Graft-versus-host disease (GVHD) is a major cause of morbidity and mortality after bone marrow transplantation (BMT). CD4+CD25+ immune regulatory T cells (Tregs), long recognized for their critical role in induction and maintenance of self-tolerance and prevention of autoimmunity, are also important in the regulation of immune responses in allogeneic bone marrow (BM) and solid organ transplantation. Published data indicate that ex vivo activated and expanded donor Tregs result in significant inhibition of lethal GVHD. This study provides a direct comparison of LSelhi and LSello Tregs for GVHD inhibition and for the promotion of allogeneic BM engraftment. Imaging of green fluorescent protein–positive effectors in GVHD control mice and LSelhi and LSello Treg-treated mice vividly illustrate the multisystemic nature of GVHD and the profound inhibition of GVHD by LSelhi Tregs. Data indicate that LSelhi Tregs interfere with the activation and expansion of GVHD effector T cells in secondary lymphoid organs early after BMT. Either donor- or host-type LSelhi, but not LSello, Tregs potently increased donor BM engraftment in sublethally irradiated mice, an event occurring independently of transforming growth factor β signaling of host T cells. These data indicate that Treg cellular therapy warrants clinical consideration for the inhibition of GVHD and the promotion of alloengraftment.
Introduction
Graft-versus-host disease (GVHD) is a major complication after bone marrow transplantation (BMT). T-cell depletion of the graft to avoid GVHD is associated with increased incidence of leukemic relapse, graft failure, and infectious complications. Despite standard prophylactic immunosuppressive therapy after T cell replete BMT, GVHD remains a major source of morbidity and mortality. Innovative therapeutic strategies that will not impair engraftment or result in the abrogation of the beneficial graft-versus-leukemia (GVL) effect are needed to minimize GVHD. Ex vivo activated and expanded CD4+CD25+ regulatory T-cell (Treg) therapy shows promise of being such a strategy. Published works by several groups, including our own, demonstrate significant inhibition of rapidly lethal GVHD by Tregs.1-3 Other studies report the inhibition of GVHD with the retention of GVL.4-6 Moreover, Tregs have been shown to mediate transplantation tolerance in murine models of skin and solid organ transplantation7-9 and more recently, antigen-specific tolerance to bone marrow (BM) allografts.10
The data presented here extend the field by providing a direct comparison of L-selectin (LSel; CD62L)hi versus LSello ex vivo activated and expanded Tregs for GVHD inhibition and, additionally, for the promotion of allogeneic BM engraftment. Direct imaging of enhanced green fluorescent protein–positive (GFP+) transgenic (Tg) T-cell effectors in GVHD control mice and LSelhi and LSello Treg-treated mice provide a dramatic illustration of the multisystemic nature of GVHD and the profound inhibition of GVHD by LSelhi Treg cellular therapy.
Materials and methods
Mice
B6.C-H2bm12/KhEg (termed bm12) (H2b) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 (termed B6) (H2b) and BALB/c (thy1.2+, H2d) were purchased from the National Institutes of Health (Bethesda, MD). B6 and bm12 (both H2b) differ at 3 amino acids due to mutations in the major histocompatibility complex (MHC) class II IA region. GFP Tg mice, on the B6 background, were obtained from the laboratory of Dr Jonathan Serody and bred at the University of Minnesota. β2-Microglobulin–deficient (β2-2m-/-) DNRII F3 mice were obtained from Drs Phil Lucas and Ron Gress and bred at the University of Minnesota.11 BALB/c Thy1.1 congenic mice were a kind gift of Dr Shimon Sakaguchi (Kyoto University, Kyoto, Japan) and provided by Dr Virginia Godfrey (University of North Carolina, Chapel Hill) and bred at the University of Minnesota. Female mice were used at 8 to 12 weeks of age. All mice were housed in a specific pathogen-free facility in microisolator cages.
CD25+ enrichment, ex vivo activation, expansion protocol, and LSel separation
To purify CD4+ T cells, lymph node (LN) single-cell suspensions were depleted of natural killer (NK) cells (hybridoma PK136, rat IgG2a) and CD8+ T cells (hybridoma 2.43, rat IgG2b) by incubation with monoclonal antibody (mAb), followed by passage through a goat antimouse and goat antirat immunoglobulin-coated column (Cellect Cell Enrichment Immunocolumns, Cedarlane, Hornby, ON, Canada). CD4+ T cells were incubated with anti–T cell receptor (TCR) γ/δ biotin (hybridoma GL3, hamster IgG2; BD PharMingen, San Diego, CA) followed by incubation with SA Dynabeads and sheep antirat Dynabeads (Dynal, Lake Success, NY). To enrich for CD25+ cells, negatively selected CD4+ cells were incubated with anti-CD25 phycoerythrin (PE; hybridoma PC61, rat IgG, BD PharMingen; or hybridoma 7D4, rat IgM, Miltenyi Biotec, Auburn, CA). After incubation with magnetically activated cell sorter (MACS) anti-PE MicroBeads, cells were positively selected on a MACS separation column (Miltenyi Biotec). Cells were determined to be 93% to 97% CD4+CD25+. Enriched CD25+ cells were activated with anti-CD3 and anti-CD28 mAb-coated magnetic microspheres (3 beads/1 cell) and cultured with recombinant human interleukin 2 (rhIL-2; 100-1000 U/mL; Amgen, Thousand Oaks, CA). Culture medium was Dulbecco complete as previously described.1 Cells expanded 20 to 50 times in 1 to 2 weeks. Immediately prior to in vivo infusion, Tregs were washed several times, magnetic beads were removed with a strong magnet, and cells were incubated with anti-LSel PE (hybridoma MEL-14, rat IgG2a; BD PharMingen). After incubation with MACS anti-PE MicroBeads, cells were positively selected on a MACS separation column (both Miltenyi Biotec) and determined to be more than 97% of the desired phenotype.
GVHD induction
Recipients were lethally irradiated with 800 to 900 cGy (39.3 cGy/min) by x-ray on the day prior to transplantation with 20 × 106 allogeneic, T cell–depleted (TCD) BM and CD25-depleted whole T cells or CD25-depleted CD4+ T cells. T cells were purified as described (see “CD25+ enrichment, ex vivo activation, expansion protocol, and LSel separation”) and CD25+ cells were depleted from effector populations by incubation with anti-CD25 (hybridoma 3C7, rat IgG; BD PharMingen) followed by incubation with sheep antirat Dynabeads (Dynal). Cohorts received either LSelhi or LSello Tregs by separate intravenous injection to avoid in vitro suppression of effectors prior to in vivo infusion. Mice were monitored daily for survival and weighed twice weekly for the first month, then once weekly thereafter as well as examined for the clinical appearance of GVHD. Representative long-term survivors were electively killed and hematoxylin and eosin-stained slides of liver, lung, colon, skin, and spleen were histologically assessed using a semiquantitative GVHD scoring system (0-4.0 grades in 0.5 increments) as published.12 Coded sections were graded by one of us (A.P.M.) without knowledge of the treatment.
Restimulation assay
B6 mice were irradiated with 900 cGy by x-ray on the day prior to transplantation with BALB/c Thy1.2+ TCD BM and BALB/c Thy1.1+ CD25-depleted T-cell effectors. Cohorts of mice received BALB/c Thy1.2+ LSelhi or LSello Tregs on days 0 and +3 at a ratio of 3 Tregs to 1 effector T cell. Spleens were harvested on day 6 after transplantation and effector (Thy1.1+) T cells and Tregs (Thy1.2+) were enumerated and phenotyped with directly conjugated fluorochromes to anti-Thy1.2, -Thy1.1, -CD4, -CD8, -CD25, -LSel, and isotype controls (BD PharMingen). For secondary restimulation assays, 3 × 104 CD4+Thy1.1+ T cells were cultured with 105 irradiated (2000 cGy) BALB/c or B6 splenocytes/well (replicates of 9 in 96-well round bottom plates; Costar, Corning, NY) in Dulbecco modified Eagle medium (DMEM) complete for 1 to 3 days. Supernatants for cytokines were harvested (3 wells) and 6 wells were pulsed with tritiated thymidine (1 μCi/well [0.037 MBq]; Amersham Life Science, Arlington Heights, IL) on days 1 to 5 of culture for 20 to 24 hours before harvesting and counted in the absence of scintillant amplification on a β-plate reader (Packard Instrument, Meridien, CT). Allogeneic anti-B6 response in counts per minute (cpm) minus syngeneic anti-BALB/c response in cpm is graphed on day of peak proliferation (day 2 of culture). Cytokines were evaluated by multiplex analysis using the Luminex system (Austin, TX) and mouse-specific bead sets from R&D Systems, (Minneapolis, MN).
Engraftment model
Host mice were sublethally irradiated with 400 to 500 cGy by x-ray on the day prior to transplantation with 20 × 106 allogeneic, TCD BM cells. Cohorts received LSelhi or LSello Tregs by separate intravenous injection. Survival was monitored daily, and mice were weighed twice weekly for the first month after transplantation, then once weekly thereafter. Documentation of donor chimerism was done by phenotyping of peripheral blood leukocytes (PBLs) obtained by retro-orbital venipuncture at 6 weeks and 3 months after transplantation. Cells were stained with fluorochrome-conjugated antibodies (anti-CD8, -CD4, –MAC-1, -CD19, -H2d, -H2b, and isotype controls; BD PharMingen) and analyzed using CellQuest software on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA).
In vivo imaging
As reported previously, images were taken with a Magnafire color camera (Optronics, Goleta, CA) mounted onto a Leica MZFLIII stereomicroscope using a GFP2-bandpass filter and a × 0.63 transfer lens (Leica Microsystems, Bannockburn, IL).13 Zoom factors from × 3.5 to 10 were used. GVHD effector T cells were from GFP Tg B6 mice, whereas BM and Tregs were from non-GFP, wild-type B6 mice. Exposure times were optimized for GVHD control mice for each organ and identical times were used for all other groups. Mice receiving allogeneic BM only (non-GFP) served as concurrent negative controls for background autofluorescence (only dark images were seen as previously reported13 and data not shown) showing minimal autofluorescence. Images of syngeneic controls consisting of irradiated B6 mice given transplants of B6 BM and B6 GFP+ T cells are shown to distinguish homeostatic expansion from the alloantigen-induced proliferation in the GVHD control mice. Three mice per group were examined at 1 and 2 weeks after BMT and a second replicate experiment was performed. Mice within a group yielded very similar results at any given time point so a representative image is illustrated. Although all GVHD control mice were dead by 3 weeks, survivors in other groups were also imaged at 3 weeks.
Statistics
Survival data in GVHD experiments were analyzed by life-table methods, and actuarial survival rates are shown. Group comparisons were made by log-rank test statistics. P < .05 was considered significant. Weight data were analyzed at each time point by Student t test. Group comparisons of GVHD scores and total splenic effector CD4+ and CD8+ T cells were analyzed by Student t test. P < .05 was considered significant. To assess engraftment data, group comparisons of percentage donor chimerism were analyzed by Student t test. Group comparisons of rates of engraftment were analyzed by χ2 test. P < .05 was considered significant.
Results
LSelhi Treg infusions reduce GVHD mortality
In prior studies we demonstrated that both donor and endogenous host CD4+CD25+ Tregs played an important role in the regulation of GVHD generation.1 We and others showed that ex vivo activated and expanded donor CD4+CD25+ regulatory cells significantly inhibited lethal GVHD.1-3
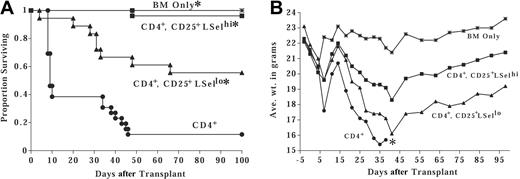
In these studies, we examined the potential for GVHD inhibition and alloengraftment promotion of the LSelhi and LSello subsets of ex vivo activated and expanded CD4+CD25+ T cells. Although both subsets have been shown to inhibit in vitro alloresponses, in vivo homing and trafficking differences may affect therapeutic efficacy. The preferential homing of LSelhi Tregs to secondary lymphoid organs may permit greater inhibition of effector alloresponses at the putative sites of alloantigen recognition thereby blunting initial T-cell activation and expansion (afferent phase). Alternatively, LSello Tregs may be able to home directly to GVHD target tissue and suppress effector T-cell function in situ (efferent phase). To evaluate the effect of LSelhi versus LSello Tregs on GVHD inhibition, BALB/c CD4+CD25+ cells were activated and expanded with anti-CD3 and anti-CD28 antibody-coated microspheres and high-dose IL-2 for 1 to 2 weeks of culture. Tregs were separated into LSelhi and LSello fractions after culture and infused with BALB/c TCD BM and CD25-depleted effector T cells into lethally irradiated allogeneic B6 mice. A second infusion of Tregs was given on day 3 after transplantation to facilitate suppression. All control mice receiving only TCD BM survived for the duration of the experiment (7 months). Seven of 8 mice receiving effector T cells died by day 80 and had a median survival time (MST) of 51 days (Figure 1A). The administration of one CD4+CD25+LSelhi Treg to one effector significantly reduced GVHD lethality, resulting in 75% long-term survivors. All mice receiving a 3-fold higher dose of LSelhi Tregs survived until they were electively killed at 7 months after transplantation. In contrast, the infusion of LSello Tregs did not protect mice from lethal GVHD (MST of 47 days).
LSelhi, but not LSello, Tregs protect against GVHD-associated mortality and morbidity by interfering with the activation and expansion of GVHD effector T cells. Lethally irradiated B6 mice were infused with BALB/c BM. Cohorts received 5 × 106 BALB/c CD25-depleted effector T cells alone or with ex vivo activated and expanded BALB/c LSelhi or LSello Tregs at the indicated T/reg ratio. Experiments illustrated in panels C-E used a ratio of 1 T cell to 3 Tregs. (A) Survival is indicated (n = 8/group;*P = .008 compared to T cells). (B) Average weight in grams of mice from panel A is indicated. *Only one mouse survived after this time point. (C) Spleens were harvested and enumerated 6 days after transplantation. Use of BALB/c Thy1 congenics allowed Thy1.1+ effectors to be distinguished from Thy1.2+ Tregs. The average total number of effector CD4+ and CD8+ T cells per spleen is shown. Error bars indicate standard deviation (n = 4 separate pools of 2 spleens/pool for each group; *P < .05). Data for panels C-E were taken from one of 2 replicate experiments. (D) Splenocytes from panel C were phenotyped. Shown is LSel expression on gated GVHD effector thy1.1+ CD4+ and CD8+ T cells. Shown are data from one of 4 representative splenic pools per treatment group. Percentage of LSel+ cells is indicated in the top right quadrant. (E) Splenocytes from panels C and D were stimulated in vitro with irradiated host-type splenic stimulators. Shown are peak secondary antihost proliferative responses of 4 separate pools of 2 spleens/pool from GVHD control mice, LSelhi and LSello Treg-treated mice. Cultures consisted of 3 × 104 effector Thy1.1+ CD4+ T cells restimulated with 105 irradiated B6 splenocytes, plated in replicates of 6. Note that infused Tregs are not depleted from the culture.
LSelhi, but not LSello, Tregs protect against GVHD-associated mortality and morbidity by interfering with the activation and expansion of GVHD effector T cells. Lethally irradiated B6 mice were infused with BALB/c BM. Cohorts received 5 × 106 BALB/c CD25-depleted effector T cells alone or with ex vivo activated and expanded BALB/c LSelhi or LSello Tregs at the indicated T/reg ratio. Experiments illustrated in panels C-E used a ratio of 1 T cell to 3 Tregs. (A) Survival is indicated (n = 8/group;*P = .008 compared to T cells). (B) Average weight in grams of mice from panel A is indicated. *Only one mouse survived after this time point. (C) Spleens were harvested and enumerated 6 days after transplantation. Use of BALB/c Thy1 congenics allowed Thy1.1+ effectors to be distinguished from Thy1.2+ Tregs. The average total number of effector CD4+ and CD8+ T cells per spleen is shown. Error bars indicate standard deviation (n = 4 separate pools of 2 spleens/pool for each group; *P < .05). Data for panels C-E were taken from one of 2 replicate experiments. (D) Splenocytes from panel C were phenotyped. Shown is LSel expression on gated GVHD effector thy1.1+ CD4+ and CD8+ T cells. Shown are data from one of 4 representative splenic pools per treatment group. Percentage of LSel+ cells is indicated in the top right quadrant. (E) Splenocytes from panels C and D were stimulated in vitro with irradiated host-type splenic stimulators. Shown are peak secondary antihost proliferative responses of 4 separate pools of 2 spleens/pool from GVHD control mice, LSelhi and LSello Treg-treated mice. Cultures consisted of 3 × 104 effector Thy1.1+ CD4+ T cells restimulated with 105 irradiated B6 splenocytes, plated in replicates of 6. Note that infused Tregs are not depleted from the culture.
Severe weight loss is a reliable indicator of GVHD. As compared to recipients of BM only, mice receiving effector T cells alone or in combination with LSello Tregs had significantly lower weights at all time points after day 20 (Figure 1B). In contrast, weights of mice receiving the higher dose of LSelhi Tregs were not significantly different, and weights of mice receiving the lower dose of LSelhi Tregs were only intermittently significantly different from weights of BM-only control mice. Although there were no overt signs of clinical GVHD (eg, hunched posture, erythema, greasy rough hair coat, alopecia, diarrhea, reduced activity, skin lesions), histopathologic examination of mice at 7 months revealed evidence of GVHD in target tissues of mice treated with LSelhi Tregs (Table 1). These data indicate that although LSelhi Tregs had a profound inhibitory effect on lethality, GVHD was not entirely abrogated.
Long-term survivors of LSelhi Tregs have sublethal GVHD
T effectors/LSelhi Tregs* . | GVHD scores . | Average ± SD . |
|---|---|---|
| Liver | ||
| None | 0, 1, 0, 0, 0 | 0.2 ± 0.45 |
| 1:1 | 1, 2, 1, 2.5, 1.5 | 1.6 ± 0.65† |
| 1:3 | 0.5, 0.5, 1.5, 1, 1 | 0.9 ± 0.42† |
| Lung | ||
| None | 1, 1, 1, 0, 0 | 0.6 ± 0.55 |
| 1:1 | 1, 1.5, 2, 3, 2 | 1.9 ± 0.74† |
| 1:3 | 2, 0.5, 2.5, 3.5, 3 | 2.3 ± 1.15† |
| Colon | ||
| None | 1, 1, 1, 0.5, 0.5 | 0.8 ± 0.27 |
| 1:1 | 1.5, 2, 1, 1.5, 1.5 | 1.5 ± 0.35† |
| 1:3 | 2, 2, 2, 2.5, 2 | 2.1 ± 0.22† |
| Skin | ||
| None | 0, 0, 0, 0, 0 | 0.0 ± 0.0 |
| 1:1 | 0.5, 0.5, 0.5, 0.5, 1 | 0.6 ± 0.22† |
| 1:3 | 0.5, 0.5, 0.5, 0.5, 0.5 | 0.5 ± 0.0† |
| Spleen | ||
| None | 0, 0, 0, 0.5, 0 | 0.1 ± 0.22 |
| 1:1 | 1.5, 2, 1, 2, 3 | 1.9 ± 0.74† |
| 1:3 | 1.5, 2.5, 3, 1, 2 | 2.0 ± 0.79† |
T effectors/LSelhi Tregs* . | GVHD scores . | Average ± SD . |
|---|---|---|
| Liver | ||
| None | 0, 1, 0, 0, 0 | 0.2 ± 0.45 |
| 1:1 | 1, 2, 1, 2.5, 1.5 | 1.6 ± 0.65† |
| 1:3 | 0.5, 0.5, 1.5, 1, 1 | 0.9 ± 0.42† |
| Lung | ||
| None | 1, 1, 1, 0, 0 | 0.6 ± 0.55 |
| 1:1 | 1, 1.5, 2, 3, 2 | 1.9 ± 0.74† |
| 1:3 | 2, 0.5, 2.5, 3.5, 3 | 2.3 ± 1.15† |
| Colon | ||
| None | 1, 1, 1, 0.5, 0.5 | 0.8 ± 0.27 |
| 1:1 | 1.5, 2, 1, 1.5, 1.5 | 1.5 ± 0.35† |
| 1:3 | 2, 2, 2, 2.5, 2 | 2.1 ± 0.22† |
| Skin | ||
| None | 0, 0, 0, 0, 0 | 0.0 ± 0.0 |
| 1:1 | 0.5, 0.5, 0.5, 0.5, 1 | 0.6 ± 0.22† |
| 1:3 | 0.5, 0.5, 0.5, 0.5, 0.5 | 0.5 ± 0.0† |
| Spleen | ||
| None | 0, 0, 0, 0.5, 0 | 0.1 ± 0.22 |
| 1:1 | 1.5, 2, 1, 2, 3 | 1.9 ± 0.74† |
| 1:3 | 1.5, 2.5, 3, 1, 2 | 2.0 ± 0.79† |
Survivors (n - 5/group) from Figure 1A were electively killed 7 months after transplantation and indicated GVHD target tissues were harvested, sectioned, and stained with hematoxylin and eosin and scored for GVHD histopathology. Shown are individual and average scores of a maximum score of 4.0.
Indicates the ratio of effector T cells to LSelhi Tregs
P = .018 vs “None”
LSelhi Tregs inhibit the activation and expansion of effector T cells in the spleen early after BMT
To further investigate the effect by Tregs on effector T cells, the described model was modified to use BALB/c Thy1.1+ effector T cells and BALB/c Thy1.2+ congenic CD25+LSelhi or CD25+LSello Tregs allowing for the phenotypic distinction of effector T cells and Tregs. Mice were analyzed 6 days after BMT, a time previously determined to be the peak of donor effector T-cell expansion in the spleen. Spleens were harvested and splenic effector T cells and Tregs enumerated, phenotyped, and studied for in vitro alloresponsiveness. Although the administration of LSelhi Tregs resulted in only a modest reduction of total effector CD4+ splenic T cells, total effector CD8+ splenic T cells were reduced by 90% (Figure 1C). In contrast to the LSelhi Tregs, LSello Tregs had no effect on CD4+ or CD8+ effector splenic T-cell number.
To characterize the activation status of effector T cells, LSel and CD25 expression were evaluated on effector CD4+ (Thy1.1+CD4+ gate) and CD8+ (Thy1.1+CD8+ gate) T cells (Figure 1D and data not shown). As compared to controls, recipients of LSelhi Tregs had significantly increased LSel (84% versus 37%) and reduced CD25 expression (7% versus 20%) on effector CD4+ T cell (Figure 1C-D and data not shown). Despite these data indicating inhibition of full T-cell activation by LSelhi Tregs, the significantly higher forward and side scatter values of effector CD4+ T cells were suggestive of blastogenesis, an early event in activation (data not shown). LSello Tregs had an intermediate effect on reducing the down-regulation of LSel (57%) and the up-regulation of CD25 (13%) on effector CD4+ T cells (Figure 1C-D and data not shown).
In addition to the 90% reduction in total CD8+ T-cell number, recipients of LSelhi Tregs had increased LSel (56% versus 8%) and reduced CD25 expression (17% versus 31%) on effector CD8+ T cells as compared to controls (Figure 1C-D and data not shown). LSello Tregs had no significant effect on LSel and CD25 expression on CD8+ effector T cells (Figure 1D and data not shown).
Equivalent numbers of Thy1.2+ LSelhi (3.9 million) or LSello (4.0 million) Tregs were recovered from spleens of treated mice. Although LSelhi Tregs were more than 97% positive for both CD25 and LSel at time of infusion into mice on day 0, LSelhi Tregs recovered from spleens on day 6 were 65% LSel+ and 55% CD25+. LSello Tregs, which were more than 97% CD25+ and LSello at time of infusion, retained their LSello status but down-regulated CD25 on about 75% of cells recovered from spleens on day 6 after BMT (data not shown).
In vitro proliferative alloresponses were evaluated from spleens of control and LSelhi-treated and LSello-treated mice 6 days after BMT (Figure 1E). Peak proliferation to host-type alloantigen restimulation was reduced by 90% in spleens from LSelhi-treated mice and by 40% in spleens from LSello-treated mice as compared to controls. Of note, LSelhi Tregs were present in the secondary restimulation culture at an approximate ratio of 2 Tregs to 1 effector CD4+ T cell. Evaluation of supernatants for cytokines in cultures from LSelhi versus LSello Treg-treated mice revealed a reduction of 73% versus 33%, respectively, in IL-2, and 97% versus 50%, respectively, in interferon γ (IFN-γ) levels, compared to GVHD control mice. Similar low levels of IL-10 were present in all cultures (data not shown).
Collectively, these data indicate that ex vivo activated and expanded LSelhi Tregs had a profound inhibitory effect on the full activation and subsequent proliferation of GVHD effector T cells in the spleen early after transplantation. Although allowing for long-term, relatively symptom-free survival, histopathology revealed moderate GVHD in target tissues in LSelhi Treg-treated survivors.
LSelhi Tregs reduce the number of effector T cells in secondary lymphoid organs and GVHD target organs
To further investigate the effect of Tregs on effector T-cell trafficking to GVHD target organs, in vivo imaging studies with enhanced GFP (eGFP) Tg effector T cells were performed. First, the inhibition of GVHD by LSelhi Tregs was verified in a different strain combination that would allow for the eventual tracking of B6 GFP effector T cells. B6 Tregs were isolated and cultured with anti-CD3 and anti-CD28 antibody-coated microspheres and IL-2 for 1 to 2 weeks. Ex vivo activated and expanded Tregs were separated into LSelhi and LSello fractions immediately prior to infusion with B6 BM and CD25-depleted CD4+ effector T cells into lethally irradiated MHC class II–disparate bm12 mice. Although both LSelhi and LSello fractions resulted in potent in vitro suppression of alloresponses (data not shown), LSelhi Tregs were superior for in vivo GVHD inhibition (Figure 2A). Sixty percent of mice receiving only effector CD4+ T cells (1 × 106) died of GVHD by day 10 with only 10% surviving at day 50 after BMT (Figure 2A). In contrast, 96% of LSelhi Treg-treated mice (1 effector to 3 Tregs) survived long-term. Recipients of LSello Tregs had an intermediate long-term survival rate of 55%. Weights of GVHD control mice and LSelhi- or LSello-treated mice were significantly lower than those recipients that received TCD BM only (Figure 2B). However, LSelhi-treated mice had significantly higher weights than LSello-treated mice or GVHD control mice. Histologic examination of survivors electively killed at 5 months revealed moderate GVHD pathology in liver, lung, skin, and spleen, but not colon of LSelhi and LSello Treg-treated mice (data not shown). Histologic evidence of GVHD included perivascular cuffing and parenchymal infiltration of inflammatory cells in the liver and lung and mononuclear cell infiltration in the epidermis of the skin with epidermal hyperplasia and mild to moderate keratosis. Therefore, despite a profound survival advantage in LSelhi Treg-treated mice in a rapidly lethal GVHD model, long-term survivors did have histologic evidence consistent with subacute GVHD.
LSelhi Tregs optimally protect against GVHD mortality and associated weight loss. Lethally irradiated bm12 mice were infused with MHC class II–disparate B6 BM. Cohorts of mice received 1 × 106 B6 CD25-depleted CD4+ effector T cells alone or with ex vivo activated and expanded B6 LSelhi or LSello Tregs in a ratio of 1 T to 3 Tregs. (A) Survival is indicated. A pool of 3 experiments is shown (n = 18-26 per group; *P < .005 compared to CD4+ T cells). (B) Average weight in grams of mice from one representative experiment from panel A is indicated. *Only one mouse survived after this time point.
LSelhi Tregs optimally protect against GVHD mortality and associated weight loss. Lethally irradiated bm12 mice were infused with MHC class II–disparate B6 BM. Cohorts of mice received 1 × 106 B6 CD25-depleted CD4+ effector T cells alone or with ex vivo activated and expanded B6 LSelhi or LSello Tregs in a ratio of 1 T to 3 Tregs. (A) Survival is indicated. A pool of 3 experiments is shown (n = 18-26 per group; *P < .005 compared to CD4+ T cells). (B) Average weight in grams of mice from one representative experiment from panel A is indicated. *Only one mouse survived after this time point.
For imaging studies, purified CD25-depleted CD4+ effector T cells were obtained from B6 eGFP Tg mice, combined with B6 wild-type BM and B6 wild-type ex vivo activated and expanded LSelhi or LSello Tregs and infused into lethally irradiated bm12 mice. As an additional imaging control, eGFP+ effector T cells and wild-type BM were infused into lethally irradiated syngeneic B6 mice to compare normal homeostatic expansion of eGFP T cells to alloantigen-driven proliferation of GFP+ effector T cells that occurs during GVHD. bm12 mice received BM only (no GFP+ effectors) as a negative control for imaging to verify lack of autofluorescence. It was previously determined that day 7 was the first optimal time point for detecting GFP+ cells in this strain combination (P.A.T. unpublished data, 2004). By 1 week after BMT, LNs, Peyer patches, and spleen were very bright in GVHD control mice receiving only eGFP+ effector CD4+ T cells (Figure 3A). In contrast, there were far fewer GFP+ cells in LSelhi Treg-treated mice that were only slightly brighter than syngeneic control mice. LSello Tregs did not significantly inhibit expansion of effectors in LNs. Moreover, Peyer patches of LSello Treg-treated mice were brighter and larger than in GVHD control mice. GFP intensity in secondary lymphoid organs was increased in GVHD controls and LSello Treg-treated mice at 2 weeks, whereas LSelhi Treg-treated mice remained dim, similar to or even less bright than syngeneic controls. Although all control mice were dead by 3 weeks, LSelhi Treg-treated mice were imaged and were not increased in brightness suggesting that effector expansion was inhibited and not merely delayed (data not shown).
LSelhi Tregs inhibit effectors in secondary lymphoid organs and GVHD target tissues. Lethally irradiated MHC class II–disparate bm12 and syngeneic B6 mice were infused with B6 BM. Cohorts of mice received 2 × 106 B6 GFP+ CD25-depleted CD4+ effector T cells alone or with ex vivo activated and expanded B6 (non-GFP) LSelhi or LSello Tregs in a ratio of 1 to 3 Tregs. Mice were imaged 1, 2, and 3 weeks after BMT. Representative images from 1 of 3 mice per group at 1 and 2 weeks after BMT are shown. Data were replicated in a second experiment. (A) Images of lymphoid organs including inguinal and mesenteric LNs, Peyer patch, and spleen. (B) Images of GVHD target tissues including skin, gingiva, liver, lung, ileum, and colon. Stereomicroscope was set to × 3.2 zoom factor for inguinal LNs and skin; × 4.0 for mouth and mesenteric LNs; × 4.5 for colon; × 7.0 for Peyer patch, ileum, liver, and spleen; and × 10.0 for lung. Exposure times were optimized for GVHD control mice for each organ and identical times were used for all other groups. The following exposure times were used for organs shown: 154 msec, inguinal LN; 80 msec, mesenteric LN; 110 msec, Peyer patch; 870 msec, spleen; 1.0 sec, skin; 490 msec, gingiva; 910 msec, liver; 540 msec, lung; 110 msec, ileum; 120 msec, colon. Negative controls of mice not receiving GFP+ effectors to verify lack of autofluorescence resulted in dark images at indicated exposure times (not shown).
LSelhi Tregs inhibit effectors in secondary lymphoid organs and GVHD target tissues. Lethally irradiated MHC class II–disparate bm12 and syngeneic B6 mice were infused with B6 BM. Cohorts of mice received 2 × 106 B6 GFP+ CD25-depleted CD4+ effector T cells alone or with ex vivo activated and expanded B6 (non-GFP) LSelhi or LSello Tregs in a ratio of 1 to 3 Tregs. Mice were imaged 1, 2, and 3 weeks after BMT. Representative images from 1 of 3 mice per group at 1 and 2 weeks after BMT are shown. Data were replicated in a second experiment. (A) Images of lymphoid organs including inguinal and mesenteric LNs, Peyer patch, and spleen. (B) Images of GVHD target tissues including skin, gingiva, liver, lung, ileum, and colon. Stereomicroscope was set to × 3.2 zoom factor for inguinal LNs and skin; × 4.0 for mouth and mesenteric LNs; × 4.5 for colon; × 7.0 for Peyer patch, ileum, liver, and spleen; and × 10.0 for lung. Exposure times were optimized for GVHD control mice for each organ and identical times were used for all other groups. The following exposure times were used for organs shown: 154 msec, inguinal LN; 80 msec, mesenteric LN; 110 msec, Peyer patch; 870 msec, spleen; 1.0 sec, skin; 490 msec, gingiva; 910 msec, liver; 540 msec, lung; 110 msec, ileum; 120 msec, colon. Negative controls of mice not receiving GFP+ effectors to verify lack of autofluorescence resulted in dark images at indicated exposure times (not shown).
Imaging of the skin adjacent to the inguinal LN revealed many GFP+ cells in skin lymphatics allowing for the striking visualization of the branching lymphatic vasculature in GVHD control mice and LSello Treg-treated mice (Figure 3B). GFP+ cells were greatly increased at 2 weeks in both the lymphatic vessels and in diffuse foci in the dermis but were not evident in blood vessels in the skin. This pattern was generalized throughout the skin of the body of affected mice. In profound contrast, only a rare GFP+ cell was found in the skin of LSelhi Treg-treated mice or syngeneic controls. As we showed in a different GVHD strain combination, the oral gingiva was a target site for allogeneic T-cell trafficking. Large GFP+ infiltrations were prominent in the oral cavity and thickened gingiva around the teeth in both GVHD control mice and LSello-treated mice (Figure 3B). Although present, GFP+ cells were greatly reduced in number in LSelhi Treg-treated mice and syngeneic controls at both 1 and 2 weeks.
Liver and lung are important parenchymal GVHD target organs. Multifocal GFP+ infiltrates were scattered through the liver and lung of GVHD control mice and LSello Treg-treated mice and increased in number and size from 1 to 2 weeks (Figure 3B). Although reduced in LSelhi-treated mice, GFP+ infiltrates were evident in the lung and especially the liver. These infiltrates were not increased in number or size at 3 weeks in LSelhi-treated mice (data not shown).
The gastrointestinal tract is a critical target tissue of GVHD. The loss of gut integrity can result in diarrhea with life-threatening fluid and electrolyte losses and leakage of endotoxin into the peritoneal cavity as well as malabsorption and cachexia. By 1 week after BMT, GVHD control mice have a pronounced diffuse GFP+ infiltration in the small intestine (Figure 3B). This was dramatically reduced in LSelhi-treated mice and, interestingly, also in LSello-treated mice. These latter findings provide a possible explanation for the survival advantage, albeit inferior to LSelhi Tregs, conferred by LSello Tregs. By 2 weeks after BMT, small intestinal infiltration was present in LSello Treg-treated mice in contrast to LSelhi Treg-treated mice that were still largely free of GFP+ cells except for the occasional small foci (Figure 3B). The colon was infiltrated in GVHD control mice by day 7. There was a modest reduction of GFP+ cells in LSello Treg-treated mice and a near absence in LSelhi Treg-treated mice although an adjacent LN was infiltrated (Figure 3B). Although intensity appeared less bright in both ileum and colon in moribund GVHD control mice at 2 weeks compared to 1 week, the gut was more dilated and flaccid and the wall appeared to be thinner, indicative of substantial mucosal injury.
Consistent with our previous report, in vivo imaging conveyed a vivid sense of the multisystemic nature of GVHD.13 In addition to those organs illustrated here, GFP+ effector infiltration was also noted in the stomach, ureter, uterus, kidney, and bone marrow cavity of femurs of GVHD control mice. LSelhi, but not LSello, Tregs inhibited the infiltration of effectors at all observed sites (data not shown).
Donor- or host-type LSelhi Tregs increase donor BM engraftment
Collectively, these data indicate that LSelhi Tregs inhibit activation, expansion, and multiorgan infiltration of GVHD effector T cells. An important consideration of any potential therapeutic modality for the prevention of GVHD is the effect on BM engraftment. To directly address this issue, donor- or host-type ex vivo activated and expanded LSelhi and LSello Tregs were tested in an allogeneic donor BM engraftment model (Table 2). B6 mice were sublethally irradiated on day -1 and infused with allogeneic TCD BALB/c BM on day 0. Cohorts received BALB/c LSelhi or LSello Tregs on day 0 and +3. Radiation and BM cell doses were chosen to guarantee rejection of donor BM in the control mice. The infusion of donor-type LSelhi Tregs greatly increased engraftment from none to all of 10 recipients with an average donor chimerism of 87% (Table 2 experiment 1). Engraftment was stable, multilineage, and long-term (> 3 months; data not shown). In contrast, LSello Tregs did not promote engraftment. These data were reproduced in a second experiment with a single lower dose of LSelhi Tregs on day 0 (Table 2 experiment 2). Although as few as 3.5 × 106 LSelhi Tregs significantly increased donor engraftment, as many as 20 × 106 LSello Tregs did not (data not shown). Donor chimeras had no evidence of clinical GVHD at any time and weights at 3 months exceeded pretransplant weights by over 30%.
Ex vivo activated and expanded donor- or host-type BALB/c CD25+ LSelhi, but not LSello, cell infusion increases donor BM engraftment and TGF-β signaling is not required for engraftment promotion
Donor . | Host . | CD25+ origin . | CD25+ no. . | No. chimeric . | % Donor . |
|---|---|---|---|---|---|
| Exp no. 1 | |||||
| BALB/c | B6 | NA | None | 0/10 | 0 ± 0 |
| BALB/c | B6 | Donor | 10 × 106 LSelhi | 10/10* | 87 ± 6* |
| BALB/c | B6 | Donor | 10 × 106 LSello | 3/10 | 3 ± 4 |
| Exp no. 2 | |||||
| BALB/c | B6 | NA | None | 0/10 | 0 ± 0 |
| BALB/c | B6 | Donor | 6 × 106 LSelhi | 9/9* | 70 ± 10* |
| Exp no. 3 | |||||
| B6 | BALB/c | NA | None | 0/8 | 0 ± 0 |
| B6 | BALB/c | Host | 8.5 × 106 LSelhi | 10/10* | 69 ± 4* |
| B6 | BALB/c | Host | 8.5 × 106 LSello | 0/8 | 0 ± 0 |
| Exp no. 4 | |||||
| BALB/c | B6 β2m-/- DNRII | NA | None | 3/9 | 8 ± 7 |
| BALB/c | B6 β2m-/- DNRII | Donor | 13 × 106 LSelhi | 7/9 | 37 ± 11* |
| Exp no. 5 | |||||
| BALB/c | B6 β2m-/- DNRII | NA | None | 6/8 | 21 ± 10 |
| BALB/c | B6 β2m-/- DNRII | Donor | 10 × 106 LSelhi | 9/9 | 74 ± 3* |
Donor . | Host . | CD25+ origin . | CD25+ no. . | No. chimeric . | % Donor . |
|---|---|---|---|---|---|
| Exp no. 1 | |||||
| BALB/c | B6 | NA | None | 0/10 | 0 ± 0 |
| BALB/c | B6 | Donor | 10 × 106 LSelhi | 10/10* | 87 ± 6* |
| BALB/c | B6 | Donor | 10 × 106 LSello | 3/10 | 3 ± 4 |
| Exp no. 2 | |||||
| BALB/c | B6 | NA | None | 0/10 | 0 ± 0 |
| BALB/c | B6 | Donor | 6 × 106 LSelhi | 9/9* | 70 ± 10* |
| Exp no. 3 | |||||
| B6 | BALB/c | NA | None | 0/8 | 0 ± 0 |
| B6 | BALB/c | Host | 8.5 × 106 LSelhi | 10/10* | 69 ± 4* |
| B6 | BALB/c | Host | 8.5 × 106 LSello | 0/8 | 0 ± 0 |
| Exp no. 4 | |||||
| BALB/c | B6 β2m-/- DNRII | NA | None | 3/9 | 8 ± 7 |
| BALB/c | B6 β2m-/- DNRII | Donor | 13 × 106 LSelhi | 7/9 | 37 ± 11* |
| Exp no. 5 | |||||
| BALB/c | B6 β2m-/- DNRII | NA | None | 6/8 | 21 ± 10 |
| BALB/c | B6 β2m-/- DNRII | Donor | 10 × 106 LSelhi | 9/9 | 74 ± 3* |
Host mice were sublethally irradiated on day-1 and infused with 10 × 106 allogeneic donor BM on day 0. Donor- or host-type BALB/c CD25+ cells were activated and expanded by anti-CD3/CD28-coated beads and IL-2 (100 U/mL) and separated into LSelhi and LSello fractions immediately prior to infusion at the indicated number on day 0 (an additional infusion was given on day 3 in experiment 1 only). PBLs were typed for percentage donor-host at 3 months after BMT. Chimeric is defined as having more than 3% donor PBLs. Percentage donor is defined as average percentage donor cells of all mice in the group ± 1 SEM.
Exp indicates experiment; NA, not applicable.
P < .02 compared to control mice receiving no CD25+ cells
Because the LSelhi Tregs were preactivated with a global T-cell stimulus prior to infusion and would be predicted to nonspecifically suppress a host antidonor T-cell response, we hypothesized that host-type Tregs would also increase engraftment. To test this, BALB/c mice were sublethally irradiated and infused with B6 BM and BALB/c LSelhi or LSello Tregs (Table 2 experiment 3). No control mice or recipients of LSello Tregs had any evidence of donor BM engraftment. In contrast, all recipients of host-type LSelhi Tregs engrafted with an average donor chimerism of 74% (Table 2 experiment 3).
TGF-β signaling to host T cells is not required for promotion of donor BM engraftment
The role of TGF-β as a mechanism for the suppressor function of Tregs is controversial and, according to accumulating data, is likely to be model-dependent.14-17 To investigate the role of TGF-β as a potential mechanism for BM engraftment promotion by LSelhi Tregs, B6 β -2m-/-DNRII mice were used as recipients for allogeneic BALB/c BM (Table 2 experiments 4-5). The absence of β2m precludes CD8+ T-cell development and the early onset of autoimmunity found in DNRII mice. The CD4+ T cells in this strain express a dominant-negative TGF-βRII that precludes effective TGF-β signaling of host T cells.11 The CD4+ T cells in these host mice are unable to respond to TGF-β putatively produced by donor-type BALB/c LSelhi Tregs. In the absence of TGF-β signaling to host T cells, LSelhi Tregs still significantly increased donor chimerism indicating that TGF-β signaling of host T cells by donor Tregs is not an absolute requirement for engraftment promotion (Table 2 experiments 4-5).
Discussion
In this study, we directly compared LSello and LSelhi Tregs for the prevention of GVHD and graft rejection and we observed profound protection from lethal GVHD and promotion of BM engraftment by the LSelhi, but not the LSello, fraction of ex vivo activated and expanded Tregs. The in vivo imaging studies offer a vivid visualization of the inhibitory effect of LSelhi Tregs on the massive expansion and widespread multiorgan dissemination of alloreactive T cells in the lymphopenic and proinflammatory environment during the early period after transplantation.
Our recently published kinetic imaging studies revealed that GFP+ T cells immediately localized to the LNs and spleen, a process independent of irradiation, proinflammation, or alloantigen recognition.13 After initial homing to LNs, donor alloreactive T cells presumably recognize host alloantigen under ideal conditions for the activation and rapid expansion of GVHD effectors. These alloantigen-activated effector T cells then exit the secondary lymphoid organs and migrate to GVHD target organs in response to chemokine signals. GVHD is a rapid cascade of events that, once in motion, is more difficult to turn off. As such, prevention or early intervention during priming events would be predicted to result in greater therapeutic efficacy than later intervention at the effector phase of GVHD. Although both LSelhi and LSello Treg subsets have in vitro suppressor function,18-21 we hypothesized that the LSelhi Tregs would have superior therapeutic benefit due to their ability to home to peripheral LNs via the binding of the LSel molecule to determinants on high endothelial venules in these LNs. Initial homing of LSelhi Tregs to the LNs would optimize the likelihood of interfering with the priming and expansion of effector T cells thereby inhibiting an early phase of GVHD. Consistent with this idea, Szanya et al found that CD4+CD25+LSelhi but not LSello T cells delayed diabetes and postulated that the Tregs inhibited the activation of islet-reactive lymphocytes in draining pancreatic LNs.20 However, the expression of LSel on Tregs was not required for the prevention of autoimmune gastritis and colitis in another study, suggesting that there is not a universal requirement for LN entry for the prevention of autoimmune disease by Tregs.21
Imaging studies demonstrated that LSelhi Tregs reduced numbers of GFP+ effector T cells in many types of tissues besides lymphoid organs. Although it is reasonable to surmise that the reduced number of GFP+ effectors in the skin, liver, lung, gastrointestinal tract, and other organs was primarily an indirect effect of initial inhibition of activation events and subsequent expansion in lymphoid organs, we do not rule out the possibility that Tregs were present and active at the sites of effector function. The data also do not preclude the possibility of priming, activation, and expansion of effector cells directly in GVHD target tissues. However, despite the presumed ability of LSello Tregs to migrate directly to sites of inflammation in response to chemokines, imaging data indicate little demonstrable effect on the accumulation of GFP+ T cells in GVHD target organs in LSello-treated mice.
Although the data incriminate early inhibition of effectors in lymphoid organs as a key event in successful GVHD inhibition, the later administration of unseparated Tregs on day 6 after allogeneic BMT, a time when priming and expansion as well as migration to target organs have already occurred, led to a 40% survival rate at 5 months compared with a 0% survival rate at 2 months in controls (P = .002, published data, 2003, P.A.T.). These data are consistent with another study that showed that Tregs significantly inhibited CD8+ T cell–mediated GVHD in minor histocompatibility antigen–disparate recipients even when administered after priming has occurred.5 Of note, survivors in our GVHD therapy study had significant signs of GVHD including severe weight loss, diarrhea, generalized erythema, and greasy rough fur coat. Collectively, these data indicate that Tregs can inhibit GVHD after priming has occurred but that, not surprisingly, early administration leads to a superior therapeutic outcome.
As well as inhibiting donor antihost alloresponses, LSelhi, but not LSello, Tregs potently inhibited host antidonor alloresponses resulting in engraftment promotion in sublethally irradiated mice. Although donor-type Tregs may be more clinically feasible, either donor- or host-type LSelhi Tregs promoted donor chimerism. The finding that exogenous host-type Tregs promote allogeneic donor BM engraftment is consistent with the finding that depletion of endogenous host CD25+ cells prior to BMT with a depleting anti-CD25 antibody resulted in reduced levels of donor engraftment (P.A.T., unpublished data, 2003). TGF-β signaling of host T cells by LSelhi Tregs was not essential for engraftment promotion as evidenced by increased donor chimerism in DNRII recipients (Table 2 experiments 4-5). These data are consistent with in vitro data demonstrating that responder T cells from DNRII mice still could be suppressed by Tregs17 but do not rule out the possibility that TGF-β, produced by Tregs, may increase alloengraftment via mechanisms that do not involve direct suppression of host antidonor T-cell immune-mediated rejection per se.
Although we tend to attribute the superior therapeutic effect of LSelhi Tregs to their ability to home to LNs and suppress early events in alloantigen T-cell priming responsible for both GVHD generation and host antidonor rejection, we cannot exclude the possibility that there may be additional reasons for the greater in vivo suppressor function of LSelhi Tregs. LSelhi and LSello Tregs may differ in more ways than LN homing. For example, one study demonstrated that LSelhi and LSello Treg subsets differed in their cell contact- and CTLA4-dependency for in vitro suppression indicating that the 2 subsets can use different mechanisms of inhibition.21 Another study showed that differential expression of adhesion molecules and chemokine receptors on Treg subsets resulted in distinct migratory properties in vivo.22 These studies indicate that Tregs are heterogeneous in more ways than just their level of LSel expression.
Of important clinical significance, others have shown that Tregs inhibit GVHD but do not inhibit a GVL effect in models using acute myeloid leukemia or lymphoma cell lines.4-6 Trenado et al demonstrated that ex vivo expanded LSelhi Tregs controlled GVHD but did not inhibit a graft-versus-tumor (GVT) effect against A20 leukemia cells, whereas this effect was lost against P815 mastocytoma cells.6 Collectively, these preclinical data indicate that Tregs inhibit GVHD and BM graft rejection with the retention of GVL in models using acute myeloid or lymphoid leukemia cell lines. Additional preclinical and ultimately clinical studies will be required to determine whether Tregs will have an adverse effect on some types of malignancies.
Recent publications describe protocols indicating that human Tregs can be readily expanded in sufficient number for clinical use.23,24 The expanded human Tregs were shown to maintain expression of L-selectin and be highly suppressive in vitro.23,24 Our data support these studies by illustrating the in vivo differences of LSelhi and LSello Tregs and indicate that strategies that optimize the selection or retention of L-selectin are likely to be desirable for the inhibition of GVHD and the promotion of alloengraftment.
Prepublished online as Blood First Edition Paper, August 3, 2004; DOI 10.1182/blood-2004-05-1850.
Supported in part by National Institutes of Health grants R01 AI 34495, 2 R37 HL56067, HL55209, R01 HL63452, R01 HL66308, and CA102052-02.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Matthew O'Shaughnessy and Christine Vogtenhuber for helpful discussions.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal