the importance of different MAPK family members in inhibiting apoptosis in other cell settings,5-7 these kinases did not regulate CCR7-mediated survival in DCs (Figure 4), suggesting that signals that regulate programmed cell death depend on the surface receptor and cell type involved.
Our results indicate that the transcription factor NFκB plays an important role in the antiapoptotic signaling induced from CCR7 based on the following experimental evidence. First, 2 NFκB inhibitors blunted the effects of chemokines on survival (Figure 5A). Second, CCL19 and CCL21 induced down-regulation of IκB, an NFκB inhibitor whose levels are inversely and tightly correlated to the activation of NFκB (Figure 5B). As such, CCR7-dependent down-regulation of IκB was not completely abrogated by inhibition of PI3K/Akt1 (Figure 5B); our results suggest that alternative signaling pathways not involving these kinases may regulate IκB. Interestingly, after 30 minutes of stimulation with CCL19 or CCL21 we observed a recovery and subsequent increase in the levels of IκB (not shown), consistent with previous reports indicating that NFκB regulates the transcription of IκB.41 Third, by carrying out EMSA experiments we demonstrate that stimulation of CCR7 induces direct activation of NFκB (Figure 6A). Fourth, by transfecting DCs with a construct encoding the NFκB subunit p65-GFP we show that stimulation of DCs with CCL19 and CCL21 induced a rapid translocation of NFκB to the nucleus (Figure 6B). Because it is known that NFκB translocation takes place when this transcriptional factor is activated, these experiments also indicate that stimulation of CCR7 induces activation of NFκB.
Mature DCs display a variety of molecular mechanisms that increase their survival, including reduced sensibility to proapoptotic signals induced from death receptors and cytotoxic cells.42,43 Our results suggest that signaling from CCR7 may also contribute to the apoptotic-resistant phenotype of mature DCs. The antiapoptotic signaling from CCR7 may have an important impact on the immune response. On their way to the lymph node, chemokine CCL21, that is expressed constitutively by endothelial cells lining lymphatic vessels and high endothelial venules and which it is also secreted, along with CCL19, from lymph node areas, by stimulating CCR7 may contribute to increase the probability of DCs to reach the node regions.11-13 Moreover, as CCL19 and CCL21 are present at high levels in T-cell zones, once DCs reach these regions, CCR7 can also increase the survival of DCs. This could have an important impact on the immune response. Because it has been shown that DCs terminate their life cycle in the lymph node,44-47 an extended longevity, favored by signaling from CCR7, may increase the probability of antigen presentation and stimulation of antigen-specific T cells.
Prepublished online as Blood First Edition Paper, April 1, 2004; DOI 10.1182/blood-2003-11-3943.
Supported by grants BFI-2001-228, CAM08.3/0040/2001.1, and PI021058 (J.L.R.-F) and SAF2002-04615-C02-02 (P.S.-M.); Fondo de Investigación Sanitaria and Ramón y Cajal programs (J.L.R.-F.); and a scholarship associated to grant PI021058 (L.R.-B.). N.S.-S. is recipient of a fellowship, Formación del Profesorado Universitario (FPU), conferred by the Ministerio de Educación (Spain).
N.S.-S. and L.R.-B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We acknowledge Julia Villarejo and Isabel Treviño for their help, Patricio Aller for advice on Hoechst 33342 staining, Eduardo Muñoz for the p65-GFP construct, and Robert Lefkovitch for providing the dominant negative βARK-CT construct.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal