Abstract
The actin cytoskeleton plays a major role in platelet function. In contrast, its precise role in the function of megakaryocytes (MKs) is less understood but may be important for a chemoattractive response and an efficient proplatelet formation. In the marrow microenvironment, mature MKs are in contact with the extracellular matrix, including fibrillar collagen type I. MKs express α2β1 integrin and the immunoglobulin superfamily member glycoprotein VI (GPVI), the main receptors for collagen. Using function-blocking antibodies or specific ligands, we investigated in primary human MKs how α2β1 integrin and GPVI regulate stress fiber formation, the primary actin structures needed for cell contraction. Stress fiber assembly requires synergistic activation of the MAPK/Erk1/2 pathway and the small guanosine triphosphatase Rho via its effector, Rho-associated coiled-coil kinase (ROCK). α2β1 integrin is crucial for stress fiber formation, whereas GPVI triggers rapid and sustained activation of the Erk1/2 pathway. Strikingly, after a longer adhesion time, proplatelet formation was significantly inhibited by the engagement of α2β1 integrin, not by GPVI, likely through the Rho/ROCK pathway. Thus, proplatelet formation in human MKs could be tightly regulated by differential interactions with their collagen receptors. We propose that this interaction with collagen prevents proplatelet formation within the marrow.
Introduction
Blood platelets play a crucial role in hemostasis by their capacities for adhesion, activation, and aggregation, which are essentially mediated by receptors for the extracellular matrix (ECM). Fibrillar collagen type I, an important component of the bone marrow environment,1 is crucial for platelet adhesion and activation.2 The main receptors interacting directly with collagen I are the integrin α2β1 and glycoprotein VI (GPVI), a recently characterized member of the immunoglobulin (Ig) superfamily.3,4 Elegant studies emphasized the differential roles of collagen receptors α2β1 and GPVI in platelet aggregation. The “2-step” model initially depicted proposed that α2β1 integrin would be required for adhesion, whereas GPVI would be needed for activation processes. However, recent data attribute a pivotal role to GPVI in platelet activation and adhesion to collagen.2 Indeed, the initial interaction between collagen and platelets is mediated by GPVI, which leads to subsequent α2β1 activation by an “inside-out” signaling.5,6 A recent report has focused on the early times of collagen-platelet interaction pathways with respect to actin cytoskeleton reorganization and platelet shape changes.7
GPVI, whose expression is restricted to platelets and megakaryocytes (MKs), is synthesized early during MK differentiation, but becomes fully functional only in mature MKs.8 GPVI is noncovalently associated with the FcRγ chain, which serves to transduce similar signaling as in platelets. Surprisingly, the integrin α2β1, which displays a relatively ubiquitous expression, has a pattern of expression similar to GPVI during MK maturation.8 However, despite the expression of these 2 collagen receptors on MKs, interaction between collagen I and MKs, as well as its consequences on MK maturation, have been poorly studied. Hence, the respective roles of α2β1 integrin and GPVI on megakaryocytopoiesis are largely unknown. MK maturation leads to the formation of platelets by the fragmentation of the MK cytoplasm in the blood flow. There are presently 2 hypotheses: either the entire MK transmigrates and fragments in the circulation, or platelets are released from proplatelets, elongation of cytoplasmic processes that penetrate the bone marrow sinus. The MK cytoskeleton is critical for maturation, migration, elongation, and fragmentation into platelets.9-11 At this stage of differentiation, it is expected that the ECM may play an important role in regulation of proplatelet formation because it occurs when MKs exit from the bone marrow and lose their interaction with the bone marrow microenvironment. Thus, the ECM may both influence the migration of MKs and the formation of proplatelets.
A complex array of biochemical events is triggered upon ligation of adhesion receptors to ECM,12 such as activation of the Rho family of small guanosine triphosphatases (GTPases) Rho, Rac, and Cdc42, which play an essential role in adhesion, membrane protrusion, migration, and other cellular activities involving cytoskeleton reorganization.13,14 In fibroblasts, Rho activation leads to the assembly of actin stress fibers,15 long bundles that support actomyosin contractility involved in the translocation of the cell body and therefore migration.16 These structures converge at focal adhesions, multimolecular complexes where integrin clusters sustain mechanically firm attachment to the underlying matrix.17 One of the multiple downstream effectors of Rho GTPase is Rho-associated coiled-coil kinase (ROCK), a kinase recruited to the plasma membrane by active Rho, increasing thereby phosphorylation of myosin light chain, association of myosin with actin filaments, and stress fiber formation.18 Other signaling processes, such as activation of serine threonine kinases, the extracellular signal-regulated kinases (Erks), intermingle closely with the Rho GTPase cascade and regulate actin cytoskeleton reorganization.19-21 Activation of myosin light-chain kinase (MLCK), which is a common downstream target of Rho and Erk1/2 pathways, leads to phosphorylation of myosin light chains (MLCs) and cell contraction.22 Interestingly, the mitogen-activated protein kinase (MAPK)/Erk pathway also plays an important role in MK polyploidization and differentiation following thrombopoietin (TPO) stimulation.23
In this study, we analyzed the respective roles of α2β1 integrin and GPVI in collagen I–induced actin organization and proplatelet formation of primary human MKs derived from CD34+ cells. We show here that α2β1 integrin is critically required for stress fiber formation induced by collagen in primary human MKs. The Erk pathway and Rho, via its downstream effector ROCK, are required to induce optimal actin stress fiber and adhesion complex assembly, mainly through the engagement of α2β1 integrin. Furthermore, adherence of MKs to collagen markedly inhibited proplatelet formation, a mechanism that was essentially mediated through ligation of the α2β1 integrin and activation of the Rho/ROCK pathway.
Materials and methods
Antibodies and reagents
Fibrillar collagen type I (Horm) from equine tendons was purchased from Nycomed (Munich, Germany), and poly-l-lysine (PLL) was from Sigma (St Quentin, France). Convulxin (CVX) and TAT-C3 exotoxin (TAT peptide fused to botulinum toxin C3) were purified as previously described.24,25 The triple-helical GFOGER peptide (GPC[GPP]5GFOGER[GPP]5GPC) was synthesized as the carboxyl-terminal amide on TentaGel R RAM resin in an Applied Biosystems (Hertford, United Kingdom) Pioneer automated peptide synthesizer.26 A control TAT peptide was synthesized as described, with a scrambled part-sequence of GPVI tail attached (Fl-Tat VI-4-Scr). Y-27632, PD98059, and U0126 were purchased from Calbiochem (Meudon, France). The anti–human integrin α2 subunit monoclonal antibody (mAb) 6F127 was provided by Dr B. S. Coller (Mount Sinai Medical Center, New York, NY). The anti-GPVI mAb and Fab-derived fragments (clone 9O12.2) were recently characterized by Lecut et al.28 The murine mAb LJ-Ib129 was from Dr Z. Ruggeri (The Scripps Research Institute, La Jolla, CA). Antihuman vinculin mAb and tetramethyl rhodamine isothiocyanate (TRITC)–conjugated phalloidin were from Sigma, those for phospho-ERK1/2 (pThr202/pTyr204) and total Erk from Cell Signaling Technology (Beverly, MA), and rabbit anti–von Willebrand factor (VWF) antibody from Dakocytomation (Trappes, France). Secondary antibodies were fluorescein isothiocyanate (FITC) goat anti–mouse or anti–rabbit IgGs (Jackson Immunoresearch, West Grove, PA). The peroxidase-coupled sheep anti–mouse and anti–rabbit IgG, the chemiluminescent reagent ECL, and Hybond nitrocellulose membranes were from Amersham Biosciences Europe (Orsay, France). Protease inhibitor cocktail and Bio-Rad DC protein assay kit were purchased from Roche Diagnostics (Meylan, France) and Bio-Rad Laboratories (Hercules, CA), respectively.
In vitro growth of MKs from CD34+ cells
CD34+ cells were obtained either from the bone marrow of healthy patients undergoing hip surgery, with their informed consent, or from aliquots of cytapheresis from the peripheral blood of patients after mobilization. CD34+ cells were purified by positive immunomagnetic selection (Miltenyi, Bergisch Gladbach, Germany) and were grown as previously reported in the presence of TPO (10 ng/mL; Kirin Brewery, Tokyo, Japan) for 9 to 10 days in “serum-free” medium.30
Fluorescence microscopy
Fibrillar collagen type I (50 μg/mL) or CVX (15 μg/mL) was incubated on coverslips overnight at 4°C. GFOGER peptide or PLL (20 μg/mL) was incubated 1 hour at room temperature (RT). After washing, the coverslips were blocked with 1% fatty acid-free purified bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 1 hour at RT and washed with PBS.
Primary MKs grown in serum-free medium were preincubated with function-blocking antibodies directed against the α2β1 (6F1, 10 μg/mL) and GPVI (monovalent Fab, 20 μg/mL) or the isotype-matched controls using similar concentrations. Cells were then plated on collagen I in the presence of the blocking antibody. In other cases, cells were pretreated in suspension for 24 hours by TAT-C3 exotoxin or TAT-peptide (Fl-Tat VI-4-Scr; 25 μg/mL) or with the inhibitors Y-27632 (10 μM; for 1 hour), PD98059 or UO126 (20 μM, 30 minutes at 37°C), or dimethyl sulfoxide (DMSO; control). The pretreated cells were then seeded in serum-free medium on coated coverslips for the indicated times in the presence of the inhibitor. Cells were then fixed in 2% paraformaldehyde, permeabilized with 0.2% Triton X-100 for 5 minutes, incubated with antivinculin antibody or anti-VWF antibody, followed by incubation with rhodamine-labeled phalloidin and FITC-conjugated goat anti–mouse or anti–rabbit IgG for 1 hour. The number of cells exhibiting stress fibers and showing focal adhesion sites was counted in at least 300 to 350 adherent MKs in 3 independent sets of experiments.
Cells were examined under a Zeiss laser scanning microscope (LSM 510 Zeiss, Jena, Germany) using a 63 ×/1.4 NA apochromat plan objective (Carl Zeiss, Le Pecq, France). Both projection view and optical sections were processed digitally using CLSM5 Zeiss Browse Image software.
Retroviral infections
Human dominant-negative form of MEK1 cDNA was cloned upstream from the internal ribosome entry site (IRES) of the MSCV-IRES-GFP (MIGR) retrovirus. Retrovirus (MSCV-MEK1 DN-IRES-GFP) or control vector (MSCV-IRES-GFP) stocks were produced as previously described.31 Virus stocks containing 107 infectious particles per milliliter or more were used to infect cell populations isolated from donor marrow and cultured in presence of TPO. Adhesion and immunofluorescence experiments were performed as described (see “Fluorescence microscopy”).
Immunoblotting
Cells (5 × 105) were harvested after the indicated times, washed with PBS, and lysed in modified radioimmunoprecipitation (RIPA) buffer. Equal amounts of extracts were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. The proteins were electrotransferred to nitrocellulose membranes (Hybond; Amersham Biosciences) and blocked 2 hours at RT in PBS, 0.1% Tween, containing 5% nonfat dried milk. Blots were incubated with dually phosphorylated ERK-specific antibody (pErk1/2; Cell Signaling Technology) and the same filters were stripped, blocked, and incubated with polyclonal antibody specific for total Erk1/2 and detected with appropriate secondary antibodies conjugated with horseradish peroxidase. Filters were developed with enhanced chemiluminescence (ECL; Amersham Biosciences). Each figure panel shows results of ECL from the same filters with comparable exposure times.
Pull-down assay of GTP-loaded Rho
RhoA activity assay was performed using a Rho activation assay kit (Upstate Biotechnology, Souffelweyersheim, France). MKs either in suspension or plated onto collagen I-coated dishes for various times were rinsed with ice-cold triethanolamine-buffered saline (TBS) and then lysed by scraping. The lysates were clarified by centrifugation at 14 000g for 5 minutes at 4°C. Cleared lysates were processed as described by the manufacturer with the Rho activation kit. Total amount of Rho in cell lysates was also determined by immunoblotting in the same experiment.
Statistical analysis
The results are presented as mean ± SD. The data were analyzed with the 2-tailed Student t test.
Results
Adhesion to fibrillar collagen I induces formation of stress fibers and focal adhesions accompanied by Rho activation in primary human MKs
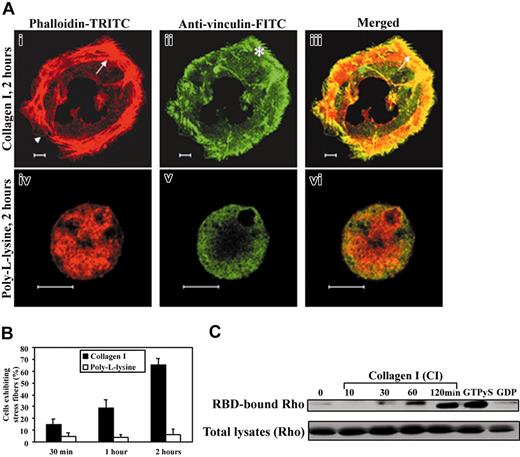
Addition of TPO to human CD34+ cells in a serum-free liquid culture induces their differentiation into MKs in 8 to 10 days.8,32 Expression of α2β1 and GPVI increases similarly, supporting MK adhesion to collagen I after 8 to 10 days of culture.8 To characterize the cytoskeletal changes induced by fibrillar collagen type I, primary human MKs cultured for 9 to 10 days were allowed to attach to collagen-coated coverslips and stained for F-actin and vinculin to visualize focal adhesions (Figure 1A). Within 2 hours, confocal analysis showed bundles of actin filaments (Figure 1Ai) anchored at the cell periphery in vinculin-containing focal adhesions (Figure 1Aii-iii). In contrast, MKs adherent to PLL substrate either displayed short filopodial projections or maintained a round shape with actin aggregates located close to the basal surface (Figure 1Aiv) and diffuse vinculin staining (Figure 1Av-vi). Thus, adhesion to collagen I for 2 hours induced stress fiber assembly in 65% ± 5% of MKs, whereas only a negligible proportion of MKs exhibited stress fibers on PLL substrate (Figure 1B).
Fibrillar collagen I induces stress fiber and focal adhesion assembly and Rho GTP activation in primary human MKs. (A) MKs were grown for 10 days in the presence of TPO. Cells were then plated onto collagen or PLL-coated coverslips in serum-free medium and incubated for 2 hours. Adherent cells were fixed, permeabilized, and stained with rhodamine-phalloidin (i,iv) and monoclonal antivinculin FITC antibody (ii,v), as described in “Materials and methods.” Photomicrographs were taken with a Zeiss Axiovert confocal microscope (× 60). The cells shown in each panel are representative of predominant morphologies observed in 4 separate experiments. Lamellipodium and stress fibers (i,iii) are observed at the periphery of spread cells (arrowhead and arrow, respectively). Vinculin-rich focal adhesions (indicated by an asterisk) colocalize with the extremities of stress fibers (iii, arrow) in the first basal section (0.8 μm). MKs adherent to PLL substrate display mainly actin aggregates (iv,vi) and diffuse vinculin staining (v,vi) without any substantial spreading. Bar equals 10 μm. (B) The graph illustrates the mean ± SD of adherent MKs showing stress fibers on collagen I or PLL substrates for the indicated times. At least 300 cells were counted in 8 to 10 different fields in 4 separate experiments. (C) Levels of GTP-loaded Rho in primary human MKs kept in suspension or allowed to attach to collagen I-coated dishes for the indicated times. GTP-loaded form of Rho was recovered by precipitation with GST-RBD beads. Non hydrolyzable form of GTP (GTPγS) and guanosine diphosphate (GDP) were loaded as positive and negative controls, respectively. The precipitates (top panel) and total lysates (bottom panel) were subjected to SDS-PAGE and immunoblotting with polyclonal anti-Rho antibody.
Fibrillar collagen I induces stress fiber and focal adhesion assembly and Rho GTP activation in primary human MKs. (A) MKs were grown for 10 days in the presence of TPO. Cells were then plated onto collagen or PLL-coated coverslips in serum-free medium and incubated for 2 hours. Adherent cells were fixed, permeabilized, and stained with rhodamine-phalloidin (i,iv) and monoclonal antivinculin FITC antibody (ii,v), as described in “Materials and methods.” Photomicrographs were taken with a Zeiss Axiovert confocal microscope (× 60). The cells shown in each panel are representative of predominant morphologies observed in 4 separate experiments. Lamellipodium and stress fibers (i,iii) are observed at the periphery of spread cells (arrowhead and arrow, respectively). Vinculin-rich focal adhesions (indicated by an asterisk) colocalize with the extremities of stress fibers (iii, arrow) in the first basal section (0.8 μm). MKs adherent to PLL substrate display mainly actin aggregates (iv,vi) and diffuse vinculin staining (v,vi) without any substantial spreading. Bar equals 10 μm. (B) The graph illustrates the mean ± SD of adherent MKs showing stress fibers on collagen I or PLL substrates for the indicated times. At least 300 cells were counted in 8 to 10 different fields in 4 separate experiments. (C) Levels of GTP-loaded Rho in primary human MKs kept in suspension or allowed to attach to collagen I-coated dishes for the indicated times. GTP-loaded form of Rho was recovered by precipitation with GST-RBD beads. Non hydrolyzable form of GTP (GTPγS) and guanosine diphosphate (GDP) were loaded as positive and negative controls, respectively. The precipitates (top panel) and total lysates (bottom panel) were subjected to SDS-PAGE and immunoblotting with polyclonal anti-Rho antibody.
It has been previously shown that reorganization of actin cytoskeleton into stress fibers is regulated by the small Rho GTPase.15 To investigate whether collagen I triggers Rho GTPase activation in primary human MKs, we performed pull-down experiments to evaluate the quantity of GTP-loaded Rho in suspended or adherent MKs to fibrillar collagen I for various times (Figure 1C). We used the GST-RBD (a fusion protein containing Rho-binding domain of Rhotekin), which selectively binds to the GTP-bound form of RhoA.33 Basal level of GTP-bound Rho was low in suspended MKs or those adherent for 10 or 30 minutes, whereas Rho GTP increased later 2 hours following adhesion to fibrillar collagen I (Figure 1C upper panel). The amount of total Rho in whole cell lysates did not vary between the conditions (Figure 1C lower panel).
Thus, during the initial phase of low Rho activation (30 minutes), a minority of cells exhibited stress fibers and focal adhesions (Figure 1B), and displayed mainly filopodia with small focal complexes. In contrast, higher cellular Rho activity induced by adhesion to collagen I for 2 hours accompanied cytoskeletal reorganization into stress fibers in a majority of MKs.
Interaction of α2β1 integrin and GPVI with collagen type I is required for optimal actin stress fiber and focal adhesion formation
To explore the respective roles of α2β1 integrin and GPVI in actin reorganization, we first pretreated MKs with the α2-blocking antibody 6F127 (10 μg/mL for 1 hour) or the anti-GPVI Fab fragments, 9O12.2 clone28 (20 μg/mL for 1 hour) before MK adhesion to collagen I.
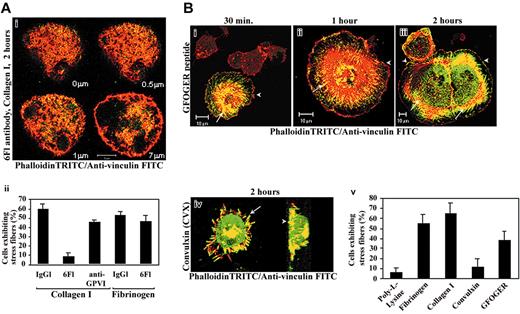
MKs treated with the isotype-matched control IgG1 and plated on collagen underwent similar changes as untreated MKs (not shown). Cells displayed important cell spreading, numerous stress fibers, and focal adhesions. Conversely, MKs pretreated by 6F1 blocking antibody retained a rounded morphology 2 hours after being plated on collagen. Stress fiber assembly and the size and number of vinculin-containing focal adhesions were greatly reduced in all sections (Figure 2Ai). Conversely, the number of cells showing stress fibers did not significantly decrease when MKs were pretreated with 6F1 and plated on fibrinogen (Figure 2Aii). The anti-GPVI Fab fragments had a moderate but reproducible effect on actin reorganization. In contrast to 6F1, which inhibited fiber stress formation in 85% of the MKs, Fab fragments against GPVI had similar effects in only 30% of MKs (Figure 2Aii). Indeed, 45% ± 3% of the MKs exhibited stress fibers after treatment with the anti-GPVI Fab fragments, versus 65% ± 5% in controls. Thus, α2β1 integrin plays a major role in stress fiber formation during MK adhesion to collagen.
Optimal stress fiber and focal adhesion assembly requires α2β1 integrin and GPVI ligation. (A) MKs preincubated with function-blocking antibody directed against α2β1 integrin (6F1; 10 μg/mL) were plated on collagen I in serum-free medium (i). Cells were allowed to attach and spread for 2 hours before fixation and staining for actin cytoskeleton (rhodamine-phalloidin, red) or vinculin (FITC antibody, green). Serial images were captured starting from the basal level of the adherent cell. Sections are indicated on the right bottom of each image. Note that stress fiber and focal adhesion formation is completely impaired by pretreatment with anti-α2β1 mAb in all examined sections of a representative cell (compare with Figure 1A). Bar equals 10 μm. In panel Aii, the data shown are the mean values ± SD of 300 cells showing stress fibers in 3 independent experiments after treatment by blocking antibodies α2β1 (6F1; 10 μg/mL), monovalent GPVI Fab (clone 9O12.2; 20 μg/mL), or the isotype-matched control (20 μg/mL) and adhesion to collagen I or fibrinogen matrices. (B) Time course of stress fiber and focal adhesion organization after attachment of primary human MKs to slides coated by GFOGER peptide (20 μg/mL), the specific substrate of α2β1 integrin. In each panel, a projection of representative horizontal section of cells stained by TRITC-phalloidin and vinculin (i-iii) showing lamellipodia (arrowheads) and stress fibers (arrows). In panel Biv, MKs were allowed to adhere for 2 hours on CVX. Horizontal confocal section of a projection gradually rotated by an angle of 5° shows the colocalization of actin (red and yellow) and vinculin (green and yellow) in filopodia (arrow) and the attachment plan (arrowhead) in a representative cell (x-y projection view). In panel Bv, the mean ± SD of 300 cells adherent to the indicated substrates for 2 hours and showing stress fibers in 3 independent experiments is shown.
Optimal stress fiber and focal adhesion assembly requires α2β1 integrin and GPVI ligation. (A) MKs preincubated with function-blocking antibody directed against α2β1 integrin (6F1; 10 μg/mL) were plated on collagen I in serum-free medium (i). Cells were allowed to attach and spread for 2 hours before fixation and staining for actin cytoskeleton (rhodamine-phalloidin, red) or vinculin (FITC antibody, green). Serial images were captured starting from the basal level of the adherent cell. Sections are indicated on the right bottom of each image. Note that stress fiber and focal adhesion formation is completely impaired by pretreatment with anti-α2β1 mAb in all examined sections of a representative cell (compare with Figure 1A). Bar equals 10 μm. In panel Aii, the data shown are the mean values ± SD of 300 cells showing stress fibers in 3 independent experiments after treatment by blocking antibodies α2β1 (6F1; 10 μg/mL), monovalent GPVI Fab (clone 9O12.2; 20 μg/mL), or the isotype-matched control (20 μg/mL) and adhesion to collagen I or fibrinogen matrices. (B) Time course of stress fiber and focal adhesion organization after attachment of primary human MKs to slides coated by GFOGER peptide (20 μg/mL), the specific substrate of α2β1 integrin. In each panel, a projection of representative horizontal section of cells stained by TRITC-phalloidin and vinculin (i-iii) showing lamellipodia (arrowheads) and stress fibers (arrows). In panel Biv, MKs were allowed to adhere for 2 hours on CVX. Horizontal confocal section of a projection gradually rotated by an angle of 5° shows the colocalization of actin (red and yellow) and vinculin (green and yellow) in filopodia (arrow) and the attachment plan (arrowhead) in a representative cell (x-y projection view). In panel Bv, the mean ± SD of 300 cells adherent to the indicated substrates for 2 hours and showing stress fibers in 3 independent experiments is shown.
To confirm these findings, we used the high-affinity GPVI agonist, CVX,8,34 or the α2β1-specific triple-helical peptide GFOGER.26 In 30 minutes, adhesion to GFOGER induced the formation of radial stress fibers emanating from the central part of the cell around the nucleus and finely delineated by lamellipodia in an average of 15% of MKs. In other cells, actin was organized in dotlike structures and few long filopodia in the cell periphery (Figure 2Bi). Adhesion for 1 hour did not increase the number of cells exhibiting stress fibers, whereas greater cell spreading occurred (Figure 2Bii). After 2 hours, actin stress fibers and focal adhesions were organized throughout the cell body in 40% ± 5% of adherent MKs (Figure 2Biii,v), a percentage very similar to that observed in MKs adherent to collagen after anti-GPVI Fab fragment treatment. In contrast, even after long incubation on CVX-coated coverslips, actin and vinculin were colocalized both in the extremities of thick and numerous filopodia and in the basal side of attached cells (Figure 2Biv), whereas the number of cells displaying stress fibers did not exceed 10% ± 5% (Figure 2Bv). After adhesion to PLL, only a negligible proportion of MKs (5% ± 3%) displayed stress fiber formation (Figure 2Bv).
Altogether, these results suggest that ligation of α2β1 integrin is sufficient for stress fiber and focal adhesion assembly on collagen I, whereas GPVI induces the formation of filopodia. However, optimal stress fiber formation requires the ligation of both α2β1 integrin and GPVI.
Rho/ROCK pathway is involved in collagen I–induced stress fibers and focal adhesions
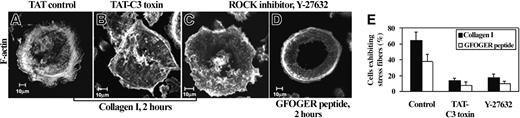
Rho GTPase activation has been directly involved in stress fiber formation in different cell types.15,17 To examine whether Rho GTPase mediates this process in MKs, we used the botulinum toxin C3 transferase coupled to TAT peptide (TAT-C3), which allows rapid and efficient entry into treated cells.25 We first ensured that introduction of TAT peptide by itself did not affect cytoskeletal reorganization in MKs plated on collagen I (Figure 3A). Inactivation of Rho by TAT-C3 treatment (25 μg/mL for 24 hours) completely prevented the formation of actin stress fibers and focal adhesions in 78% ± 5% of adherent MKs (Figure 3B,E).
Rho-ROCK pathway is involved in stress fiber formation induced by collagen I and GFOGER peptide. (A) MKs were preincubated with a control TAT peptide that contains the TAT sequence fused to a scrambled sequence of the GPVI tail (Fl-Tat VI-4-Scr), (B) the specific inhibitor of RhoGTPase TAT-C3 toxin (25 μg/ml, for 24 hours), or (C) ROCK inhibitor Y-27632 (10 μM, for 1 hour). Cells were then plated on collagen I or GFOGER peptide for 2 hours. A representative photomicrograph (A) shows actin stress fibers in the basal section (0.75 μm) of an MK pretreated by TAT-control, whereas pretreatment by TAT-C3 toxin or Y-27632 inhibited actin stress fibers assembly in MKs plated on collagen I (B,C) or GFOGER peptide (D). Scale bar equals 10 μm in panels A-D. (E) Each bar represents the mean ± SD of 300 cells showing stress fibers in collagen I or GFOGER peptide without and with TAT-C3 or Y-27632 treatment in 3 independent experiments.
Rho-ROCK pathway is involved in stress fiber formation induced by collagen I and GFOGER peptide. (A) MKs were preincubated with a control TAT peptide that contains the TAT sequence fused to a scrambled sequence of the GPVI tail (Fl-Tat VI-4-Scr), (B) the specific inhibitor of RhoGTPase TAT-C3 toxin (25 μg/ml, for 24 hours), or (C) ROCK inhibitor Y-27632 (10 μM, for 1 hour). Cells were then plated on collagen I or GFOGER peptide for 2 hours. A representative photomicrograph (A) shows actin stress fibers in the basal section (0.75 μm) of an MK pretreated by TAT-control, whereas pretreatment by TAT-C3 toxin or Y-27632 inhibited actin stress fibers assembly in MKs plated on collagen I (B,C) or GFOGER peptide (D). Scale bar equals 10 μm in panels A-D. (E) Each bar represents the mean ± SD of 300 cells showing stress fibers in collagen I or GFOGER peptide without and with TAT-C3 or Y-27632 treatment in 3 independent experiments.
ROCK was previously shown to mediate the formation of stress fibers and focal adhesions.18 To investigate the role of ROCK, we used a pharmacologic inhibitor, the pyridine derivative Y-27632. Formation of stress fibers stimulated by collagen I was inhibited by Y-27632 treatment (10 μM for 1 hour) to the same extent as observed with TAT-C3 (Figure 3C,E).
Interestingly, stress fiber formation induced by GFOGER peptide was similarly impaired by either TAT-C3 toxin or Y-27632 inhibitor treatment, suggesting the involvement of Rho/ROCK pathway in α2 integrin-mediated stress fiber formation (Figure 3D,E).
In contrast, spreading was not altered by TAT-C3 and Y-27362 treatment suggesting that GTPases other than Rho are involved in this phenomenon.
Collagen I induced sustained and strong activation of the MAPK/Erk1/2 pathway
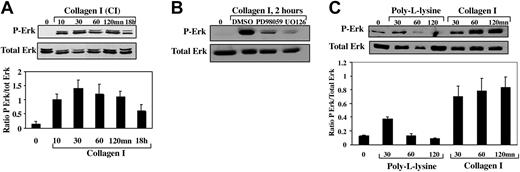
To assess whether stress fiber formation could be regulated by Erk1/2 pathway, we first investigated the role of collagen I in Erk1/2 activation using a phospho-specific antibody that recognizes the dually activated forms of p42 and p44 MAPKs (Figure 4A). MKs plated on BSA-coated surface showed barely detectable Erk phosphorylation (Figure 4A lane 1). A strong and rapid activation of Erk1/2 (12-fold in comparison to the control cells) was observed 10 minutes after plating the cells on collagen (Figure 4A lane 2). This level of Erk activation was sustained for 2 hours (Figure 4A lanes 3-5) and slightly decreased to 8-fold after 18 hours (Figure 4A lane 6). Pretreatment of MKs with 2 pharmacologic agents, PD98059 or UO126 (20 μM, 30 minutes) known to inhibit the MAPK pathway,35,36 reduced significantly Erk1/2 phosphorylation in MKs adherent to collagen I for 2 hours (Figure 4B lanes 3 and 4, respectively).
Collagen I induced rapid and sustained activation of Erk1/2 signaling pathway. MKs were kept in suspension in BSA-coated dishes (A-C lane 1), or plated on fibrillar collagen I (A lanes 2-6; B lanes 2-4; C lanes 5-7) or PLL substrates (C lanes 2-4) for the indicated times. MKs pretreated by PD98059 or UO126 (B lanes 3,4; 20 μM for 30 minutes) were seeded on collagen I–coated dishes for 2 hours. Cells were lysed as described in “Materials and methods.” Proteins were analyzed by Western blot analysis using mAbs against phosphorylated Erk or total Erk (A-C top and bottom panels, respectively). The graphs illustrate levels of phosphorylated Erk relative to the levels of total Erk present in each sample.
Collagen I induced rapid and sustained activation of Erk1/2 signaling pathway. MKs were kept in suspension in BSA-coated dishes (A-C lane 1), or plated on fibrillar collagen I (A lanes 2-6; B lanes 2-4; C lanes 5-7) or PLL substrates (C lanes 2-4) for the indicated times. MKs pretreated by PD98059 or UO126 (B lanes 3,4; 20 μM for 30 minutes) were seeded on collagen I–coated dishes for 2 hours. Cells were lysed as described in “Materials and methods.” Proteins were analyzed by Western blot analysis using mAbs against phosphorylated Erk or total Erk (A-C top and bottom panels, respectively). The graphs illustrate levels of phosphorylated Erk relative to the levels of total Erk present in each sample.
In contrast to collagen I (Figure 4C lanes 6-7), MK adherence to PLL only induced a transient 2-fold increase in Erk1/2 phosphorylation at 30 minutes (Figure 4C, lane 2).
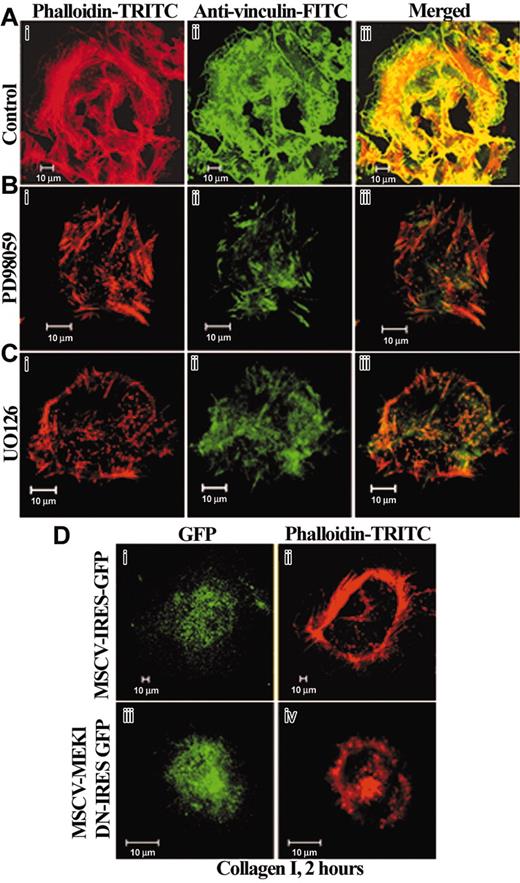
To examine the potential role of MAPK/Erk1/2 signaling in stress fiber formation, we inhibited these signaling pathways with PD98059 (Figure 5B) or UO126 (Figure 5C). MKs were pretreated by DMSO (Figure 5A), PD98059 (Figure 5B), or UO126 (Figure 5C) and then allowed to attach to collagen-coated slides. The formation of stress fibers was prevented in 60% ± 7% of MKs pretreated by PD98059 (Figure 5Bi,iii) or UO126 (Figure 5Ci,iii), but not in the control (Figure 5Ai,iii). Dotlike actin structures were discernible in the basal surface of MKs treated by the inhibitors. Similarly, irregular and diffuse vinculin staining was observed, suggesting that focal adhesions were misassembled (Figure 5B,Cii-iii).
MAPK/Erk1/2 kinase activation is required for optimal stress fiber formation on fibrillar collagen I. Confocal sections show F-actin distribution in MKs preincubated with DMSO (A), PD98059 (B), or UO126 (C), 20 μM for 30 minutes, seeded on collagen I for 2 hours, fixed, permeabilized, and stained as indicated, with rhodamine-phalloidin and monoclonal antivinculin FITC antibody. Note the presence of punctuate F-actin and very short and disorganized peripheral actin filaments in MKs treated by PD98059 (B) or UO126 (C). Also, in contrast with the control (Aiii), actin and vinculin are not any longer colocalized in MKs treated by the inhibitors (Biii,Ciii). (D) Confocal sections of a representative image of a primary MK infected by the retroviral vector MSCV-IRES-GFP used as a control (Di) or the retrovirus containing the dominant-negative (DN) form of MEK1 (Diii) and visualized through GFP expression. MKs were plated on collagen I for 2 hours, fixed, and stained for actin with rhodamine-labeled phalloidin (Dii,iv). Note the presence of stress fibers (Dii) in the MK infected by the retroviral vector control, whereas the MK infected by the DN-MEK1 shows the presence of dense bundles of actin aggregates (Div).
MAPK/Erk1/2 kinase activation is required for optimal stress fiber formation on fibrillar collagen I. Confocal sections show F-actin distribution in MKs preincubated with DMSO (A), PD98059 (B), or UO126 (C), 20 μM for 30 minutes, seeded on collagen I for 2 hours, fixed, permeabilized, and stained as indicated, with rhodamine-phalloidin and monoclonal antivinculin FITC antibody. Note the presence of punctuate F-actin and very short and disorganized peripheral actin filaments in MKs treated by PD98059 (B) or UO126 (C). Also, in contrast with the control (Aiii), actin and vinculin are not any longer colocalized in MKs treated by the inhibitors (Biii,Ciii). (D) Confocal sections of a representative image of a primary MK infected by the retroviral vector MSCV-IRES-GFP used as a control (Di) or the retrovirus containing the dominant-negative (DN) form of MEK1 (Diii) and visualized through GFP expression. MKs were plated on collagen I for 2 hours, fixed, and stained for actin with rhodamine-labeled phalloidin (Dii,iv). Note the presence of stress fibers (Dii) in the MK infected by the retroviral vector control, whereas the MK infected by the DN-MEK1 shows the presence of dense bundles of actin aggregates (Div).
To confirm these results, we infected primary MKs by a bi-cistronic retrovirus containing the dominant-negative (DN) form of MEK1 and GFP (MSCV-MEK1 DN-IRES-GFP). Thus, infected cells coexpress the MEK1 DN and GFP, and can be visualized by fluorescent microscopy. In these experiments about 35% of the MKs were infected by the retrovirus and could be compared to noninfected cells present in the same culture. As a control, we used a retrovirus encoding only GFP (MSCV-IRES-GFP; Figure 5D). MKs infected with the control GFP vector (Figure 5Di) exhibited stress fibers (Figure 5Dii), suggesting that GFP has no effect on actin cytoskeleton reorganization induced by collagen I. In contrast, expression of DN MEK1, visualized by GFP (Figure 5Diii) impaired actin reorganization into stress fibers (Figure 5Div). A defect in stress fiber formation was observed in almost all DN MEK1-infected cells.
Hence, it appears that MEK/Erk1/2 kinase signaling is required for optimum collagen-induced stress fiber formation and focal adhesion organization in primary human MKs.
Collagen I induced robust activation of Erk1/2 through GPVI but not α2 ligation
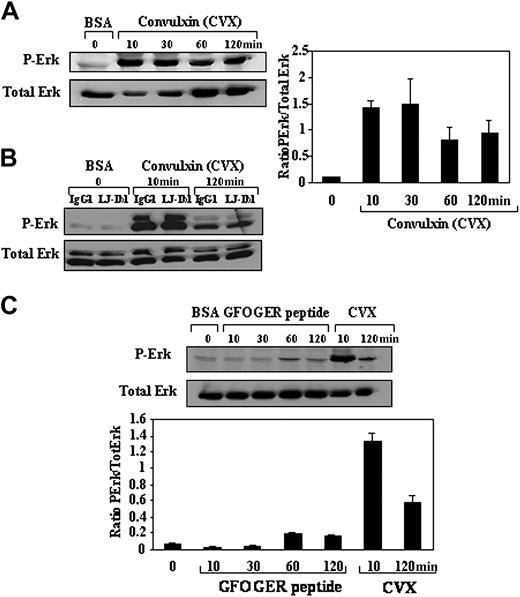
To determine which of the 2 collagen receptors, GPVI or α2β1 integrin, was responsible for Erk1/2 phosphorylation, we used their respective ligands CVX or GFOGER peptide.
MK adherence to CVX induced Erk1/2 phosphorylation (Figure 6A), which peaked to 14-fold at 10 minutes and 30 minutes (Figure 6A lanes 2-3). Recent work has shown that human glycoprotein Ibα (GPIbα) cDNA transfected in Chinese hamster ovary (CHO) cell line binds to CVX and raised the possibility that CVX may not be entirely specific for GPVI receptor.37 To explore whether GPIb could act as a mediator in Erk1/2 activation in our model, we used the function-blocking anti-GPIbα LJ-Ib1 antibody, which has been shown to inhibit CVX binding to GPIbα.37 Erk phosphorylation did not vary in primary human MKs preincubated with LJ-Ib1 blocking antibody (Figure 6B lanes 2, 4, and 6) or the isotype-matched control (Figure 6B lanes 1, 3, and 5) either in suspended cells (Figure 6B lanes 1-2) or MKs adherent to CVX substrate for 10 minutes (Figure 6B lanes 3-4) or 2 hours (Figure 6B lanes 5-6).
Erk1/2 activation is mainly induced by GPVI but not α2 ligation. (A,C) MKs were kept in suspension in BSA-coated dishes, allowed to adhere to CVX, the high-affinity substrate for GPVI or GFOGER peptide, the specific ligand of alpha2 integrin for different times. (B) MKs preincubated with the function-blocking anti-GPIbα (LJ-Ib1; lanes 2, 4, and 6) or the isotype-matched control (IgG1; lanes 1, 3, and 5) were kept in suspension (lanes 1-2) or allowed to attach to CVX substrate for 10 minutes (lanes 3-4) or 2 hours (lanes 5-6) in the presence of the antibodies (100 μg/mL). Suspended or adherent cells were lysed and subjected to Western blot analysis using an anti-phospho-Erk1/2 (P-Erk) antibody as indicated in “Materials and methods.” Equal loading was assayed by labeling with an anti-Erk antibody (Total Erk). Note that Erk phosphorylation induced by CVX was not affected by GPIbα blocking antibody (LJ-Ib1). The graphs illustrate densitometric quantitation of the ratio of phospho-Erk to total Erk in each condition. The mean ± SD of 3 independent experiments is shown.
Erk1/2 activation is mainly induced by GPVI but not α2 ligation. (A,C) MKs were kept in suspension in BSA-coated dishes, allowed to adhere to CVX, the high-affinity substrate for GPVI or GFOGER peptide, the specific ligand of alpha2 integrin for different times. (B) MKs preincubated with the function-blocking anti-GPIbα (LJ-Ib1; lanes 2, 4, and 6) or the isotype-matched control (IgG1; lanes 1, 3, and 5) were kept in suspension (lanes 1-2) or allowed to attach to CVX substrate for 10 minutes (lanes 3-4) or 2 hours (lanes 5-6) in the presence of the antibodies (100 μg/mL). Suspended or adherent cells were lysed and subjected to Western blot analysis using an anti-phospho-Erk1/2 (P-Erk) antibody as indicated in “Materials and methods.” Equal loading was assayed by labeling with an anti-Erk antibody (Total Erk). Note that Erk phosphorylation induced by CVX was not affected by GPIbα blocking antibody (LJ-Ib1). The graphs illustrate densitometric quantitation of the ratio of phospho-Erk to total Erk in each condition. The mean ± SD of 3 independent experiments is shown.
In striking contrast with CVX substrate, adhesion to GFOGER peptide induced only a slight and transient increase of Erk/2 phosphorylation at 1 hour (Figure 6C).
Taken together, these results suggest that collagen-mediated Erk phosphorylation is mainly elicited by GPVI ligation.
Proplatelet formation is negatively regulated by α2 integrin likely through Rho/ROCK pathway
To investigate the biologic relevance of our findings, we examined whether engagement of α2β1 or GPVI could affect proplatelet formation. When MKs were allowed to attach for a longer adhesion time (18 hours), the proportion of MKs exhibiting stress fibers on collagen and GFOGER-coated slides decreased (Figure 7B). To make the distinction between cytoplasmic extensions induced by cell spreading and the formation of proplatelets, we performed double immunofluorescence staining to visualize F-actin and VWF, to ensure that the extensions contain α granules. MKs adherent to PLL substrate formed typical proplatelets (Figure 7Ai-ii). Interestingly, compared with PLL, adhesion to fibrillar collagen I reduced by 3-fold the number of MKs forming proplatelets (Figure 7C).
Rho/ROCK pathway negatively regulates proplatelet formation mainly through α2 integrin. (A) MKs were plated onto PLL (Ai-ii) or GFOGER-coated coverslips in serum-free medium and incubated for 18 hours without (Aiii-iv) or with Y-27632 (Av-vi). Adherent cells were fixed, permeabilized, and stained with polyclonal anti-VWF antibody (Ai,iii,v) and phalloidin (Aii,iv,vi). Phase contrast images merged with VWF staining show the distribution of VWF (Ai,iii,v) in MKs forming proplatelets. Phalloidin staining shows irregular F-actin distribution in the same cells (Aii,iv,vi). The arrowheads indicate branching emanating from VWF positive proplatelets (Av-vi). The graphs represent the mean ± SD of 300 cells showing stress fibers (B) or VWF+ proplatelets (C) in the indicated substrates without and with TAT-C3 or Y-27632 treatment in 3 independent experiments. Scale bar equals 10 μm.
Rho/ROCK pathway negatively regulates proplatelet formation mainly through α2 integrin. (A) MKs were plated onto PLL (Ai-ii) or GFOGER-coated coverslips in serum-free medium and incubated for 18 hours without (Aiii-iv) or with Y-27632 (Av-vi). Adherent cells were fixed, permeabilized, and stained with polyclonal anti-VWF antibody (Ai,iii,v) and phalloidin (Aii,iv,vi). Phase contrast images merged with VWF staining show the distribution of VWF (Ai,iii,v) in MKs forming proplatelets. Phalloidin staining shows irregular F-actin distribution in the same cells (Aii,iv,vi). The arrowheads indicate branching emanating from VWF positive proplatelets (Av-vi). The graphs represent the mean ± SD of 300 cells showing stress fibers (B) or VWF+ proplatelets (C) in the indicated substrates without and with TAT-C3 or Y-27632 treatment in 3 independent experiments. Scale bar equals 10 μm.
To explore how the engagement of either GPVI or α2β1 could affect actin cytoskeletal organization and proplatelet formation, we quantified the proportion of MKs forming VWF+ proplatelets on CVX- or GFOGER peptide-coated surfaces. Although adhesion to CVX did not quantitatively alter this process (Figure 7C), we found that GPVI engagement induced important spreading of the cell body and the formation of long and numerous cytoplasmic projections, which were completely devoid of VWF (data not shown). In contrast, adhesion to GFOGER peptide (Figure 7Aiii-iv) significantly reduced the number of MKs forming proplatelets (Figure 7C).
We used the pharmacologic inhibitors, TAT-C3 toxin and Y-27632, to analyze the potential role of the Rho/ROCK pathway in proplatelet formation. Strikingly, the number of MKs forming VWF+ proplatelets increased significantly, but only when MKs were allowed to spread on GFOGER-coated slides (Figure 7C). Moreover, treated cells displayed an increasing number of cytoplasmic extensions, which also displayed bifurcations emanating from the same extension (Figure 7Av-vi, arrows). In contrast, TAT-C3 toxin and Y-27632 were not able to significantly increase proplatelet formation by MKs adherent to collagen I, whereas they inhibited stress fiber formation (Figure 7B-C), demonstrating their efficiency in inactivating the Rho/ROCK pathway.
Taken together, these data suggest that α2 integrin ligation can exert negative regulation of proplatelet formation through the Rho/ROCK pathway and raises the possibility that collagen I inhibits proplatelet formation by the simultaneous activation of several signaling pathways.
Discussion
Reorganization of the cytoskeleton plays a major role in proplatelet formation and the release of nascent platelets.9-11 However, most studies addressing this issue have been performed in murine MKs or human cell lines grown in suspension, whereas cytoskeletal reorganization can be profoundly affected by the interaction of ECM receptors with the bone marrow microenvironment. As demonstrated by the Rho GTPase pull-down assay33,38 and MAPK/Erk1/2 phosphorylation, we show that fibrillar collagen I is able to trigger activation of both pathways in primary human MKs. We provide 2 main arguments supporting the requirement for α2β1-integrin engagement to induce the assembly of stress fibers and adhesion complexes. First, we observed an inhibition of these processes using a specific antagonist of the α2β1 integrin. Second, the exclusive engagement of GPVI was not sufficient to trigger stress fiber formation. The “outside-in” activation of α2β1 integrin by the specific peptide GFOGER allowed stress fiber and focal adhesion assembly through Rho/ROCK activation independently of GPVI. This surprising outcome could be controversial, with the emerging concept that GPVI is required for the “inside-out” signaling leading to α2β1 activation in mouse platelets,5,6 although recent data from both mouse and human studies indicate that α2β1 can remain competent to bind collagen and be active in the signaling process despite GPVI blockade39 or knockout.40 The GFOGER peptide provides also a specific and direct α2 integrin-mediated “outside-in” activation leading to filopodial and lamellipodial extension in platelets. This could be due to the high density of GFOGER sequences, which would stabilize adhesion of the low-affinity α2 integrin to its specific substrate.7 At least 2 mechanisms could account for Rho/ROCK activation required for stress fiber assembly. First, formation of filopodia, which is a hallmark of Cdc42 activation, was induced following GPVI ligation by CVX and during the first phase of collagen or GFOGER-mediated adhesion. We cannot therefore exclude that stress fiber formation could be due to sequential activation of Rho by Cdc42/Rac activation through direct ligation of either GPVI or α2β1 receptors. Indeed, in platelets, CVX induced lamellae spreading following rapid activation of Rac and PAK.41 Second, α2β1 integrin may activate the Rho/ROCK pathway directly. Recent work using platelets demonstrates that RhoA is activated in response to soluble agonists (thrombin, adenosine diphosphate, collagen), immobilized proteins (VWF, fibrinogen), and high shear stress.42 Further studies are needed to validate the concept that GPVI and α2β1-integrin engagement regulate directly and differentially the activation state of Rho and Cdc42 GTPases.
Our results also show that fibrillar collagen I mediates a strong and sustained activation of Erk1/2 pathway in primary human MKs, which was not found in suspended or adherent cells to a nonspecific substrate such as PLL. In suspension murine MKs, TPO induced Erk activation that peaked at 10 minutes and clearly decreased over the following 3 hours.23 The differences noted with our system reveal the importance of adhesion receptors in the sustained activation of the MAPK/Erk1/2 pathway. Importantly, this pathway seems to be crucial for TPO-induced endomitosis23 or TPO-induced MK differentiation of human CD34+ hematopoietic progenitor cells generated from cord blood.43,44 The mechanism by which GPVI is able to activate the Erk pathway in MKs could be mediated by the FcRγ chain immunoreceptor tyrosine-based activation motif (ITAM). One attractive model for GPVI signaling would be that the Syk pathway triggers the rapid and persistent Erk1/2 activation in MKs and platelets. Phosphoinositide 3-kinase (PI3K) is another component of the multimolecular signaling machinery that binds to the phosphorylated ITAM of FcRγ after platelet activation induced by collagen or CVX.45 Recently, it was shown that GPVI mediates Rap1 activation through a PI3K-dependent mechanism46 and that Rap1 activation induces a prolonged Erk1 activation in megakaryocytic cell lines.47
In contrast, α2β1 was unable to activate the MAPK pathway. A conventional means by which integrins can activate Erks involves Src kinases in association with either focal adhesion kinase (FAK) or caveolin, leading to tyrosine phosphorylation of binding sites for the SH2 domain of Grb2/Sos and recruitment of the RasGTPase/Raf pathway.48 Integrins can also activate the Ras/MAPK pathway via the tyrosine kinase Fyn and the adaptor protein Shc.49,50 We only detected a late and transient activation of the Erk pathway, which could be related to a gradual modulation of α2β1-integrin affinity/avidity due to the high density of GFOGER sequences in the peptide-coated surface. Also, it was recently shown that tyrosine phosphorylation of FAK and phospholipase Cγ2 (PLCγ2) through a Src kinase-dependent pathway can be transduced by α2β1 independently of GPVI in platelets.7 However, this pathway seems not to be efficient enough to transduce a sustained activation for Erk1/2 through α2β1 integrin in primary human MKs.
Finally, adhesion to collagen I significantly inhibited the process of proplatelet formation by MKs through activation of the α2β1 integrin: proplatelet formation was markedly inhibited by adhesion to GFOGER peptide, but not to CVX. This inhibition of proplatelets was mediated through the Rho pathway because treatment by Y-27632 or TAT-C3 restored proplatelet formation. However, inhibition of stress fiber formation per se was not sufficient to induce proplatelets because Y-27632 or TAT-C3 inhibited stress fiber formation for MKs adherent on collagen I without inducing proplatelet formation. This suggests that the Rho/ROCK pathway may have other effectors that regulate the process of proplatelet formation. Given the importance of microtubules (MTs) in the first phases of proplatelet elongation,9,10 the modulation of proplatelet formation by adhesion to collagen matrix could also be related to a specific regulation of MT dynamics by collagen receptors. It has been shown that the Rho GTPase pathway selectively stabilizes MTs during cell polarization.51 Thus, the findings that Rho/ROCK pathway is involved in the negative regulation of proplatelet formation through α2 integrin could be due to downstream effects of this pathway in MT assembly and/or stabilization.
In contrast, blockade of the Rho pathway was unable to induce proplatelet formation from MK adherent on collagen I. This suggests that other signaling pathways activated by collagen I, independently of the α2β1-mediated signaling, contribute to this inhibition. Apparently this is not related to the MAPK/Erk pathway because UO126 or PD98059 used alone or together with Y-27632 did not significantly reverse the inhibitory effects of collagen I (data not shown). Further studies are required to identify these pathways.
Previously, it has been shown that MK adherence to stromal cells also inhibited proplatelet formation, but the mechanism was not determined.52 It was hypothesized that this inhibitory mechanism prevents platelet shedding in the bone marrow microenvironment. Thus, negative regulation of proplatelet formation by collagen I may participate in this regulation. However, it has to be emphasized that neither α2 nor GPVI knockout mice exhibited changes in platelet count.40,53 Several explanations may account for these differences between the in vivo and in vitro approach, such as: (1) a simultaneous defect in both collagen receptors may be required to modify the platelet count, (2) in vivo several mechanisms may compensate the effect on platelet production of an α2β1 defect, and (3) regulation of platelet production may be different between humans and mice, as previously suggested.54
Altogether these findings could be of great interest to dissect MK-matrix interactions, which remain poorly defined in pathophysiologic situations, such as myelofibrosis and congenital thrombocytopenia.
Prepublished online as Blood First Edition Paper, July 20, 2004; DOI 10.1182/blood-2003-12-4398.
Supported by European Commission (contract no. QLG1CT 1999-01090, WASPNEST program), La Ligue Nationale contre le cancer (équipe labellisée 2000/2004), and a contract of the French Ministry of Research (ACI). S.S. was supported by fellowships from the European community and la Fondation de France and R.W.F. by the British Heart Foundation and Medical Research Council. Approval was obtained from INSERM Institutional Review Board.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Barry Coller for the generous gift of 6F1 antibody, Dr Jacques Pouysegur (Centre Antoine Lacassagne, Nice, France) for the construct (DN MEK1), and Dr Zaverio Ruggeri and Dr Tom Kunicki (The Scripps Research Institute, La Jolla, CA) for the generous gift of LJ-Ib1 antibody. We thank Dr Olivier Albagli for critical reading of the manuscript and Pascal Roux (Pasteur Institute) and Abdelali Jalil (Institut Gustave Roussy) for their assistance for confocal imaging. We greatly acknowledge Cathy Crouin and Béatrice Comba for their technical help.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal