Abstract
CD157, a glycosylphosphatidylinositol (GPI)–anchored protein encoded by a member of the CD38 NADase/ADP-ribosyl cyclase gene family, is expressed on the surface of most human circulating neutrophils. This work demonstrates that CD157 is a receptor that induces reorganization of the cytoskeleton and significant changes in cell shape, and that signals mediated by CD157 act through modulation of cytosolic Ca2+ concentration. These signals are independent of the products of CD157's enzymatic activities (ie, cyclic adenosine diphosphate [ADP]–ribose and ADP-ribose). Indeed, the enzymatic activities of CD157 in circulating neutrophils as well as in dimethyl sulfoxide (DMSO)–differentiated (CD157+/CD38-) HL-60 cells, are hardly detectable. This work also shows that the receptorial activity relies on cross-talk between CD157 and β2 integrin. CD157 localizes in GM1-enriched lipid rafts and, upon activation, it migrates to the uropod, a structure specialized in motility and adhesive functions. Indeed, CD157 is involved in adhesion to extracellular matrix proteins and in chemotaxis induced in vitro by formyl-methionyl-leucyl-phenylalanine (fMLP). These findings were consistent with the results obtained in neutrophils from patients with paroxysmal nocturnal hemoglobinuria (PNH), in which CD157 is deficient. These neutrophils showed constant defects in adhesion and migration. Our data attribute specific and crucial roles to CD157 in the regulation of innate immunity during inflammation.
Introduction
Human CD157 is a glycosylphosphatidylinositol (GPI)–linked glycoprotein encoded by a member of the NADase/adenosine diphosphate (ADP)–ribosyl cyclase gene family, which also includes CD38.1 The CD157 and CD38 molecules are pleiotropic in function, acting both as ectoenzymes and receptors.2-4 CD157 is abundantly expressed by myeloid lineage from precursors to differentiated stages5 by bone marrow stromal cells, synovial cells, endothelial cells, and other cell types.6 Functional analysis by means of agonistic monoclonal antibodies (mAbs) mimicking natural ligand(s) suggested that CD157 could act as a receptor with signal transduction capacity. However, as CD157 lacks a cytoplasmic domain, it must associate with some “conventional” receptor(s) to transduce signals; much in the same way as CD38, which has a short intracellular domain, CD157 acts as a parasite on specific receptors to compensate for its structural ineptitude to exert receptorial functions.7,8
In concert with monocytes and tissue macrophages, neutrophils carry out many of the major functional responses of the innate immune system. They participate in inflammatory responses, such as host resistance to infection, and in detrimental host inflammatory reactions, including arthritis, septic shock, stroke, and transplantation rejection. The regulation of neutrophil recruitment into the inflammatory sites and neutrophil clearance are critical processes assuring effective host defense without tissue injury. Neutrophil extravasation is a multistep process dependent on the coordination of many cellular functions. To achieve motility, a cell must acquire and maintain spatial and functional asymmetry characterized by the leading edge, which protrudes, and the uropod, which retracts. This process, termed polarization, is driven by specific interactions between the plasma membrane and the actin cytoskeleton through actin-membrane binding proteins.9
An increasing number of membrane proteins expressed in selected microdomains composed of sphingolipids, cholesterol, and GPI-anchored proteins have been attributed a role in the regulation of neutrophil behavior.10 GPI-anchored proteins are ubiquitous and are involved in several biologic processes such as immune recognition, proteolysis of the extracellular matrix, complement regulation, myelin biosynthesis, and prion pathogenesis.11
Notwithstanding the expression of CD157 on neutrophils and endothelial cells (2 key players in host defense against pathogens), the role of CD157 in the regulation of innate immune response has never been explored. This work demonstrates that CD157 is one of the orchestrators of signal transduction pathways essential to neutrophil adherence and migration.
Materials and methods
Antibodies and reagents
Anti-human CD3 mAb CBT3G (IgG2a), anti-HLA class I mAb O1.65 (IgG2a), anti-CD11b mAb 107 (IgG1),12 anti-CD18 mAb TS1/18 (IgG1, kindly provided by A. Arnout, Harvard Medical School, Boston, MA), and anti-CD38 mAb IB4 (IgG2a)13 were affinity purified as described.14 Anti-CD157 mAbs RF3 (IgG2a), Bec-7 (IgG1), and SG2 (IgG2a) were provided by K. Ishihara and T. Hirano (University of Osaka, Japan),15 and mAb Mo5 (IgG2a) was provided by R. Todd III (University of Michigan Health System, Ann Arbor, MI).5 An affinity-purified, F(ab')2 fraction of rabbit antibody to mouse IgG (F(ab')2-RaMIgG), fluorescein isothiocyanate (FITC)–labeled F(ab')2-RaMIg (F(ab')2-RaMIg-FITC), Texas red–conjugated F(ab')2-RaMIg (F(ab')2-RaMIg-Texas red), and purified murine IgG Fc fragments, were purchased from Jackson ImmunoResearch (West Grove, PA).
Anti-CD11b-phycoerythrin was from Becton-Dickinson (Milano, Italy) and anti-CD157 (RF3-FITC) was from Immunotech (Marseille, France). EGTA (ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid), formyl-methionyl-leucyl-phenylalanine (fMLP), all-trans-retinoic acid (ATRA), dimethyl sulfoxide (DMSO), nicotinamide guanine dinucleotide (NGD+), phalloidin-FITC and rhodamine isothiocyanate (TRITC), FITC-labeled cholera toxin B subunit (CTX-FITC), TRITC-labeled streptavidin, 8-Br-cyclic ADP ribose (8-Br-cADPR), wortmannin, and staurosporin were provided by Sigma (Milano, Italy). Fluo3-am and pluronic F-127 were from Molecular Probes (Eugene, OR).
Purification of human neutrophils
Peripheral blood was obtained from healthy blood donors or patients with paroxysmal nocturnal hemoglobinuria (PNH) and centrifuged through Ficoll-Paque (Amersham Pharmacia, Milano, Italy). Polymorphonuclear cells (PMNs) were isolated to more than 95% purity by sedimentation in 1% gelatin in Ca2+/Mg2+-free phosphate-buffered saline (PBS), followed by hypotonic lysis of erythrocytes with H2O for 20 seconds. Informed consent was obtained from patients with PNH enrolled in the study before each blood sample collection. Samples were collected according to the protocol approved by the review board of the National Institute for Cancer Research (Genova, Italy).
Immunofluorescence flow cytometry
PMNs (3 × 105/sample) were suspended in PBS with 0.5% bovine serum albumin (BSA) and incubated for 30 minutes at 4°C with 5 μg/mL of the selected mAb. After washing, cells were incubated for 30 minutes at 4°C with F(ab')2-RaMIg-FITC and fluorescence was analyzed using a FACSCalibur flow cytometer and CellQuest software (Becton Dickinson). Background mAb binding was estimated by means of isotype-matched negative control mAbs. Where indicated, experiments were performed on whole blood treated with NH4Cl-EDTA lysis buffer (15 minutes at 20°C) before undergoing fluorescence-activated cell sorting (FACS) analysis.
HL-60 promyelocytic leukemia cells were cultured in vitro in RPMI 1640 medium (Sigma) supplemented with 5% fetal calf serum (FCS), 50 μg/mL streptomycin, 2 U/mL penicillin, and 2 mM l-glutamine (Sigma). For differentiation, cells (3 × 105/mL) were incubated in the presence of 1.2% DMSO or 1 μM ATRA for 4 days.
Evaluation of GDP-ribosyl cyclase activity
The enzymatic activity present on the membrane of live PMNs was determined as described.16 Briefly, PMNs were suspended in PBS and incubated for 20 minutes at 37°C in the presence or in the absence (controls) of 0.1 mM NGD+. After centrifugation, supernatants were collected to evaluate the conversion of NGD+ to the fluorescent compound cyclic guanosine diphosphate ribose (cGDPR) and analyzed by fluorescence spectrometer set at excitation 300 nm and emission 410 nm.
Intracellular calcium measurements
Changes in [Ca2+]i were measured by using fluo3-AM fluorescent dye. PMNs were washed in PBS containing 5 mM KCl, 5 mM glucose, and 1% FCS, and incubated for 30 minutes at 30°C with 4 μM fluo3-AM to minimize compartmentalization in the presence of 0.01% pluronic F-127 detergent. Then, cells were washed and suspended (2 × 106/mL) in Hanks balanced salt solution (HBSS) with or without 1 mM CaCl2, 0.5 mM MgCl2.
PMNs loaded with fluo3-AM were incubated with selected primary mAbs (5 μg/mL-10 μg/mL) for 10 minutes at 37°C, then cell stimulation was induced by cross-linking with F(ab')2-RaMIgG (20 μg/mL-100 μg/mL). Cells were first incubated with murine IgG Fc fragments (150 μg/mL) at 4°C for 15 minutes to block binding of the primary mAb to Fc receptors.17
Cells were incubated with an irrelevant isotype-matched mAb and with F(ab')2-RaMIgG alone to serve as negative controls. Analysis was performed by FACSCalibur flow cytometer. To avoid signal inaccuracy, the temperature was strictly maintained at 37°C throughout the experiments and 525 nm emission was measured on a linear scale.
Laser confocal microscopy
PMNs were treated for 30 minutes at 4°C with saturating amounts of biotin-labeled mAb, cross-linked with streptavidin-FITC for 10 minutes at 4°C, then brought to 37°C for the indicated time, and washed, and fixed with 4% paraformaldehyde.
The effects of fMLP stimulation on cell polarization and actin polymerization were analyzed as described.18 Briefly, PMNs were incubated with anti-CD157 (or control mAb) for 30 minutes at 4°C, then washed and stained with F(ab')2-GaMIg–Texas red for 30 minutes at 4°C. PMNs were stimulated with fMLP (100 nM) for 5 minutes at 37°C, fixed and labeled with CTX-FITC, or labeled for F-actin by the addition of 4% paraformaldehyde, 0.1% glutaraldehyde, 0.5 mg/mL saponin, and 0.1 μg/mL phalloidin-TRITC.
The slides were analyzed using an Olympus FV300 confocal microscope equipped with a Green Helium Neon (543 nm) laser, a Blue Argon (488 nm) laser, and FluoView 300 software. In double-label experiments, the 2 channels were scanned alternatively, using only one laser and one detector at any given time to avoid cross-talk.
Integrin engagement
Engagement of β2 integrins was induced by adhesion of PMNs to immobilized fibrinogen (Sigma) or serum. Briefly, 50 μL PMNs (5 × 106/mL) in HBSS were seeded onto microscope slides coated with fibrinogen (50 μg/mL) or FCS at 4°C overnight, followed by extensive washes. Adhesion was induced at 37°C in the presence of 1 mM CaCl2 and 1 mM MgCl2. Adherent cells were fixed with 4% paraformaldehyde for 30 minutes at 4°C, washed, stained with mAb, and analyzed using a confocal laser microscope.
Chemotaxis assays
Neutrophil chemotactic activity was assayed in 48-well microchemotaxis chambers (Neuro Probe, Bethesda, MD). The bottom wells of the chamber were filled with 30 μL chemotactic stimulus or medium. A 10-mm-thick polyvinylpyrrolidone-free polycarbonate filter with 5-μm pores was placed over the samples and 50 μL of the cell suspension (1.2 × 106/mL) was placed into the upper wells. The chambers were incubated in humidified air with 5% CO2 at 37°C for one hour. After washing, the filter was fixed, stained, and mounted on a glass slide. Cells that had completely migrated through the filter were counted using light microscopy.
Neutrophil adhesion assay
Polystyrene strips were coated with fibrinogen (50 μg/mL) in PBS overnight at 4°C and counter-coated with 1% BSA. PMNs were labeled with 51Cr for one hour at 37°C, as described,19 then washed, suspended (1.5 × 106/mL) in RPMI 1640, and incubated for 15 minutes at 4°C with 150 μg/mL purified murine IgG Fc fragments. Labeled PMNs were plated onto precoated wells (50 μL/well, in triplicate) in the presence or in the absence of mAb (10 μg/mL) and incubated at 37°C. Nonadherent cells were removed by washing; the bound cells were lysed in 100 μL of 2% Triton X-100 and the counts per minute were determined in a γ-counter.
Statistical analysis
Analysis of the statistical significance of differences between 2 mean values was determined using the Student t test and the Mann Whitney U test. P values less than .05 were considered significant.
Results
CD157 is expressed on human PMNs but is enzymatically inactive
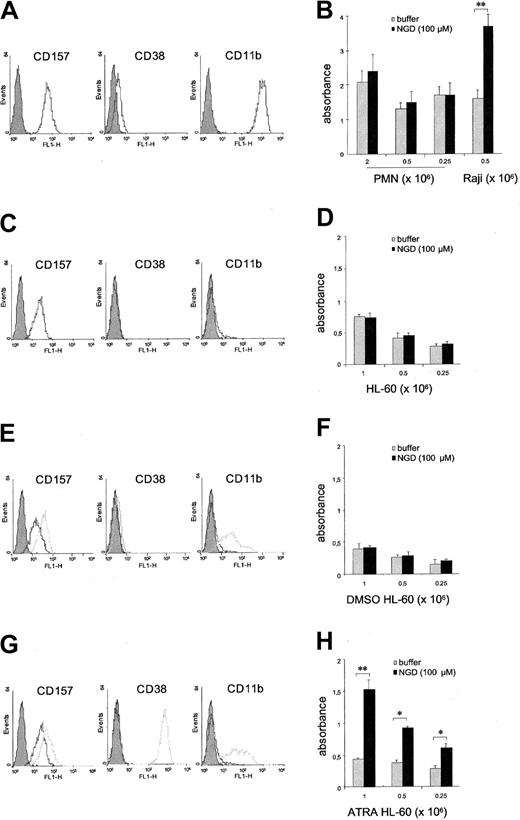
Flow cytometric analysis demonstrated that CD157 is expressed by all circulating PMNs at high epitope density. Its paralogue, CD38, is absent or expressed at very low levels by a small percentage of PMNs, with significant interdonor variability ranging from 0% to 25% (Figure 1A).
Analysis of the expression and cyclase activity of CD157. PMNs purified from (A) peripheral blood, (C) HL-60 cells, (E) DMSO-induced HL-60 cells, and (G) ATRA-induced HL-60 cells were incubated with anti-CD157, anti-CD38, or anti-CD11b mAbs and F(ab′)2-RaMIg-FITC and analyzed by flow cytometry. (E, G) Analysis of basal expression (black profiles) and of the expression induced following treatment with DMSO or ATRA for 4 days (gray profiles). Gray-filled profiles are the isotype control mAb. Number of cells tested was 7 000. Results are representative of 5 experiments. (B) Evaluation of the cyclase activity of PMNs. The cyclase activity of PMNs was measured by analyzing the ability of increasing concentrations of cells to produce cGDPR in the absence (▦) or presence (▪) of NGD+ using a fluorimetric assay. Raji B cells were used as a positive control. Evaluation of the cyclase activity of (D) HL-60, (F) DMSO-induced HL-60 cells, and (H) ATRA-induced HL-60 cells. Cells were incubated in the absence ( ) or in the presence (▪) of NGD+. The formation of the fluorescent product cGDPR was measured by a fluorescence spectrometer, set at excitation 300 nm and emission 410 nm. Each experiment was performed in triplicate, and results are expressed as means of absorbance obtained from 3 different experiments ± the standard deviation (SD). *P < .05; **P < .001.
) or in the presence (▪) of NGD+. The formation of the fluorescent product cGDPR was measured by a fluorescence spectrometer, set at excitation 300 nm and emission 410 nm. Each experiment was performed in triplicate, and results are expressed as means of absorbance obtained from 3 different experiments ± the standard deviation (SD). *P < .05; **P < .001.
Analysis of the expression and cyclase activity of CD157. PMNs purified from (A) peripheral blood, (C) HL-60 cells, (E) DMSO-induced HL-60 cells, and (G) ATRA-induced HL-60 cells were incubated with anti-CD157, anti-CD38, or anti-CD11b mAbs and F(ab′)2-RaMIg-FITC and analyzed by flow cytometry. (E, G) Analysis of basal expression (black profiles) and of the expression induced following treatment with DMSO or ATRA for 4 days (gray profiles). Gray-filled profiles are the isotype control mAb. Number of cells tested was 7 000. Results are representative of 5 experiments. (B) Evaluation of the cyclase activity of PMNs. The cyclase activity of PMNs was measured by analyzing the ability of increasing concentrations of cells to produce cGDPR in the absence (▦) or presence (▪) of NGD+ using a fluorimetric assay. Raji B cells were used as a positive control. Evaluation of the cyclase activity of (D) HL-60, (F) DMSO-induced HL-60 cells, and (H) ATRA-induced HL-60 cells. Cells were incubated in the absence ( ) or in the presence (▪) of NGD+. The formation of the fluorescent product cGDPR was measured by a fluorescence spectrometer, set at excitation 300 nm and emission 410 nm. Each experiment was performed in triplicate, and results are expressed as means of absorbance obtained from 3 different experiments ± the standard deviation (SD). *P < .05; **P < .001.
) or in the presence (▪) of NGD+. The formation of the fluorescent product cGDPR was measured by a fluorescence spectrometer, set at excitation 300 nm and emission 410 nm. Each experiment was performed in triplicate, and results are expressed as means of absorbance obtained from 3 different experiments ± the standard deviation (SD). *P < .05; **P < .001.
To test whether CD157+ PMNs are able to catalyze the cyclase reaction, we analyzed their ability to produce the fluorescent compound cGDPR in the presence of NGD+. No significant amounts of cGDPR were observed even in the presence of 2 × 106 PMNs/sample. Control Raji B cells (which express CD38 at high epitope density, but lack CD157) showed marked cyclase activity (Figure 1B). To rule out any intrinsic incapability of PMNs to exert the cyclase activity due to handling during the purification procedures, we performed similar experiments using HL-60 cells in conventional culture conditions and also following treatment with DMSO or ATRA.20 HL-60 cells cultured in the presence of DMSO or ATRA showed progressive morphologic evidence of granulocytic maturation, expressed lineage-specific differentiation markers, and acquired migratory properties.21 Cytofluorimetric analysis showed that CD157 is constitutively expressed by HL-60 cells at low epitope density, whereas CD38 is undetectable (Figure 1C). The expression of CD157 is enhanced by both DMSO- and ATRA-induced granulocytic maturation. CD38 is induced by ATRA but not by DMSO differentiation (Figure 1E,G). The results demonstrated that HL-60 cells metabolize NGD+, thus giving origin to cGDPR, only after ATRA-induced differentiation (Figure 1H). Indeed, neither HL-60 nor DMSO-treated cells (CD157+/CD38-) showed any detectable cyclase activity (Figure 1D,F).
CD157 ligation increases intracellular Ca2+ concentration
Two routes for stimulating Ca2+ signals in PMNs are believed to exist: one is ruled by 7 transmembrane receptors for chemokines, whereas the other is mediated by specific receptors, such as Fc receptors, integrins, and selected GPI-anchored proteins. Our working hypothesis was that CD157 may belong to the GPI-anchored molecules involved in early PMN activation steps. The results demonstrated that ligation of CD157 by 4 different mAbs and successive cross-linking with an F(ab')2-RaMIgG induces Ca2+ currents in PMNs. The profile is characterized by a significant increase in [Ca2+]i, although with a major time lapse and a lower amplitude than that observed following incubation with fMLP (100 nM). To prevent potential aggregation mediated by engagement of FcR with the Fc domain of the anti-CD157 mAb, PMNs were preincubated with 150 μg/mL murine IgG Fc fragments. Moreover, a possible interference of Fc receptor–mediated signaling notwithstanding the block of the receptor was excluded by the observation that an isotype-matched anti-CD3 mAb elicited no Ca2+ currents (Figure 2A, bottom panel). To establish a possible relationship between Ca2+ signaling and CD157 clustering efficiency,22 PMNs were treated with saturating amounts of anti-CD157 (5 μg/mL) and increasing concentrations (20 μg/mL-100 μg/mL) of cross-linking F(ab')2-RaMIgG. Under these experimental conditions, the magnitude of Ca2+ flux and the percentage of responding cells (not shown) proved strictly dependent on the extent of CD157 cross-linking (Figure 2A, top panel).
Effects of CD157 cross-linking on intracellular Ca2+ concentration. Purified PMNs were first incubated with murine IgG Fc fragments (150 μg/mL) at 4°C for 15 minutes to block binding of the primary mAb to Fc receptors,17 then loaded with fluo3-AM; after calibration of the baseline fluorescence, the different stimuli were added (arrow) and cell events acquired. Dynamic changes in [Ca2+]i were monitored continuously by plotting the shift in the fluo3-AM fluorescence over a 516-second time course. Data are representative of 3 separate experiments. (A) Top panel: PMNs were treated with saturating amounts of anti-CD157 Mo5 mAb (5 μg/mL) and increasing concentrations (20 μg/mL-100 μg/mL) of F(ab′)2-RaMIgG. Bottom panel: a significant increase in [Ca2+]i was observed following incubation with fMLP (100 nM), whereas a control isotype-matched anti-CD3 mAb did not elicit any Ca2+ currents. (B) The mobilization of Ca2+ after CD157 cross-linking (top) or fMLP stimulation (bottom) was monitored after treatment for 5 minutes with 3 mM EGTA. (C) Dye-loaded PMNs were preincubated in the presence or absence of 100 μM 8-Br-cADPR and then stimulated with Mo5 mAb and F(ab′)2-RaMIgG or 100 nM fMLP (arrow). (D) The effects of CD157 and CD18 cross-linking or fMLP stimulation were analyzed in PMNs pretreated with 100 nM wortmannin or 500 nM staurosporin. Data are representative of 3 experiments with cells from different donors.
Effects of CD157 cross-linking on intracellular Ca2+ concentration. Purified PMNs were first incubated with murine IgG Fc fragments (150 μg/mL) at 4°C for 15 minutes to block binding of the primary mAb to Fc receptors,17 then loaded with fluo3-AM; after calibration of the baseline fluorescence, the different stimuli were added (arrow) and cell events acquired. Dynamic changes in [Ca2+]i were monitored continuously by plotting the shift in the fluo3-AM fluorescence over a 516-second time course. Data are representative of 3 separate experiments. (A) Top panel: PMNs were treated with saturating amounts of anti-CD157 Mo5 mAb (5 μg/mL) and increasing concentrations (20 μg/mL-100 μg/mL) of F(ab′)2-RaMIgG. Bottom panel: a significant increase in [Ca2+]i was observed following incubation with fMLP (100 nM), whereas a control isotype-matched anti-CD3 mAb did not elicit any Ca2+ currents. (B) The mobilization of Ca2+ after CD157 cross-linking (top) or fMLP stimulation (bottom) was monitored after treatment for 5 minutes with 3 mM EGTA. (C) Dye-loaded PMNs were preincubated in the presence or absence of 100 μM 8-Br-cADPR and then stimulated with Mo5 mAb and F(ab′)2-RaMIgG or 100 nM fMLP (arrow). (D) The effects of CD157 and CD18 cross-linking or fMLP stimulation were analyzed in PMNs pretreated with 100 nM wortmannin or 500 nM staurosporin. Data are representative of 3 experiments with cells from different donors.
Determining whether the increase in intracellular Ca2+ was due to release from intracellular stores or to calcium entering the cell through the plasma membrane was achieved by monitoring the mobilization of Ca2+ after removing extracellular Ca2+ with EGTA. Removal of external Ca2+ partly reduces signals elicited via CD157 (Figure 2B), indicating that the Ca2+ flux drawn by CD157 has both an extracellular and an intracellular component.
Similar experiments were performed in fMLP- and CD157-stimulated PMNs pretreated with the cyclic ADP-ribose (cADPR) antagonist 8-Br-cADPR, to establish whether cADPR is involved in CD157-induced Ca2+ mobilization notwithstanding the inefficient cyclase activity displayed by CD157. Addition of 8-Br-cADPR had no effect on the [Ca2+]i, suggesting that in these experimental conditions, cADPR is not involved in Ca2+ mobilization (Figure 2C).
Further insight into the CD157-driven signaling pathway was obtained by investigating the effects of wortmannin and staurosporin. The intracellular Ca2+ increase upon cross-linking of CD157 is extremely sensitive to 100 nM wortmannin, an inhibitor of phosphoinositide 3-kinase (PI3-K). Comparable results were obtained upon cross-linking of CD18. However, wortmannin does not alter the response to fMLP (Figure 2D).
Staurosporin, which inhibits both tyrosine and ser/thr kinases, does not influence Ca2+ mobilization in response to CD157 ligation, suggesting that this signaling pathway does not require protein kinase phosphorylation (Figure 2D).
CD157 ligation induces a change of cell shape and F-actin distribution in neutrophils
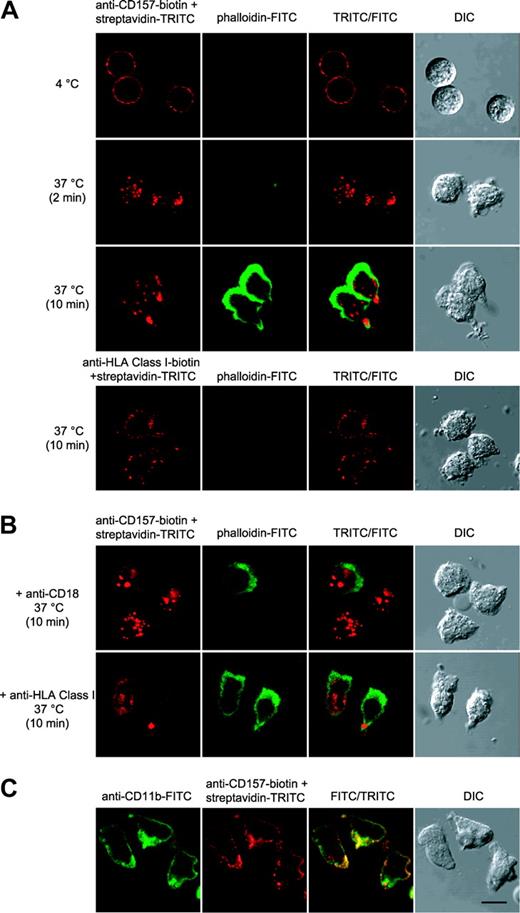
The effects of CD157 ligation on cytoskeletal organization were analyzed by tracking the localization of F-actin in neutrophils. Our previous observations indicated that CD157-mediated signals are strictly dependent on the extent of CD157 cross-linking. Therefore, these experiments were done using biotin-labeled Mo5 and streptavidin-TRITC. In these conditions, CD157 ligation is followed by a rapid clustering of the molecule and successive changes in cell shape and an increase in F-actin content, as made evident by the staining with phalloidin-FITC. The effects are specific since no change of cell shape and F-actin distribution were induced by temperature or by anti-HLA class I mAb used in the same experimental conditions (Figure 3A, bottom panels). CD157 migrates prevalently to the rear of the cells whereas F-actin is localized at the opposite pole (Figure 3A). The changes in cell shape and in F-actin distribution induced by CD157 cross-linking are strongly inhibited by preincubation of neutrophils with saturating amounts of anti-CD18 mAb but not by an isotype-matched anti-HLA class I mAb (Figure 3B), suggesting that CD157 is involved in β2 integrin–dependent cytoskeletal remodeling.
CD157 cross-linking induces a change of cell shape and F-actin distribution in neutrophils. (A-B) Neutrophils were incubated with anti–CD157-biotin (or anti–HLA class I–biotin) and streptavidin-TRITC under the indicated conditions, fixed, permeabilized, and stained with phalloidin-FITC. Then, samples were observed by differential interference contrast (DIC) and fluorescence confocal microscopy. CD157 cross-linking progressively induces neutrophil polarization, whereas HLA class I cross-linking has no effects. (B) The effects following CD157 cross-linking were inhibited when the experiment was performed in the presence of 10 μg/mL anti-CD18, but not in the presence of anti-HLA class I mAb, used in the same experimental conditions. (C) Clustering of the CD11b/CD18 complex induces spatial colocalization of CD157. CD11b/CD18 clustering was induced by incubation of PMNs (30 minutes at 37°C) on microscope slides coated with FCS. Cells were fixed and stained with anti-CD11b-FITC, anti-CD157-biotin and streptavidin-TRITC. Cells were mounted onto slides and imaged at 20°C using a confocal scanning laser microscope (FV300) mounted on an IX71 inverted microscope (both from Olympus, Hamburg, Germany) with a PlanApo 60 × oil, 1.4 numerical aperture (NA) objective lens. Data are representative of 5 experiments with cells from different donors. Bar, 10 μm.
CD157 cross-linking induces a change of cell shape and F-actin distribution in neutrophils. (A-B) Neutrophils were incubated with anti–CD157-biotin (or anti–HLA class I–biotin) and streptavidin-TRITC under the indicated conditions, fixed, permeabilized, and stained with phalloidin-FITC. Then, samples were observed by differential interference contrast (DIC) and fluorescence confocal microscopy. CD157 cross-linking progressively induces neutrophil polarization, whereas HLA class I cross-linking has no effects. (B) The effects following CD157 cross-linking were inhibited when the experiment was performed in the presence of 10 μg/mL anti-CD18, but not in the presence of anti-HLA class I mAb, used in the same experimental conditions. (C) Clustering of the CD11b/CD18 complex induces spatial colocalization of CD157. CD11b/CD18 clustering was induced by incubation of PMNs (30 minutes at 37°C) on microscope slides coated with FCS. Cells were fixed and stained with anti-CD11b-FITC, anti-CD157-biotin and streptavidin-TRITC. Cells were mounted onto slides and imaged at 20°C using a confocal scanning laser microscope (FV300) mounted on an IX71 inverted microscope (both from Olympus, Hamburg, Germany) with a PlanApo 60 × oil, 1.4 numerical aperture (NA) objective lens. Data are representative of 5 experiments with cells from different donors. Bar, 10 μm.
The lateral association of CD157 and β2 integrin was analyzed after integrin clustering induced by adhesion of PMNs to microscope slides precoated with fibrinogen or FCS.23 The results demonstrated that CD157 and β2 integrin are closely associated spatially. Indeed, ligand binding of β2 integrin induces its clustering in adherent PMNs, as inferred by CD11b and CD18 (not shown) confocal analysis, and CD157 staining colocalizes to identical regions of the membrane in the majority of cells (Figure 3C).
fMLP induces overexpression and membrane compartmentalization of CD157
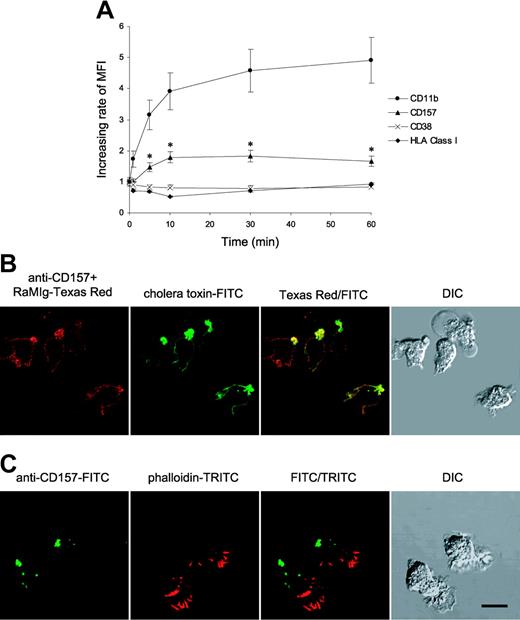
The expression of CD157 and CD38 by PMNs was examined during stimulation with fMLP and compared with the expression of CD11b, known to rapidly increase upon PMN activation.24 CD157 surface expression rapidly increased nearly 2-fold after stimulation with fMLP, reaching a plateau after 10 minutes and showing kinetic similarities to CD11b. No appreciable modulation of CD38 expression occurred at any time point considered. As expected, CD11b rapidly increased following fMLP treatment (Figure 4A).
Effects of fMLP on CD157 expression and compartmentalization. (A) The expression of CD157, CD38, and CD11b was examined during stimulation of PMNs with fMLP. All the experiments were performed on whole blood to minimize the artifactual modulation attributable to cell purification procedures. Blood (100 μL) was treated with fMLP (10 nM, 37°C) for the indicated times, fixed, and incubated with anti-CD157, anti-CD38, anti-CD11b, and anti-HLA class I mAbs. After labeling with F(ab′)2-RaMIg-FITC, samples were treated with lysis buffer and analyzed by FACS. Forward- and right-angle scatters were used to selectively gate PMNs. Results showing the increasing rate of mean fluorescence intensity (MFI) are expressed as fMLP-induced expression of CD157, CD38, CD11b, or HLA class I/basal surface expression of each molecule at 37°C. The results are expressed as means of 3 separate experiments ± SD. (B) Confocal microscopy analysis of PMNs treated with fMLP. PMNs were incubated for 5 minutes at 37°C in the presence of 100 nM fMLP, then fixed and stained with anti-CD157 and cholera toxin-FITC. (C) PMNs treated with fMLP were fixed, permeabilized, and stained with anti-CD157-FITC and phalloidin-TRITC to visualize F-actin polarization. Samples were observed by differential interference contrast (DIC) and fluorescence confocal microscopy. Cells were mounted onto slides and imaged at 20°C using a confocal scanning laser microscope (FV300) mounted on an IX71 inverted microscope (both from Olympus) with a PlanApo 60 × oil, 1.4 numerical aperture (NA) objective lens. Data are representative of 5 experiments with cells from different donors. Bar, 10 μm.
Effects of fMLP on CD157 expression and compartmentalization. (A) The expression of CD157, CD38, and CD11b was examined during stimulation of PMNs with fMLP. All the experiments were performed on whole blood to minimize the artifactual modulation attributable to cell purification procedures. Blood (100 μL) was treated with fMLP (10 nM, 37°C) for the indicated times, fixed, and incubated with anti-CD157, anti-CD38, anti-CD11b, and anti-HLA class I mAbs. After labeling with F(ab′)2-RaMIg-FITC, samples were treated with lysis buffer and analyzed by FACS. Forward- and right-angle scatters were used to selectively gate PMNs. Results showing the increasing rate of mean fluorescence intensity (MFI) are expressed as fMLP-induced expression of CD157, CD38, CD11b, or HLA class I/basal surface expression of each molecule at 37°C. The results are expressed as means of 3 separate experiments ± SD. (B) Confocal microscopy analysis of PMNs treated with fMLP. PMNs were incubated for 5 minutes at 37°C in the presence of 100 nM fMLP, then fixed and stained with anti-CD157 and cholera toxin-FITC. (C) PMNs treated with fMLP were fixed, permeabilized, and stained with anti-CD157-FITC and phalloidin-TRITC to visualize F-actin polarization. Samples were observed by differential interference contrast (DIC) and fluorescence confocal microscopy. Cells were mounted onto slides and imaged at 20°C using a confocal scanning laser microscope (FV300) mounted on an IX71 inverted microscope (both from Olympus) with a PlanApo 60 × oil, 1.4 numerical aperture (NA) objective lens. Data are representative of 5 experiments with cells from different donors. Bar, 10 μm.
In response to a chemoattractant (such as fMLP), an endogenous polarity and asymmetry is established in migrating neutrophils with the generation of specialized domains: the chemokine receptors are prevalently located at the leading edge, whereas several proteins (including adhesion receptors, transmembrane CD44, and several GPI-anchored molecules) are concentrated at the uropod.9 The acquisition of the spatial and functional asymmetries between the rear and the front of the PMN is a prelude to cell chemotaxis. To establish the effects of fMLP on CD157 cell membrane redistribution, PMNs were incubated for 5 minutes at 37°C in the presence of 100 nM fMLP, and the localization of CD157 was monitored by confocal microscopy analysis. In response to fMLP, CD157 undergoes redistribution and migrates preferentially to the rear of the polarized PMNs, as revealed by colocalization with cholera toxin, a specific marker for the glycosphingolipid GM1,25 preferentially localized at the uropod (Figure 4B). fMLP stimulation induces actin polymerization almost exclusively at the leading edge, resulting in a dramatic accumulation of F-actin at the front of the cells (Figure 4C).
CD157 is involved in neutrophil chemotaxis
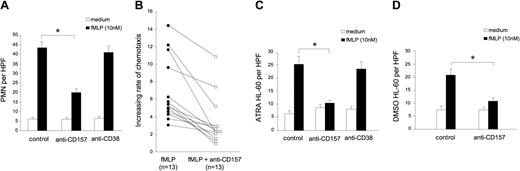
Neutrophils migrate to the site of infection in response to gradients of chemokines that are produced by the local cells and by pathogens. To investigate whether CD157 is involved in migration, neutrophils were pretreated with a panel of anti-CD157 mAbs and successively exposed to an fMLP gradient. The percentage of migrated cells was evaluated after a one-hour incubation at 37°C and compared with that of isotype-matched mAb-treated cells. All of the anti-CD157 mAbs analyzed displayed a significant inhibitory effect on PMN migration, whereas the anti-CD38 mAbs showed no appreciable effects (Figure 5A). Inhibition was independent from the amount of fMLP used in the experiments, and was evident even at suboptimal concentrations of fMLP (data not shown). So far, the analysis of all the healthy blood donors confirmed the effects induced by CD157 ligation, although the interdonor variability was not negligible (Figure 5B).
CD157 regulates neutrophil chemotaxis in response to fMLP. (A) PMNs treated with an isotype-matched Ig, anti-CD157, or anti-CD38 (5 μg/mL, 15 minutes at 37°C) were exposed to medium (□) or 10 nM fMLP (▪). There were 5 fields counted per well, and neutrophil chemotactic response was expressed as the mean number of migrated cells per high-power field (HPF; 100×) from triplicate wells (PMNs per HPF), ± SD. Data are the average of 13 donors analyzed. *P < .05. (B) Inhibitory effects of anti-CD157 on fMLP-induced neutrophil chemotaxis in 13 donors. The increasing rate of fMLP-induced chemotaxis was evaluated as fMLP-induced migration/basal migration in the absence (•) or in the presence (○) of anti-CD157 mAb. P = .004. (C) fMLP-induced chemotaxis of ATRA-treated HL-60 cells and (D) DMSO-treated HL-60 cells are reduced by CD157 ligation. Number of migrated cells was calculated as in panel A. Data are the average of 3 separate experiments. *P < .05.
CD157 regulates neutrophil chemotaxis in response to fMLP. (A) PMNs treated with an isotype-matched Ig, anti-CD157, or anti-CD38 (5 μg/mL, 15 minutes at 37°C) were exposed to medium (□) or 10 nM fMLP (▪). There were 5 fields counted per well, and neutrophil chemotactic response was expressed as the mean number of migrated cells per high-power field (HPF; 100×) from triplicate wells (PMNs per HPF), ± SD. Data are the average of 13 donors analyzed. *P < .05. (B) Inhibitory effects of anti-CD157 on fMLP-induced neutrophil chemotaxis in 13 donors. The increasing rate of fMLP-induced chemotaxis was evaluated as fMLP-induced migration/basal migration in the absence (•) or in the presence (○) of anti-CD157 mAb. P = .004. (C) fMLP-induced chemotaxis of ATRA-treated HL-60 cells and (D) DMSO-treated HL-60 cells are reduced by CD157 ligation. Number of migrated cells was calculated as in panel A. Data are the average of 3 separate experiments. *P < .05.
The analysis of the role of CD157 on chemotaxis was extended to differentiated HL-60 cells. In spite of their different phenotypes, both ATRA- and DMSO-induced HL-60 cells express comparable levels of fMLP receptors (E.O., unpublished data, June 24, 2003), and acquire the ability to migrate in response to fMLP, with almost equal efficiency. In both cases, migration proved to be reduced by CD157 ligation (Figure 5C-D), while no effects were attributable to CD38 ligation on ATRA-differentiated HL-60 cells (Figure 5C).
PMN migration is impaired in patients with PNH
Lipid rafts and GPI-anchored proteins are potential regulators of PMN adhesion to (and transmigration through) the endothelium, thus providing the spatial and temporal regulation necessary for neutrophil transmigration, and facilitating morphologic changes, signaling events, and cytoskeletal organization.10,26
PNH is an acquired clonal disease of hematopoiesis in which a variable proportion of blood cells are deficient in GPI-linked proteins,27 and therefore, in CD157. Thus, PMNs from patients with PNH could serve to cross-check the functional role of this molecule.
In 12 patients with PNH expressing CD157 on a variable percentage of cells (from 0% to 27%; Figure 6A) we observed a reduced chemotactic response in the presence of 10 nM fMLP (as well as 1 nM fMLP, data not shown), producing a variable degree of impaired migration of PMNs (Figure 6B).
Chemotactic response to fMLP of neutrophils from patients with PNH. (A) PMNs isolated from the peripheral blood of 12 patients were evaluated for the expression of CD157 using RF3-FITC mAb. Cytofluorimetric analysis was performed on at least 10 000 cells. The results are expressed as percentage of CD157+ PMNs. (B) Summary of the results of migration of neutrophil from 12 patients with PNH (•)in response to 10 nM fMLP analyzed in parallel with 13 healthy donors (○). The increasing rate of fMLP-induced chemotaxis was evaluated as fMLP-induced migration/basal migration. P = .001. (C) Inhibitory effect of anti-CD157 mAb on neutrophil chemotaxis. The results are the average of 13 healthy donors analyzed and of 3 patients with PNH with a virtually absent (05, 09, 10), low (< 15%) (02, 06, 11), or significant (> 20%) (03, 04, 07) percentage of PMNs expressing CD157. Results are expressed as percentage of inhibition of fMLP-induced chemotaxis following CD157 ligation. (D) PMNs from the PNH07 patient with -30% normal neutrophils were enriched in GPI- PMNs, by negative selection with anti-CD157–coated magnetic beads. (E) The impaired migration of neutrophils from the PNH07 patient (▪) is increased in the CD157-deprived population (▦). There were 5 fields counted per well, and neutrophil chemotactic response was expressed as the mean number of migrated cells per high power field from triplicate wells ± SD. *P < .05.
Chemotactic response to fMLP of neutrophils from patients with PNH. (A) PMNs isolated from the peripheral blood of 12 patients were evaluated for the expression of CD157 using RF3-FITC mAb. Cytofluorimetric analysis was performed on at least 10 000 cells. The results are expressed as percentage of CD157+ PMNs. (B) Summary of the results of migration of neutrophil from 12 patients with PNH (•)in response to 10 nM fMLP analyzed in parallel with 13 healthy donors (○). The increasing rate of fMLP-induced chemotaxis was evaluated as fMLP-induced migration/basal migration. P = .001. (C) Inhibitory effect of anti-CD157 mAb on neutrophil chemotaxis. The results are the average of 13 healthy donors analyzed and of 3 patients with PNH with a virtually absent (05, 09, 10), low (< 15%) (02, 06, 11), or significant (> 20%) (03, 04, 07) percentage of PMNs expressing CD157. Results are expressed as percentage of inhibition of fMLP-induced chemotaxis following CD157 ligation. (D) PMNs from the PNH07 patient with -30% normal neutrophils were enriched in GPI- PMNs, by negative selection with anti-CD157–coated magnetic beads. (E) The impaired migration of neutrophils from the PNH07 patient (▪) is increased in the CD157-deprived population (▦). There were 5 fields counted per well, and neutrophil chemotactic response was expressed as the mean number of migrated cells per high power field from triplicate wells ± SD. *P < .05.
Ligation of CD157 shows an appreciable inhibitory effect in those patients expressing CD157 on a significant subset of PMNs, while it is ineffective on patients expressing GPI-anchored molecules on less than 10% of PMNs (Figure 6C). In 2 patients who had 70% GPI- PMNs, the enrichment in GPI- PMNs, by negative selection with immune-magnetic beads coated with anti-CD157 mAb (Figure 6D), reduced further the chemotactic response (Figure 6E). This confirmed that the GPI- population is indeed the one that is defective in the ability to migrate.
CD157 ligation inhibits integrin-dependent adhesion of neutrophils to fibrinogen
The role of CD157 in regulating the adhesion of neutrophils to extracellular matrix proteins was assessed by performing the experiments in the presence of selected mAbs. β2 integrin is involved in the adhesion of neutrophils to fibrinogen (in resting conditions as well as fMLP-activated conditions), as revealed by the inhibitory effects constantly induced by incubation of cells with anti-CD18 mAb. Mo5 mAb interferes with the ability of neutrophils to adhere to fibrinogen in a dose-dependent manner (Figure 7A-B), whereas an isotype-matched mAb to HLA class I has no effect. The same effects were obtained using Bec-7 or RF3 anti-CD157 mAbs (A.F., unpublished data, November 10, 2003). Comparable results were obtained in the presence of fMLP. Neutrophils obtained from 3 patients with PNH completely lacking CD157 showed impaired adhesion to fibrinogen at all times considered (Figure 7C).
Effect of CD157 on the adhesion of neutrophils to fibrinogen. (A) 51Cr-labeled neutrophils were plated in fibrinogen-coated wells (in triplicate samples) in the presence of 10 μg/mL anti-CD157, anti-CD18, or anti-HLA class I mAbs and incubated at 37°C for increasing amounts of time. (B) 51Cr-labeled neutrophils were plated in fibrinogen-coated wells in the presence of increasing concentrations of anti-CD157 or anti-CD18 mAbs and incubated for 1 hour at 37°C. After washing, adherent neutrophils were lysed with 2% Triton X-100 (100 μL) and the released radioactivity was quantified in a γ-counter. Percent inhibition of adherence was calculated using the following formula: [(cpm in the presence of control IgG - cpm in the presence of relevant mAb / cpm in the presence of control IgG) × 100]. Results represent the mean ± SD of 3 independent experiments. (C) 51Cr-labeled PMNs from healthy donors (▦) or patients with PNH (▪) were plated in fibrinogen-coated wells (in triplicate samples) and incubated at 37°C for increasing time. After washing to remove nonadherent cells, adherent PMNs were lysed with 2% Triton X-100 (100 μL) and the released radioactivity was quantified in a γ-counter. Percent specific adhesion was calculated using the following formula: [(51Cr released from adherent cells / total 51Cr added to each well) × 100]. Results represent the mean ± SD of 3 independent experiments. *P < .05.
Effect of CD157 on the adhesion of neutrophils to fibrinogen. (A) 51Cr-labeled neutrophils were plated in fibrinogen-coated wells (in triplicate samples) in the presence of 10 μg/mL anti-CD157, anti-CD18, or anti-HLA class I mAbs and incubated at 37°C for increasing amounts of time. (B) 51Cr-labeled neutrophils were plated in fibrinogen-coated wells in the presence of increasing concentrations of anti-CD157 or anti-CD18 mAbs and incubated for 1 hour at 37°C. After washing, adherent neutrophils were lysed with 2% Triton X-100 (100 μL) and the released radioactivity was quantified in a γ-counter. Percent inhibition of adherence was calculated using the following formula: [(cpm in the presence of control IgG - cpm in the presence of relevant mAb / cpm in the presence of control IgG) × 100]. Results represent the mean ± SD of 3 independent experiments. (C) 51Cr-labeled PMNs from healthy donors (▦) or patients with PNH (▪) were plated in fibrinogen-coated wells (in triplicate samples) and incubated at 37°C for increasing time. After washing to remove nonadherent cells, adherent PMNs were lysed with 2% Triton X-100 (100 μL) and the released radioactivity was quantified in a γ-counter. Percent specific adhesion was calculated using the following formula: [(51Cr released from adherent cells / total 51Cr added to each well) × 100]. Results represent the mean ± SD of 3 independent experiments. *P < .05.
Discussion
Ectoenzymes represent almost 4% of the surface molecules on human leukocytes. They are highly conserved in phylogeny and are generally pleiotropic in function, that is, they exert enzymatic activities along with receptorial functions. Experience gathered over the years with human CD382,28,29 was transferred to CD157, a second member of the NADase/ADP-ribosyl cyclase family.2,30 Both are involved in the metabolism of NAD+, although their tissue distributions are strikingly different.
CD157—originally identified as a bone marrow stromal cell molecule as BST-131 —within the hematopoietic system is prevalently expressed by the myeloid lineage.3,32 Our results demonstrate that it is expressed by the majority of circulating PMNs and that its expression is enhanced in the presence of fMLP. CD38 is expressed at low epitope density by only a small percentage of cells and is not induced by fMLP stimulation.
We found that circulating human neutrophils do not display efficient enzymatic activity, as inferred from their inability to utilize NGD+ as a substrate for generating cGDPR. This observation indicates that the cyclase activity of CD157 on intact PMNs lies below the threshold of sensitivity of the method adopted and that the expression of CD38 is too low to sustain detectable amounts of cGDPR. This assumption was strengthened following experiments using differentiated HL-60 myeloid cells.33 DMSO-induced (CD157+/CD38-) HL-60 cells display no detectable cyclase activity, whereas ATRA-induced (CD157+/CD38+) HL-60 cells catalyze the production of cGDPR. CD157 is a poor cyclase on PMNs, as demonstrated in other cell lineages34 and predicted in all physiologic situations on the basis of the behavior of the recombinant soluble molecules.35
These initial results suggested that more attention should be paid to CD157 as a receptor and not only as an ectoenzyme. Efforts in this direction were undertaken by exploiting a panel of specific mAbs, which may mimic the natural ligand(s) still waiting to be identified. Using this approach, which has been widely used to mimic the ligands of a number of receptors including CD3813 and many GPI-anchored molecules,17,22,23 we demonstrated that CD157 orchestrates a signal transduction pathway crucial to the function of human neutrophils. Indeed, CD157 cross-linking regulates calcium homeostasis in neutrophils, confirming its receptorial function.3,36,37 The Ca2+ flux elicited by CD157 is both of extracellular and intracellular origin and its amplitude is dependent on the extent of cross-linking, suggesting that the redistribution of the molecule is crucial to the creation of signaling-competent microdomains. The observation that wortmannin inhibits the increase in intracellular Ca2+ mediated by CD157 engagement suggests that the downstream signal transduction pathway involves PI3-K. Indeed, it is known that the lipid products of PI3-K control polarity and motility in neutrophils.38-40
The CD157-dependent Ca2+ mobilization in human neutrophils is not regulated by the products of the enzymatic functions of the molecules (ie, cADPR and ADPR), since it is unaffected by 8Br-cADPR.41 More generally, cADPR is not required for chemotaxis of human neutrophils induced through the high-affinity fMLP receptor, as demonstrated by the efficient chemotaxis of DMSO-induced HL-60 cells, whose cyclase activity is undetectable. This observation differs from data presented by Partida-Sanchez et al,42 who demonstrated that cADPR is a crucial element in the regulation of intracellular Ca2+ homeostasis and in fMLP-induced neutrophil chemotaxis in the mouse model. In line with our results, the same group recently reported that cADPR is not involved in the chemotactic response of human neutrophils to ligands selective for the fMLP receptor.43 It is conceivable that human and mouse neutrophils behave in different ways. Otherwise, it may be that the cell populations reflecting the distinct differentiation stages used in the 2 models (ie, circulating neutrophils in human, versus bone marrow neutrophils in mouse) account for the functional discrepancy observed in vitro.
Beside Ca2+ mobilization, CD157-mediated signals are associated with a very rapid clustering of CD157 and subsequent profound modifications in cytoskeletal organization and cell shape. Within minutes, cells become polarized through formation of lamellipodia at the leading edge, and of a uropod at the rear of the cell. Polarization is central to chemotaxis, because it makes movement more efficient and it provides a mechanism whereby the cell perceives the existence of a gradient of a chemoattractant. Therefore, any molecule that promotes polarization must be regarded as a key coordinator of the process.
When fMLP-activated neutrophils rapidly transform from resting cells to migratory ones,44,45 CD157 localizes into GM1-enriched rafts and migrates preferentially to the uropod. Rafts are platforms in which activated receptors and signal transduction partners interact and to which selected cytosolic signaling molecules are recruited.46
Localization to the rear of the polarized neutrophil is a characteristic shared by many GPI-anchored proteins and selected transmembrane molecules.10,25,47 Moreover, a plethora of adhesion molecules (such as CD43, CD44, and intercellular adhesion molecules [ICAMs]) localize at the uropod during cell migration; thus, the uropod is an important adhesive structure, able to anchor the cell to the extracellular matrix and to control leukocyte recruitment and transendothelial migration.48
CD157 regulates chemotaxis stimulated through the high-affinity fMLP receptor.49 Indeed, ligation of CD157 by specific mAbs is followed by a significantly reduced chemotactic response in all the donors analyzed so far, as well as in HL-60 cells differentiated with both DMSO and ATRA. All the mAbs used showed the same inhibitory effects because they recognize the same or very close epitopes, as inferred by binding competition experiments (A.F., unpublished data, March 26, 2004).
Like all GPI-anchored molecules, CD157 lacks a cytoplasmic domain; therefore, it is structurally unsuitable to transduce signals. A reasonable model is that CD157 exploits its lateral mobility to establish functional interactions with conventional receptors. Our results suggest that CD157 associates to the CD11b/CD18 complex for signal transduction. Indeed, β2 integrin and CD157 appear to be closely associated spatially, as ligand-induced clustering of β2 integrin causes colocalization with CD157. Furthermore, the changes in cell shape and in actin polymerization following CD157 engagement are prevented by preincubation of cells with anti-CD18 mAb.
Cocapping, lateral diffusion, and fluorescent resonance energy transfer studies have revealed that β2 integrin physically associates with several GPI-anchored proteins that modulate neutrophil functions, namely CD14, CD16, CD87, and GPI-80.50-54 Integrins mediate cell adhesion, migration, phagocytosis, and virtually all PMN functions. To accomplish their roles, integrins must act not only as individual receptors, but also as components of supra-molecular complexes at the plasma membrane.55 A prerequisite for integrin-mediated signaling is a conformational switch from an inactive (ie, low affinity for ligands) to an active (ie, high affinity for ligands) state. Along with inside-out and outside-in signaling,54,56 lateral association with other membrane proteins is one priming mechanism able to effect this switch.57,58
To corroborate these findings on the physiology of CD157, we analyzed neutrophils from patients with PNH, a disease that entails failure of expression of all GPI-anchored molecules on the plasma membrane.59,60 The results demonstrated that neutrophils derived from 12 patients with PNH featured constant defective in vitro adhesion and migration. This finding is in line with previous evidence that (1) the migration of PNH neutrophils across endothelium is significantly impaired,61 and (2) the acquisition of the active conformation of the CD11b/CD18 complex is significantly reduced by treatment with phosphatidylinositol-specific phospholipase C, which removes approximately 70% of cell surface GPI-anchored proteins.62 It is likely that the absence of cross-talk between the CD11b/CD18 complex and selected GPI-anchored molecules is the reason why neutrophils from patients with PNH display functional defects. Thus, the blockage of CD157 through appropriate mAbs on normal neutrophils or the absence of CD157 (due to a somatic mutation) on PNH neutrophils has the same functional consequences.
However, the possibility that GPI-anchored molecules other than CD157 may be also involved in defective neutrophil functions cannot be ruled out. It is not yet clear whether enhanced susceptibility to bacterial infections in patients with PNH is related only to neutropenia or also to the impairment of neutrophil functions here demonstrated.
Nonetheless, patients with PNH do not necessarily show enhanced susceptibility for infections, whereas in vitro neutrophils are constantly impaired in function. Possible explanations might be that (1) in the acquired clonal disease PNH,63 a variable portion of unaffected cells may establish a sufficient host defense; or (2) circulating soluble GPI-anchored molecules (such as CD157 and CD87) may compensate for the lack of membrane-anchored molecules.
CD157, like many other GPI-anchored molecules, is frequently shed from the cell membrane through cleavage by cell surface proteases, producing soluble forms.64 This implies that CD157 may also be able to bind to the membrane of CD157- cells. The presence of CD157 on cells that do not constitutively express it raises intriguing questions about its function and suggests that GPI-anchored proteins may serve as hormones or cytokines in endocrine- or paracrine-like systems.
The finding that CD157 and other GPI-anchored molecules coordinate neutrophil adhesion and migration offers new perspectives for the design of treatment strategies in inflammatory conditions, in which aberrant recruitment of neutrophils results in tissue damage.
Prepublished online as Blood First Edition Paper, August 31, 2004; DOI 10.1182/blood-2004-06-2129.
Supported by the Associazione Italiana Ricerca Cancro (Milano, Italy); the Fondo Investimenti Ricerca di Base (Ministero dell'Istruzione, dell'Universitá e della Ricerca), and by the Special Project “Oncologia” Compagnia SanPaolo (Torino, Italy). The Compagnia SanPaolo, the Regione Piemonte, and FIRMS (International Foundation for Researches in Experimental Medicine) provided valuable financial contributions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr R. F. Todd III for generously supplying the Mo5 mAb; Dr K. Ishihara and Dr T. Hirano for kindly providing RF3, Bec-7, and SG2 mAbs; and Dr E. Hirsch and Dr O. Azzolin for helpful discussions and advice. Sincere thanks to the patients with PNH for their generous and enthusiastic participation in this study. E.O. and B.F. are students in the PhD program “Human Oncology,” at the University of Torino Medical School, Italy.

![Figure 2. Effects of CD157 cross-linking on intracellular Ca2+ concentration. Purified PMNs were first incubated with murine IgG Fc fragments (150 μg/mL) at 4°C for 15 minutes to block binding of the primary mAb to Fc receptors,17 then loaded with fluo3-AM; after calibration of the baseline fluorescence, the different stimuli were added (arrow) and cell events acquired. Dynamic changes in [Ca2+]i were monitored continuously by plotting the shift in the fluo3-AM fluorescence over a 516-second time course. Data are representative of 3 separate experiments. (A) Top panel: PMNs were treated with saturating amounts of anti-CD157 Mo5 mAb (5 μg/mL) and increasing concentrations (20 μg/mL-100 μg/mL) of F(ab′)2-RaMIgG. Bottom panel: a significant increase in [Ca2+]i was observed following incubation with fMLP (100 nM), whereas a control isotype-matched anti-CD3 mAb did not elicit any Ca2+ currents. (B) The mobilization of Ca2+ after CD157 cross-linking (top) or fMLP stimulation (bottom) was monitored after treatment for 5 minutes with 3 mM EGTA. (C) Dye-loaded PMNs were preincubated in the presence or absence of 100 μM 8-Br-cADPR and then stimulated with Mo5 mAb and F(ab′)2-RaMIgG or 100 nM fMLP (arrow). (D) The effects of CD157 and CD18 cross-linking or fMLP stimulation were analyzed in PMNs pretreated with 100 nM wortmannin or 500 nM staurosporin. Data are representative of 3 experiments with cells from different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-06-2129/6/m_zh80240470960002.jpeg?Expires=1770476971&Signature=Zg5l5P1Tcwn9NUbDzBvIUFiIKZqwVcKDInbl5aBoYf2uKf8Nu2V28mCvt5tmPetn3tiiQP4AQ4dzTUg1gLYsYeEP0Yj-vRwMisOK3~FwaaEXvmYpT-Joy~TnQEYDoN9C7c5ihuUW9LFgazzJ1WEPfYAq0DvY8TeIAr3aA-n7dal-SkzCp9amkYqHMvZiV5PQR2je5Jp0K6pJToOOaEdp16dQZHQSR9PYaIa8v7o-MN0HSU~S-K3lGs3puJnlZjDMZs~VXnapMUmSonmygLGBR2l4w5oJ02Dk1Xv0tBU1nz65Q3Ow75fjCiklFuGG24TLvEuCg0e8lQPJt8jfLjSR0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. Effect of CD157 on the adhesion of neutrophils to fibrinogen. (A) 51Cr-labeled neutrophils were plated in fibrinogen-coated wells (in triplicate samples) in the presence of 10 μg/mL anti-CD157, anti-CD18, or anti-HLA class I mAbs and incubated at 37°C for increasing amounts of time. (B) 51Cr-labeled neutrophils were plated in fibrinogen-coated wells in the presence of increasing concentrations of anti-CD157 or anti-CD18 mAbs and incubated for 1 hour at 37°C. After washing, adherent neutrophils were lysed with 2% Triton X-100 (100 μL) and the released radioactivity was quantified in a γ-counter. Percent inhibition of adherence was calculated using the following formula: [(cpm in the presence of control IgG - cpm in the presence of relevant mAb / cpm in the presence of control IgG) × 100]. Results represent the mean ± SD of 3 independent experiments. (C) 51Cr-labeled PMNs from healthy donors (▦) or patients with PNH (▪) were plated in fibrinogen-coated wells (in triplicate samples) and incubated at 37°C for increasing time. After washing to remove nonadherent cells, adherent PMNs were lysed with 2% Triton X-100 (100 μL) and the released radioactivity was quantified in a γ-counter. Percent specific adhesion was calculated using the following formula: [(51Cr released from adherent cells / total 51Cr added to each well) × 100]. Results represent the mean ± SD of 3 independent experiments. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-06-2129/6/m_zh80240470960007.jpeg?Expires=1770476971&Signature=E2H-xDvdZJTD8NwYI2Wvw9q7Nh-Off4Gx~fZiqjJtYFXE2Sa79e9SWOyeDUq6d8jROVzRej1POyhbWLrIdnwjERl9xuAV9QKPjMAfvT5StEZqxfRoSH4tSxsBkuHcbOVT8tGxH28CMPkeAk7PH0WNw6isS9qYlzFsbOsbBZ~CU3QDPLKqonG5okHZCnfmO814UTHvoaAjHLIjKjzK9dKgWeLG-dyW7hKirYe1V-H6BmnvDe34rhjc5PFEMnEBfUs4kgJte3bsn5tOUGbOXZrOaGk824CcHFSt3TZwGODKf9f5w7NrsOevirEkNOMLaQIh8lVA3LirjpTMhEX8Ax1pA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal