The possible role of physiologic stress hormones in enhancing adhesion of sickle erythrocytes (SS RBCs) to endothelial cells (ECs) in sickle cell disease (SCD) has not been previously explored. We have now found that up-regulation of intracellular cyclic adenosine monophosphate (cAMP)–dependent protein kinase A (PKA) by epinephrine significantly increased sickle but not normal erythrocyte adhesion to both primary and immortalized ECs. Inhibition of serine/threonine phosphatases also enhanced sickle erythrocyte adhesion at least partially through a PKA-dependent mechanism. Adhesion was mediated through LW (intercellular adhesion molecule-4 [ICAM-4], CD242) blood group glycoprotein, and immunoprecipitation studies showed that LW on sickle but not on normal erythrocytes undergoes increased PKA-dependent serine phosphorylation as a result of activation. The major counter receptor for LW was identified as the αvβ3 integrin on ECs. These data suggest that adrenergic hormones such as epinephrine may initiate or exacerbate vaso-occlusion and thus contribute to the association of vaso-occlusive events with physiologic stress.
Introduction
In sickle cell disease (SCD), abnormal adherence of sickle erythrocytes (SS RBCs) to endothelial cells (ECs) has been postulated to be important in the initiation and/or progression of vaso-occlusive crises.1-3 Although many vaso-occlusive episodes are associated with intercurrent illnesses, and thus with release of endothelium-activating cytokines such as tumor necrosis factor α (TNF-α), other vaso-occlusive episodes are associated with other types of stressors. We are interested in exploring whether physiologic stress may precipitate vaso-occlusion via activation of red cell adhesion.
Human erythrocytes are equipped with a diversity of receptors and effectors that are known to mediate well-characterized signal transduction pathways in nonerythroid cells.4 Although activation of adhesion receptors via signal transduction is well accepted in nonerythroid cells, until recently, little attention had been paid to the possibility that abnormal SS RBC adhesion might be mediated via signaling mechanisms inducing activation of RBC adhesion receptors.5,6 Nevertheless, such events could promote, or even initiate, vaso-occlusion.
A variety of stimuli, including epinephrine, have been shown to activate the basal cell adhesion molecule-Lutheran (B-CAM/LU) laminin receptor on SS RBCs through a cyclic adenosine monophosphate (cAMP)–dependent protein kinase A (PKA) pathway.7 Epinephrine is a major stress mediator that elevates cAMP in SS RBCs through stimulation of adrenergic receptors.7 Cyclic AMP mediates at least some of its effects through activation of PKA. Based on these observations, we postulated that activation of SS RBC adhesion via known signaling pathways might lead to up-regulation of SS RBC adhesion to endothelium, even in the absence of plasma bridging proteins. Study of up-regulation of cAMP-dependent PKA signaling pathway by epinephrine in SS RBCs has now led to the identification of LW as the erythrocyte receptor for the endothelial integrin previously shown to be the most important in vaso-occlusion, αvβ3.8 Demonstration of the effect of epinephrine on adhesive function of SS RBCs further supports the hypothesis that physiologic stress may promote vaso-occlusion in SCD.
Materials and methods
Endothelial cells
Primary human umbilical vein endothelial cells (HUVECs) were grown as monolayers in Eagle basal medium 2 (EBM2; Clonetics, Walkersville, MD) supplemented with endothelial growth medium 2 (EGM2; Clonetics). The immortalized HUVEC line, EC-RF24,9 was obtained from H. Pannekoek (University of Amsterdam) and maintained in EBM (Clonetics) supplemented with EGM (Clonetics). EC-RF24 cells maintain key characteristics of HUVECs.10,11 Endothelial cell passage was accomplished with trypsinization, as required. For flow chamber experiments, ECs were cultured until they reached confluence on clear glass slides precoated with 2% gelatin. HUVECs were used to confirm all key results obtained with EC-RF24 cells.
Antibodies
Monoclonal antibodies (mAbs) used included 5E9 (antihuman transferrin receptor, ascitic fluid diluted 1:100, generously provided by Dr Barton F. Haynes, Duke University, Durham, NC)12 ; BS46 (anti-LW)13 ; MP30-1 (anti-CD47)14 ; 7E3 (anti-αvβ3 and αIIbβ3 integrins); and 10E5 (specific for αIIbβ3 integrin), generously provided by Dr Barry S. Coller, Rockefeller University, NY; LM609 (specific for αvβ3 integrin, generously provided by Dr David Cheresh, Scripps Institute, La Jolla, CA); 1951Z-20 (anti-β1 integrin; Chemicon International, Temecula, CA); and A3D8 (anti-CD44).15 Rabbit antihuman laminin was from Gibco BRL (Gaithersburg, MD). The murine myeloma protein P3x63/Ag8 (P3 ascitic fluid diluted 1:500) was used as a nonreactive control murine immunoglobulin (Ig) for mAbs.16 In all studies, mAbs were used at saturating dilutions unless otherwise indicated. Fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse Ig and FITC-conjugated goat anti–rabbit Ig were from Jackson Immunoresearch (West Grove, PA) and Sigma Immunochemicals (St Louis, MO), respectively.
Collection, preparation, and treatment of RBCs
Blood samples from patients homozygous for hemoglobin S and from healthy controls were collected into citrate tubes. All RBCs were washed in phosphate-buffered saline (PBS, pH 7.4), with removal of the plasma and buffy coat. Prior to adhesion assays, aliquots of cells were treated with various reagents to affect intracellular cAMP signaling or protein phosphorylation; sham-treated cells were incubated with the same buffer and vehicle, but without the active agent. Unless otherwise indicated, cells were treated for one hour at 37°C with one or more of the following: 0.2 mM phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX; Sigma), 176 μM N6, 2′-O-dibutyryladenosine 3′:5′-cyclic monophosphate (db-cAMP; Sigma), 176 μM 8-bromoadenosine 3′:5′-cyclic monophosphate (Br-cAMP; Sigma),7 80 μM forskolin (Sigma), 0.5 to 2 μg/mL Pertussis toxin (Calbiochem), 30 nM protein kinase A inhibitor 14-22 amide (PKAI; Calbiochem, La Jolla, CA), and 15 nM okadeic acid (serine/threonine protein phosphatases PP1 and PP2A inhibitor; Calbiochem). RBCs were also treated with 20 nM epinephrine for 1 minute at 37°C unless otherwise indicated. Normal RBCs were used as controls. Treated and sham-treated RBCs were labeled with PKH 26 red fluorescent cell linker kit (Sigma), following the manufacturer's instructions, before adhesion assays.
Flow chamber assays
Graduated height flow chambers were used to quantitate the adhesion of RBCs to ECs. Slides coated with ECs were washed with Hanks balanced saline solution (HBSS) with 1.26 mM Ca2+, 0.9 mM Mg2+ (Gibco, Grand Island, NY) warmed previously to 37°C and then fit into a variable height flow chamber, as previously described.17,18 The flow chamber was mounted on the stage of an inverted phase contrast microscope (Diaphot; Nikon, Melville, NY) connected to a thermoplate (Tokai Hit, Fujinomiya-shi, Japan) set at 37°C. Cells were observed using a video camera (RS Photometrics, Tucson, AZ) attached to the microscope and connected to a Macintosh G4 computer (Apple, Cupertino, CA). RBCs (3 mL) suspended at 0.2% (vol/vol) in HBSS with Ca2+, Mg2+ were infused into the flow chamber and allowed to adhere to the slide for 15 minutes without flow. Before exposure to flow, a minimum of 3 fields at each of 7 different locations along a line oriented normal to future flow were examined for the total number of fluorescent cells. Fluid flow (HBSS with Ca2+, Mg2+) was then started using a calibrated syringe pump. After exposure to flow, the fields were again examined and the number of adherent cells counted. The fraction of adherent cells was presented as follows: Number of cells attached after exposure to flow/Cells present per field before flow. The wall shear stress was calculated as follows: τw = (6μQ)/(wH [x]2), in which τw indicates wall shear stress (dyne/cm2); Q, volumetric flow rate (cm3/s); μ, media viscosity; w, width of the flow channel; and H(x), height of the flow chamber as a function of position along the microscope slide.17 Several investigators have shown that blood flow in small vessels may be continuous, with shear stresses of 1 to 2 dynes/cm2, or flow may be intermittent.19 Our data were obtained using intermittent flow conditions, although we have shown increased epinephrine-treated washed SS RBC adhesion to ECs during continuous flow as well.
Inhibition assays
Antibody inhibition assays were performed to identify the RBC adhesion receptor for ECs. SS RBCs were first incubated for 30 minutes with RBC-reactive IgG, washed, and then treated for 1 minute with 20 nM epinephrine, before additional washing and adhesion assays using HUVECs. SS RBCs were also treated with 0.2 mM IBMX in the presence of 176 μM db-cAMP for one hour at 37°C, washed with HBSS, and then incubated for an additional 30 minutes with 10 or 100 μg/mL RBC-reactive IgG and washed again before adhesion assays to EC-RF24 cells. Negative controls always included purified mAbs reactive with RBC surface structures rather than only nonreactive IgG, in order to control for a nonspecific inhibitory effect of RBC-bound IgG. For experiments using recombinant Fc proteins, HUVECs were washed with HBSS and then incubated for one hour at 37°C with 25 μg/mL recombinant LW-Fc20 or recombinant CD44-Fc21 before adhesion assays.
The ligand on ECs was identified by incubating HUVECs or EC-RF24 cells with 10 μg/mL 7E3, 10 μg/mL anti-β1 integrin, 10 μg/mL LM609, 10 μg/mL 10E5, 10 μg/mL A3D8, or 40 μg/mL rabbit anti–laminin IgG for one hour at 37°C, before adhesion assays.
To confirm the identity of the SS RBC receptor for αvβ3, EC-RF24 cells were activated with 10 ng/mL TNF-α for 4 hours, then incubated for one hour at 37°C with 25 μg/mL recombinant LW-Fc or CD44-Fc before testing EC interaction with nontreated SS RBCs. For antibody inhibition assays, nontreated SS RBCs were incubated for one hour with 10 μg/mL anti-LW or anti-CD44 IgG, prior to use in adhesion assays with TNF-α–treated EC-RF24 cells.
Reticulocyte enrichment and depletion
Separation of reticulocytes from mature SS RBCs was accomplished using antitransferrin receptor mAb 5E9 and goat anti–mouse IgG-coated microbead affinity columns (MACS; Miltenyi Biotec, Auburn, CA), following the manufacturer's instructions.
Density centrifugation of SS RBCs
SS RBCs were density-fractionated using a high potassium buffer and discontinuous arabinogalactan density separation medium on the same day of blood collection, according to the manufacturer's reticulocyte separation protocol (Cellsep; Larex, St Paul, MN).
Flow cytometric analysis
Transferrin receptor expression of RBC subpopulations, LW expression on high (mature SS RBCs) and low (reticulocytes) density cells, and surface antigen expression on both HUVECs and EC-RF24 cells were tested by flow cytometric analysis as previously described.18
32P erythrocyte labeling
Phospholabeling of RBC proteins was performed on packed normal and SS RBCs, as described by Brunati et al.22 To define whether the cytoplasmic serine of LW and other membrane proteins undergo phosphorylation, RBCs were incubated for 30 minutes with or without phosphatase cocktail inhibitor I (Sigma), specific for serine/threonine (Ser/Thr) protein phosphatases such as PP1 and PP2A, before being treated or sham-treated with forskolin. To further confirm that LW serine phosphorylation is PKA-dependent, cells were first incubated with 30 nM PKAI, followed by treatment with forskolin and phosphatase cocktail inhibitor I.
Anti-LW immunoprecipitation
Phospholabeled RBCs and nonlabeled forskolin-treated or sham-treated packed RBCs were incubated for 30 minutes at room temperature with anti-LW antibody or P3. Cells were washed with cold PBS and lysed for one hour at 4°C with lysis buffer (10 mM EDTA [ethylenediaminetetraacetic acid], 20 mM Tris [tris(hydroxymethyl)aminomethane],110 mM NaCl, pH 7.5) containing 2 mM phenylmethylsulphonylfluoride (PMSF), 1% Triton X-100, phosphatase cocktail inhibitor I, and protease inhibitor cocktail (Sigma). Immune complexes were obtained using protein A–Sepharose 4B (Amersham Biosciences, Piscataway, NJ). Immunoprecipitates were washed with lysis buffer, containing 0.1% Triton X-100, then resuspended in 50 μL nonreducing sodium dodecyl sulfate (SDS) sample buffer. For membrane protein phosphorylation, extensively washed treated and nontreated phospholabeled RBCs were lysed with ghosting buffer (5 mM Na2HPO4 + 0.1 M EDTA + 0.1% NaN3, pH 8). Immunoprecipitated proteins and equal amounts of total membrane proteins were separated by polyacrylamide gel electrophoresis before transfer to nitrocellulose membrane.23 LW protein was detected by immunoblotting using anti-LW antibody. Phosphorylation of LW and other membrane proteins was detected by PhosphorImager (Molecular Dynamics, Storm 840, Sunnyvale, CA) and quantified using ImageQuant Software version 1.2 (Amersham).
Results
Increased intracellular cAMP stimulates SS RBC adhesion through a PKA-dependent mechanism
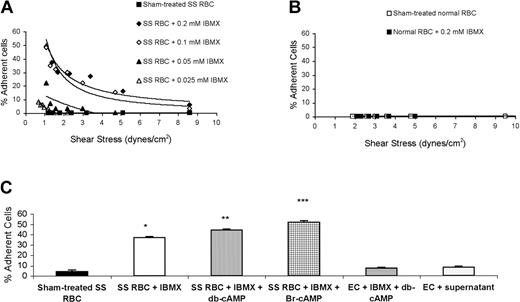
When phosphodiesterase is inhibited by IBMX, intracellular cAMP rises. Since epinephrine increases intracellular RBC cAMP,7 we first tested the effect of IBMX on SS RBC adhesion to EC-RF24 cells. Less than 5% of nontreated SS or normal RBCs were able to adhere to ECs (Figure 1A-B). IBMX increased SS RBC adhesion to nonactivated ECs in a dose-dependent fashion (Figure 1A). Treatment of SS RBCs with 0.2 mM IBMX alone or IBMX in the presence of db-cAMP or Br-cAMP induced 9-, 11-, and 13-fold increases in SS RBC adhesion over baseline, respectively (Figure 1C). However, IBMX did not increase normal RBC adhesion (Figure 1B).
Increased intracellular cAMP induces SS RBC adhesion to EC-RF24 cells. (A) SS RBCs were sham treated or treated with 0.025 to 0.2 mM IBMX and tested for adhesion to EC-RF24 cells at different shear stresses. (B) Sham-treated or IBMX-treated normal RBCs were tested for their ability to adhere to EC-RF24 cells. Each graph is representative of 2 different experiments. (C) SS RBCs were sham treated (n = 8) or treated with 0.2 mM IBMX (n = 5), 0.2 mM IBMX + 176 μM db-cAMP (n = 9), or 0.2 mM IBMX + 176 μM Br-cAMP (n = 9). As controls, EC-RF24 cells were incubated with 0.2 mM IBMX + 176 μM db-cAMP (n = 3) or with the supernatant obtained from the last wash of SS RBCs treated with 0.2 mM IBMX + 176 μM db-cAMP (n = 3). Adhesion was assayed at a shear stress of 2 dynes/cm2. Error bars in panel C show standard deviation (SD). *P < .0002 compared with sham-treated SS RBCs; **P < .002 compared with SS RBCs + IBMX; and ***P < .0002 compared with SS RBCs + IBMX.
Increased intracellular cAMP induces SS RBC adhesion to EC-RF24 cells. (A) SS RBCs were sham treated or treated with 0.025 to 0.2 mM IBMX and tested for adhesion to EC-RF24 cells at different shear stresses. (B) Sham-treated or IBMX-treated normal RBCs were tested for their ability to adhere to EC-RF24 cells. Each graph is representative of 2 different experiments. (C) SS RBCs were sham treated (n = 8) or treated with 0.2 mM IBMX (n = 5), 0.2 mM IBMX + 176 μM db-cAMP (n = 9), or 0.2 mM IBMX + 176 μM Br-cAMP (n = 9). As controls, EC-RF24 cells were incubated with 0.2 mM IBMX + 176 μM db-cAMP (n = 3) or with the supernatant obtained from the last wash of SS RBCs treated with 0.2 mM IBMX + 176 μM db-cAMP (n = 3). Adhesion was assayed at a shear stress of 2 dynes/cm2. Error bars in panel C show standard deviation (SD). *P < .0002 compared with sham-treated SS RBCs; **P < .002 compared with SS RBCs + IBMX; and ***P < .0002 compared with SS RBCs + IBMX.
The possible effect of residual IBMX and cAMP analogs on ECs was tested first by treating ECs with IBMX and db-cAMP, and secondly by incubating ECs with the supernatant obtained from the last wash of SS RBC treatment with IBMX and db-cAMP. Adhesion assays were then performed using nontreated SS RBCs. Exposure of ECs to db-cAMP and IBMX or to the supernatant from the last wash of SS RBC treatment with IBMX and db-cAMP only slightly increased adhesion of nontreated SS RBCs, compared with adhesion of nontreated SS RBCs to nontreated ECs (Figure 1C), demonstrating that IBMX and db-cAMP are not mediating significant effects through stimulation of ECs.
Pertussis toxin, which inhibits Gαi-mediated suppression of adenylyl cyclase, also significantly increased SS RBC adhesion in a dose-dependent manner (Figure 2A). Similarly, forskolin, an activator of adenylyl cyclase, up-regulated SS RBC adhesion (Figure 2B). An even greater increase was observed when SS RBCs were treated with forskolin and IBMX. These data confirm the ability of endogenous cAMP to modulate SS RBC adhesion.
Up-regulation of endogenous cAMP induces SS RBC adhesion to EC-RF24 cells through a PKA-dependent pathway. (A) SS RBCs were sham treated or treated with Pertussis toxin (0.5 μg/mL, black symbols; 1 μg/mL, white symbols; 2 μg/mL, gray symbols) and tested for adhesion at a shear stress of 2 dynes/cm2. All 3 patients were tested once for adhesion. SS RBCs from each patient were treated with the 3 different concentrations of Pertussis toxin. (B) Adhesion of sham-treated, forskolin-treated (80 μM), or forskolin + IBMX (0.2 mM)–treated SS RBCs was compared (n = 3). (C) Inhibition by PKAI of adhesion of SS RBCs activated with 0.2 mM IBMX + 176 μM db-cAMP was assayed at a shear stress of 2 dynes/cm2. Results are presented as percent inhibition of IBMX + db-cAMP–stimulated SS RBC adhesion (n = 3). Black bar: inhibitory effect of 30 nM PKAI present at the same time as stimulatory agents. White bar: effect of pretreatment of SS RBCs with 30 nM PKAI, followed by washing and treatment with IBMX and db-cAMP. (D) SS RBCs were sham treated or treated with 0.2 mM IBMX + 80 μM forskolin, 15 nM okadeic acid, 0.2 mM IBMX + 80 μM forskolin + 15 nM okadeic acid, or okadeic acid + PKAI (30 nM). Adhesion to EC-RF24 cells was assayed at a shear stress of 1 dyne/cm2. In panels B and C, error bars show SD.
Up-regulation of endogenous cAMP induces SS RBC adhesion to EC-RF24 cells through a PKA-dependent pathway. (A) SS RBCs were sham treated or treated with Pertussis toxin (0.5 μg/mL, black symbols; 1 μg/mL, white symbols; 2 μg/mL, gray symbols) and tested for adhesion at a shear stress of 2 dynes/cm2. All 3 patients were tested once for adhesion. SS RBCs from each patient were treated with the 3 different concentrations of Pertussis toxin. (B) Adhesion of sham-treated, forskolin-treated (80 μM), or forskolin + IBMX (0.2 mM)–treated SS RBCs was compared (n = 3). (C) Inhibition by PKAI of adhesion of SS RBCs activated with 0.2 mM IBMX + 176 μM db-cAMP was assayed at a shear stress of 2 dynes/cm2. Results are presented as percent inhibition of IBMX + db-cAMP–stimulated SS RBC adhesion (n = 3). Black bar: inhibitory effect of 30 nM PKAI present at the same time as stimulatory agents. White bar: effect of pretreatment of SS RBCs with 30 nM PKAI, followed by washing and treatment with IBMX and db-cAMP. (D) SS RBCs were sham treated or treated with 0.2 mM IBMX + 80 μM forskolin, 15 nM okadeic acid, 0.2 mM IBMX + 80 μM forskolin + 15 nM okadeic acid, or okadeic acid + PKAI (30 nM). Adhesion to EC-RF24 cells was assayed at a shear stress of 1 dyne/cm2. In panels B and C, error bars show SD.
PKA acts as a downstream effector of cAMP. Exposure of SS RBCs to IBMX and db-cAMP in combination with PKAI markedly blocked SS RBC adhesion by 82%. Slightly less inhibition (55% inhibition) of SS RBC adhesion was obtained when SS RBCs were first pretreated with PKAI, washed, and then activated with IBMX and db-cAMP (Figure 2C). These data show that cAMP induces SS RBC adhesion at least in part through PKA.
Since PKA phosphorylates serine/threonine residues often but not always contained in motifs fitting the consensus RXXS/T or RXS/T, we further tested whether SS RBC adhesion is increased by PKA-dependent Ser/Thr phosphorylation. Treatment of SS RBCs with okadeic acid, which enhances Ser/Thr phosphorylation, increased SS RBC adhesion approximately 10-fold, similar to the degree of SS RBC adhesion observed when SS RBCs were treated with forskolin and IBMX (Figure 2D). Okadeic acid in the presence of IBMX and forskolin caused an even greater increase in SS RBC adhesion. However, PKAI blocked SS RBC adhesion induced by okadeic acid by 69 ± 0.6% (Figure 2D), suggesting that PKA-dependent phosphorylation of Ser/Thr is one of the mechanisms necessary for increased SS RBC adhesion.
Epinephrine increases SS RBC adhesion to ECs
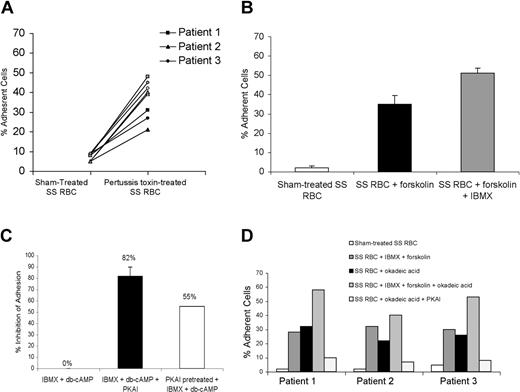
Vaso-occlusion is associated with various types of physiologic stress and epinephrine, a physiologic mediator of stress, elevates cAMP in SS RBCs through stimulation of adrenergic receptors.7 Epinephrine rapidly induced SS RBC adhesion to ECs after just 1 minute of incubation (Figure 3A-B). For all patient samples, SS RBC adhesion decreased with longer exposure of SS RBCs to epinephrine (Figure 3A). However, PKAI significantly reduced (83 ± 4.6% inhibition) epinephrine-stimulated SS RBC adhesion to HUVECs (Figure 3B).
Epinephrine modulates SS RBC adhesion to ECs. Adhesion of SS and normal RBCs to both EC-RF24 cells (A) and HUVECs (B-D) was tested, and results are presented as percent of cells adherent at a shear stress of 2 dynes/cm2. (A) SS RBCs were sham treated or stimulated with 20 nM epinephrine for 1 to 15 minutes. (B) SS RBCs were sham treated, activated with 20 nM epinephrine for 1 minute, or preincubated with 30 nM PKAI, washed, and then activated with 20 nM epinephrine for 1 minute prior to adhesion assays (n = 3). As a control, HUVECs were incubated with the supernatant obtained from the last wash of SS RBC treatment with 20 nM epinephrine for 1 minute (n = 3), washed, and then tested for their ability to support adhesion of nontreated SS RBCs. (C) Normal RBCs were sham treated or activated with 20 nM epinephrine (1 minute) and then used for adhesion assays (n = 3). (D) Separation of reticulocytes and mature RBCs was accomplished using antitransferrin receptor mAb 5E9 and goat anti–mouse IgG–coated magnetic microbeads as described in “Materials and methods.” Adhesion assays to HUVECs were then performed after incubation of unselected SS RBCs, mature SS RBCs, or SS reticulocytes with 20 nM epinephrine for 1 minute. In panels B-D, error bars show SD of 3 different experiments measuring adhesion at a shear stress of 2 dynes/cm2.
Epinephrine modulates SS RBC adhesion to ECs. Adhesion of SS and normal RBCs to both EC-RF24 cells (A) and HUVECs (B-D) was tested, and results are presented as percent of cells adherent at a shear stress of 2 dynes/cm2. (A) SS RBCs were sham treated or stimulated with 20 nM epinephrine for 1 to 15 minutes. (B) SS RBCs were sham treated, activated with 20 nM epinephrine for 1 minute, or preincubated with 30 nM PKAI, washed, and then activated with 20 nM epinephrine for 1 minute prior to adhesion assays (n = 3). As a control, HUVECs were incubated with the supernatant obtained from the last wash of SS RBC treatment with 20 nM epinephrine for 1 minute (n = 3), washed, and then tested for their ability to support adhesion of nontreated SS RBCs. (C) Normal RBCs were sham treated or activated with 20 nM epinephrine (1 minute) and then used for adhesion assays (n = 3). (D) Separation of reticulocytes and mature RBCs was accomplished using antitransferrin receptor mAb 5E9 and goat anti–mouse IgG–coated magnetic microbeads as described in “Materials and methods.” Adhesion assays to HUVECs were then performed after incubation of unselected SS RBCs, mature SS RBCs, or SS reticulocytes with 20 nM epinephrine for 1 minute. In panels B-D, error bars show SD of 3 different experiments measuring adhesion at a shear stress of 2 dynes/cm2.
To confirm that SS RBCs treated with epinephrine were thoroughly washed prior to exposure to ECs, the effect of residual epinephrine on ECs was tested by incubating ECs with the supernatant obtained from the last wash of SS RBC treatment with epinephrine. Exposure of HUVECs to the supernatant obtained from the last wash of SS RBC treatment with epinephrine had no effect on nontreated SS RBC adhesion to treated ECs, compared with nontreated SS RBC adhesion to nontreated ECs, demonstrating that epinephrine was not mediating its effects through stimulation of ECs (Figure 3B).
Epinephrine was also not able to induce a marked increase in adhesion of normal RBCs to HUVECs (Figure 3C), consistent with the known reduced basal and stimulated cAMP levels of normal RBCs.7 Taken together, these data suggest that epinephrine is capable of significantly increasing SS RBC but not normal RBC adhesion at least partially through a PKA-dependent pathway.
To determine if only the youngest cell population (reticulocytes) responded to this cAMP-dependent signaling pathway, reticulocyte-enriched and -depleted SS RBCs were analyzed for adhesion response to epinephrine. In each of 3 experiments, flow cytometric analysis of unseparated SS RBCs showed that up to 16% of SS RBCs expressed the transferrin receptor, a marker of reticulocytes. After separation, more than 90% of the reticulocyte-enriched cells expressed the transferrin receptor, while the reticulocyte-depleted population reacted with the antitransferrin receptor antibody no more strongly than with the negative control immunoglobulin.
Epinephrine similarly increased adhesion to HUVECs by all 3 SS RBC populations—reticulocyte enriched, reticulocyte depleted, and unseparated (Figure 3D). Epinephrine also similarly increased adhesion to EC-RF24 cells by all 3 SS RBC populations (data not shown). These data suggest that SS reticulocytes and mature SS RBCs have the signal transduction components required to activate adhesion in response to epinephrine.
Identification of LW as the activatable SS RBC receptor involved in adhesion to ECs
Because epinephrine caused adhesion to ECs of both reticulocytes and mature SS RBCs, we focused only on putative adhesion molecules expressed by mature RBCs. Antibody to LW (10 μg/mL), which binds to a variety of leukocyte β1 and β2 integrins as well as αvβ1,24,25 markedly inhibited SS RBC adhesion to EC-RF24 cells by 69 ± 1.2%, while antibody to the CD47 thrombospondin receptor (100 μg/mL) did not inhibit activated SS RBC adhesion (Figure 4A). A polyclonal antibody to B-CAM/LU that blocked SS RBC adhesion to laminin also failed to inhibit stimulated SS RBC adhesion to ECs, as did antibody to the CD44 hyaluronan receptor (data not shown). Similarly, anti-LW antibody (10 μg/mL) significantly inhibited epinephrine-activated SS RBC adhesion to HUVECs by 75 ± 7.2%, while anti-CD47 antibody (100 μg/mL) did not (Figure 4B).
Adhesion of activated SS RBCs to ECs is mediated through the LW receptor. Inhibition of adhesion with antibody and recombinant protein was performed as described in “Materials and methods,” using EC-RF24 (A) and HUVECs (B-C). For all experiments, SS RBC controls were sham treated as described in “Materials and methods.” Results are presented as percent of cells adherent at a shear stress of 2 dynes/cm2 (n = 3 for A-C). (A) SS RBCs activated with 0.2 mM IBMX + 176 μM db-cAMP were incubated without mAb, with 10 μg/mL anti-LW, or with 100 μg/mL anti-CD47 mAb IgG, and then washed before adhesion assays were performed. (B) SS RBCs were incubated without mAb, with 10 μg/mL anti-LW, or with 100 μg/mL anti-CD47 mAb, washed, treated with 20 nM epinephrine for 1 minute, and then washed before adhesion assays were performed. (C) Confluent cultures of HUVECs were incubated without recombinant protein or with 25 μg/mL recombinant LW-Fc or CD44-Fc protein, washed, and then tested for their ability to support adhesion of sham-treated SS RBCs or SS RBCs treated with 20 nM epinephrine for 1 minute. In panels A through C, error bars show SD.
Adhesion of activated SS RBCs to ECs is mediated through the LW receptor. Inhibition of adhesion with antibody and recombinant protein was performed as described in “Materials and methods,” using EC-RF24 (A) and HUVECs (B-C). For all experiments, SS RBC controls were sham treated as described in “Materials and methods.” Results are presented as percent of cells adherent at a shear stress of 2 dynes/cm2 (n = 3 for A-C). (A) SS RBCs activated with 0.2 mM IBMX + 176 μM db-cAMP were incubated without mAb, with 10 μg/mL anti-LW, or with 100 μg/mL anti-CD47 mAb IgG, and then washed before adhesion assays were performed. (B) SS RBCs were incubated without mAb, with 10 μg/mL anti-LW, or with 100 μg/mL anti-CD47 mAb, washed, treated with 20 nM epinephrine for 1 minute, and then washed before adhesion assays were performed. (C) Confluent cultures of HUVECs were incubated without recombinant protein or with 25 μg/mL recombinant LW-Fc or CD44-Fc protein, washed, and then tested for their ability to support adhesion of sham-treated SS RBCs or SS RBCs treated with 20 nM epinephrine for 1 minute. In panels A through C, error bars show SD.
Recombinant LW-Fc protein (25 μg/mL) also effectively inhibited epinephrine-activated SS RBC adhesion to HUVECs by 74 ± 3.2% (Figure 4C). In contrast, recombinant CD44-Fc protein failed to block adhesion. These results strongly argue that LW mediates epinephrine-activated SS RBC adhesion to ECs.
Since both reticulocytes and mature SS RBCs showed increased adhesion in response to epinephrine, and the activated receptor was identified as LW, we also analyzed the expression of LW on SS RBCs separated by density. After separation, 20% to 54% of cells in the low-density fraction were reticulocytes, while the high-density fraction contained less than 2% reticulocytes. Flow cytometric analysis on high- and low-density fractions separated from SS RBCs obtained from 5 different patients showed that high-density SS RBCs expressed 86 ± 13.2% as much LW as the low-density population. Thus, similar levels of LW expression may partly explain why both reticulocytes and mature SS RBCs can adhere to ECs in response to epinephrine.
LW serine phosphorylation on activated SS RBCs
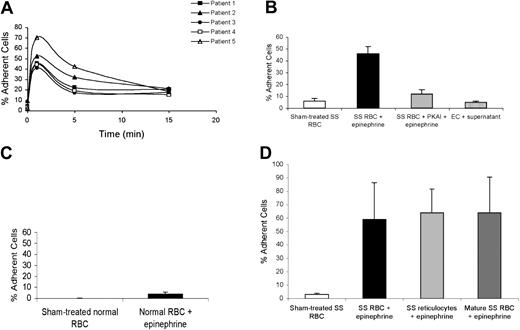
The LW protein possesses only one serine, one tyrosine, and no threonine within the 12 amino acids of its cytoplasmic tail; it does not contain a typical PKA target consensus motif. Since activation of SS RBC adhesion requires Ser/Thr phosphorylation at least partly through a PKA-dependent mechanism, we asked whether the cytoplasmic serine of the LW receptor undergoes PKA-dependent phosphorylation in response to stimulation. PhosphorImager analysis of immunoprecipitated 32P-radiolabeled LW and negative control immune complexes showed that LW of nonstimulated SS RBCs (Figure 5A, lane 1) is modestly phosphorylated. Inhibition of Ser/Thr protein phosphatases (Figure 5A, lane 2) resulted in a 2.9-fold increase in phosphorylation of LW, suggesting that increased LW phosphorylation is a result of serine phosphorylation, as tyrosine phosphatase inhibitors were not present.
LW undergoes serine phosphorylation. (A-B) Inorganic 32P-radiolabeled intact SS RBCs (A) and normal RBCs (B) were incubated in the absence (lanes 1,3) or presence (lanes 2,4) of Ser/Thr protein phosphatase inhibitors (SPIs), followed by treatment either without (lanes 1-2) or with 80 μM forskolin (lanes 3-4). (A, lane 5) SS RBCs were first incubated with 30 nM PKAI, followed by treatment with 80 μM forskolin and SPIs. The cpm shown are the cpm representative of 1 of 3 experiments, calculated by subtraction of cpm present in a lane (not shown) containing immunoprecipitate obtained using negative control P3 rather than anti-LW mAb from cpm obtained using anti-LW mAb for immunoprecipitation under each set of conditions indicated. (C-D) SS RBCs (C) and normal RBCs (D) were incubated without (lanes 1,3) or with (lanes 2,4) 80 μM forskolin. Lanes 3-4 were immunoprecipitated with anti-LW mAb, while lanes 1-2 were immunoprecipitated with the negative control immunoglobulin P3; all lanes for both panels C and D were then immunostained with anti-LW.
LW undergoes serine phosphorylation. (A-B) Inorganic 32P-radiolabeled intact SS RBCs (A) and normal RBCs (B) were incubated in the absence (lanes 1,3) or presence (lanes 2,4) of Ser/Thr protein phosphatase inhibitors (SPIs), followed by treatment either without (lanes 1-2) or with 80 μM forskolin (lanes 3-4). (A, lane 5) SS RBCs were first incubated with 30 nM PKAI, followed by treatment with 80 μM forskolin and SPIs. The cpm shown are the cpm representative of 1 of 3 experiments, calculated by subtraction of cpm present in a lane (not shown) containing immunoprecipitate obtained using negative control P3 rather than anti-LW mAb from cpm obtained using anti-LW mAb for immunoprecipitation under each set of conditions indicated. (C-D) SS RBCs (C) and normal RBCs (D) were incubated without (lanes 1,3) or with (lanes 2,4) 80 μM forskolin. Lanes 3-4 were immunoprecipitated with anti-LW mAb, while lanes 1-2 were immunoprecipitated with the negative control immunoglobulin P3; all lanes for both panels C and D were then immunostained with anti-LW.
Stimulation of SS RBCs with forskolin (Figure 5A, lane 3) also resulted in increased LW phosphorylation. When SS RBCs were treated with both serine protein phosphatase inhibitors (SPIs) and forskolin, a marked increase in LW phosphorylation was observed (Figure 5A, lane 4) over cells not treated with either SPI or forskolin (Figure 5A, lane 1) or treated with either alone (Figure 5A, lanes 2-3). However, PKAI completely blocked the ability of forskolin and SPIs to up-regulate LW phosphorylation (Figure 5A, lane 5).
LW receptor phosphorylation was also tested in normal RBCs. PhosphorImager analysis of immunoprecipitated LW showed that LW of normal RBCs was not significantly phosphorylated, even in the presence of SPIs or forskolin (Figure 5B, lanes 1-3). However, when cells were treated with both SPIs and forskolin, a very small degree of serine phosphorylation of LW, lower than that seen in unstimulated SS RBCs (Figure 5A, lane 1), was detectable (Figure 5B, lane 4).
Immunoblots of LW immunoprecipitates from forskolin-stimulated and nonstimulated SS RBCs (Figure 5C) and normal RBCs (Figure 5D) with anti-LW antibody indicated that a similar amount of LW was immunoprecipitated from both forskolin-stimulated and nonstimulated RBCs.
These results demonstrate that LW on stimulated SS RBCs undergoes a significant increase in serine phosphorylation due to up-regulation of an intracellular cAMP and PKA-dependent pathway. In contrast, LW on normal RBCs undergoes only minimal serine phosphorylation in response to the same reagents.
We also examined whether other proteins in SS RBCs showed elevated levels of phosphorylation after stimulation. Phosphor-Imager analysis of total membrane proteins showed that treatment of SS RBCs with both SPIs and forskolin induced 1.2- to 2.1-fold increases over baseline in phosphorylation of spectrins α and β, band 3, band 4.2, band 5, and band 6, suggesting that numerous membrane-associated proteins undergo phosphorylation as a result of PKA activation.
αvβ3 integrin is the EC ligand involved in the adhesion of SS RBCs
In order to define the molecule(s) on ECs involved in SS RBC adhesion, we first examined expression of adhesion molecules by HUVECs and EC-RF24 cells using flow cytometric analysis. As shown in Table 1, most EC-RF24 cells (more than 80% of the population) strongly expressed αvβ3, β1, CD44, and CD47; expression of these antigens by HUVECs was slightly lower. In contrast to HUVECs, which did not express laminin, about half of EC-RF24 cells reacted with rabbit antilaminin, suggesting that they produced a laminin isoform that was then bound to their surface. LW protein was not expressed on the surface of either HUVECs or EC-RF24 cells.
Target ligand expression on EC-RF24 cells and HUVECs by flow cytometry
Antibody (target) . | % Positive EC-RF24 cells (MFI) . | % Positive HUVECs (MFI) . |
|---|---|---|
| P3 (negative control) | 1 (6) | 1 (8) |
| 7E3 (αvβ3 + αIIbβ3) | 90 (117) | 53 (101) |
| LM609 (αvβ3) | 89 (112) | 48 (81) |
| 10E5 (αIIbβ3) | 2 (26) | 2 (12) |
| 1951Z-20 (β1 integrin) | 99 (119) | 63 (96) |
| A3D8 (CD44) | 81 (233) | 67 (316) |
| MP30-1 (CD4-7) | 99 (115) | 39 (51) |
| Rabbit antilaminin | 52 (56) | 7 (9) |
| BS46 (LW) | 1 (6) | 1 (21) |
Antibody (target) . | % Positive EC-RF24 cells (MFI) . | % Positive HUVECs (MFI) . |
|---|---|---|
| P3 (negative control) | 1 (6) | 1 (8) |
| 7E3 (αvβ3 + αIIbβ3) | 90 (117) | 53 (101) |
| LM609 (αvβ3) | 89 (112) | 48 (81) |
| 10E5 (αIIbβ3) | 2 (26) | 2 (12) |
| 1951Z-20 (β1 integrin) | 99 (119) | 63 (96) |
| A3D8 (CD44) | 81 (233) | 67 (316) |
| MP30-1 (CD4-7) | 99 (115) | 39 (51) |
| Rabbit antilaminin | 52 (56) | 7 (9) |
| BS46 (LW) | 1 (6) | 1 (21) |
Indirect immunofluorescence assays were performed on EC-RF24 cells and HUVECs. 7E3 (anti-αVβ3 and -αIIβ3), LM609 (anti-αvβ3), 10E5 (anti-αIIbβ3), 1951Z-20 (anti-β1), A3D8 (anti-CD44), MP30-1 (anti-CD47), and anti-LW mouse antihuman mAbs were used at 5 μg/mL. P3 (as a negative control) was used as ascitic fluid diluted 1:500. Rabbit antihuman laminin mAb was diluted 1:500. Percentage and mean fluorescence intensity (MFI) of positive cells are shown.
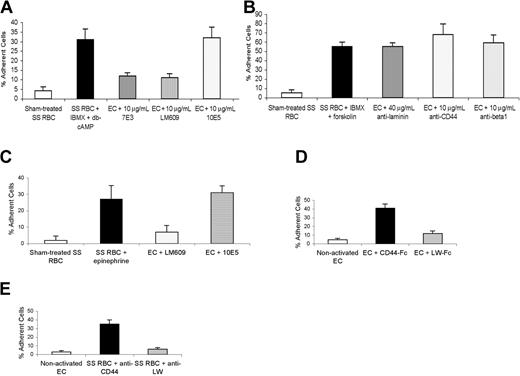
Based on the previous work of Kaul et al8 and data showing that LW bound to a variety of integrins,24,25 we hypothesized that LW on activated SS RBCs might recognize the αvβ3 integrin on ECs. Antibody-blocking assays showed that antibody to β1 integrin, as well as to CD44 and laminin, failed to inhibit SS RBC adhesion to EC-RF24 cells (Figure 6A-B). (In control experiments, not shown, the rabbit antilaminin efficiently blocked SS RBC adhesion to immobilized laminin.) In contrast, significant inhibition of adhesion was achieved with antibodies 7E3, which reacts with both αvβ3 and αIIbβ3 integrins, and LM609, a mAb specific for αvβ3, but not with 10E5, a mAb specific for αIIbβ3 (Figure 6A). Similarly, anti-αvβ3 but not anti-αIIbβ3 antibody blocked epinephrine-activated SS RBC adhesion to HUVECs by 77 ± 8% (Figure 6C). These data show that αvβ3 integrin is the major ligand on both primary and immortalized ECs involved in activated SS RBC adhesion and is thus the major counter receptor for LW.
Endothelial cell αvβ3 integrin is the ligand involved in adhesion of both activated and nonactivated SS RBCs. Adhesion of sham-treated and activated SS RBCs was assayed with and without incubation of nonactivated EC-RF24 cells (A-B) and HUVECs (C) with various mAbs. Panels D-E show adhesion of nontreated SS RBCs to activated EC-RF24 cells. Error bars show SD of 3 different experiments measuring adhesion at a shear stress of 2 dynes/cm2 (n = 3 for each of A-E). (A) EC-RF24 cells were incubated without or with 10 μg/mL anti-αvβ3 and αIIbβ3 integrin mAb 7E3, 10 μg/mL anti-αvβ3 integrin mAb LM609, or 10 μg/mL anti-αIIbβ3 integrin mAb 10E5, washed, and then tested for their ability to support adhesion of sham-treated SS RBCs or SS RBCs treated with 0.2 mM IBMX + 176 μM db-cAMP. (B) EC-RF24 cells were incubated without or with 40 μg/mL mAb, 10 μg/mL anti–laminin-10/11 mAb, anti-CD44 mAb, or anti-β1 integrin mAb, and then washed before being tested for their ability to support adhesion of sham-treated SS RBCs or SS RBCs treated with 0.2 mM IBMX + 80 μM forskolin. (C) HUVECs were incubated without mAb or with 10 μg/mL LM609 or 10E5, then washed before being tested for their ability to support adhesion of sham-treated SS RBCs or SS RBCs treated with 20 nM epinephrine for 1 minute. (D) EC-RF24 cells were first activated by exposure to 10 ng/mL TNF-α, incubated with 25 μg/mL recombinant CD44-Fc or LW-Fc protein, and then washed before being tested for their ability to support adhesion of nontreated SS RBCs at a shear stress of 2 dynes/cm2. (E) Nontreated SS RBCs were incubated with 10 μg/mL anti-CD44 or anti-LW mAb, washed, and then tested for adhesion to EC-RF24 cells activated with 10 ng/mL TNF-α.
Endothelial cell αvβ3 integrin is the ligand involved in adhesion of both activated and nonactivated SS RBCs. Adhesion of sham-treated and activated SS RBCs was assayed with and without incubation of nonactivated EC-RF24 cells (A-B) and HUVECs (C) with various mAbs. Panels D-E show adhesion of nontreated SS RBCs to activated EC-RF24 cells. Error bars show SD of 3 different experiments measuring adhesion at a shear stress of 2 dynes/cm2 (n = 3 for each of A-E). (A) EC-RF24 cells were incubated without or with 10 μg/mL anti-αvβ3 and αIIbβ3 integrin mAb 7E3, 10 μg/mL anti-αvβ3 integrin mAb LM609, or 10 μg/mL anti-αIIbβ3 integrin mAb 10E5, washed, and then tested for their ability to support adhesion of sham-treated SS RBCs or SS RBCs treated with 0.2 mM IBMX + 176 μM db-cAMP. (B) EC-RF24 cells were incubated without or with 40 μg/mL mAb, 10 μg/mL anti–laminin-10/11 mAb, anti-CD44 mAb, or anti-β1 integrin mAb, and then washed before being tested for their ability to support adhesion of sham-treated SS RBCs or SS RBCs treated with 0.2 mM IBMX + 80 μM forskolin. (C) HUVECs were incubated without mAb or with 10 μg/mL LM609 or 10E5, then washed before being tested for their ability to support adhesion of sham-treated SS RBCs or SS RBCs treated with 20 nM epinephrine for 1 minute. (D) EC-RF24 cells were first activated by exposure to 10 ng/mL TNF-α, incubated with 25 μg/mL recombinant CD44-Fc or LW-Fc protein, and then washed before being tested for their ability to support adhesion of nontreated SS RBCs at a shear stress of 2 dynes/cm2. (E) Nontreated SS RBCs were incubated with 10 μg/mL anti-CD44 or anti-LW mAb, washed, and then tested for adhesion to EC-RF24 cells activated with 10 ng/mL TNF-α.
Since Kaul et al8 have reported that human SS RBCs adhered to cytokine-stimulated postcapillary endothelium through the αvβ3 integrin in the absence of plasma, we asked whether LW on nonstimulated SS RBCs is also the receptor for the αvβ3 integrin on activated ECs. Both recombinant LW-Fc protein (Figure 6D) and anti-LW mAb (Figure 6E) completely blocked nonstimulated SS RBC adhesion to TNF-α–activated EC-RF24 cells, whereas recombinant CD44-Fc protein (Figure 6D) and anti-CD44 mAb (Figure 6E) did not. These data confirm that LW on SS RBCs is also the counter receptor for αvβ3 integrin on activated ECs. We conclude that SS RBC adhesion to ECs appears only to require activation either of LW (Figures 6A-C) or of the αvβ3 integrin through cytokines (Figures 6D-E).
Discussion
Abnormal adherence of SS RBCs to endothelium is believed to contribute to sickle cell vaso-occlusive events.26 We have now identified an SS RBC adhesion receptor (LW) that can be activated by epinephrine, at least partially via a cAMP-dependent PKA pathway, to mediate adhesion to ECs through at least one direct ligand, αvβ3 integrin. This work suggests new and potentially pathologic roles for both epinephrine and LW in SCD, since the LW counter receptor—the αvβ3 integrin—has already been shown to play a critical pathophysiologic role in vaso-occlusion.8 Our results also bring to light the ability of at least this member of the intercellular adhesion molecule (ICAM) protein family to undergo functional activation, as well as serine phosphorylation through a PKA-dependent pathway. We hypothesize that phosphorylation-induced changes in the LW cytoplasmic domain induce a conformational change in its extracellular domain. Thus, these data make LW a potential target against which one might be able to develop antiadhesive reagents that specifically bind to activated LW in order to prevent vaso-occlusion. In addition, the ability of stress hormones such as epinephrine to activate SS RBC adhesion warrants further study in animal models.
Characterization of the SS RBC adhesive response to epinephrine
It is particularly interesting that epinephrine was able to significantly increase adhesion to ECs of both reticulocytes and mature SS RBCs but not of normal RBCs. β adrenergic receptors were identified on normal mature RBCs several decades ago,27-29 and epinephrine was shown to decrease normal RBC filterability,29 suggesting that even mature normal RBCs have at least some intact signaling downstream of adrenergic receptors. However, normal RBCs, which have a much longer life span as well as perhaps lower LW expression30 compared with SS RBCs, showed minimally increased adhesion in response to epinephrine. This observation may be due to the previously described age-related loss of multiple protein kinase activities, including PKA, PKC, and casein kinases.31 Mature SS RBCs also show a blunted cAMP response to epinephrine compared with SS reticulocytes.7 Nevertheless, our data suggest that mature SS RBCs, unlike mature normal RBCs, have adequately conserved adenylyl cyclase and downstream signaling molecules to lead to activation of the LW adhesion receptor. We hypothesize that it is the overall younger age of SS RBCs that contributes to an increased SS RBC adhesive response to epinephrine compared with normal RBCs.
Prolonged exposure times to epinephrine induced decreased SS RBC adhesion. This seemingly paradoxical finding may be due to increasing desensitization with prolonged exposure to epinephrine via association of adrenergic receptors with Gαi protein.32 Moreover, different patients have variable adhesive responses to epinephrine (Figure 3A). This variability may be related to the well-described polymorphisms of adrenergic receptors,33,34 and this possibility is being actively investigated. Similar variability was also reported when the effect of epinephrine on B-CAM/LU-mediated adhesion to laminin was studied.7
LW is an activatable SS RBC receptor that mediates adhesion to ECs
We have demonstrated that LW on SS RBCs is activated by epinephrine to mediate adhesion to ECs. Importantly, as epinephrine has recently been shown to increase B-CAM/LU-mediated adhesion of SS RBCs to immobilized laminin,7 we have demonstrated that the adhesion of SS RBCs to ECs is not dependent on laminin, as both antilaminin (Figure 6B) and anti–B-CAM/LU (data not shown) polyclonal antibodies that block SS RBC adhesion to laminin failed to inhibit activated SS RBC adhesion to ECs. Thus, epinephrine appears to increase at least 2 adhesive characteristics of SS RBCs: adhesion to laminin mediated through B-CAM/LU and adhesion to a nonlaminin component of endothelial cells mediated through LW.
LW has previously been described to bind to leukocyte β1 and β2 integrins,24,25 as well as to αvβ1 and αvβ5 integrins.24 Thus, while some evidence had previously indicated that LW might bind to αv integrins activated by inflammatory cytokines and be involved in SS RBC adhesion to injured endothelial cells,35,36 we now show that LW binds to endothelial cell αvβ3. Recently, Hermand et al37 have also shown that LW on nonactivated normal RBCs interacts with the platelet fibrinogen receptor αIIbβ3 integrin (platelet glycoprotein IIb [GPIIb] to IIIa) following platelet receptor activation. Our data and Hermand et al's data37 strongly argue that LW interactions with both endothelial cells and platelets may have physiologic significance.
LW undergoes serine phosphorylation through a PKA-dependent pathway
Our data demonstrate that LW on SS RBCs is serine phosphorylated to some extent, which may reflect the ability of SS RBCs, in contrast to normal RBCs, to adhere to some degree to ECs in the absence of any activation. Increased intracellular cAMP in SS RBCs induces up-regulation of LW serine phosphorylation through a PKA-dependent mechanism, implying that modulation of LW-mediated SS RBC adhesion might be due at least in part to LW serine phosphorylation. In contrast, the minimal degree of LW phosphorylation observable in normal RBCs may be due to an age-related reduction in the cell's ability to produce cAMP and thereby up-regulate PKA activity in response to stimulation.7 Thus, weak phosphorylation may also explain the negligible adhesion of normal RBCs when stimulated with epinephrine (Figure 3C). Our results obtained with normal RBCs are in agreement with those of Porzig et al,38 who showed that response of erythroid precursors to adrenergic stimuli decreased during differentiation. Similarly, Baumann et al39 showed progressive loss of response to β-adrenergic receptor agonists and forskolin in embryonic RBCs during the transition from reticulocytes to mature erythrocytes. Our findings thus show for the first time that a member of the ICAM family can undergo both phosphorylation and adhesive activation. However, we do not yet know whether it is PKA or another downstream kinase that directly phosphorylates LW.
Moreover, several membrane-associated proteins showed increased phosphorylation after stimulation of SS RBCs. However, we do not know whether phosphorylation of these proteins also contributes to up-regulation of SS RBC adhesion.
Identification of αvβ3 integrin as the major EC ligand for LW
Our results strongly suggest that αvβ3 integrin on ECs is recognized by the LW receptor both when the receptor on SS RBCs is activated as well as when nonstimulated SS RBCs come into contact with activated ECs. LW-mediated SS RBC adhesion to αvβ3 integrin on activated ECs is probably due to alteration or up-regulation of integrin expression by TNF-α,40,41 which could promote interaction of LW with its endothelial ligand.35,36 Abnormally high expression of LW on SS RBCs could also partly account for their adhesion to activated ECs in this setting. Conversely, when SS RBCs are activated through adrenergic signaling, LW becomes phosphorylated. This may induce conformational changes in its binding site(s), leading to an increase in its affinity for αvβ3 integrin without requiring the latter also to be activated. Kaul et al8 showed that vaso-occlusion produced by SS RBCs in ex vivo models was prevented by the same anti-αvβ3 antibodies we have used. Spring et al24 have also suggested that soluble recombinant LW-Fc binds to nonhematopoietic cells through αv integrin(s), which they tentatively identified as αvβ1 and αvβ5 but not αvβ3.
In conclusion, LW appears to be the SS RBC receptor that mediates binding to at least one endothelial integrin, αvβ3. Furthermore, stress hormones, such as epinephrine, may contribute to vaso-occlusion by activating LW to mediate adhesion at least in part through cAMP/PKA-dependent signaling pathways. These data bring to light several novel features of LW as a member of the ICAM subfamily. Therefore, we speculate that LW may be a suitable target for future development of antiadhesive therapy in SCD.
Prepublished online as Blood First Edition Paper, August 12, 2004; DOI 10.1182/blood-2004-01-0042.
Supported by grants HL58939, HL63409, and HL070769 from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), and by a grant from the March of Dimes. R.Z. was supported by T32 HL07057 from NHLBI, NIH and DK065040 from the National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to acknowledge P. Bailly and P. Hermand (INSERM U76, Paris) for help in the production of LW reagents.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal