In paroxysmal nocturnal hemoglobinuria (PNH), an acquired mutation of the PIGA gene results in the absence of glycosylphosphatidylinositol (GPI)–anchored cell surface membrane proteins in affected hematopoietic cells. Absence of GPI-anchored proteins on erythrocytes is responsible for their increased sensitivity to complement-mediated lysis, resulting in hemolytic anemia. Cell-to-cell transfer of CD55 and CD59, 2 GPI-anchored proteins, by red cell microvesicles has been demonstrated in vitro, with retention of their function. Because red cell units stored for transfusion contain many erythrocyte microvesicles, transfused blood could potentially serve as a source of CD55 and CD59. We examined whether GPI-anchored proteins could be transferred in vivo to deficient cells following transfusions given to 6 patients with PNH. All patients were group A1 blood type. Each was given transfusions of 3 U of compatible, washed group O blood. Patient group A1 cells were distinguished from the transfused group O cells by flow cytometry and staining with a labeled lectin, Dolichos biflorus, which specifically binds to group A1 erythrocytes. Increased surface CD59 was measured on recipient red cells and granulocytes 1, 3, and 7 days following transfusion in all 6 patients. Our data suggest a potential therapeutic role for GPI-anchored protein transfer for severe PNH.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a clonal bone marrow disorder resulting from an acquired, somatic mutation of the X chromosome PIGA gene in a hematopoietic stem cell.1-3 Absence of PIG-A function in a cell prevents synthesis of the glycosylphosphatidylinositol (GPI) moiety, which anchors many different types of proteins to the cell membrane.4,5 Intravascular red blood cell (RBC) destruction, the hallmark of the disorder, is caused by susceptibility of the abnormal erythrocyte to complement-mediated lysis; this sensitivity is due to lack of CD59, a potent inhibitor of the late components of complement and reactive lysis.6-8
Transfer of GPI-anchored proteins to deficient cells has been demonstrated in tissue culture and in a few animal models. Cell-to-cell transfer of CD59 from erythrocytes to endothelial cells occurred in mice made transgenic for this GPI-anchored protein,9 as well as of Thy-1 in chimeric murine embryoid bodies composed of normal and PIG-A “knock-out” cells.10 Others have demonstrated transfer of the variant surface glycoprotein (VSG) from trypanosomal membranes to the RBCs of parasitemic patients,11 transfer of CD59 and CD55 (a second GPI-anchored protein that also functions in complement resistance) from seminal fluid to prostasomes,12 and transfer of CD55 from high-density lipoproteins to deficient cells.13
Our laboratory has shown that RBC microvesicles, derived from outdated blood units during storage, can be a source of CD59 and CD55 and alter the susceptibility of RBCs to lysis.14 We hypothesized that transfusion of normal RBCs to patients with PNH might effect transfer of CD55 and CD59 to GPI-anchored protein-deficient cells in these patients. Should such transfer occur, transfusion could potentially increase cell surface CD55 and CD59 on patients'cells, decreasing their sensitivity to complement and slowing the rate of intravascular hemolysis.
Many GPI-anchored proteins can be easily detected on the cell surface by flow cytometry using fluorescent-labeled monoclonal antibodies. To test our idea, we required a further distinguishing assay for patient and donor erythrocytes, which was provided by Dolichos biflorus, a lectin specific for cells of group A1 blood that is routinely used in a fluorescein-isothiocyanate (FITC)–conjugated form to resolve some clinical problems in transfusion medicine.15 In this study, patients with PNH with blood group A1 were given transfusions with compatible RBCs of blood group O. Pretransfusion and posttransfusion samples were analyzed by flow cytometry for CD55 and CD59 expression on recipient cells identified by lectin labeling.
Patients, materials, and methods
Patient selection
Samples of venous blood were obtained and analyzed from 6 healthy volunteers, 6 patients with PNH who had not had transfusions for the previous 4 months, and 4 patients having many transfusions (non-PNH) receiving RBCs. Informed consent was obtained according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute. The diagnosis of PNH was based on clinical signs of intravascular hemolysis and flow cytometric evidence for deficient granulocyte expression of CD16 and CD66 by flow cytometry. All 6 patients with PNH were transfusion-dependent.
Blood transfusion
Blood was drawn from group O volunteer donors who met the standards of the American Association of Blood Banks (AABB) for donation. RBC units were stored in Adsol buffer and washed to remove plasma-containing complement. Six PNH patients with blood group A1 blood who required transfusion as judged by their treating physician were given 3 U of washed, compatible group O blood.
Sample preparation
Erythrocytes from patients were obtained before and after transfusion, washed 3 times with phosphate-buffered saline (PBS), adjusted to a concentration of 2 × 104/μL, and incubated in the dark and at room temperature with phycoerythrin (PE)–conjugated CD55- and CD59-specific monoclonal antibodies (mAbs; Monosan, Caltag, San Francisco, CA). Samples were then incubated for 30 minutes at 4°C with fluorescein isothiocyanate (FITC)–conjugated lectin, Dolichos biflorus (which binds specifically to RBCs of group A1; Sigma, St Louis, MO). For myeloid cells, unseparated whole blood was diluted and incubated with FITC-conjugated CD15 mAb (Becton Dickinson, San Jose, CA), which binds specifically to granulocytes, and then stained with either PE-conjugated CD55- or CD59-specific mAbs (Becton Dickinson). In a separate set of experiments, RBC-derived microvesicles reacting with antiglycophorin were examined and distinguished from intact erythrocytes on the basis of size and granularity in a flow cytometer and from cellular debris by use of specific fluorescent mAb binding (glycophorin). Binding of CD59 and CD-55 PE-conjugated mAb to RBCs or RBC-derived microvesicles was expressed as mean channel fluorescence (MCF) intensity or as a percentage of the particles binding mAb.
Flow cytometry
Samples were analyzed using an EPICS XL-MCL flow cytometer (Beckman Coulter, Miami, FL) equipped with a 15-MW air-cooled argon ion laser operating at 488 nm. Single-cell suspensions were first analyzed for size and granularity by forward and side scatter, using 488 laser light to establish an RBC gate. The FITC-conjugated lectin was measured by a fluorescent detector equipped with 525-BP filter while blocking the 488 laser light with a 488 blocking filter; the PE florescence was measured by the detector equipped with 575-nmBP filter. To assess binding of CD55 and CD59, the analysis was confined to lectin-positive cells (> 99%). PE- or FITC-conjugated mouse isotype-matched mAb served as controls (Becton Dickinson). Light scatter properties were used to establish a granulocyte gate when appropriate.
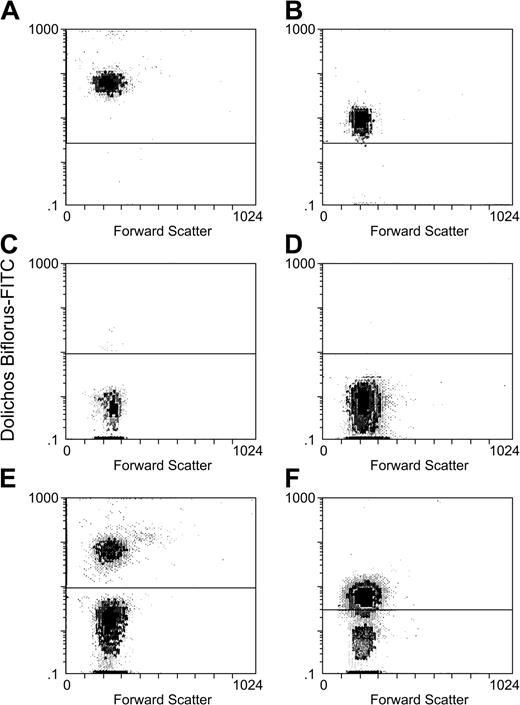
To determine if FITC–Dolichos biflorus distinguished group O and group A1 blood cells, we stained individual samples of patient and donor blood, mixed these cells in defined proportions, and performed flow cytometric analysis (Figure 1).
Group O and A1 RBCs can be clearly distinguished by Dolichos biflorus–FITC staining and flow cytometry. A sample of washed group A1 blood from patient 1 (A) was mixed with washed group O blood from donor 1 (C) in a ratio of 1:3 (patient 1 and donor 1 blood mixture is seen in panel E). A sample of patient 2 group A1 blood (B) was mixed with donor 2 group O blood (D) in a 1:1 ratio (mixture of patient group A1 blood and donor 2 group O blood is seen in panel F). All were stained with Dolichos biflorus–FITC and analyzed by flow cytometry. Above are scattergrams (E-F) showing that samples of the 2 blood groups can be clearly discriminated by flow cytometry.
Group O and A1 RBCs can be clearly distinguished by Dolichos biflorus–FITC staining and flow cytometry. A sample of washed group A1 blood from patient 1 (A) was mixed with washed group O blood from donor 1 (C) in a ratio of 1:3 (patient 1 and donor 1 blood mixture is seen in panel E). A sample of patient 2 group A1 blood (B) was mixed with donor 2 group O blood (D) in a 1:1 ratio (mixture of patient group A1 blood and donor 2 group O blood is seen in panel F). All were stained with Dolichos biflorus–FITC and analyzed by flow cytometry. Above are scattergrams (E-F) showing that samples of the 2 blood groups can be clearly discriminated by flow cytometry.
Multiple control experiments were performed to validate the reproducibility of the experiments. The optimal amount of anti-CD59 mAb used to stain a fixed number of RBCs was determined by titration; subsequently an amount in excess was used to stain patient cells. Similar experiments were performed using labeled lectin, but it was not possible to add an excess of the reagent to the RBC mixture because it produced agglutination. Even though we were cautious to avoid agglutination, RBC gates were drawn very tightly, based on side scatter/forward scatter to exclude clumped cells. The number of group O cells staining with lectin was always less than 0.5%; however, the number of group A1 cells that did not stain with the lectin varied from 0% to 8% (because it was not possible to add excess lectin to ensure binding to all cells and because of differential expression of A1 antigen16 ). The small number of recipient A1 cells that failed to bind lectin was not considered significant for our study because lectin binding was used only to positively identify group A1 cells and to exclude group O cells.
Animal model
To verify that RBC microvesicles were responsible for transfer of CD59 to deficient RBCs, we transfused human RBCs and RBC microvesicles prepared from outdated group O erythrocyte units for transfusion into nonobese/severe combined immune deficiency (NOD/SCID) mice. Microvesicles were obtained from stored RBCs by sonication for 10 seconds at 4°C and then separated from intact erythrocytes by differential centrifugation, followed by 5 cycles of washing in PBS. Varying numbers of microvesicles or RBCs were transfused into 7 NOD/SCID mice; another 2 mice received RBCs only. We used flow cytometry and a CD59-specific mAb that did not cross-react with mouse cells to assess transfer of human CD59 to mouse cells; we positively identified mouse erythrocytes by staining with TER119 and CD24 and excluded human erythrocytes by staining with mAb to human glycophorin.
Statistics
Correlations between tests were measured using the Pearson coefficient of correlation or Fisher exact test where appropriate. The Fisher exact test was used to determine statistical significance.
Results
Transfusion of normal RBCs increase CD55 and CD59 on deficient erythrocytes from patients
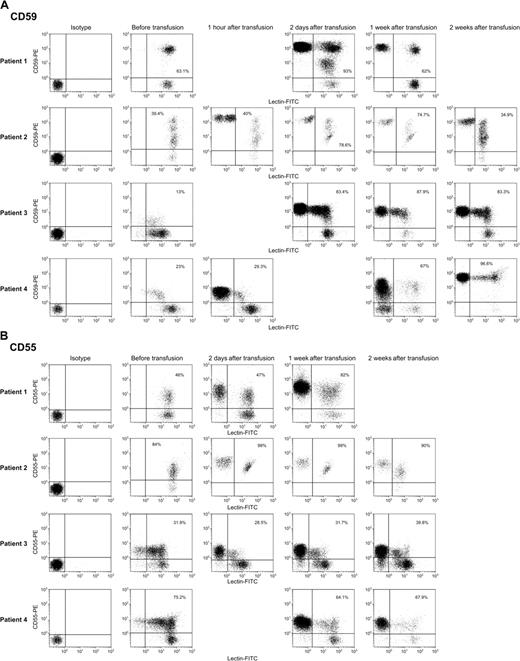
Six patients with PNH with episodic severe hemolysis and anemia required periodic RBC transfusion, but none had received blood during the 4 months previous to infusion of group O donor erythrocytes. By flow cytometry, donor and patient blood cells could be distinguished by staining with FITC-labeled Dolichos biflorus (Figure 1). Before transfusion, all circulating RBCs in the patients stained with FITC-labeled lectin, but following transfusion, a distinct lectin-negative population was readily observed. When evaluation gates were drawn to include only the labeled groupA1 (recipient) cells for further analysis, there were significant increases in the level of binding of mAbs specific for GPI-anchored proteins CD59 (and CD55 in some samples) as early as 1 to 2 days following transfusion (Figure 2A-B), but no significant change in staining was observed 1 hour after transfusion. The proportion of
CD59, and to a lesser extent CD55, are transferred to deficient RBCs in PNH patients following transfusion. Erythrocytes were washed as described in “Patients, materials, and methods” and stained with FITC-conjugated Dolichos biflorus and either PE-conjugated mAbs to CD55 or CD59. Above are scattergrams showing isotype controls stained with PE-IgG and staining of group A1 donor cells with PE-CD59 mAb (A) and PE-CD55 mAb (B) before transfusion and at varying intervals following transfusion. The transfused cells, which were lectin negative, appear in the posttransfusion samples, but transfer was not seen in the samples obtained 1 hour after transfusion. The percentages in the right upper quadrant of the scattergrams reflect lectin-positive cells (ie, group A1 patient cells), which are CD59+ or CD55+. Transfer of CD59 appeared to be more efficient than transfer of CD55.
CD59, and to a lesser extent CD55, are transferred to deficient RBCs in PNH patients following transfusion. Erythrocytes were washed as described in “Patients, materials, and methods” and stained with FITC-conjugated Dolichos biflorus and either PE-conjugated mAbs to CD55 or CD59. Above are scattergrams showing isotype controls stained with PE-IgG and staining of group A1 donor cells with PE-CD59 mAb (A) and PE-CD55 mAb (B) before transfusion and at varying intervals following transfusion. The transfused cells, which were lectin negative, appear in the posttransfusion samples, but transfer was not seen in the samples obtained 1 hour after transfusion. The percentages in the right upper quadrant of the scattergrams reflect lectin-positive cells (ie, group A1 patient cells), which are CD59+ or CD55+. Transfer of CD59 appeared to be more efficient than transfer of CD55.
CD55 and CD59 on PNH patient RBCs at intervals following transfusion
. | Start . | 1 h after transfusion . | 1-2 d after transfusion . | 1 wk after transfusion . | 2 wk after transfusion . |
|---|---|---|---|---|---|
| Patient 1 | |||||
| CD59, % | 62 ± 2.2 | ND | 92 ± 1.2 | 61 ± 2.2 | ND |
| CD55, % | 46 ± 0.4 | ND | 49 ± 2.4 | 82 ± 1.0 | ND |
| Hgb, g/dL | 5g/dL | 8.2 | 8.5 | 8.6 | ND |
| Reticulocytes/μL | 200 000 | ND | 222 000 | ND | ND |
| Group A1, % | 96 ± 0.6 | 55 ± 2.8 | 55 ± 1.4 | 68 ± 2.6 | ND |
| Patient 2 | |||||
| CD59, % | 39 ± 0.4 | 40 ± 1.0 | 79 ± 0.7 | 74 ± 0.2 | 35 ± 0.0 |
| CD55, % | 83 ± 1.5 | ND | 99 ± 0.0 | 99 ± 0.2 | 90 ± 0.0 |
| Hgb, g/dL | 6.3 | 10.0 | 10.2 | 9.8 | 10.6 |
| Reticulocytes/μL | 245 000 | ND | 176,000 | ND | ND |
| Group A1, % | 99 ± 0.4 | 54 ± 3.0 | 52 ± 1.4 | 52 ± 1.0 | 74 ± 4.3 |
| Patient 3 | |||||
| CD59, % | 15 ± 0.3 | 17 ± 1.2 | 83 ± 0.0 | 87 ± 0.7 | 83 ± 0.7 |
| CD55, % | 32 ± 0.7 | 26 ± 1.4 | 34 ± 2.8 | 31 ± 0.7 | 40 ± 0.8 |
| Hgb, g/dL | 8.9 | 12.2 | 12.5 | 12.3 | 12.0 |
| Reticulocytes/μL | 298 000 | ND | ND | ND | ND |
| Group A1, % | 90 ± 0.5 | 51 ± 2.5 | 57 ± 1.9 | 54 ± 2.2 | 58 ± 4.6 |
| Patient 4 | |||||
| CD59, % | 26 ± 3.8 | 25 ± 2.0 | ND | 67 ± 0.0 | 97 ± 0.5 |
| CD55, % | 73 ± 3.2 | 72 ± 1.5 | ND | 66 ± 2.3 | 67 ± 1.5 |
| Hgb, g/dL | 5.0 | 8.2 | ND | 8.8 | 9.0 |
| Reticulocytes/μL | 24 000 | ND | ND | ND | ND |
| Group A1, % | 99 ± 2.5 | 54 ± 2.0 | ND | 55 ± 5.4 | 59 ± 2.5 |
| Patient 5 | |||||
| CD59, % | 56 ± 0.2 | 52 ± 0.3 | 97 ± 1.0 | 83 ± 1.7 | 86 ± 0.0 |
| CD55, % | 32 ± 1.4 | 38 ± 0.5 | 41 ± 2.0 | 45 ± 0.3 | 42 ± 0.6 |
| Hgb, g/dL | 6.2 | 9.0 | 9.2 | 9.5 | ND |
| Reticulocytes/μL | 54 000 | ND | 24 000 | ND | ND |
| Group A1, % | 99 ± 0.0 | 62 ± 2.4 | 66 ± 2.5 | 63 ± 2.4 | 69 ± 1.2 |
| Patient 6* | |||||
| CD59, % | 26 ± 1.5 | 11 ± 0.6 | ND | 26 ± 1.4 | 53 ± 0.8 |
| CD55, % | 44 ± 5.0 | 42 ± 0.7 | ND | 42 ± 0.6 | 61 ± 0.6 |
| Hgb, g/dL | 6.5 | 9.4 | 10.0 | 10.1 | 9.8 |
| Reticulocytes/μL | 233 000 | ND | 150 000 | ND | ND |
| Group A1, % | 97 ± 5.8 | 41 ± 3.4 | ND | 60 ± 2.2 | 61 ± 5.2 |
. | Start . | 1 h after transfusion . | 1-2 d after transfusion . | 1 wk after transfusion . | 2 wk after transfusion . |
|---|---|---|---|---|---|
| Patient 1 | |||||
| CD59, % | 62 ± 2.2 | ND | 92 ± 1.2 | 61 ± 2.2 | ND |
| CD55, % | 46 ± 0.4 | ND | 49 ± 2.4 | 82 ± 1.0 | ND |
| Hgb, g/dL | 5g/dL | 8.2 | 8.5 | 8.6 | ND |
| Reticulocytes/μL | 200 000 | ND | 222 000 | ND | ND |
| Group A1, % | 96 ± 0.6 | 55 ± 2.8 | 55 ± 1.4 | 68 ± 2.6 | ND |
| Patient 2 | |||||
| CD59, % | 39 ± 0.4 | 40 ± 1.0 | 79 ± 0.7 | 74 ± 0.2 | 35 ± 0.0 |
| CD55, % | 83 ± 1.5 | ND | 99 ± 0.0 | 99 ± 0.2 | 90 ± 0.0 |
| Hgb, g/dL | 6.3 | 10.0 | 10.2 | 9.8 | 10.6 |
| Reticulocytes/μL | 245 000 | ND | 176,000 | ND | ND |
| Group A1, % | 99 ± 0.4 | 54 ± 3.0 | 52 ± 1.4 | 52 ± 1.0 | 74 ± 4.3 |
| Patient 3 | |||||
| CD59, % | 15 ± 0.3 | 17 ± 1.2 | 83 ± 0.0 | 87 ± 0.7 | 83 ± 0.7 |
| CD55, % | 32 ± 0.7 | 26 ± 1.4 | 34 ± 2.8 | 31 ± 0.7 | 40 ± 0.8 |
| Hgb, g/dL | 8.9 | 12.2 | 12.5 | 12.3 | 12.0 |
| Reticulocytes/μL | 298 000 | ND | ND | ND | ND |
| Group A1, % | 90 ± 0.5 | 51 ± 2.5 | 57 ± 1.9 | 54 ± 2.2 | 58 ± 4.6 |
| Patient 4 | |||||
| CD59, % | 26 ± 3.8 | 25 ± 2.0 | ND | 67 ± 0.0 | 97 ± 0.5 |
| CD55, % | 73 ± 3.2 | 72 ± 1.5 | ND | 66 ± 2.3 | 67 ± 1.5 |
| Hgb, g/dL | 5.0 | 8.2 | ND | 8.8 | 9.0 |
| Reticulocytes/μL | 24 000 | ND | ND | ND | ND |
| Group A1, % | 99 ± 2.5 | 54 ± 2.0 | ND | 55 ± 5.4 | 59 ± 2.5 |
| Patient 5 | |||||
| CD59, % | 56 ± 0.2 | 52 ± 0.3 | 97 ± 1.0 | 83 ± 1.7 | 86 ± 0.0 |
| CD55, % | 32 ± 1.4 | 38 ± 0.5 | 41 ± 2.0 | 45 ± 0.3 | 42 ± 0.6 |
| Hgb, g/dL | 6.2 | 9.0 | 9.2 | 9.5 | ND |
| Reticulocytes/μL | 54 000 | ND | 24 000 | ND | ND |
| Group A1, % | 99 ± 0.0 | 62 ± 2.4 | 66 ± 2.5 | 63 ± 2.4 | 69 ± 1.2 |
| Patient 6* | |||||
| CD59, % | 26 ± 1.5 | 11 ± 0.6 | ND | 26 ± 1.4 | 53 ± 0.8 |
| CD55, % | 44 ± 5.0 | 42 ± 0.7 | ND | 42 ± 0.6 | 61 ± 0.6 |
| Hgb, g/dL | 6.5 | 9.4 | 10.0 | 10.1 | 9.8 |
| Reticulocytes/μL | 233 000 | ND | 150 000 | ND | ND |
| Group A1, % | 97 ± 5.8 | 41 ± 3.4 | ND | 60 ± 2.2 | 61 ± 5.2 |
Numbers for CD55, CD59, and A1 represent the mean and SD for at least 3 samples run at each time period.
ND indicates not determined; Hgb, hemoglobin.
Patient was also receiving corticosteroids for active hemolysis
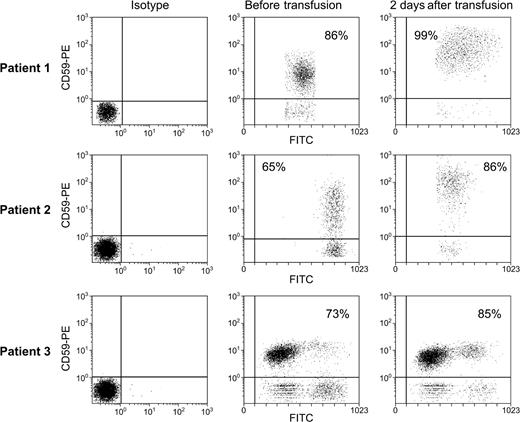
CD59 is transferred to patient granulocytes following transfusion. To exclude hemolysis as an explanation for the RBC results, we examined transfer of a GPI–anchored protein, CD59, to granulocytes. Whole blood was diluted as described in “Patients, materials, and methods” and stained with FITC-conjugated CD15-specific mAb and PE-CD59-specific mAb. Gates were drawn to include only cells staining with FITC (cells of group A1 blood). Above are scattergrams showing staining of patient granulocytes with PE-conjugated antibody to CD59.
CD59 is transferred to patient granulocytes following transfusion. To exclude hemolysis as an explanation for the RBC results, we examined transfer of a GPI–anchored protein, CD59, to granulocytes. Whole blood was diluted as described in “Patients, materials, and methods” and stained with FITC-conjugated CD15-specific mAb and PE-CD59-specific mAb. Gates were drawn to include only cells staining with FITC (cells of group A1 blood). Above are scattergrams showing staining of patient granulocytes with PE-conjugated antibody to CD59.
Transfused cells transfer CD59 to myeloid cells
Although selective hemolysis seemed an unlikely explanation for the increases in CD59 expression on PNH patient RBCs, we examined myeloid cells to rigorously exclude this possibility because granulocytes are not present in appreciable numbers in leukocyte-depleted RBC units used for transfusion (< 106/U blood). CD16, a GPI-anchored protein on granulocytes but absent from erythrocytes, showed no increases following transfusion, as expected (data not shown). However, granulocytes, defined flow cytometrically by staining with a mAb to CD15, showed increased CD59 following transfusion in all cases (Figure 3).
Transfused RBCs are rich in CD59-expressing microvesicles, as is blood from patients receiving transfusions chronically but not in healthy controls
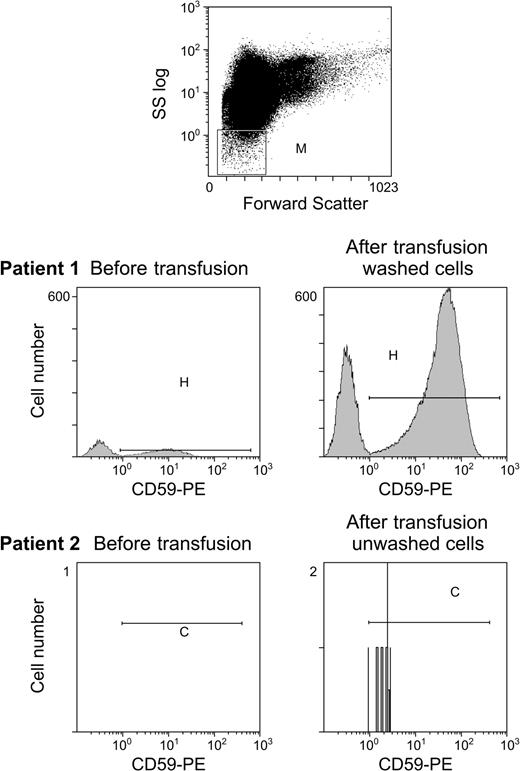
In vitro GPI-anchored protein transfer from microvesicles to deficient cells is more efficient than cell-to-cell transfer.14 Although microvesicles are relatively abundant in stored blood,17 where vesiculation is promoted by low pH, decreased adenosine triphosphate (ATP) levels, and the anticoagulant, they are infrequent in circulating blood14 where vesiculation primarily occurs extravascularly in the spleen.18 When we identified microvesicles by size and staining with antiglycophorin and anti-CD59 mAb and assessed microvesicle number in healthy nontransfused controls (n = 5), we found a mean of only 6 microvesicles/μL blood (data not shown). Patients receiving frequent transfusions (aplastic anemia patients without PNH who received an average of 2 U twice monthly) had a mean of 20-fold more microvesicles than did healthy controls and this number increased more than 2-fold at 1 hour after transfusion of unwashed blood. The patients with PNH described in this study who received washed blood showed a 10- to 100-fold increase in microvesicles the day following transfusion (n = 4; Figure 4).
RBC microvesicles increase following transfusion in patients receiving washed RBCs. Our previous data demonstrated that transfer from RBC microvesicles was much more effective than cell-to-cell transfer. We examined the number of circulating RBC microvesicles in healthy controls and following transfusion of washed and unwashed RBCs. Gating was performed to include a microvesicle population that was smaller than erythrocytes and staining with antiglycophorin (top panel). Although the number of RBC microvesicles increased following unwashed RBCs (patient 2), there was a marked increase following transfusion of washed RBCs (patient 1) where the number of microvesicles increased from 10 microvesicles/μL to 1000 microvesicles/μL.
RBC microvesicles increase following transfusion in patients receiving washed RBCs. Our previous data demonstrated that transfer from RBC microvesicles was much more effective than cell-to-cell transfer. We examined the number of circulating RBC microvesicles in healthy controls and following transfusion of washed and unwashed RBCs. Gating was performed to include a microvesicle population that was smaller than erythrocytes and staining with antiglycophorin (top panel). Although the number of RBC microvesicles increased following unwashed RBCs (patient 2), there was a marked increase following transfusion of washed RBCs (patient 1) where the number of microvesicles increased from 10 microvesicles/μL to 1000 microvesicles/μL.
We next assessed the number and GPI-anchored protein content of RBC microvesicles in the units of blood that were transfused and then examined microvesicles in stored blood following washing in unbuffered normal saline (a standard blood banking procedure). When RBC microvesicles were identified by size and antiglycophorin staining and stained with CD55- and CD59-specific mAbs, CD59 expression predominated (Figure 5A); washing blood resulted in a 2- to 4-fold increase in the number of microvesicles in the product (Figure 5B) with a 3-fold increase in CD59 staining of those microvesicles (n = 4). Figure 5C shows an electron micrograph of CD59, antiglycophorin-positive microvesicles purified by flow cytometry sorting.
Microvesicles from stored RBC units transfused to patients express primarily CD59 on their surface. To assess the GPI-anchored protein content of erythrocyte microvesicles, we examined aliquots from 3 U blood received by a patient. Gating was performed to include a microvesicle population that was smaller than erythrocytes and staining with antiglycophorin. (A) Scattergrams of microvesicles binding to antiglycophorin and anti-CD55 and anti-CD59 showing primarily expression of CD59. (B) Stored RBC units were washed 3 times per blood bank procedure in unbuffered saline and the number and composition of microvesicles assessed in the washed and unwashed preparations. More CD59-rich microvesicles were found in the washed preparation. Microvesicle number per 10 mL washed and nonwashed blood is seen above. (C) RBC microvesicles were sorted by flow cytometry on the basis of staining with anti–CD59mAb-PE and antiglycophorin-FITC. Electron microscopy was performed on the positive preparation at × 18 000 magnification using a Zeiss model Axioskop microscope, a Zeiss camera (model Mc80 DX), with a × 60 objective.
Microvesicles from stored RBC units transfused to patients express primarily CD59 on their surface. To assess the GPI-anchored protein content of erythrocyte microvesicles, we examined aliquots from 3 U blood received by a patient. Gating was performed to include a microvesicle population that was smaller than erythrocytes and staining with antiglycophorin. (A) Scattergrams of microvesicles binding to antiglycophorin and anti-CD55 and anti-CD59 showing primarily expression of CD59. (B) Stored RBC units were washed 3 times per blood bank procedure in unbuffered saline and the number and composition of microvesicles assessed in the washed and unwashed preparations. More CD59-rich microvesicles were found in the washed preparation. Microvesicle number per 10 mL washed and nonwashed blood is seen above. (C) RBC microvesicles were sorted by flow cytometry on the basis of staining with anti–CD59mAb-PE and antiglycophorin-FITC. Electron microscopy was performed on the positive preparation at × 18 000 magnification using a Zeiss model Axioskop microscope, a Zeiss camera (model Mc80 DX), with a × 60 objective.
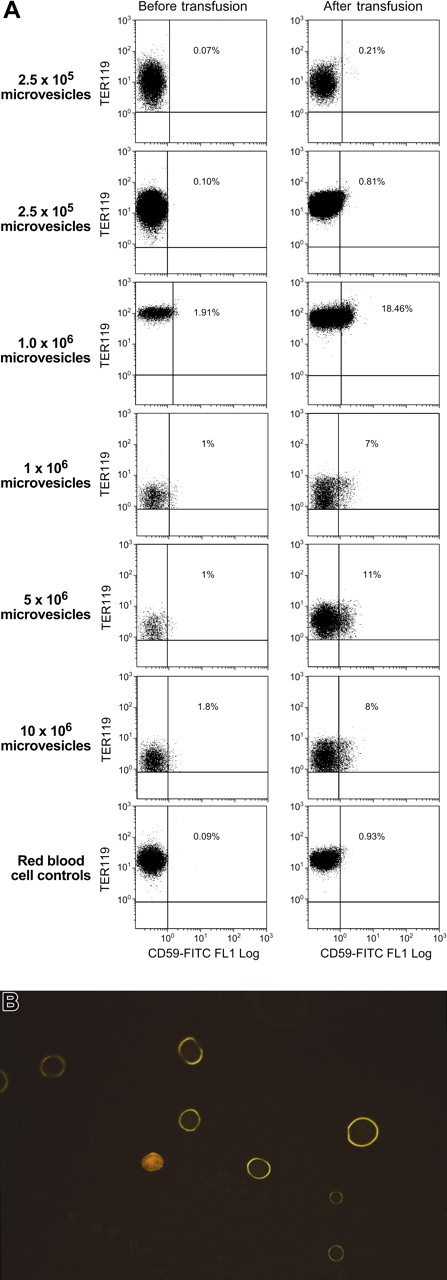
Transfusion with RBC microvesicles from outdated RBC units is associated with transfer of human CD59 to mouse RBCs in vivo
Six NOD/SCID mice were given transfusions with a mixture of microvesicles derived from outdated units of human blood; another 2 mice were given transfusions of human RBCs. In this model we used flow cytometry and CD59-specific mAbs, which did not cross-react with mouse cells, to assess transfer of human CD59 to mouse cells; we positively identified mouse erythrocytes by staining with TER119 and CD24 and excluded human erythrocytes by staining with mAb to human glycophorin. Small but consistent increases in staining with CD59 mAb were seen in all 6 mice, whereas no significant change in CD59 binding was seen in mice given intact RBCs. The extent of increase in CD59 was roughly proportional to the number of microvesicles transfused. Data from representative mice are seen in Figure 6, which shows scattergrams of TER119-PE+ mouse cells staining with human CD59. When double-positive (TER119-PE/CD59-FITC) mouse cells were sorted and examined by fluorescent light microscopy (× 60), mouse RBCs showed homogeneous membrane staining under the FITC filter.
Transfusion of human RBC microvesicles to mice results in transfer of CD59. Six NOD/SCID mice were administered transfusions of a mixture of microvesicles derived from outdated units of human blood. An additional 2 received RBCs. CD59 mAbs that did not cross-react with mouse cells was used to stain TER119 and CD24+ mouse erythrocytes. Data from 7 representative mice are seen above (A). When double-positive–stained (TER119-PE/CD59-FITC) mouse cells were sorted and looked at using a fluorescent light microscope (× 60) mouse RBCs showed homogeneous membrane staining under the FITC filter, suggesting uniform transfer (B).
Transfusion of human RBC microvesicles to mice results in transfer of CD59. Six NOD/SCID mice were administered transfusions of a mixture of microvesicles derived from outdated units of human blood. An additional 2 received RBCs. CD59 mAbs that did not cross-react with mouse cells was used to stain TER119 and CD24+ mouse erythrocytes. Data from 7 representative mice are seen above (A). When double-positive–stained (TER119-PE/CD59-FITC) mouse cells were sorted and looked at using a fluorescent light microscope (× 60) mouse RBCs showed homogeneous membrane staining under the FITC filter, suggesting uniform transfer (B).
Discussion
Cell-to-cell transfer of functionally active GPI-anchored protein has been reported in a variety of in vitro systems. Transferred protein appears to be stable and biologically functional.6,10,12,19,20 For example, transfer of CD55 and CD59 to RBCs confers resistance to complement-mediated lysis in the Ham test.14 For effective transfer to occur, both the GPI anchor and the protein moiety must be intact.21 Transferred molecules are inserted into the outer leaflet of the plasma membrane by the acyl/alkyl chains on the GPI moiety. GPI-anchored molecules are clustered in membrane microdomains that resist extraction by detergents.22 These GPI-anchored proteins actively take part in membrane vesicle formation, resulting in vesicles rich in GPI-anchored protein.22,23 Some laboratory evidence suggests that direct cell-to-cell transfer may neither occur nor be required; GPI-anchored proteins may be initially transferred from vesicles or liposomes originating from the donor cell.24 Our previously published data were consistent with these reports because we could demonstrate little transfer between normal and abnormal cells after short incubation times in vitro, whereas significant transfer was observed when deficient cells were incubated with microvesicles obtained by sonication of normal erythrocytes.14 The absence of direct cell-to-cell transfer and the relative rarity of naturally occurring circulating microvesicles25 may explain the lack of significant in vivo transfer of GPI-anchored proteins between patients' normal cells and the PNH clone and the persistence of a clear phenotypically defined population of deficient cells. RBCs stored for transfusion readily form microvesicles, which may facilitate transfer of GPI-anchored proteins following transfusion.26-29
In this study, we observed 2 GPI-anchored proteins, CD55 and CD59, to be increased on recipient erythrocytes and granulocytes after blood transfusion. Significant transfer could be demonstrated as early as 1 day following transfusion and persisted several days. Increased concentrations of CD59 on patient granulocytes, but no transfer of a GPI-anchored protein (CD16) absent from RBCs, suggest that these findings were unlikely to be a result of selective hemolysis or destruction of recipient cells. Furthermore, the proportion of patient group A1 blood remained constant from 1 hour to 1 day and 1 week following transfusion, and the calculated amount of hemolysis required to explain observed increases in CD59 expression would have had to be large and likely to be obvious. The absence of similar CD55 increases in many patients also argues against hemolysis.
Extrapolation of our observations to clinical practice should be undertaken with caution. In principle, if in vivo transfer, like in vitro transfer, confers functional activity on recipient cells, a program of regular transfusion might decrease hemolysis through partial correction of the PNH cell membrane defect in complement inactivation. However, even a reduction in the frequency or volume of transfusion might be counterbalanced by transfusional iron overload if intravascular RBC destruction and iron loss were entirely avoided. Hemolysis is frequently associated with a variety of symptoms that do not correlate with the level of anemia, prominently disproportionate fatigue, and also dyspnea and impotence, which might improve with transfusion. Eculizumab,30 a humanized antibody that inhibits the activation of terminal complement components, may potentially be useful in inhibiting hemolysis in these patients in the future and clinical trials will soon be underway.
Of potentially greater impact than hemolysis is the striking thrombotic proclivity of PNH.31 Venous thrombosis at unusual sites is a major cause of morbidity and mortality in PNH,32 and this complication is often refractory to conventional anticoagulation therapies and an indication for allogeneic stem cell transplantation.33 The mechanism of thrombophilia in PNH is unknown, but if protein transfer were effective in correcting GPI-anchored protein deficiencies on platelets34 or if erythrocyte lytic products were themselves thrombogenic,35,36 regular transfusion could be prophylactic. Washed cells, historically administered in PNH to remove complement,37 might provide RBC microvesicles more effectively and require further study.
Prepublished online as Blood First Edition Paper August 10, 2004; DOI 10.1182/blood-2004-02-0645.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal