Abstract
Invasion by the human malaria parasite, Plasmodium falciparum, is associated with marked yet selective increases in red blood cell (RBC) membrane permeability. We previously identified an unusual voltage-dependent ion channel, the plasmodial surface anion channel (PSAC), which may account for these increases. Since then, controversy has arisen about whether there are additional parasite-induced anion channels on the RBC membrane and whether these channels are parasite-encoded proteins or the result of modifications of an endogenous host protein. Here, we used genetically divergent parasite isolates and quantitative transport measurements to examine these questions. Our studies indicate that PSAC alone can adequately account for the increased permeability of infected RBCs to key solutes. Two distinct parasite isolates, grown in RBCs from a single donor, exhibit channel activity with measurably different voltage-dependent gating, a finding difficult to reconcile with simple activation or modification of a host protein. Instead, this difference in channel gating can be conservatively explained by a small number of polymorphisms in a parasite gene that encodes PSAC. The absence of known eukaryotic ion channel homologues in the completed P falciparum genome suggests a novel channel gene, and substantiates PSAC as a target for antimalarial development.
Introduction
The altered membrane transport properties of red blood cells (RBCs) infected with the malaria parasite were first identified more than 50 years ago.1 A flurry of studies, carried out in several laboratories, have established the precise rates and rank order of increased permeability for anions,2 sugars,3 amino acids,4 purines,5 vitamins,6 and some cations,7 using radioisotope flux and osmotic fragility assays. In addition, these studies discovered several structurally diverse small-molecule inhibitors of this increased permeability. Although these inhibitors were nonspecific and had poorly understood mechanisms of ion channel blockade in other systems, they suggested one or more ion channels induced by the parasite to mediate an increased uptake of certain solutes,2 many needed for growth of the parasite.8
Working from this foundation, we recently used the cell-attached and whole-cell patch-clamp methods to identify an unusual ion channel on the RBC membrane of infected cells.9 This channel, the plasmodial surface anion channel (PSAC), exhibits an anion selectivity matching that of previous isotope flux measurements. Whole-cell measurements with known antagonists suggested that PSAC also has the same gross pharmacologic profile of inhibition seen in the previous flux measurements. Single-channel recordings revealed a small 20 picosiemens (pS) conductance in 1.15 molar Cl- solutions and fast flickering openings with a mean open time less than or equal to 0.5 milliseconds. PSAC exhibited markedly lower open probabilities (Po) at positive membrane potentials (Vm) that correlated with significantly smaller absolute whole-cell currents at Vm = +100 millivolts (mV) than at Vm = -100 mV, despite equal but opposite driving forces for ion movement. Spectral analysis of single-channel and whole-cell measurements revealed parallel 1/frequency (1/f) profiles, indicating PSAC is the predominant Cl- conductive pathway in the infected RBC membrane and that it is present in some 1000 to 2000 functional copies per cell.
Three subsequent electrophysiological studies produced somewhat different results. In one,10 whole-cell patch-clamp suggested 2 distinct anion conductive pathways, with the predominant one having a “outward-rectifying” voltage-dependence, opposite that of PSAC. Both pathways were partially inhibited by treatment with the reducing agent dithioerythritol (DTE). Both could also be induced on uninfected RBCs by the oxidizing agent t-butyl hydroperoxide. Single-channel patch-clamp was not performed in this study. Based on the similarities between their uninfected and infected RBC recordings, this study proposed that the intracellular parasite activates quiescent channels endogenous to the human RBC membrane via oxidative stress.
In the second electrophysiological study,11 whole-cell and single-channel patch-clamp revealed a single inward-rectifying channel that differs significantly from both PSAC and the 2 pathways reported above. In their examination of uninfected RBCs, these workers saw 2 voltage-dependent anion channels, one inward-rectifying and one outward-rectifying. The inward-rectifying channel was inactive unless stimulated by stretch or the combined application of protein kinase A, adenosine triphosphate (ATP), and theophylline. This activated channel had a chord conductance, selectivity, and pharmacology matching that of the channel seen on infected RBCs. Thus, these workers also proposed that the intracellular parasite activates an endogenous channel, but via the action of parasite-encoded kinases present in RBC cytosol.
In the third study,12 whole-cell patch-clamp experiments using infected RBCs revealed inward-rectifying currents that were absent when parasites were cultured in RBCs from cystic fibrosis donors with the homozygous ΔF508 mutation in the cystic fibrosis transmembrane regulator (CFTR) chloride channel. Despite loss of Cl- channel activity, these infected RBCs retained an increased permeability to organic solutes with preserved osmotic lysis in isotonic glucose, choline chloride, and alanine. From these findings, the authors proposed that the parasite activates an endogenous chloride channel tightly linked to CFTR and that a separate channel or transporter mediates the increased permeability of infected RBCs to organic solutes.
Significant confusion and controversy have arisen because many findings in these various studies are mutually incompatible. The debate revolves primarily around 2 important questions. First, how many distinct anion channels does the parasite induce on the RBC membrane and what are their relative contributions to anion and key nutrient solute uptake? Second, do these channels result from the activation of host proteins or are they parasite-encoded proteins trafficked out to the RBC membrane? The answers to these questions are obviously fundamental to understanding parasite biology and to determining if these channels are suitable targets for antimalarial development.
A major goal of our study9 and the subsequent electrophysiological studies10-12 was to provide mechanistic molecular explanations for the parasite-induced increase in solute permeability. Because patch-clamp, isotope flux, and osmotic lysis methods endeavor to study the same phenomena and should produce comparable results, we have now used all 3 methods to examine the above questions. Our quantitative examination indicates that a single inward-rectifying anion channel, PSAC, can adequately explain the permeability changes seen after infection. Our study also supports a parasite-encoded channel over an activated endogenous protein for the following reasons: (1) PSAC was not affected by RBC redox status, energy status, or protein kinase/phosphatase balance; (2) PSAC-like activity could not be induced on uninfected RBCs with any of a range of pharmacologic manipulations; (3) parasites cultured in RBCs from cystic fibrosis donors induced indistinguishable PSAC activity with unchanged functional copy number per cell; (4) PSAC activity was preserved on prolonged cold storage of infected RBCs, suggesting that it is an irreversible modification of the RBC membrane not dependent on sustained parasite viability; and (5) two divergent parasite isolates, grown in RBCs from a single donor, exhibited clear differences in single-channel gating properties that are most simply explained by polymorphisms in a parasite-encoded ion channel.
Materials and methods
Materials
Sp-adenosine-3′,5′-cyclic monophosphorothioate (Sp-cAMPS), Rp-adenosine-3′,5′-cyclic monophosphorothioate (Rp-cAMPS), and okadaic acid were purchased from BioMol Research Labs (Plymouth Meeting, PA); isoproterenol was from Calbiochem (La Jolla, CA). All other reagents were from Sigma (St Louis, MO). Blood for parasite culture was obtained with informed consent from healthy donors and from 6 separate cystic fibrosis donors with confirmed CFTRΔF508/Δ F508 (homozygous) genotypes after approval by the National Institutes of Health (NIH) institutional review board.
Osmotic lysis assays
The kinetics of infected RBC osmotic lysis in sorbitol solutions was followed as described previously.13 In brief, trophozoite-infected RBCs were grown by standard culture methods, enriched to greater than 95% by percoll/sorbitol separation,14 washed in phosphate-buffered saline (PBS; 150 mM NaCl, 20 mM Na-phosphate, pH 7.5) pretreated with reagents as indicated, and resuspended to 0.5% hematocrit (hct) in sorbitol lysis solution (280 mM sorbitol, 20 mM Na-HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.1 mg/mL bovine serum albumin [BSA], pH 7.4) at 37°C. The transmittance of 700 nm light (%T) through this cell suspension was then continuously followed as a marker of osmotic swelling and lysis. This method produces estimates of permeation rates through PSAC that quantitatively match those obtained with both radioisotope flux and patch-clamp.13
Electrophysiology
Single-channel and whole-cell patch-clamp recordings of infected RBCs were obtained as previously described.9 Unless otherwise indicated, these experiments used symmetrical bath and pipette solutions of 1000 mM choline-Cl, 115 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 20 mM Na-HEPES, pH 7.4 (solution A). Pipettes were pulled from quartz glass to tip diameters less than 0.5 μm and resistances of 1 to 4 MΩ. Recordings were filtered at 5 kHz with an 8-pole Bessel filter and digitized at 100 kHz.
To determine mean single-channel open probabilities (Po), we pooled 17 to 31 seconds of recordings at a Vm of -100 mV from 2 to 4 single-channel patches at each furosemide concentration, used locally developed code that identifies transitions between channel open and closed states by the 50% threshold-crossing technique, and normalized the results to the measured Po in the absence of furosemide, 43% ± 2%.
Dwell-time distributions were obtained using code that detects midthreshold crossings, uses linear interpolation of adjacent sample times,15 and corrects for a Gaussian filter risetime of 66.4 μs as described.16 Histogram ordinate values were normalized to the percent of the total number of events detected under each condition and are displayed on a square root-logarithmic plot.15
Because PSAC openings at Vm of +100 mV are short and poorly resolved, we used integration of currents relative to the closed channel baseline to estimate transport rates at this voltage instead of the standard Po algorithm above.
Radioisotope uptake
Infected RBCs were enriched as described above, washed, and used for uptake of 14C-lactate (8.5 μCi [0.31 MBq]/mL) at 10% hct in 150 mM NaCl, 20 mM Na-phosphate, 1 mM Na-lactate, pH 7.4 with the indicated furosemide concentrations. Uptake was performed at 4°C to minimize uptake of lactate via endogenous carriers. At timed intervals up to 90 seconds, uptake was terminated by transfer of 100 μL cell suspension to 1 mL tracer-free uptake buffer with 1 mM furosemide, and centrifugation at 14 000g through dibutyl phthalate. Cell pellets were digested and counted as previously described.17 Rates were corrected for non-PSAC–mediated uptake by subtracting the rate in 1 mM furosemide.
Cold storage experiment
Infected RBCs were enriched as described in “Osmotic lysis assays,”, washed, and stored at 0.4% hct in PBS with 100 μM 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB), 10 μg/mL gentamicin, and 2% dialyzed heat-inactivated serum at 4°C for 7 days. Serum, added to improve RBC membrane integrity during storage, was extensively dialyzed against PBS (150 mM NaCl, 20 mM Na-phosphate, pH 7.5; molecular weight cut-off of 7000 Da) to remove all nutrients. NPPB was included in the storage medium to prevent osmotic lysis due to slow but measurable Na+ uptake via PSAC,18 but was not essential for preserving PSAC activity. Cold-stored infected RBCs were then washed in PBS and used for osmotic lysis measurements.
To test the effects of percoll/sorbitol harvest and cold storage on parasite viability, enriched infected RBCs were seeded with 2 volumes of uninfected RBCs into standard in vitro cultures in RPMI 1640 supplemented with 10% human serum; viability was then evaluated by daily microscopic examination of Giemsa-stained thin smears.
Results
Furosemide dose-responses
We examined the dose-response of furosemide, an established antagonist of the increased permeability of infected RBCs. Figure 1A shows single PSAC recordings obtained in the cell-attached patch-clamp configuration. As previously described,9 PSAC exhibits a small 20 pS chord conductance in symmetrical 1.1 M Cl- solutions, bursts of fast-flickering open events, and voltage-dependent gating with significantly fewer openings at +100 mV than at -100 mV imposed Vm. Addition of furosemide to the recording solutions superimposed intermittent periods of inactivity with no effect on open channel amplitude (Figure 1B), suggesting channel block with intermediate kinetics.19 To quantify this inhibition, the mean open probability in single-channel recordings at a Vm of -100 mV was determined for concentrations up to 25 μM (Figure 1D, filled triangles). The resulting single-channel dose-response matched precisely the macroscopic dose-response for inhibition of osmotic lysis of infected RBCs in sorbitol (Figure 1D, open circles).
Inhibition by furosemide. (A) Single-channel recordings with solution A in the bath and pipette. Seal resistance equal to 490 GΩ; imposed Vm as indicated. (B) Single-channel recordings with indicated furosemide concentrations added to the bath and pipette. Vm equal to -100 mV. Opening events (downward deflections) are less frequent as furosemide concentration increases, but their amplitudes are not affected. (C) Whole-cell recordings with 0 μM or25 μM furosemide in identical bath and pipette solutions of 115 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 20 mM Na-HEPES, pH 7.4. In each group of traces, 50-ms steps from Vm of 0 mV to values between -100 mV and +100 in 20-mV increments are superimposed. Vertical scale bar represents 2 pA (A-B) and 713 pA (C); horizontal bar is 50 ms (A), 100 ms (B), and 18.6 ms (C). (D) Furosemide dose-responses determined for PSAC single-channel open probabilities (▴, mean ± standard error of the mean [SEM]), whole-cell currents (□), osmotic lysis in sorbitol (○, calculated from the data in Wagner et al13 ), and 14C-lactate- accumulation (•), each normalized to 1.0 at 0 μM furosemide to allow comparison. Solid curve represents a least-squares fit to y = Km/(Km + x) with a Km of 2.7 μM.
Inhibition by furosemide. (A) Single-channel recordings with solution A in the bath and pipette. Seal resistance equal to 490 GΩ; imposed Vm as indicated. (B) Single-channel recordings with indicated furosemide concentrations added to the bath and pipette. Vm equal to -100 mV. Opening events (downward deflections) are less frequent as furosemide concentration increases, but their amplitudes are not affected. (C) Whole-cell recordings with 0 μM or25 μM furosemide in identical bath and pipette solutions of 115 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 20 mM Na-HEPES, pH 7.4. In each group of traces, 50-ms steps from Vm of 0 mV to values between -100 mV and +100 in 20-mV increments are superimposed. Vertical scale bar represents 2 pA (A-B) and 713 pA (C); horizontal bar is 50 ms (A), 100 ms (B), and 18.6 ms (C). (D) Furosemide dose-responses determined for PSAC single-channel open probabilities (▴, mean ± standard error of the mean [SEM]), whole-cell currents (□), osmotic lysis in sorbitol (○, calculated from the data in Wagner et al13 ), and 14C-lactate- accumulation (•), each normalized to 1.0 at 0 μM furosemide to allow comparison. Solid curve represents a least-squares fit to y = Km/(Km + x) with a Km of 2.7 μM.
Because PSAC might not be the only ion channel on infected RBCs, we also examined furosemide's dose-response with whole-cell current measurements (Figure 1C) and added the corresponding whole-cell chord conductance to the dose-response (Figure 1D, open squares). Finally, we measured and included 14C-lactate uptake into infected cells in the presence of furosemide (Figure 1D, filled circles).
The identical dose-response profiles obtained with these disparate measurements is most conservatively explained with a single ion channel mediating the transport of sorbitol, lactate, and Cl- into infected RBCs. The identical affinity of furosemide for inhibiting each of these permeabilities, 2.7 μM ± 0.5 μM, is strong evidence for a single channel for 2 reasons. First, although a number of transporters in other systems are inhibited by furosemide, its affinity for other transporters varies over a wide range, with published inhibitory concentration (IC50) values from nano- to millimolar.20-22 Second, the data in Figure 1D were adequately fitted by the Michaelis-Menten equation (solid line), implicating a 1:1 stoichiometry of furosemide binding to PSAC and further supporting the overwhelming predominance of a single ion channel type.
Mechanism of furosemide inhibition
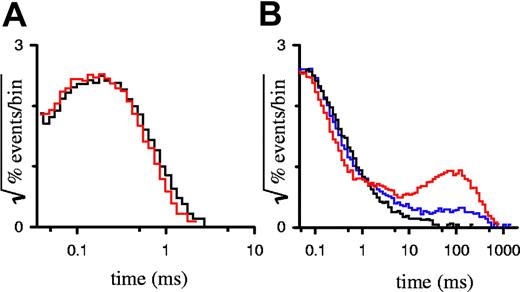
Because furosemide is a hydrophobic anion, it may inhibit PSAC by entry into and plugging of the channel's pore. Alternatively, furosemide may bind to sites distant from the pore and stabilize one or more closed states. To distinguish among these and more complicated mechanisms of inhibition, we analyzed the durations of open and closed events, known as dwell times, in single PSAC recordings with furosemide. The simplest kinetic scheme for an ion channel would have one open state and one closed state; the durations of open and closed events for such a channel would each have a dwell time distribution that decays as a single exponential. PSAC gating behavior reveals a single open state because open dwell distributions are adequately explained with a single exponential,15 visible as a single peak on a square root–logarithmic plot (Figure 2A). Furosemide had no effect on this open dwell distribution, even at a high 25 μM concentration, where PSAC open probability is reduced by 93%. This lack of effect on open durations excludes models where furosemide inhibits PSAC predominantly by plugging an open pore.
Dwell-time distributions for single-channel recordings. Open (A) and closed (B) dwell time distributions for single-channel recordings with 0 μM (black curves), 4 μM (blue curve), or 25 μM (red curves) furosemide. Number of events in each histogram ranged from 33 000 to 48 000, tallied from up to 530 seconds of recording.
Dwell-time distributions for single-channel recordings. Open (A) and closed (B) dwell time distributions for single-channel recordings with 0 μM (black curves), 4 μM (blue curve), or 25 μM (red curves) furosemide. Number of events in each histogram ranged from 33 000 to 48 000, tallied from up to 530 seconds of recording.
Examination of PSAC closed dwell time distributions reveals a more complicated picture with multiple closed states, which probably explain both the bursting behavior characteristic of single-channel recordings and their 1/f power spectrum.9 Furosemide-induced inhibition, apparent in our single-channel recordings (Figure 1B), added a distinct population to the closed dwell distribution with an exponential time constant of approximately 100 milliseconds (ms) (Figure 2B). Importantly, this time constant did not vary with furosemide concentration. PSAC's intrinsic closings (events of < 10 ms duration in Figure 2B) were not affected by the addition of furosemide. These findings indicate that furosemide is an allosteric inhibitor rather than a direct pore blocker; it likely imposes a single blocked state by stabilizing one of PSAC's closed channel conformations.
Effects of redox, RBC energy status, CFTRΔF508/ ΔF508, and parasite viability
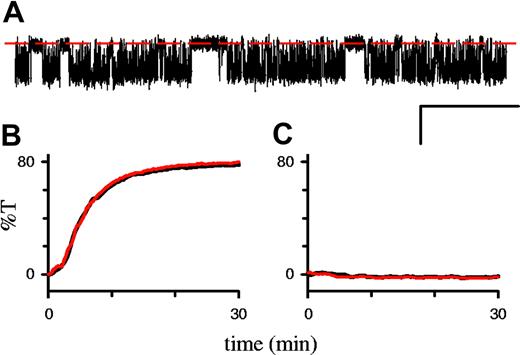
In single-channel recordings on infected RBCs, 1 mM DTE, added to both the bath and pipette, had no effect on PSAC's conductance, gating properties, or voltage dependence (Figure 3A). There were also no changes in the rate of osmotic lysis in sorbitol (Figure 3B). Finally, oxidation of uninfected RBCs with 1 mM t-butyl hydroperoxide failed to increase sorbitol permeability in our hands (Figure 3C).
Effects of redox status on permeability. (A) Single PSAC recording on an infected RBC in solution A with 1 mM DTE in the bath and pipette. Seal resistance equal to 230 GΩ; Vm equal to -100 mV; scale bar 2 pA/100 ms. Additional analyses revealed unchanged single-channel chord conductance, voltage dependence, and open/closed dwell-time distributions (not shown). (B) Osmotic lysis of infected cells in sorbitol lysis solution with (red curve) or without (black) 1 mM DTE. Increasing the DTE concentration to 50 mM or adding preincubation with DTE also had no effect; similar results were obtained with the 8 parasite isolates listed in “Results” and in 14C-sorbitol uptake studies (not shown). (C) Osmotic lysis of uninfected RBCs in sorbitol lysis solution after preincubation with (red curve) or without (black curve) 1 mM t-butyl hydroperoxide for 15 minutes with a 2.5-hour postincubation period at 37°C, as in Huber et al.10 Increasing the postincubation period to 24 hours also had no effect (not shown). Although the transmittance values are presented in arbitrary units, their scaling is conserved in panels B and C so that %T equal to 80 corresponds to complete osmotic lysis.
Effects of redox status on permeability. (A) Single PSAC recording on an infected RBC in solution A with 1 mM DTE in the bath and pipette. Seal resistance equal to 230 GΩ; Vm equal to -100 mV; scale bar 2 pA/100 ms. Additional analyses revealed unchanged single-channel chord conductance, voltage dependence, and open/closed dwell-time distributions (not shown). (B) Osmotic lysis of infected cells in sorbitol lysis solution with (red curve) or without (black) 1 mM DTE. Increasing the DTE concentration to 50 mM or adding preincubation with DTE also had no effect; similar results were obtained with the 8 parasite isolates listed in “Results” and in 14C-sorbitol uptake studies (not shown). (C) Osmotic lysis of uninfected RBCs in sorbitol lysis solution after preincubation with (red curve) or without (black curve) 1 mM t-butyl hydroperoxide for 15 minutes with a 2.5-hour postincubation period at 37°C, as in Huber et al.10 Increasing the postincubation period to 24 hours also had no effect (not shown). Although the transmittance values are presented in arbitrary units, their scaling is conserved in panels B and C so that %T equal to 80 corresponds to complete osmotic lysis.
We examined a broad range of reagents that alter either RBC cytosolic ATP concentration or the balance of cellular protein kinase and phosphatase activities with our kinetic osmotic lysis assay (Table 1). None of these manipulations had a measurable effect on the rate of osmotic lysis of infected RBCs; similarly, treatment of uninfected RBCs with these reagents was without effect on their very low sorbitol permeability.
PSAC activity does not depend on energy or phosphorylation status
Agent . | Mechanism of action . |
|---|---|
| Glucose, 10 mM | Energy source |
| Na3VO4, 1 mM and 5 mM | Inhibitor of ATPases and kinases |
| Sp-cAMPS, 10 μM | cAMP-dependent kinase agonist |
| Rp-cAMPS, 20 μM | cAMP-dependent kinase antagonist |
| Okadaic acid, 5 μM | Protein phosphatase inhibitor |
| Theophylline, 100 μM | Phosphodiesterase inhibitor |
| Isoproterenol, 20 μM | β-adrenergic receptor agonist |
| Staurosporine, 3 μM | Nonspecific protein kinase inhibitor |
Agent . | Mechanism of action . |
|---|---|
| Glucose, 10 mM | Energy source |
| Na3VO4, 1 mM and 5 mM | Inhibitor of ATPases and kinases |
| Sp-cAMPS, 10 μM | cAMP-dependent kinase agonist |
| Rp-cAMPS, 20 μM | cAMP-dependent kinase antagonist |
| Okadaic acid, 5 μM | Protein phosphatase inhibitor |
| Theophylline, 100 μM | Phosphodiesterase inhibitor |
| Isoproterenol, 20 μM | β-adrenergic receptor agonist |
| Staurosporine, 3 μM | Nonspecific protein kinase inhibitor |
Each agent was tested for its effects on infected and uninfected RBCs using the osmotic lysis assay after a 15-minute preincubation period at the indicated concentrations. Glucose and Na3VO4 were also evaluated and found to have no effect in single-channel patch-clamp studies.
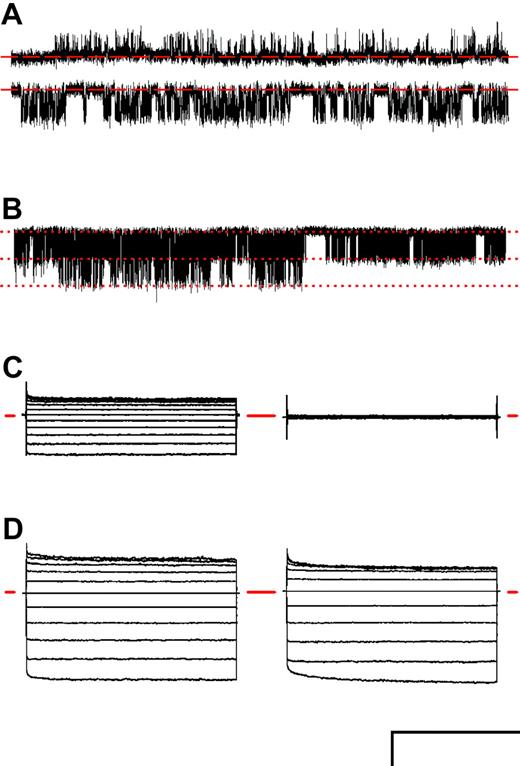
To test if PSAC activity is linked to the CFTR chloride channel, we cultured parasites in RBCs from cystic fibrosis donors with the CFTRΔF508/ ΔF508 mutation.23 In contrast to a previous report,12 we found that PSAC activity was preserved in our cell-attached recordings (Figure 4A-B). Whole-cell recordings in either near-physiologic or hypertonic saline solutions (Figure 4C-D) demonstrated that PSAC's voltage-dependence, inhibition by furosemide, and functional copy number are also not affected by the host CFTRΔF508/ ΔF508 mutation.
PSAC activity on RBCs from CFTRΔF508/ΔF508 donors. (A) Single-channel recordings on an RBC from a CFTRΔF508/ΔF508 donor infected with a trophozoite-stage parasite. Bath and pipette contained solution A. Vm was +100 or -100 mV (upper and lower traces, respectively). Closed channel levels are indicated by dashed red lines. (B) Recording from a 2-channel patch on an infected CFTRΔF508/ΔF508 RBC in solution A at a Vm of -100 mV. Downward transitions represent the opening of either one or both channels; corresponding levels are marked with dotted lines. (C) Whole-cell recording on an infected CFTRΔF508/ΔF508 RBC with near physiologic bath and pipette solutions of 165 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 20 mM Na-HEPES, pH 7.4, before or after addition of 200 μM furosemide (left and right groups, respectively). (D) Whole-cell currents with solution A in bath and pipette from infected RBCs using healthy (left traces) and CFTRΔF508/ΔF508 donors (right traces). In panels C and D, traces for 50-ms steps to Vm between -100 mV and +100 mV in 20-mV increments are superimposed to demonstrate PSAC's voltage dependence; the zero current levels are marked with horizontal red lines. Scale bars represent 2.9 pA/127 milliseconds (A), 3.1 pA/500 ms (B), 1590 pA/30 ms (C), and 3000 pA/30 ms (D). Similar PSAC activity was recorded from each of 6 CFTRΔF508/ΔF508 donors after in vitro parasite culture.
PSAC activity on RBCs from CFTRΔF508/ΔF508 donors. (A) Single-channel recordings on an RBC from a CFTRΔF508/ΔF508 donor infected with a trophozoite-stage parasite. Bath and pipette contained solution A. Vm was +100 or -100 mV (upper and lower traces, respectively). Closed channel levels are indicated by dashed red lines. (B) Recording from a 2-channel patch on an infected CFTRΔF508/ΔF508 RBC in solution A at a Vm of -100 mV. Downward transitions represent the opening of either one or both channels; corresponding levels are marked with dotted lines. (C) Whole-cell recording on an infected CFTRΔF508/ΔF508 RBC with near physiologic bath and pipette solutions of 165 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 20 mM Na-HEPES, pH 7.4, before or after addition of 200 μM furosemide (left and right groups, respectively). (D) Whole-cell currents with solution A in bath and pipette from infected RBCs using healthy (left traces) and CFTRΔF508/ΔF508 donors (right traces). In panels C and D, traces for 50-ms steps to Vm between -100 mV and +100 mV in 20-mV increments are superimposed to demonstrate PSAC's voltage dependence; the zero current levels are marked with horizontal red lines. Scale bars represent 2.9 pA/127 milliseconds (A), 3.1 pA/500 ms (B), 1590 pA/30 ms (C), and 3000 pA/30 ms (D). Similar PSAC activity was recorded from each of 6 CFTRΔF508/ΔF508 donors after in vitro parasite culture.
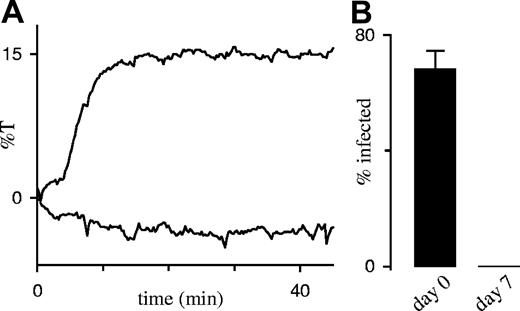
If PSAC activity were induced by a reversible modification of endogenous membrane proteins, it should be abrogated by reduced parasite metabolism or by death of the intracellular parasite. In contrast to this prediction, we found that storage of infected RBCs in saline devoid of all nutrients at 4°C for 7 days did not reduce PSAC activity or change its gross pharmacologic properties (Figure 5A). Because these cold-stored infected RBCs failed to grow when seeded into standard in vitro culture (Figure 5B), PSAC activity represents an irreversible modification of the RBC membrane not dependent on sustained parasite viability.
PSAC activity does not require sustained parasite viability. (A) Sorbitol-induced lysis time course with and without 200 μM furosemide (lower and upper curves, respectively) after cold storage for 7 days. The half-time in this lysis experiment, 6 minutes, is unchanged from that with viable parasites (Figure 3B and Wagner et al13 ). (B) Cold storage kills P falciparum trophozoites. Cultures were seeded immediately after percoll-sorbitol enrichment (day 0) or after storage at 4°C for 7 days; viability was assessed after 24 hours by counting the percentage of RBCs infected with ring-stage parasites (mean ± SEM, n = 3). The cold-stored culture was maintained for an additional week without the appearance of any parasites, verifying complete killing.
PSAC activity does not require sustained parasite viability. (A) Sorbitol-induced lysis time course with and without 200 μM furosemide (lower and upper curves, respectively) after cold storage for 7 days. The half-time in this lysis experiment, 6 minutes, is unchanged from that with viable parasites (Figure 3B and Wagner et al13 ). (B) Cold storage kills P falciparum trophozoites. Cultures were seeded immediately after percoll-sorbitol enrichment (day 0) or after storage at 4°C for 7 days; viability was assessed after 24 hours by counting the percentage of RBCs infected with ring-stage parasites (mean ± SEM, n = 3). The cold-stored culture was maintained for an additional week without the appearance of any parasites, verifying complete killing.
Is PSAC parasite-encoded or a modified endogenous RBC membrane protein?
Failure to induce PSAC-like activity in uninfected RBCs does not definitively exclude a modified host protein: yet unexplored biochemical or enzymatic manipulations might be used by the parasite to irreversibly activate quiescent host channels. Taking an alternative approach to this question, we examined the functional properties of PSAC in genetically divergent parasite isolates. Examination of sorbitol lysis rates with our quantitative light scattering assay13 revealed experimentally indistinguishable sorbitol permeability coefficients (not shown) for a geographically diverse collection of parasite isolates: Indo 1 (from a patient in Indochina), D10 (Papua New Guinea), Dd2 (Southeast Asia), FCR-3 (probably Southeast Asia), 3D7 (probably Africa), GB4 (Ghana), 7G8 (Brazil), and HB3 (Honduras).
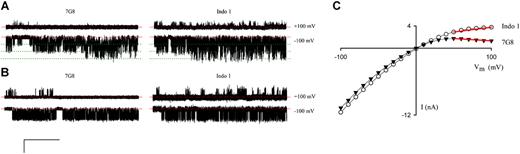
Because there may be subtle polymorphisms in PSAC not revealed by macroscopic osmotic lysis rate measurements, we performed selected single-channel patch-clamp and found a significant difference in the voltage-dependence of PSAC's gating between the Indo 1 and 7G8 isolates. Figure 6A demonstrates this difference with cell-attached patches containing 3 functional channels each. At a Vm of -100 mV (lower traces), PSAC molecules from both isolates exhibited fast-flickering gating with indistinguishable conductances and open probabilities. At a Vm of +100 mV, however, these 3-channel patches reveal reduced open probabilities with dramatically different gating: far fewer opening events (brief up-going transitions from the baseline, upper traces in Figure 6A) were seen on the 7G8-infected cell than on the Indo 1–infected cell.
Functional polymorphisms in PSAC gating. (A) Three-channel patches from 7G8- and Indo 1–infected cells, left and right sweeps respectively. (B) Single-channel recordings. The scale bars represent 4 pA/400 ms in panel A and 3 pA/525 ms in panel B. Identical Vm values were imposed for each isolate, as indicated. Dashed red and dotted green lines represent closed and open channel levels, respectively. PSAC behavior in these isolates differed at a Vm of +100 mV with more frequent openings (brief upward spikes in upper traces) on Indo 1 than on 7G8. To avoid bias, these traces were selected because they have open probabilities matching the average for their corresponding isolates (10 single-channel patches each). (C) Whole-cell current-voltage profiles for single RBCs infected with either an Indo 1 parasite (○) or a 7G8 parasite (▾). Red lines illustrate the differing chord conductances between +50 mV and +100 mV. For each isolate, similar results were obtained using blood from approximately 9 separate donors.
Functional polymorphisms in PSAC gating. (A) Three-channel patches from 7G8- and Indo 1–infected cells, left and right sweeps respectively. (B) Single-channel recordings. The scale bars represent 4 pA/400 ms in panel A and 3 pA/525 ms in panel B. Identical Vm values were imposed for each isolate, as indicated. Dashed red and dotted green lines represent closed and open channel levels, respectively. PSAC behavior in these isolates differed at a Vm of +100 mV with more frequent openings (brief upward spikes in upper traces) on Indo 1 than on 7G8. To avoid bias, these traces were selected because they have open probabilities matching the average for their corresponding isolates (10 single-channel patches each). (C) Whole-cell current-voltage profiles for single RBCs infected with either an Indo 1 parasite (○) or a 7G8 parasite (▾). Red lines illustrate the differing chord conductances between +50 mV and +100 mV. For each isolate, similar results were obtained using blood from approximately 9 separate donors.
To test the statistical significance of this difference, we obtained and analyzed single-channel recordings from these 2 isolates (Figure 6B). Because the fast, poorly resolved openings at Vm of +100 mV complicate standard open probability determinations, we integrated the total currents from single-channel patches, revealing PSAC-mediated currents of 24 ± 5 femtoamperes (fA) and 92 ± 22 fA from 7G8 (mean ± SEM, n = 10) and Indo 1 (n = 10) patches, respectively. This difference, identified from 580 seconds of recording, was statistically significant with a P value of .0067 (unpaired 2-tailed Student t test).
If PSAC is the only anion channel present on infected RBCs, the observed difference in single-channel currents should produce measurable differences in whole-cell currents on RBCs infected with these 2 isolates. Indeed, whole-cell currents were comparable at a Vm of -100 mV, but significantly smaller at a Vm of +100 mV for the 7G8 isolate, producing a more strongly inward rectifying current-voltage profile (Figure 6C). We used the chord conductance calculated between Vm of +50 mV and +100 mV to quantify this inward rectification and corrected for cell-to-cell variation in PSAC expression with division by the chord conductance between Vm of -50 mV and -100 mV. Estimates for this ratio were -0.013 ± 0.009 for 7G8 (n = 43 cells) and 0.18 ± 0.02 for Indo 1 (n = 30 cells). This difference was also statistically significant with a P value less than 10-9 (2-tailed Student t test), further confirming the difference between these isolates.
Effects of Cl- concentration on PSAC gating and single-channel conductance
PSAC's fast-flickering gating (mean open and closed durations < 1 millisecond in Figure 2) and its small single-channel conductance require the use of hypertonic solutions for unambiguous detection. To test the effects of our recording conditions on the channel's behavior, we performed cell-attached patch-clamp experiments with 3 solutions of different osmolarity added symmetrically to the bath and pipette. PSAC gating was measurably identical in these 3 solutions, but the amplitude of channel openings increased with Cl- concentration (Figure 7A). Chord conductances, calculated from these amplitudes, indicate that the channel saturates only at high Cl- activities (Figure 7B).
Single PSAC recordings at different [Cl-]. (A) Single-channel recordings with bath and pipette solutions of 115 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 20 mM Na-HEPES, and 185 mM (top trace), 595 mM (middle), or 1000 mM (bottom) choline chloride, pH 7.4. Vm equal to -100 mV; scale bar represents 2 pA/200 ms. The nominal Cl concentration in each experiment, as indicated in mM, determines the single-channel amplitude; openings are reflected by downward transitions. (B) Single-channel chord conductances, calculated between Vm of -100 mV and 0 mV, for the 3 solutions in panel A. Cl- activities (ordinate) were measured with a Cl- electrode. Solid line represents the best fit to y = a*x/(x + b). Unambiguous single PSAC measurements at physiologic [Cl-] of 145 mM are not possible because their predicted amplitudes are comparable to that of an optimally minimized baseline noise.
Single PSAC recordings at different [Cl-]. (A) Single-channel recordings with bath and pipette solutions of 115 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 20 mM Na-HEPES, and 185 mM (top trace), 595 mM (middle), or 1000 mM (bottom) choline chloride, pH 7.4. Vm equal to -100 mV; scale bar represents 2 pA/200 ms. The nominal Cl concentration in each experiment, as indicated in mM, determines the single-channel amplitude; openings are reflected by downward transitions. (B) Single-channel chord conductances, calculated between Vm of -100 mV and 0 mV, for the 3 solutions in panel A. Cl- activities (ordinate) were measured with a Cl- electrode. Solid line represents the best fit to y = a*x/(x + b). Unambiguous single PSAC measurements at physiologic [Cl-] of 145 mM are not possible because their predicted amplitudes are comparable to that of an optimally minimized baseline noise.
Discussion
The molecular mechanisms responsible for the increased permeability of infected RBCs have recently been probed with electrophysiological methods. Rather than providing simple answers, this examination has spurred debate and raised new, enlivening questions.
An important first question is the number of distinct ion channels induced on the infected RBC membrane to mediate the increased uptake of anions, amino acids, sugars, purines, and some cations. Because the increased uptake of these diverse solutes could be abrogated by a small list of admittedly nonspecific blockers and because of a desire for parsimony, most early models proposed a single molecular mechanism.2,3 The first electrophysiological study of these permeability changes9 supported this model in that (1) only one type of channel, PSAC, was seen in the cell-attached patch-clamp configuration, (2) the whole-cell current had a voltage-dependence similar to the voltage-dependence of PSAC, and (3) most rigorously, spectral analysis of whole-cell and single-channel recordings exhibited parallel 1/f profiles, unlikely with any model invoking 2 or more distinct Cl- conductive pathways in the RBC membrane. The data presented here provide additional evidence for a single parasite-induced anion channel. Michaelis-Menten type kinetics for inhibition by furosemide (Figure 1D) favors a one-to-one interaction with a single transporter type: multiple channels with differing affinities for furosemide would presumably produce more complex kinetics. Furthermore, quantitative concordance of this furosemide dose-response for inhibition of Cl- currents in patch-clamp recordings, sorbitol-induced osmotic lysis, and 14C-lactate accumulation measurements strongly suggests that PSAC alone can fully account for the increases in Cl-, sorbitol, and lactate permeabilities.
Do these various measurements exclude other anion transport mechanisms on the infected RBC membrane? Exchange-restricted mechanisms, such as those that apply to the predominant component of transport via the
While important for other often-studied phenotypes, differences between parasite isolates are an unlikely explanation for the differing results of recent patch-clamp studies on infected RBCs9-12 ; our survey of isolates from around the world revealed only PSAC-like behavior with only subtle variations in voltage dependence (Figure 6). Instead, these differences are probably explained by the technical difficulties associated with patch-clamp of the small, deformable human RBC. To minimize artifacts arising from these difficulties, we developed and adhered to several guidelines. First, we have insisted on patch-clamp seal resistances of at least 100 gigaohm (GΩ) in our studies, considerably higher than values regarded acceptable for other cell types. Seal resistances up to 1000 GΩ can routinely be achieved on RBCs with strict adherence to solution and pipette cleanliness.25 Second, we have restricted single-channel measurements to nonphysiologic hypertonic solutions containing up to 1.1 M Cl-. When combined with a minimum seal resistance of 100 GΩ, use of this hypertonic solution produces a markedly higher signal-to-noise ratio, essential for detection of this small conductance channel. Third, while acceptable recordings can be obtained with pipettes fabricated from borosilicate glass, we generally prefer quartz because its lower dissipation factor26 further reduces noise. Fourth, while bulk perfusion of the bath is routinely used with other cell types, we do not use continuous perfusion because it invariably leads to deterioration of the seal quality on the fragile RBC. Finally, because patch-clamp data on RBCs are prone to various technical artifacts, we have insisted, where possible, on verifying patch-clamp results with osmotic lysis and/or tracer flux experiments (Figure 1D).
Even with the high seal resistance and low capacitive noise we achieved, detection of Cl- transport through single PSAC molecules requires high Cl- concentrations possible only with hypertonic salt solutions. We worried about the effects of these nonphysiologic solutions on the channel's properties. The identical furosemide dose-response in 1145 mM Cl- (single-channel recordings), physiologic saline (whole-cell currents and 14C-lactate- accumulation), and Cl--free solutions (sorbitol-induced lysis) indicate that PSAC's pharmacologic properties do not depend on solution composition (Figure 1D). Similarly, channel block induced by covalent modification with various N-hydroxysulfosuccinimide esters also is independent of solution composition.18 Single-channel events in less hypertonic solutions (Figure 7) suggest that PSAC's gating properties are also unaffected. Finally, its spectral properties, halide selectivity, and voltage dependence are grossly unaffected by osmolarities between 300 mOsm and 2000 mOsm.9 As new functional properties of PSAC are identified in hypertonic solutions, possible effects of the recording conditions should be critically considered.
While anion-conductive pathways induced by the intracellular parasite other than PSAC are essentially excluded by these data, separate parasite-induced transport mechanisms for other solutes almost certainly exist. For example, there are dramatic increases in the Ca++ permeability of infected RBCs,27-30 though there are fundamental debates on its mechanism and on the conditions required for its activation. Because furosemide does not inhibit this increase,28 it is presumably not mediated by PSAC. Another example is the increased uptake of phosphatidyl-choline analogs and lucifer yellow by infected RBCs31 via an incompletely understood, furosemide-resistant32 mechanism.
A second fundamental question is whether PSAC represents a modified host protein or a parasite-encoded ion channel trafficked to the host membrane. We were unable to reproduce the reported activation of anion channels on uninfected RBCs with various pharmacologic manipulations or membrane stretch. Moreover, PSAC's functional properties—for example, its stringent exclusion of Na+ despite permeability to neutral and cationic organic solutes, pH dependence of cation permeability,18 its unusual 1/f spectral properties, and its atypical anion selectivity of SCN- > I- > Br- > Cl-—differ sufficiently from those of known eukaryotic anion channels to cast doubt on models invoking simple up-regulation of endogenous channels.
A parasite-encoded protein for PSAC activity is strongly favored by the finding that 2 isolates exhibit measurable differences in gating, despite culture in identical RBCs. Gating, which refers to the conformational changes in a channel protein during transitions between its open and closed states, generally involves movement of key residues in the electric field of the membrane. Thus, the observed difference in PSAC's voltage-dependent gating between isolates is best explained by one or more polymorphisms in PSAC's gene(s) that alter either the net charge or the distance through the electric field those residues must move during its conformational changes. Given that gating of anion channels may involve as little as the movement of a single residue into the channel's pore,33 this difference may well result from only one nucleotide polymorphism.
Because definitive proof of the channel's origin will require the difficult cloning and functional reconstitution of PSAC activity, we considered other explanations for the observed differences in gating at positive Vm. Because these differences were seen in both single-channel and whole-cell recordings, differential modulation of channel activity by soluble components in the RBC cytosol of the 2 isolates can be excluded: these components are quickly removed after achieving the whole-cell configuration. A possibility that cannot be formally excluded is that these channels may contain more than one polypeptide or lipidic subunit, with at least one subunit each contributed by parasite and human gene products. In such a scenario, the identified polymorphism in PSAC gating would result from genetic variations in the parasite subunit. Another possible explanation is that PSAC is entirely a modified host protein specifically activated by a parasite-encoded enzyme. Here, the differences in gating would result from polymorphisms in this enzyme (for example, a protease). This model has a number of unappealing constraints. It would require polymorphisms in the parasite-encoded enzyme between the 2 isolates that convert a common host protein into 2 distinct anion channels. These 2 channels would need to differ in gating at a Vm of +100 mV and yet have indistinguishable single-channel amplitudes, selectivity properties, copy number per infected RBC (see the final paragraph below), and gating at Vm of -100 mV. This model would also require irreversible modification of the putative host protein (Figure 5).
Although the voltage-dependence of PSAC is a useful marker in single-channel and whole-cell recordings, it is probably not of direct physiologic significance because the RBC membrane potential remains very near -10 mV throughout the parasite's habitation. Instead, this epiphenomenon presumably reflects structural constraints in the channel protein required by its unique selectivity properties: PSAC is highly permeant to anions, uncharged organic solutes, and some cations, but must effectively exclude sodium ions.18 Poor conservation in the degree of PSAC's voltage-dependence (Figure 6) supports this view.
In our search for variations in the permeability phenotype due to genetic polymorphisms, we screened the 8 divergent P falciparum isolates listed in “Results.” These particular isolates were chosen because microsatellite marker analyses suggest that they are highly divergent.34 Although we successfully identified a functional polymorphism in channel gating between 2 of these isolates (Figure 6), we found that the mean half-times for sorbitol-induced osmotic lysis in all these isolates were indistinguishable (not shown). This quantitative indicator of sorbitol permeability13 suggests a conserved copy number of functional PSAC molecules on the RBC membrane after infection by each of these isolates, implicating conserved regulation of PSAC expression levels. Because PSAC is likely involved in parasite nutrient acquisition,9 mechanisms that produce this regulation may have evolved to maintain precise control of the permeability of key nutrients.
Prepublished online as Blood First Edition Paper, August 19, 2004; DOI 10.1182/blood-2004-05-2047.
An InsideBlood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. Kang, G. Lisk, and T. Wellems for critical reading of this manuscript. We thank M. Chernick, J. Moss, and their staff, for help with obtaining blood from cystic fibrosis donors.

![Figure 1. Inhibition by furosemide. (A) Single-channel recordings with solution A in the bath and pipette. Seal resistance equal to 490 GΩ; imposed Vm as indicated. (B) Single-channel recordings with indicated furosemide concentrations added to the bath and pipette. Vm equal to -100 mV. Opening events (downward deflections) are less frequent as furosemide concentration increases, but their amplitudes are not affected. (C) Whole-cell recordings with 0 μM or25 μM furosemide in identical bath and pipette solutions of 115 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 20 mM Na-HEPES, pH 7.4. In each group of traces, 50-ms steps from Vm of 0 mV to values between -100 mV and +100 in 20-mV increments are superimposed. Vertical scale bar represents 2 pA (A-B) and 713 pA (C); horizontal bar is 50 ms (A), 100 ms (B), and 18.6 ms (C). (D) Furosemide dose-responses determined for PSAC single-channel open probabilities (▴, mean ± standard error of the mean [SEM]), whole-cell currents (□), osmotic lysis in sorbitol (○, calculated from the data in Wagner et al13), and 14C-lactate- accumulation (•), each normalized to 1.0 at 0 μM furosemide to allow comparison. Solid curve represents a least-squares fit to y = Km/(Km + x) with a Km of 2.7 μM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-05-2047/6/m_zh80240471180001.jpeg?Expires=1769100359&Signature=T24en4v~8WBb1Fh9bESX8~7FEK4Dyh3AvvJFW~gBuTA1nH4tYyKWSre2YKN1DK0FmYHg5v5fMaTlzSeqAWYsPd6-50ALRVnV8do6aCxjCRTrs-TxvN015lIVAShqCmGRkIqOc~jnwfk9xZ1WGlYDlcPuVTF8ShSKxa5qryuXf7~XG2PqK9mevmuo1shNEJEJuZpolg27XcFnLVNqfoczOEz0pul8M26rRUG4xb3UNI-6m4P4knG5IEOQE9qr6lKcNigNzkOLntL7gSxLdVC~BE5VbZRECukTBPZ-3ivJSYdn691~HDdkNPSKD5j56C-mcQlwb-6hIYA97VOkRy3dOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Single PSAC recordings at different [Cl-]. (A) Single-channel recordings with bath and pipette solutions of 115 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 20 mM Na-HEPES, and 185 mM (top trace), 595 mM (middle), or 1000 mM (bottom) choline chloride, pH 7.4. Vm equal to -100 mV; scale bar represents 2 pA/200 ms. The nominal Cl concentration in each experiment, as indicated in mM, determines the single-channel amplitude; openings are reflected by downward transitions. (B) Single-channel chord conductances, calculated between Vm of -100 mV and 0 mV, for the 3 solutions in panel A. Cl- activities (ordinate) were measured with a Cl- electrode. Solid line represents the best fit to y = a*x/(x + b). Unambiguous single PSAC measurements at physiologic [Cl-] of 145 mM are not possible because their predicted amplitudes are comparable to that of an optimally minimized baseline noise.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-05-2047/6/m_zh80240471180007.jpeg?Expires=1769100359&Signature=zQkDrx3ZeaBzOghmsuS0Ip8dWtj6T79SrpofFiPgRci1GLbFJNX95gPx5IqBPkhU43KtdktjW82vRycHBn0ebozjh17BIjslBU47Xo93JXYmr~MAcn-IUH9h5qdALUEG4OJBHHhDx~wvzL4bwg0wdqDa1RlHM9ZI9edPLWYZ6ceLgw~MZWLDgm81iZZlMmoognJFoqhuMOAImSe2TcGWo8NsPdpd8NVKvyhl7vNLG-5CKEXUV82sfbeGVUfwg1tD15DYNw~n4388Xh4aCc~~KQyqAySGHn9fHFjr5zzXETjmZz0~lbRqxL07FJ3t5PegoaTWK16ZR~UXH~L7xOj6eg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal