Myeloablative allogeneic hematopoietic stem cell transplantation (allo-HSCT) is increasingly used in patients with lymphoma who experience disease relapse after autologous hematopoietic stem cell transplantation (auto-HSCT) because the allograft is tumor free and may induce a graft-versus-tumor effect. We analyzed 114 patients treated with this approach from 1990 to 1999 to assess disease progression, progression-free survival (PFS), and overall survival (OS). Cumulative incidence of disease progression at 3 years was 52%, whereas treatment-related mortality was 22%, lower than previously reported. Three-year probabilities of OS and PFS were 33% and 25%, respectively. With prolonged follow-up, however, nearly all patients experienced disease progression, and 5-year probabilities were 24% and 5%, respectively. Complete remission at the time of allo-HSCT and use of total body irradiation (TBI) in patients with non-Hodgkin lymphoma (NHL) were associated with lower rates of disease progression and higher rates of OS. In summary, allo-HSCT is feasible for patients with lymphoma who have relapses after auto-HSCT and can result in prolonged survival for some, but it is usually not curative. Most likely to benefit are patients who have HLA-matched sibling donors, are in remission, and have good performance status.
Introduction
High-dose chemotherapy with autologous hematopoietic stem cell transplantation (auto-HSCT) has an established role in the treatment of patients with lymphoid malignancies.1-3 Unfortunately, relapse or progression of lymphoma is common after auto-HSCT, and the prognosis for patients who experience relapse is poor.4-6 Conventional-dose chemotherapy can induce remission in a small proportion of these patients, but the remissions are usually short.4 Second auto-HSCT is sometimes performed, but it is most likely to benefit patients in prolonged remission after the initial auto-HSCT.7,8
Another therapeutic approach for patients who experience relapse after auto-HSCT is to perform myeloablative allogeneic hematopoietic stem cell transplantation (allo-HSCT).9 Potential advantages of allo-HSCT include the use of a tumor-free graft and immune-mediated graft-versus-tumor effects.10,11 Information is limited regarding the outcome for patients who undergo allo-HSCT after autologous transplantation for lymphoma fails. Published reports include small numbers of patients and show conflicting results.8,12-17 To more precisely determine outcomes and to further identify prognostic variables for patients who undergo allo-HSCT after failed auto-HSCT, we undertook an analysis of patients treated between 1990 and 1999 and reported to the International Bone Marrow Transplant Registry (IBMTR) and the Autologous Blood and Marrow Transplant Registry (ABMTR).
Patients, materials, and methods
Data sources
The IBMTR/ABMTR is a voluntary working group of more than 400 HSCT centers worldwide that contribute detailed data on consecutive allogeneic or autologous HSCT recipients to a Statistical Center at the Health Policy Institute of the Medical College of Wisconsin in Milwaukee. Approximately 40% of allo-HSCTs worldwide and more than 50% of auto-HSCTs in North and South America are registered with the IBMTR/ABMTR. Participating centers are required to report all transplantations consecutively; compliance is monitored by on-site audits. Patients are followed up longitudinally, with yearly follow-up. Computerized checks for errors, physician reviews of submitted data, and on-site audits of participating centers ensure data quality. Requests for data on disease progression or death for registered patients are at 1-year intervals. IBMTR observational studies are conducted with a waiver of informed consent and in compliance with HIPAA regulations as determined by the institutional review board and the privacy officer of the Medical College of Wisconsin.
Patients
We reviewed the records of all patients with lymphoma reported to the IBMTR/ABMTR who underwent allo-HSCT after relapse from auto-HSCT. We included patients with Hodgkin lymphoma (HL) or non-Hodgkin lymphoma (NHL), including low-, intermediate-, and high-grade lymphomas according to the Working Formulation for the Classification of Lymphomas.18 The allo-HSCT had to have been performed between 1990 and 1999. Patients who received nonmyeloablative conditioning regimens were excluded.
Study end points
Primary outcomes studied were disease progression, progression-free survival (PFS), and overall survival (OS). Progression was defined as any increase in size of sites of disease, development of new sites of disease, or recurrence of disease after an initial complete response. OS was defined as the time from transplantation to death from any cause. PFS was defined as time from transplantation to relapse, progressive disease, or death from any cause. Other outcomes examined were transplant-related mortality (TRM), defined as death from any cause in the first 28 days or, thereafter, death without progression, and the incidence and severity of acute and chronic graft-versus-host disease (GVHD). Acute GVHD was defined as moderate to severe (grades II-IV) using established criteria; patients surviving more than 21 days with evidence of engraftment were considered at risk. Chronic GVHD was determined by clinical criteria in patients surviving more than 90 days with evidence of engraftment.
Statistical analysis
Probabilities of OS and PFS were calculated using the Kaplan-Meier product limit estimate, and probabilities of progression, TRM, and acute and chronic GVHD were calculated using cumulative incidence to allow for competing risks. Associations between patient-, disease-, and transplant-related factors and the outcomes of interest were assessed using multivariate Cox proportional hazards regression. Variables included in the model building are listed in Table 1. For most patients, we had few data about details of auto-HSCT or treatment before auto-HSCT and, hence, could not examine these factors in model building. HL and NHL are different biologically, and initial treatment for HL commonly involves radiation, limiting the choices for allo-HSCT conditioning in HL. Consequently, we forced the factor HL versus NHL in all the models built and examined interactions between this factor and other significant covariates identified. If the interaction term was significant, indicating differential effects in HL and NHL, the final model subdivides patients according to this interaction term. For example, Tables 4, 5, and 6 subdivide patients into 4 groups: NHL with or without total body irradiation (TBI) and HL with or without TBI. All computations used the procedure PHREG in the SAS statistical package. Forward stepwise variable selection at a 0.05 significance level was used to identify covariates associated with outcomes. All variables were tested for the assumption of proportional hazards using a time-dependent covariate. All variables satisfied the proportionality assumption. First-order interactions were tested for all significant covariates. Overall covariate effects were tested using the Wald test.
Patient-, disease-, and transplant-related characteristics of patients with HL or NHL who underwent allo-HSCT following relapse after autologous transplantation, 1990 to 1999, reported to IBMTR
Variables . | N(%)* . |
|---|---|
| N | 114 |
| Age, y, median (range) | 34 (15-65)† |
| Male | 65 (57) |
| Karnofsky performance score at allo-HSCT | |
| 90%-100% | 64 (56) |
| 80% | 29 (25) |
| 70% | 16 (14) |
| 60% | 2 (2) |
| 50% or lower | 3 (3) |
| Disease type | |
| NHL | 79 (69) |
| Low grade | 16 (20) |
| Intermediate grade | 44 (56) |
| High grace | 8 (10) |
| Other‡ | 11 (14) |
| HL | 35 (31) |
| Interval from diagnosis to first, autologous, transplantation, mo, median (range) | 14 (3-130)† |
| 12 or less | 43 (38) |
| More than 12 | 71 (62) |
| Interval from auto-HSCT to allo-HSCT, mo, median (range) | 16 (2-66)† |
| 12 or less | 44 (39) |
| More than 12 | 70 (61) |
| Disease status before allo-HSCT | |
| Complete remission | 24 (21) |
| Relapse or persistent disease, chemosensitive | 39 (35) |
| Relapse or persistent disease, chemoresistant | 23 (20) |
| Relapse or persistent, untreated after auto-HSCT | 28 (24) |
| Donor type for allo-HSCT | |
| HLA-identical sibling | 70 (61) |
| Haploidentical sibling | 17 (14) |
| Unrelated | 27 (25) |
| Graft type used for allo-HSCT | |
| Bone marrow | 77 (68) |
| Peripheral blood | 37 (32) |
| TBI for allo-HSCT | 45 (39) |
| Posttransplantation use of growth factors, within 7 d | 84 (74) |
| GVHD prophylaxis | |
| MTX + CSA ± others | 74 (65) |
| CSA ± others | 24 (21) |
| T-cell depletion ± others | 16 (14) |
| Years of allo-HSCT | |
| 1990-1996 | 56 (49) |
| 1997-1999 | 58 (51) |
| No. centers reporting | 54 |
| Follow-up time of survivors, mo, median (range) | 43 (3-89)† |
| 75th percentile | 21 |
| 25th percentile | 58 |
Variables . | N(%)* . |
|---|---|
| N | 114 |
| Age, y, median (range) | 34 (15-65)† |
| Male | 65 (57) |
| Karnofsky performance score at allo-HSCT | |
| 90%-100% | 64 (56) |
| 80% | 29 (25) |
| 70% | 16 (14) |
| 60% | 2 (2) |
| 50% or lower | 3 (3) |
| Disease type | |
| NHL | 79 (69) |
| Low grade | 16 (20) |
| Intermediate grade | 44 (56) |
| High grace | 8 (10) |
| Other‡ | 11 (14) |
| HL | 35 (31) |
| Interval from diagnosis to first, autologous, transplantation, mo, median (range) | 14 (3-130)† |
| 12 or less | 43 (38) |
| More than 12 | 71 (62) |
| Interval from auto-HSCT to allo-HSCT, mo, median (range) | 16 (2-66)† |
| 12 or less | 44 (39) |
| More than 12 | 70 (61) |
| Disease status before allo-HSCT | |
| Complete remission | 24 (21) |
| Relapse or persistent disease, chemosensitive | 39 (35) |
| Relapse or persistent disease, chemoresistant | 23 (20) |
| Relapse or persistent, untreated after auto-HSCT | 28 (24) |
| Donor type for allo-HSCT | |
| HLA-identical sibling | 70 (61) |
| Haploidentical sibling | 17 (14) |
| Unrelated | 27 (25) |
| Graft type used for allo-HSCT | |
| Bone marrow | 77 (68) |
| Peripheral blood | 37 (32) |
| TBI for allo-HSCT | 45 (39) |
| Posttransplantation use of growth factors, within 7 d | 84 (74) |
| GVHD prophylaxis | |
| MTX + CSA ± others | 74 (65) |
| CSA ± others | 24 (21) |
| T-cell depletion ± others | 16 (14) |
| Years of allo-HSCT | |
| 1990-1996 | 56 (49) |
| 1997-1999 | 58 (51) |
| No. centers reporting | 54 |
| Follow-up time of survivors, mo, median (range) | 43 (3-89)† |
| 75th percentile | 21 |
| 25th percentile | 58 |
MTX indicates methotrexate; CSA, cyclosporine.
Categorical variable
Continuous variable
Includes NHL composite, mantle cell, and peripheral T-cell lymphomas
Multivariate analysis of PFS
Variables . | RR of treatment failure (95% CI) . | P . |
|---|---|---|
| Disease type and use of TBI | .002† | |
| NHL with TBI, n = 37 | 1.00 | - |
| NHL no TBI, n = 42 | 2.45 (1.49-4.02) | < .001 |
| HL with TBI, n = 8* | 1.77 (0.78-4.01) | .17 |
| HL no TBI, n = 27 | 1.97 (1.14-3.41) | .01 |
| Disease status before second transplantation | < .001† | |
| Complete remission, n = 24 | 1.00 | - |
| Relapse/primary induction failure, chemosensitive, n = 39 | 1.74 (0.99-3.03) | .06 |
| Relapse/primary induction failure, chemoresistant, n = 23 | 3.91 (2.05-7.44) | < .001 |
| Relapse/primary induction failure, untreated, n = 28 | 3.68 (1.97-6.84) | < .001 |
| Donor type | .05‡ | |
| HLA-identical sibling, n = 70 | 1.00 | - |
| Haploidentical sibling, n = 17 | 1.85 (1.04-3.31) | .04 |
| Unrelated, n = 27 | 1.47 (0.92-2.34) | .11 |
Variables . | RR of treatment failure (95% CI) . | P . |
|---|---|---|
| Disease type and use of TBI | .002† | |
| NHL with TBI, n = 37 | 1.00 | - |
| NHL no TBI, n = 42 | 2.45 (1.49-4.02) | < .001 |
| HL with TBI, n = 8* | 1.77 (0.78-4.01) | .17 |
| HL no TBI, n = 27 | 1.97 (1.14-3.41) | .01 |
| Disease status before second transplantation | < .001† | |
| Complete remission, n = 24 | 1.00 | - |
| Relapse/primary induction failure, chemosensitive, n = 39 | 1.74 (0.99-3.03) | .06 |
| Relapse/primary induction failure, chemoresistant, n = 23 | 3.91 (2.05-7.44) | < .001 |
| Relapse/primary induction failure, untreated, n = 28 | 3.68 (1.97-6.84) | < .001 |
| Donor type | .05‡ | |
| HLA-identical sibling, n = 70 | 1.00 | - |
| Haploidentical sibling, n = 17 | 1.85 (1.04-3.31) | .04 |
| Unrelated, n = 27 | 1.47 (0.92-2.34) | .11 |
Treatment failure indicates disease progression or death.
indicates not applicable
Pairwise comparison: HL with TBI versus HL with no TBI (P = .82)
3 degrees of freedom test
2 degrees of freedom test
Multivariate analysis of risk for death
Variables . | RR of death (95% CI) . | P . |
|---|---|---|
| Disease type and use of TBI | .001† | |
| NHL with TBI, n = 37 | 1.00 | - |
| NHL no TBI, n = 42 | 2.11 (1.19-3.77) | .01 |
| HL with TBI, n = 8* | 0.98 (0.33-2.85) | .96 |
| HL no TBI, n = 27 | 1.44 (0.76-2.76) | .26 |
| Karnofsky performance score at transplantation | - | |
| 90%-100%, n = 64 | 1.00 | - |
| 80% or less, n = 50 | 1.76 (1.10-2.82) | .02 |
| Disease status before second transplantation | .006† | |
| Complete remission, n = 24 | 1.00 | - |
| Relapse/primary induction failure, chemosensitive, n = 39 | 2.76 (1.27-5.96) | .01 |
| Relapse/primary induction failure, chemoresistant, n = 23 | 3.95 (1.70-9.15) | .001 |
| Relapse/primary induction failure, untreated, n = 28 | 3.74 (1.67-8.41) | .001 |
| Donor type | .004‡ | |
| HLA-identical sibling, n = 70 | 1.00 | - |
| Haploidentical sibling, n = 17 | 2.40 (1.24-4.66) | .009 |
| Unrelated, n = 27 | 2.14 (1.24-3.68) | .006 |
Variables . | RR of death (95% CI) . | P . |
|---|---|---|
| Disease type and use of TBI | .001† | |
| NHL with TBI, n = 37 | 1.00 | - |
| NHL no TBI, n = 42 | 2.11 (1.19-3.77) | .01 |
| HL with TBI, n = 8* | 0.98 (0.33-2.85) | .96 |
| HL no TBI, n = 27 | 1.44 (0.76-2.76) | .26 |
| Karnofsky performance score at transplantation | - | |
| 90%-100%, n = 64 | 1.00 | - |
| 80% or less, n = 50 | 1.76 (1.10-2.82) | .02 |
| Disease status before second transplantation | .006† | |
| Complete remission, n = 24 | 1.00 | - |
| Relapse/primary induction failure, chemosensitive, n = 39 | 2.76 (1.27-5.96) | .01 |
| Relapse/primary induction failure, chemoresistant, n = 23 | 3.95 (1.70-9.15) | .001 |
| Relapse/primary induction failure, untreated, n = 28 | 3.74 (1.67-8.41) | .001 |
| Donor type | .004‡ | |
| HLA-identical sibling, n = 70 | 1.00 | - |
| Haploidentical sibling, n = 17 | 2.40 (1.24-4.66) | .009 |
| Unrelated, n = 27 | 2.14 (1.24-3.68) | .006 |
indicates not applicable
Pairwise comparison: HL with TBI versus HL with no TBI (P = .47)
3 degrees of freedom test
2 degrees of freedom test
Results
Patient and transplant characteristics
Patient-, disease-, and transplant-related characteristics are listed in Table 1. One hundred fourteen patients from 54 different centers underwent allo-HSCT following relapse after auto-HSCT. Their median age at the time of allo-HSCT was 34 years (range, 15-65 years). Sixty-five (57%) patients were male. Fifty (44%) patients had Karnofsky performance scores of 80% or less at the time of allo-HSCT.
Seventy-nine (69%) patients had NHL, and 35 (31%) had HL. Among patients with NHL, 44 (56%) had intermediate-grade, 16 (20%) had low-grade, and 8 (10%) had high-grade lymphomas; 11 (14%) had other types of NHL not defined in the Working Formulation.18
The median time interval from diagnosis to auto-HSCT was 14 months (range, 3-130 months). Forty-three (38%) patients underwent auto-HSCT less than 1 year after the diagnosis of lymphoma. The median interval between auto-HSCT and allo-HSCT was 16 months (range, 2-66 months). Only 24 (21%) patients were in complete remission at the time of allo-HSCT. Seventy (61%) patients received transplants from an HLA-identical sibling, 17 (14%) from a haploidentical sibling, and 27 (25%) from an unrelated donor. Seventy-seven (68%) patients received bone marrow as the source of allogeneic stem cells; the remaining 37 (32%) patients received peripheral blood. TBI was used as part of the conditioning regimen for allo-HSCT in 45 (39%) patients. Eighty-four (74%) patients received hematopoietic growth factors (HGFs) within 7 days of allo-HSCT. GVHD prophylaxis included methotrexate and cyclosporine in 74 (65%) patients, combinations of cyclosporine with other agents in 24 (21%) patients, and T-cell depletion of the graft in 16 (14%) patients.
Fifty-eight (51%) allo-HSCTs were performed in the 3 years between 1997 and 1999, whereas the remaining procedures were performed in the previous 7 years, 1990 to 1996. Median follow-up of survivors was 43 months.
Outcomes
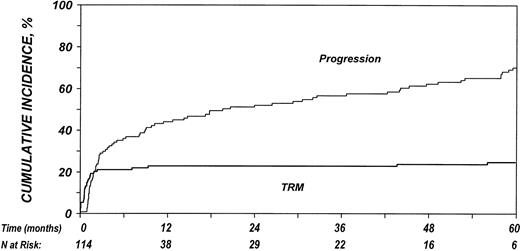
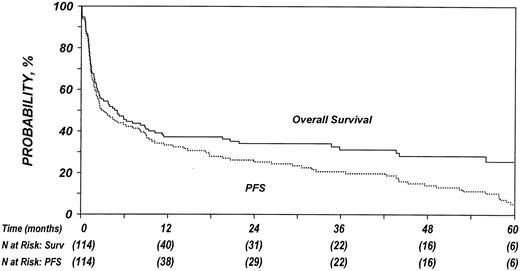
Estimated outcomes are summarized in Table 2. Of the 114 patients studied, 79 (69%) died, 31 (27%) were alive with lymphoma, and 4 (4%) were alive without evidence of lymphoma at last follow-up. The 100-day mortality rate was 21% (95% confidence interval [95% CI], 14%-29%). TRM after allo-HSCT was 22% (95% CI, 16%-31%) at 1 year and 25% (95% CI, 17%-33%) at 5 years. Twenty-four of 90 (27%) patients not in complete remission at the time of allo-HSCT achieved complete remission after allo-HSCT. Disease-progression rate was 45% (95% CI, 36%-54%) at 1 year and increased to 70% (95% CI, 61%-76%) at 5 years after transplantation. Figure 1 illustrates the cumulative incidences of progression and TRM. The probability of OS at 1 year was 37% (95% CI, 28%-46%) and decreased to 33% (95% CI, 24%-42%) at 3 years and 24% (95% CI, 14%-37%) at 5 years after transplantation (Figure 2). PFS was 32% (95% CI, 24%-41%), 25% (95% CI, 18%-33%), and 5% (95% CI, 2%-10%) at 1, 3, and 5 years after transplantation, respectively.
Outcome probabilities
. | 100 d . | 1 y . | 3 y . | 5 y . |
|---|---|---|---|---|
| Grade II-IV acute GVHD* | 29 (21-37) | - | - | - |
| Chronic GVHD* | - | 11 (6-18) | 12 (7-20) | 13 (7-20) |
| TRM* | 21 (14-29) | 22 (16-31) | 22 (16-31) | 25 (17-33) |
| Progression* | - | 45 (36-54) | 52 (43-61) | 70 (61-76) |
| Progression-free survival† | - | 32 (24-41) | 25 (18-33) | 5 (2-10) |
| Survival† | - | 37 (28-46) | 33 (24-42) | 24 (14-37) |
. | 100 d . | 1 y . | 3 y . | 5 y . |
|---|---|---|---|---|
| Grade II-IV acute GVHD* | 29 (21-37) | - | - | - |
| Chronic GVHD* | - | 11 (6-18) | 12 (7-20) | 13 (7-20) |
| TRM* | 21 (14-29) | 22 (16-31) | 22 (16-31) | 25 (17-33) |
| Progression* | - | 45 (36-54) | 52 (43-61) | 70 (61-76) |
| Progression-free survival† | - | 32 (24-41) | 25 (18-33) | 5 (2-10) |
| Survival† | - | 37 (28-46) | 33 (24-42) | 24 (14-37) |
Values in parentheses represent 95% CI.
indicates not applicable
Cumulative incidence rate
Kaplan-Meier estimate
Cumulative incidences of TRM and disease progression after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL.
Cumulative incidences of TRM and disease progression after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL.
Probabilities of PFS and OS after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL.
Probabilities of PFS and OS after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL.
The 100-day probability of developing grades II to IV acute GVHD was 29% (95% CI, 21%-37%). The 3-year probability of developing chronic GVHD was 12% (95% CI, 7%-20%). There was no difference in the risk for disease progression between patients who developed grades II to IV acute GVHD and those who did not. For patients who experienced engraftment and survived at least 21 days (n = 99), the relative risk (RR) of disease progression for patients with grades II to IV acute GVHD compared with those without acute GVHD using time-dependent Cox models was 0.93 (95% CI, 0.58-1.49; P = .76). Additionally, there was no difference in the risk for progression between patients in whom chronic GVHD developed and those in whom it did not among patients who experienced engraftment and survived at least 90 days (n = 62), with an RR of 0.81 (95% CI, 0.40-1.67l; P = .57).
There were no differences in OS, PFS, or TRM between patients with HL and NHL. In addition, there were no differences in outcome among patients with low-, intermediate-, or high-grade NHL (data not shown).
Multivariate analysis
The only factor significantly correlated with TRM risk was donor type. Patients with haploidentical sibling donors had a 3-fold increased risk for TRM (RR, 3.17; 95% CI, 1.04-3.31; P = .02), and those with unrelated donors had a 2-fold increased risk for TRM (RR, 2.15; 95% CI, 0.90-5.12; P = .08) compared with recipients of transplants from HLA-identical siblings.
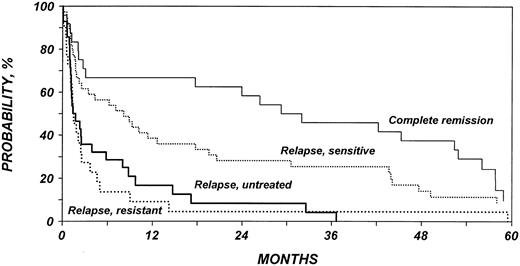
Table 3 shows the multivariate analysis of disease progression. Rate of disease progression was associated with type of conditioning regimen and disease status at allo-HSCT. The effect of conditioning was dependent on the type of lymphoma. Patients with NHL who underwent non-TBI–conditioning HSCT had a 3-fold increased rate of disease progression compared with patients who received TBI. Patients with HL had an increased rate of recurrence compared with the risk in NHL patients receiving TBI. However, among HL patients, we failed to detect any effect of TBI on recurrence. Patients who had active disease before allo-HSCT also had an increased rate of progression compared with patients who underwent transplantation while in remission. Using patients in complete remission as the reference group, the RR for progression ranged from 1.38 for patients with chemosensitive relapse to 2.90 for patients with chemotherapy-refractory relapse and 3.45 for patients with untreated relapse.
Multivariate analysis of progression
Variables . | RR of progression (95% CI) . | P . |
|---|---|---|
| Disease type and use of TBI | .002† | |
| NHL with TBI, n = 37 | 1.00 | - |
| NHL no TBI, n = 42 | 3.02 (1.71-5.32) | < .001 |
| HL with TBI, n = 8* | 2.18 (0.86-5.52) | .10 |
| HL no TBI, n = 27 | 2.15 (1.14-4.05) | .02 |
| Disease status before second transplantation | < .001† | |
| Complete remission, n = 24 | 1.00 | - |
| Relapse/primary induction failure, chemosensitive, n = 39 | 1.38 (0.77-2.46) | .28 |
| Relapse/primary induction failure, chemoresistant, n = 23 | 2.90 (1.38-6.08) | .005 |
| Relapse/primary induction failure, untreated, n = 28 | 3.45 (1.74-6.87) | < .001 |
Variables . | RR of progression (95% CI) . | P . |
|---|---|---|
| Disease type and use of TBI | .002† | |
| NHL with TBI, n = 37 | 1.00 | - |
| NHL no TBI, n = 42 | 3.02 (1.71-5.32) | < .001 |
| HL with TBI, n = 8* | 2.18 (0.86-5.52) | .10 |
| HL no TBI, n = 27 | 2.15 (1.14-4.05) | .02 |
| Disease status before second transplantation | < .001† | |
| Complete remission, n = 24 | 1.00 | - |
| Relapse/primary induction failure, chemosensitive, n = 39 | 1.38 (0.77-2.46) | .28 |
| Relapse/primary induction failure, chemoresistant, n = 23 | 2.90 (1.38-6.08) | .005 |
| Relapse/primary induction failure, untreated, n = 28 | 3.45 (1.74-6.87) | < .001 |
indicates not applicable
Pairwise comparison: HL with TBI versus HL with no TBI (P = .98)
3 degrees of freedom test
Table 4 shows the multivariate analysis of PFS (treatment failure, death, or progression). Patients with NHL who received non-TBI conditioning for allo-HSCT had an approximately 2.5-fold increased rate of treatment failure compared with patients who received TBI. Patients with HL had a higher rate of treatment failure than did patients with NHL who received TBI. However, among patients with HL, there was no association between TBI and treatment failure. Patients who had active disease before allo-HSCT also had an increased rate of treatment failure compared with patients who underwent transplantation in remission. Using patients in complete remission as the reference group, the relative risk for treatment failure ranged from 1.74 for patients with chemosensitive relapse to 3.91 for patients with chemotherapy refractory relapse and to 3.68 for patients with untreated relapse. Patients who received transplants from haploidentical siblings or from unrelated donors had a higher rate of treatment failure than those who received transplants from HLA-identical siblings.
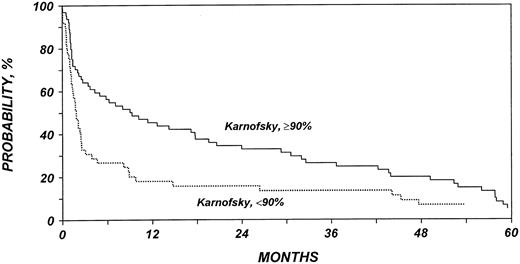
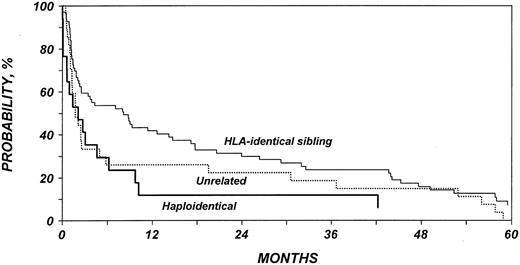
Table 5 shows the multivariate analysis of overall mortality. Patients with NHL who received non-TBI conditioning for allo-HSCT had a 2-fold increased mortality rate compared with patients who received TBI. The mortality rate for patients with HL was similar to that of patients with NHL receiving TBI. Among patients with HL, we failed to detect any association between TBI and mortality rate. Patients with low Karnofsky performance scores (80%) at the time of allo-HSCT died at an approximately 2-fold increased rate. Patients who had active disease before allo-HSCT also had a higher mortality rate than did patients who underwent transplantation while in remission. Finally, the mortality rate was higher in patients who received transplants from haploidentical siblings or from unrelated donors than in patients who received transplants from HLA-identical siblings. Figures 3, 4, 5, and 6 show probabilities of OS according to the identified risk factors.
Probability of survival after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL, according to Karnofsky performance score at allo-HSCT.
Probability of survival after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL, according to Karnofsky performance score at allo-HSCT.
Probability of survival after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL according to disease status at allo-HSCT.
Probability of survival after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL according to disease status at allo-HSCT.
Probability of survival after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL according to type of donor.
Probability of survival after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL according to type of donor.
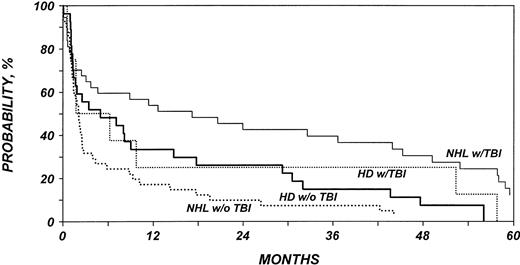
Probability of survival after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL according to disease type and use of TBI as part of conditioning regimen. NHL with TBI versus NHL with no TBI (P = .01). HL with TBI versus HL with no TBI (P = .47).
Probability of survival after allo-HSCT in patients who experience relapse after auto-HSCT for HL or NHL according to disease type and use of TBI as part of conditioning regimen. NHL with TBI versus NHL with no TBI (P = .01). HL with TBI versus HL with no TBI (P = .47).
In summary, multivariate analyses showed that TBI was associated with a decreased rate of disease progression and higher rates of PFS and OS in patients with NHL, but no such effect was shown in a smaller group of patients with HL. Chemotherapy responsiveness was also associated with decreased rate of disease progression and higher PFS and OS rates. Transplants from donors other than HLA-matched siblings were associated with higher TRM and lower PFS and OS rates, and good performance status was associated with higher OS rate.
Discussion
Recurrence occurs relatively frequently after auto-HSCT for lymphoma, and managing patients who experience relapse is difficult. Allo-HSCT is increasingly used because it has the potential advantages of a graft-versus-tumor effect and a tumor-free graft. Despite increasing use, reports describing the results of allo-HSCT are few and include small numbers of patients.8,12-17 Identifying patient- or disease-related prognostic variables in a large group of patients undergoing allo-HSCT after failure of auto-HSCT was the major purpose of this analysis. We excluded patients who underwent nonmyeloablative therapy because this technique was introduced recently and the follow-up of patients receiving nonmyeloablative regimens has been short. Diverse lymphoma histology findings were included in this analysis, but most patients had HL or aggressive NHL.
The TRM rate was relatively low (22%) and suggests that with current supportive care, allo-HSCT is feasible for this group of patients. This finding is in apparent contrast to results of several previous studies. Tsai et al12 report 85% TRM among 14 patients. Radich et al13 summarize the Seattle experience and report 78% TRM among patients with lymphoma at 2 years after second transplantation. Bierman et al15 report 16 patients with a TRM rate of 44% at 100 days. Reports of other small studies also demonstrate high TRM rates.14,17 Possible explanations for the low TRM rate in our report compared with previous ones include changes in patient selection over time and improvements in supportive care over the past decade. All patients included in our analysis underwent transplantation after 1990, and half underwent transplantation between 1997 and 1999. The reports by Tsai et al12 and by Radich et al13 include considerable numbers of patients who underwent transplantation before 1990, when growth factor support, prevention and management of opportunistic cytomegalovirus (CMV) infections, and donor selection strategies based on high-resolution HLA typing were less optimal. In addition, 15 of 18 patients reported by Radich et al13 had active disease at the time of transplantation, and 7 had HLA-mismatched or unrelated donors, features that in our current study are associated with high risk for TRM. Three of the 14 transplantations in the series by Tsai et al12 also involved donors other than HLA-matched siblings. By contrast, in the small series by de Lima et al,8 most patients had chemotherapy-responsive disease and HLA-matched sibling donors. Three of 5 patients in that series obtained durable remissions. Our data are also in agreement with those of Seropian et al,19 who recently reported a 55% PFS rate among 11 patients undergoing allo-HSCT after previous auto-HSCT failed. The comparatively low TRM in our report may also be partly explained by methodological differences from other studies, such as the use of cumulative incidence rather than Kaplan-Meier estimates.
Although TRM was within acceptable limits for allo-HSCT, there was a high rate of disease progression in this cohort. The risk for progression was 45% at 1 year after transplantation and 52% at 3 years, with a continued risk for relapse beyond 3 years. At 5 years, almost all survivors experienced lymphoma recurrence or progression, suggesting that allogeneic transplantation is rarely curative in patients for whom autologous transplantation fails. Similarly, in the study by Radich et al,13 the 4 patients who did not die of treatment-related causes experienced relapse. In the report by Tsai et al,12 only 2 of 14 patients were alive and in remission 2 years after the second transplantation, and de Lima et al8 report only 3 of 8 patients were alive and disease free at 25, 22, and 7 months after transplantation. In the report by Bierman et al,15 the OS and disease-free survival rates were estimated to be 42% at 2 years. In their experience, longer intervals between autologous and allogeneic transplantation were associated with better outcomes, a finding that was not replicated in our analysis.
Although not curative, prolonged survival was observed in a substantial proportion of patients in this study. The study then provided a unique opportunity to determine the effects of donor selection, disease type, performance score, disease stage, conditioning regimen, and potential effect of graft-versus-tumor effects and disease histology on transplantation outcome, specifically on the duration of allotransplantation-induced remissions and survival.
Not surprisingly, recipients of transplants from HLA-identical siblings had better outcomes than those with unrelated donors or related haploidentical donors, and poor performance status was associated with worse outcome. Patients with NHL who received TBI for conditioning had only one third the rate of disease progression of patients who did not receive TBI. This unexpected role of TBI in prolonging remission is consistent with our previous observations in follicular lymphoma20 and with recent data in autologous transplantation.21,22 On the other hand, it remains possible that TBI conditioning represents a surrogate for other undetected prognostic variables. No effect of TBI on recurrence rates was observed among patients with HL. The latter data should be interpreted with caution because the number of HL patients receiving TBI was small. Undergoing allo-HSCT while in remission was also associated with a decreased rate of disease progression. Among patients who underwent transplantation with active disease, the rate of disease progression was lower if the disease was chemosensitive. This is consistent with multiple previous observations in studies of allogeneic or autologous transplantation.2,23
Clinical observations of patients undergoing myeloablative and nonmyeloablative allo-HSCT suggest that the graft-versus-tumor effects may be important in inducing prolonged remission in patients with lymphoma24-27 and that it constitutes one of the rationales for considering allogeneic transplantation. Nevertheless, we were unable to clearly demonstrate a graft-versus-lymphoma effect of GVHD in this study. The rate of progression in patients with acute or chronic GVHD was similar to that in patients without GVHD. Several previous registry analyses, including a recent study of first transplantation for follicular lymphoma20 and an analysis of syngeneic transplantation,28 have similarly failed to demonstrate such an effect. However, given that only 30% of patients acquired GVHD, even our relatively large study (and other registry analyses) might not have been large enough to detect a significant graft-versus-lymphoma effect. Additionally, the lack of benefit from graft-versus-tumor effect in this group of patients at very high risk does not rule out that such effects might be operative in patients at standard risk.
We also did not observe any differences in overall outcomes among patients with low-, intermediate-, and high-grade NHL. The outcome for patients with HL was worse than that for patients with NHL receiving TBI for most outcome variables, but it was similar to that for patients with NHL receiving chemotherapy conditioning. It was previously reported that the outcome of allogeneic transplantation depends on disease histology,29 and we have shown that relapse rates after transplantation for low-grade lymphoma in particular are low.23 The failure to observe an effect of disease histology in the current study is likely attributable to the fact that study patients had disease that was resistant to numerous previous therapies, including high-dose chemotherapy, and, therefore, regardless of histology, was high risk. The small sample sizes of the subgroups of patients whose disease was of indolent histology might also have contributed to the lack of discernible histology-related effects.
The OS rate of patients included in this study appears to be superior to the reported OS rate of patients treated with conventional therapy after failed autologous transplantation. Vose et al4 reviewed the outcomes of 169 patients at the University of Nebraska who had malignant lymphoma and experienced relapse after auto-HSCT. With a median follow-up of only 1 year, 18 (11%) of these 169 patients remained alive, off therapy, and without evidence of disease progression. By contrast, in our study, 33% of patients were projected to be alive at 3 years after allo-HSCT, and 25% were projected to be alive and free of disease. This is impressive given the fact that only one fifth of the patients in our study were in remission at the time of allo-HSCT and that almost half had Karnofsky scores lower than 90%. Additionally, nearly 40% of patients received grafts from unrelated or HLA-mismatched sibling donors.
In summary, it is apparent from this study that with modern supportive care, selected young patients with lymphoma who experience relapse after autologous transplantation may benefit from allogeneic transplantation, though few will ultimately be cured. Patients who continue to exhibit disease responsiveness and who have HLA identical siblings have relatively good 3-year survival. Conditioning with TBI should be considered for patients with NHL because it seems to be associated with prolonged duration of remission. In contrast, patients with refractory disease and poor performance status scores and who lack HLA-identical sibling donors have poorer outcomes. Our observations of the impact of TBI in NHL patients and the lack of detectable GVL effects suggest that nonmyeloablative transplantation, with its emphasis on dose reduction, may not constitute an optimal long-term strategy for these patients. Recent reports using nonmyeloablative allo-HSCT after auto-HSCT demonstrate the feasibility of this approach,30-33 but longer follow-up is necessary to demonstrate its effectiveness in achieving long-term disease control.
Prepublished online as Blood First Edition Paper, July 27, 2004; DOI 10.1182/blood-2004-01-0231.
Supported by Public Health Service grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; and by grants from Allianz Life/Life Trac; American Cancer Society; American Red Cross; American Society of Clinical Oncology; Amgen, Inc; Anonymous; Aventis Pharmaceuticals; Baxter Healthcare Corp; Baxter Oncology; Berlex Oncology; Blue Cross and Blue Shield Association; The Lynde and Harry Bradley Foundation; Bristol Myers Squibb Oncology; Cedarlane Laboratories Ltd; Cell Pathways; CelMed Biosciences; Centocor, Inc; Cubist Pharmaceuticals; Darwin Medical Communications, Ltd; Dynal Biotech ASA; Edwards Lifesciences RMI; Endo Pharmaceuticals, Inc; Enzon Pharmaceuticals, Inc; Excess, Inc; Fujisawa Healthcare, Inc; Gambro BCT, Inc; GlaxoSmithKline, Inc; Human Genome Sciences; ICN Pharmaceuticals, Inc; ILEX Oncology; The Kettering Family Foundation; Kirin Brewery Company; Ligand Pharmaceuticals, Inc; Eli Lilly and Company; Nada and Herbert P. Mahler Charities; Merck & Company; Millennium Pharmaceuticals; Miller Pharmacal Group; Milliman USA, Inc; Miltenyi Biotec; Irving I. Moskowitz Foundation; National Marrow Donor Program; NeoRx; Novartis Pharmaceuticals, Inc; Novo Nordisk Pharmaceuticals; Orphan Medical, Inc; Ortho Biotech, Inc; Osiris Therapeutics, Inc; PacifiCare Health Systems; Pall Medical; Pfizer US Pharmaceuticals; Pharmacia Corporation; Pharmametrics; Pharmion Corp; Protein Design Labs; Roche Laboratories; SangStat Medical; Schering AG; StemCyte, Inc; StemCell Technologies, Inc; Stemco Biomedical; StemSoft Software, Inc; SuperGen, Inc; Sysmex; THERAKOS, a Johnson & Johnson Company; Unicare Life & Health Insurance; University of Colorado Cord Blood Bank; ViaCell, Inc; ViaCor Biotechnologies; WB Saunders Mosby Churchill; and Zymogenetics, Inc.
Presented in part as an oral presentation at the 38th annual meeting of the American Society of Clinical Oncology, Orlando, FL, May 2002.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
A complete list of the members of the Lymphoma Working Committee of the International Bone Marrow Transplant Registry appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal