Abstract
Deregulated apoptosis is a common finding in tumorigenesis. The oncogenic tyrosine kinase nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) delivers a strong survival signal in anaplastic large cell lymphomas (ALCLs). Although NPM/ALK activates multiple antiapoptotic pathways, the biologic relevance and therapeutic potential of more downstream apoptotic effectors are mostly unknown. In this report, the NPM/ALK-mediated induction of Bcl-XL (but not of Bcl-2) was identified in human ALCL-derived cells. NPM/ALK kinase activity was required to promote Bcl-XL expression and its protective effect on mitochondrial homeostasis. Down-regulation of Bcl-XL significantly reduced the antiapoptotic potential of NPM/ALK in both transformed murine Ba/F3 pro-B cells and human ALCL-derived KARPAS-299 cells. To elucidate the role of Bcl-XL in vivo, Ba/F3-NPM/ALK+ cells expressing a doxycycline (Dox)-inducible Bcl-XL antisense transgene (pTet-ON) were injected into nude mice. Doxycycline administration prevented a fatal systemic disease in 15 of 15 intravenously injected mice and the appearance of subcutaneous tumor xenografts in 9 of 12 mice; in vivo down-regulation of Bcl-XL was also documented. Our results show a pivotal role for Bcl-XL in ALK-mediated oncogenicity; a single protein placed downstream of a known oncogene can be crucial for the survival of neoplastic cells both in vitro and in vivo. Bcl-XL deserves further investigation as a possible therapeutic target in ALK+ ALCLs. (Blood. 2004;103:2787-2794)
Introduction
The broad category of non-Hodgkin lymphomas (NHLs) includes distinct neoplasms arising from clonal expansion of B or T lymphocytes at different stages of maturation and differentiation.1 The CD30/Ki-1+anaplastic large cell lymphomas (ALCLs) account for about 20% of high-grade NHLs presenting unique immunophenotypical, morphologic, and genetic features.2 Approximately 70% of ALCLs express an 80-kDa hybrid oncoprotein, named NPM/ALK, arising from the chromosomal translocation t(2;5)(p23;q35).3 The NPM/ALK chimera contains the N-terminal portion of the ubiquitously expressed nucleolar protein nucleophosmin (NPM) fused to the kinase domain of anaplastic lymphoma kinase (ALK).4 ALK is a member of the insulin receptor tyrosine kinase superfamily, whose expression is restricted to the central nervous system mainly during the neonatal period.5
As a consequence of the t(2;5) rearrangement, the strong NPM gene promoter drives the high-level ectopic expression of ALK in lymphoid ALCL cells.6,7 In addition, the oligomerization domain contained in the NPM portion promotes the constitutive trans-phosphorylation and activation of the ALK kinase domain in NPM/ALK.8 Thus, NPM/ALK can exert its oncogenic potential via abnormal stimulation of multiple cellular signaling cascades, enhancing growth factor-independent proliferation and prolonging viability. Several groups described a direct recruitment and activation by NPM/ALK of numerous intracellular signal transduction effectors such as phospholipase C-γ (PLC-γ),9 pp60 c-Src kinase,10 phosphatidylinositol-3-kinase (PI-3kinase),11,12 Janus kinases (Jaks),13-15 and signal transducers and activators of transcription (Stats),13,16,17 but the precise biologic implication of their activation in ALK+ ALCL cells remains unclear.
The Bcl-2 family members are important modulators of mitochondrially initiated apoptosis.18 This still-expanding family contains both prosurvival (Bcl-2, Bcl-XL, myeloid cell-leukemia-1 [Mcl-1]) and proapoptotic (Bad, Bak, Bax) factors that share the presence of 1 to 4 Bcl-2 homology (BH) domains.19 Of these domains, BH3 represents a key element in regulating their homo-/heterodimerization and subcellular localization. Bcl-2 and Bcl-XL exhibit both structural and functional similarity to prokaryotic pore-forming proteins, acting as ionic channels in the outer mitochondrial membrane for the control of mitochondrial swelling and loss of transmembrane potential.20,21 The “BH3-only” proapoptotic Bad, which lacks the mitochondrial-membrane insertion C-terminal signal, is reversibly regulated by phosphorylation at multiple serine (Ser132, Ser136, and Ser155) residues.22-24 In particular, phosphorylation at Ser136 is known to create a binding site for the phosphospecific binding of 14-3-3 proteins, which retain Bad in the cytoplasm preventing its translocation to the mitochondrial membrane.23,25 In fact, upon a death signal, such as withdrawal of interleukin-3 (IL-3)26 or insulin-like growth factor-1,27 dephosphorylated Bad uses its BH3 death domain to bind the hydrophobic groove in the nonmembrane inserted portion of Bcl-XL, which is then inactivated.28,29 The collapse of mitochondrial transmembrane potential caused by the Bcl-XL/Bad association occurs early in the apoptotic process, before other changes develop, such as nuclear DNA fragmentation and the exposure of phosphatidylserine (PS) on the outer surface of the cell membrane.18,19 This early event in the commitment to apoptosis leads to the subsequent release of cytocrome c from mitochondria and activation of the intracellular apoptogenic effectors caspases.28,30
Recently, it has been demonstrated that NPM/ALK blocks the activation of caspase-3 by preventing the cytosolic accumulation of cytocrome c.31,32 In addition, transcriptional induction of Bcl-XL is elicited by NPM/ALK through the constitutive activation of the Jak3/Stat3 pathway, protecting cells from drug-induced apoptosis.13,15
In this report, the expression and biologic relevance of Bcl-XL as a potential therapeutic target were evaluated in vitro and in vivo in NPM/ALK-transformed lymphoma cells.
Materials and methods
Cell cultures and reagents
The murine pro-B Ba/F3 cells stably transformed by NPM/ALK and the SUDHL-1 and SUP-M2 cell lines established from human T-cell ALCL (T-ALCL) carrying the t(2;5) were a kind gift from Dr Stephan W. Morris (St Jude Research Hospital, Memphis, TN). The t(2;5)-positive KARPAS-299 (K-299) ALCL cells were purchased from DSMZ (Berlin, Germany). Cells were cultured in RPMI-1640 (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS; GibcoBRL, Paisley, United Kingdom). Parental Ba/F3 cells were maintained in RPMI-1640 supplemented with 10% FBS and 0.2% WEHI-3B-conditioned medium as a source of IL-3 as described previously.14 All culture media contained 100 Units/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine (GibcoBRL), and the cells were incubated at 37°C with 5% CO2 atmosphere.
LY294002 (PI-3kinase pathway inhibitor) and herbimycin A (HA; nonspecific tyrosine kinase inhibitor) were purchased from Calbiochem (La Jolla, CA). Etoposide was from Sigma Chemical (St Louis, MO). All reagents were resuspended in dimethyl-sulfoxide (DMSO; Sigma Chemical) used as a solvent control in some experiments.
Plasmids and DNA transfections
The pcDNA3.1-neo expression vector (Invitrogen, Groningen, The Netherlands) bearing the human cDNA for a kinase-defective mutant of NPM/ALK, named NPM/ALK.K210R (kindly provided by Dr Stephan W. Morris),9 was transiently transfected into Ba/F3-NPM/ALK+ cells by electroporation with a Biorad Gene Pulser apparatus (Biorad Laboratories, Hercules, CA) at 270 V, 1060 μF. The human Bcl-XL expression vector pRSV-Bcl-XL was a kind gift from Dr Fabrice Gouilleux (ICGM-Institut National de la Santé et de la Recherche Médicale [INSERM], France). The full-length Bcl-XL cDNA was then subcloned into a plasmid vector enhanced green fluorescence protein (pEGFP) vector (Clontech, Palo Alto, CA) in a reverse 3′ to 5′ orientation. The resulting Bcl-XL antisense expression construct was checked by sequencing and restriction enzyme digestion and transiently transfected into K-299 cells using Lipofectamine2000 Transfection kit (Invitrogen) according to the manufacturer's instructions. In order to enhance pEGFP-cytomegalovirus (CMV) promoter activity and antisense transgene expression, K-299 cells were incubated with phytohemagglutinin L (PHA-L) and phorbol myristate acetate (PMA) at final concentration of 1 μg/mL and 50 ng/mL, respectively, for 12 hours following transfection. A sequence of 496 base pairs derived from Bcl-XL cDNA was ligated in an antisense orientation into pTRE vector (Clontech), containing a doxycycline (Dox)-responsive promoter (pTRE.ASBcl-XL). To obtain an inducible expression of the antisense Bcl-XL transgene, Ba/F3-NPM/ALK+ cells stably expressing a reverse Tet-repressor (rTetR) encoded by the pTet-ON vector (Clontech) were electroporated with 30 μg pTRE empty vector (pEV) or pTRE.ASBcl-XL (pAS), and 5 μg pTK-Hygro vector (Clontech). Double-stable Tet-cell lines (Ba/F3-N/A-pEV or Ba/F3-N/A-pAS) were selected for 3 weeks with hygromycin-B (Boehringer Mannheim, Indianapolis, IN), and individual clones were obtained by limiting dilution. Selective antisense Bcl-XL transcription was confirmed by reverse-transcriptase-polymerase chain reaction (RT-PCR) after addition of 1 μg/mL Dox in culture.
Antibodies and Western blot analysis
The monoclonal anti-ALK1 antibody was kindly provided by Dr Karen Pulford (John Radcliffe Hospital, Oxford, United Kingdom). The mouse monoclonal antiphosphotyrosine 4G10 antibody and the rabbit polyclonal total anti-Bad or anti-phosphoserine-136-Bad antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). Polyclonal antibody against Bcl-XL (H-62) and goat polyclonal antibody anti-β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Bcl-2 (Ab-2) rabbit polyclonal was from Oncogene Research Products (Boston, MA). Protein lysates, prepared as previously reported,14 were separated by 8% to 16% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Biorad Laboratories) and blotted onto Immobilon-P nitrocellulose membrane (Millipore, Bedford, MA). Membranes were blocked for one hour at room temperature in Tris (tris(hydroxymethyl)aminomethane)--buffered saline/Tween 20 (TBS-T) buffer containing 5% dried milk (for anti-ALK, anti-Bcl-XL, anti-Bad, anti-phophoserine-136-Bad blots) or with 2% bovine serum albumin (for 4G10 and anti-Bcl-2 blots). Primary antibody incubation times were 1 to 16 hours. Secondary incubations were for one hour and antibodies used were horseradish peroxidase (HRP)-conjugated antimouse or antirabbit (Amersham, Arlington, Heights, IL). Proteins were visualized by chemiluminescence as recommended by the manufacturer (Super Signal; Pierce, Rockford, IL). Relative protein levels of Bcl-XL were quantified by immunoblotting, and densitometric analysis of films was carried out on an Eagle Eye II Photodensitometer (Stratagene, La Jolla, CA).
Preparation and analysis of mitochondria extracts
The isolation of mitochondria was adapted from a previously described method.33 Briefly, cells washed twice with ice-cold phosphate-buffered saline (PBS) were resuspended in a cold hypotonic buffer (10 mM KCl; 1 mM MgCl2; 10% glycerol; 0.5 mM dithiothreitol [DTT]; 1 mM phenylmethylsulfonyl-fluoride [PMSF]; 15 mg/mL aprotinin; 3 mg/mL leupeptin/pepstatin; 2 mM Na3V04; 0.2% nonidet P-40 [NP-40] with 20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.9]) and homogenized by using a Dounce homogenizer. The homogenate was mixed with an iso-osmotic MSE buffer (210 mM mannitol; 70 mM sucrose; 5 mM Tris-HCl, and 1 mM ethylenediaminetetraacetic acid [EDTA] pH 7.5) and centrifuged at 750g for 20 minutes at 4°C to pellet nuclei and unbroken cells. The pellet containing the mitochondria was resuspended in the MSE buffer and stored at -80°C. Protein concentration was determined using the bicinchoninic acid (BCA) protein assay (Pierce), and 100 μg protein was immunoprecipitated for 16 hours with 4 μg anti-Bcl-XL polyclonal antibody (H-62). Bcl-XL immunocomplexes were captured with 60 μg protein A-Sepharose beads (Pharmacia Biotech, St Alban, United Kingdom) for 4 hours at 4°C, separated on 16% SDS-PAGE, and analyzed by Western blotting as described above.
Mitochondrial permeability transition detection
Changes in the mitochondrial transmembrane potential (MTP) were detected using a fluorescent cationic dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzamidazolocarbocyanin iodide, commonly known as JC-1-fluorochrome (BIOMOL Research Laboratories, PA). Inside healthy cells, the lipophilic positively charged JC-1 dye aggregates in mitochondria and under fluorescent light emits a red signal (J-aggregates emit at 590 nm). Upon reduction in MTP, the JC-1 fluorochrome converts to a monomeric form that fluoresces green (monomeric dye emits at 490 nm). Cells were incubated with 2 μg/mL JC-1 in media for 30 minutes at 37°C. All stained samples were analyzed using a FACScalibur and Cell-quest software (Becton Dickinson, San José, CA).
Treatment with Bcl-XL antisense oligonucleotides
The optimal conditions for Bcl-XL protein depletion were established previously.34 NPM/ALK-positive Ba/F3 cells were incubated for 3 days in the presence of either sense (S), antisense (AS), or nonsense (NS) oligodeoxynucleotides (ODNs) targeting the coding region 5′ to the translation initiation codon site for the Bcl-XL gene (100% sequence identity between mouse and human) and extending 3′ downstream for a total of 18 bases. The DNA oligonucleotides with a phosphorothioated backbone were synthesized and purified by high-performance liquid chromatography (HPLC; MWG-Biotech, Ebersberg, Germany). The antisense AS-ODN sequence used was 5′-CCGGTTGCTCTGAGACAT-3′. There were 2 additional control ODNs used: (1) a complementary sequence to the antisense in the reverse 5′ to 3′ order, (S: 5′-ATGTCTCAGAGCAACCGG-3′), and (2) a missense sequence containing a 4-base mismatched compared with the antisense sequence (NS: 5′-CCGGTTGCTGTCACAGAT-3′). The lyophilized ODNs were reconstituted in sterile distilled water to 100 mM, filter-sterilized, and stored in aliquots at -20°C as stock solutions. Fresh oligonucleotides were added at 0, 16, 24, 32, 48, and 60 hours.
Cell viability and apoptosis assays
Apoptosis was assessed by several criteria. The percentage of apoptotic cells was determined by enumerating cells undergoing a reduction of cell volume with chromatin condensation or micronuclear fragmentation in a total of at least 200 cells using the intercalating fluorescent DNA binding dye, acridine orange (AO; Sigma Chemical). Cells were stained with 5 μM AO dissolved in PBS and examined using a Zeiss fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
DNA degradation was further assessed by measuring the DNA content of individual cells by flow cytometry. Cells were washed with ice-cold PBS and fixed in 70% cold ethanol. Samples were then treated with Dnase-free Rnase (Boehringer Mannheim) and stained with 10 μg/mL propidium iodide (Sigma Chemical). Apoptosis was also detected by annexin V-fluorescein isothiocyanate (FITC) binding using the Apoptosis Detection Kit purchased from Bender MedSystems Diagnostic (Vienna, Austria). All samples were prepared following the manufacturer's instructions and analyzed using FACScalibur and Cell-quest software.
Animals and treatments
Female CD-1 nu/nu mice (7-9 weeks old) were supplied by Charles River (Calco, Como, Italy) and kept under standard laboratory conditions according to the guidelines of our institute. Animal studies were approved by the Ethics Committee for Animal Experimentation of the Istituto Nazionale Tumori, Milan, Italy. Mice were implanted with Ba/F3 cells transformed by NPM/ALK and transfected with the inducible antisense Bcl-XL transgene (Ba/F3-N/A-pAS) or bearing an empty vector as control (Ba/F3-N/A-pEV) either intravenously (10 × 106 cells/mouse) or subcutaneously (20 × 106 cells/mouse). Therapeutic efficiency of the pAS vector was assessed by the addition of doxycycline hydrochloride in the drinking water (5% sucrose, 0.2 mg Dox/mL), which was changed twice a week. Mice were monitored every other day for the appearance of tumors and for signs of disease (weight loss, adenopathies). To analyze in vivo Bcl-XL protein levels, subcutaneous tumor-bearing mice were killed and nonnecrotic tumor tissue was extracted and homogenized in a 5-fold volume of sodium dodecyl sulfate (SDS) loading buffer (50 mM Tris-HCl [pH 6.8], 2% SDS, and 5% β-mercaptoethanol). After sonication for 3 minutes, samples were centrifugated at 15 000g for 15 minutes, heated at 95°C for 10 minutes, and analyzed in Western blot as described above.
Statistical analysis
Statistical analysis was performed with Student test by use of the GraphPad software analysis program (Prism, San Diego, CA). P values of less .05 were considered to be statistically significant and were derived from 2-sided statistical tests. All data are presented as the mean (95% confidence interval [CI]). CIs are displayed when they exceed 10% of the respective mean. Correlation coefficient (CC) was calculated using the Pearson method.
Results
The Bcl-2 family members in ALK+ ALCL lymphoid cells
Bcl-2 family members are well-known cell-death regulators.18 Bcl-2 and Bcl-XL protect cells from apoptosis, whereas Bad increases susceptibility to cell death.20,23
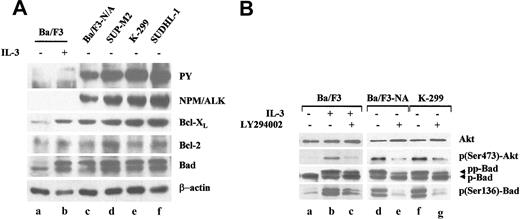
The expression levels of Bcl-XL and of Bcl-2 were evaluated in human NPM/ALK+ ALCL-derived cells carrying the t(2;5) translocation and in pro-B murine Ba/F3 transformed by NPM/ALK (Ba/F3-N/A).9,12 Analysis on total lysates from Ba/F3 cells showed that NPM/ALK promotes an IL-3-independent Bcl-XL protein induction of approximately 2.2-fold (as measured by band densitometry; Figure 1, lane c), compared with parental cells starved of IL-3 for 18 hours (Figure 1, lane a). Bcl-XL expression is induced in parental Ba/F3 cells after stimulation with the growth factor IL-3, as previously reported by Dumon et al35 (Figure 1, lane b compared with lane a). In 3 ALK+ ALCL-derived cell lines, Bcl-XL protein is detected at levels higher than in Ba/F3-N/A+ cells. A direct relationship between Bcl-XL expression levels and the intensity of NPM/ALK bands appears to be present (Figure 1, lanes c-f). The basal level of Bcl-2 in parental Ba/F3 cells remains low and unchanged after ectopic overexpression of NPM/ALK or IL-3 stimulation. These Bcl-2 levels are comparable with those detected in ALCL ALK+ cells, suggesting that NPM/ALK does not markedly affect Bcl-2 levels in these cells. The hybridization of the same filter with an anti-total Bad rabbit polyclonal antibody reveals that Bad migrates as a doublet of approximately 26 kDa, as previously described.24,25 It has been proposed that these 2 distinct forms of Bad could reflect a hyperphosphorylated (pp-Bad) and hypophosphorylated (p-Bad) status of the protein that can be modified at multiple serine residues.25 In Ba/F3 parental cells, 2 hours following withdrawal of IL-3, the hyperphosphorylated Bad was eliminated (Figure 1B, lane a), whereas 30-minute readdition of IL-3 restored the slower-migrating form of Bad (Figure 1B, lane b). The serine 136 has been identified as the major site of Bad phosphorylation by AKT.23,26 Treatment with an irreversible inhibitor of PI-3kinase, LY294002, in presence of IL-3, impaired AKT activation at serine 473 and phosphorylation of Bad at serine 136, as indicated by using 2 phospho-site specific antibodies (Figure 1B, lane c). Likewise, treatment of Ba/F3-N/A+ cells and ALK+ ALCL K-299 with LY294002 inhibited AKT and Bad phosphorylation and decreased, but not completely abolished, the hyperphosphorylated form of Bad.
Expression and activation status of Bcl-2 family members in NPM/ALK-expressing cells. (A) Expression and tyrosine phosphorylation of NPM-ALK were evaluated with an anti-ALK monoclonal and antiphosphotyrosine (PY) antibodies in total lysates from pro-B Ba/F3 parental cells cultured in the absence or presence of IL-3 for 18 hours (lanes a-b), in NPM/ALK-expressing cell lines including Ba/F3 cells transfected with NPM/ALK (Ba/F3-N/A; lane c), and the human ALCL-derived cell lines SUP-M2 (lane d), K-299 (lane e), and SUDHL-1 (lane f). Expression levels of Bcl-XL, Bcl-2, and Bad were also assessed. (B) Ba/F3 parental cells were starved without IL-3 for 2 hours (lane a) and then stimulated with IL-3 in the absence (lane b) or presence (lane c) of 25 μM 294002 for 30 minutes. Ba/F3-N/A+ and K-299 ALCL cells were incubated with the same drug concentration (lanes e, g) or vehicle alone (lanes d, f) for 30 minutes. Expression and activation status of AKT and Bad were evaluated from total lysates with anti-total AKT and Bad polyclonal antibodies as well as by using antiphosphoserine-specific antibodies (phospho-Ser473 for AKT and phospho-Ser136 for Bad).
Expression and activation status of Bcl-2 family members in NPM/ALK-expressing cells. (A) Expression and tyrosine phosphorylation of NPM-ALK were evaluated with an anti-ALK monoclonal and antiphosphotyrosine (PY) antibodies in total lysates from pro-B Ba/F3 parental cells cultured in the absence or presence of IL-3 for 18 hours (lanes a-b), in NPM/ALK-expressing cell lines including Ba/F3 cells transfected with NPM/ALK (Ba/F3-N/A; lane c), and the human ALCL-derived cell lines SUP-M2 (lane d), K-299 (lane e), and SUDHL-1 (lane f). Expression levels of Bcl-XL, Bcl-2, and Bad were also assessed. (B) Ba/F3 parental cells were starved without IL-3 for 2 hours (lane a) and then stimulated with IL-3 in the absence (lane b) or presence (lane c) of 25 μM 294002 for 30 minutes. Ba/F3-N/A+ and K-299 ALCL cells were incubated with the same drug concentration (lanes e, g) or vehicle alone (lanes d, f) for 30 minutes. Expression and activation status of AKT and Bad were evaluated from total lysates with anti-total AKT and Bad polyclonal antibodies as well as by using antiphosphoserine-specific antibodies (phospho-Ser473 for AKT and phospho-Ser136 for Bad).
It can be concluded that NPM/ALK, when expressed in ALCL cells as a result of the t(2;5) chromosomal translocation or after transfection of NPM/ALK cDNA into Ba/F3 cells, is able to up-regulate Bcl-XL (but not Bcl-2) and to promote a hyperphosphorylation of Bad that can be partially modulated by PI-3kinase/AKT signaling.
Inhibition of NPM/ALK tyrosine kinase activity induces apoptosis via modulation of the Bcl-XL/Bad death effectors
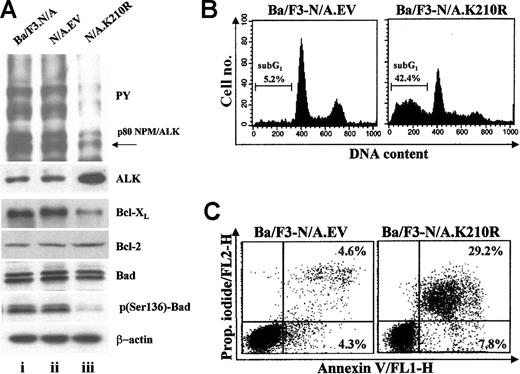
The tyrosine kinase activity of NPM/ALK is essential to elicit growth factor-independent proliferation and resistance to apoptosis in Ba/F3 cells. In fact, it is known that a kinase-defective mutant of NPM/ALK, named N/A.K210R, characterized by a point mutation in the critical adenosine triphosphate (ATP) binding site (K210R) fails to induce a transformed phenotype in Ba/F3 cells after IL-3 withdrawal.9 We used this construct as a dominant-negative inhibitor of ALK kinase activity and determined its effect on Bcl-XL expression and survival in NPM/ALK-transformed cells. The N/A.K210R mutant was transiently overexpressed in Ba/F3-N/A cells. At 48 hours after transfection, anti-ALK (ALK) and antiphosphotyrosine (PY) immunoblots were performed to evaluate the expression and activation status of NPM/ALK (Figure 2A, upper panel). Decreased tyrosine phosphorylation of NPM/ALK, as well as of several other proteins, was observed in N/A.K210R transfectants, confirming the inhibitory action of N/A.K210R on wild-type NPM/ALK. The transient transfection of N/A.K210R also caused a substantial down-regulation of Bcl-XL expression but did not change the Bcl-2 protein levels (Figure 2A, lower panel). In addition, N/A.K210R failed to promote Bad phosphorylation on serine 136 without affecting its total expression levels (Figure 2A, lower panel). Upon Bad dephosphorylation, Bcl-XL/Bad dimerization plays a significant role in promoting apoptosis by inactivating mitochondrial Bcl-XL.28,29 Correlating with the inactivation of Bad and down-regulation of Bcl-XL in Ba/F3-N/A.K210R-expressing cells, an increase in the sub-G1 peak, indicative of apoptotic cells with subdiploid DNA (42.4%), was detected in these cells compared with control vector Ba/F3-N/A.EV transfectants (5.2%; Figure 2B). In addition, morphologic changes related to the exposure of phosphatidylserine (PS) on the outer surface of Ba/F3-N/A.K210R-transfected cells undergoing apoptosis (7.8% + 29.2%) were detected using an annexin V-propidium iodide staining (Figure 2C).
Tyrosine kinase activity of NPM/ALK up-regulates Bcl-XL and promotes Bad phosphorylation. Ba/F3-NPM/ALK+ cells were transiently transfected with NPM/ALK kinase defective mutant (N/A.K210R), an empty control vector (N/A.EV), or electroporated in the absence of DNA (Ba/F3.N/A). (A) At 48 hours from transfection total lysates were separated by 7.5% SDS-PAGE and analyzed by immunoblotting with anti-ALK (ALK) and antiphosphotyrosine (PY) monoclonal antibodies (upper panel). The same cellular lysates were also separated by 12% SDS-PAGE and analyzed by immunoblotting with antibodies specifically recognizing total Bcl-XL, Bcl-2, Bad, and a phosphoserine-136 (p136-Bad) residue of Bad (lower panel). Levels of β-actin were reported as a protein loading control (lower panel). (B) Cell-cycle distribution of the transfected cells was assessed 48 hours after transfection by propidium iodide staining and flow cytometric analysis. The sub-G1 peak is indicative of the presence of apoptotic cells with subdiploid DNA content. (C) Occurrence of apoptosis was also performed by a propidium (Prop.) iodide-annexin V staining at 48 hours after transfection.
Tyrosine kinase activity of NPM/ALK up-regulates Bcl-XL and promotes Bad phosphorylation. Ba/F3-NPM/ALK+ cells were transiently transfected with NPM/ALK kinase defective mutant (N/A.K210R), an empty control vector (N/A.EV), or electroporated in the absence of DNA (Ba/F3.N/A). (A) At 48 hours from transfection total lysates were separated by 7.5% SDS-PAGE and analyzed by immunoblotting with anti-ALK (ALK) and antiphosphotyrosine (PY) monoclonal antibodies (upper panel). The same cellular lysates were also separated by 12% SDS-PAGE and analyzed by immunoblotting with antibodies specifically recognizing total Bcl-XL, Bcl-2, Bad, and a phosphoserine-136 (p136-Bad) residue of Bad (lower panel). Levels of β-actin were reported as a protein loading control (lower panel). (B) Cell-cycle distribution of the transfected cells was assessed 48 hours after transfection by propidium iodide staining and flow cytometric analysis. The sub-G1 peak is indicative of the presence of apoptotic cells with subdiploid DNA content. (C) Occurrence of apoptosis was also performed by a propidium (Prop.) iodide-annexin V staining at 48 hours after transfection.
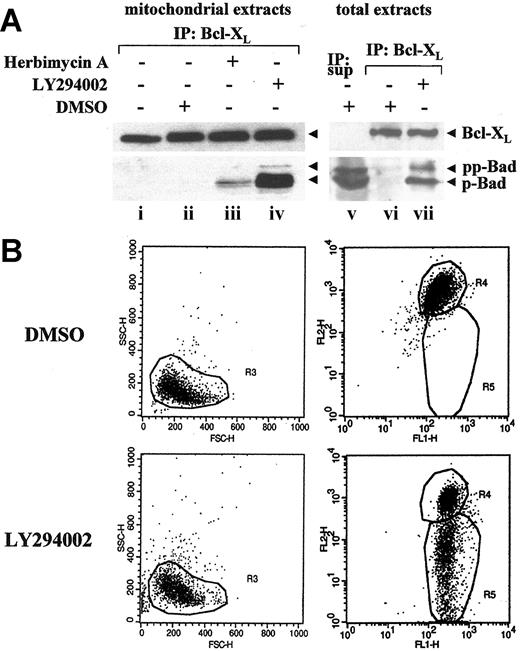
Treatment of ALK+ ALCLs with LY29400211,12 as well as the nonspecific tyrosine kinase inhibitor herbimycin A (HA)32,36 has been shown to promote induction of apoptosis. Therefore, we asked whether Bad dephosphorylation on serine 136, upon PI-3kinase/AKT pathway inactivation, was important to promote Bcl-XL/Bad heterodimerization altering the mitochondria homeostasis (Figure 3). Mitochondrial extracts from K-299 cells untreated (NT) or incubated for 8 hours with vehicle alone (DMSO), 10 μM HA, or 25 μM LY294002 were immunoprecipitated using the anti-Bcl-XL antibody and immunoblotted with anti-Bad and anti-Bcl-XL antibodies. Both proteins were detected in samples treated with HA, and to a higher extent, with LY294002, whereas in untreated and vehicle-alone samples only Bcl-XL was observed (Figure 3A). Western analysis on total lysates confirmed that Bad, mainly in its hypophosphorylated form, was coimmunoprecipitated with an anti-Bcl-XL antibody upon LY294002 treatment, while in DMSO-treated cells Bad remained detectable as a doublet in the supernatant depleted of Bcl-XL complexes (Figure 3A). This finding confirmed that a specific inhibition of PI-3kinase activity appears to dictate subcellular localization of Bad and its capacity to associate with Bcl-XL. As a consequence of Bcl-XL/Bad heterodimerization, an early event in the commitment to cell death is mitochondrial membrane depolarization, which occurs as a result of the opening of permeability transition pores.21,28,37 We therefore analyzed the collapse of the mitochondrial transmembrane potential (ΔΦm) in K-299 cells after exposure to LY294002 for 8 hours using the JC-1 dye (Figure 3B). Blocking the PI-3kinase/AKT pathway caused cells to fall into the R5 region, indicating a loss of mitochondrial homeostasis as JC-1 converts from the red-aggregated form to the green-monomeric form.
Inhibition of NPM/ALK signaling promotes Bcl-XL/Bad heterodimerization and disruption of mitochondrial homeostasis. (A) K-299 cells were incubated with vehicle alone (DMSO), herbimycin A (10 μM), LY294002 (25 μM), or left untreated (NT) for 8 hours. Mitochondrial extracts (left panels) and total lysates (right panels) were immunoprecipitated by using an anti-Bcl-XL polyclonal antibody. Supernatant (IP:sup) depleted of Bcl-XL complexes and immunoprecipitates (IP: Bcl-XL) were analyzed by Western blot with anti-Bcl-XL and Bad antibodies. (B) Disruption of mitochondrial transmembrane potential (ΔΦm) was assessed after an 8-hour incubation with LY294002 (25 μM) or the equivalent amount of DMSO by FACS analysis of JC-1 dye-stained cells. Apoptotic cells fall into the R5 region (right panel) showing a reduced red FL2-H-fluorescence: R5 = 2.1%, in the control sample (upper right panel) and 47.6% in the LY294002-treated sample (lower right panel). The left panels show cells plotted as forward scatter (FSC) against side scatter (SSC) showing no substantial alteration in cell morphology at this early stage of apoptosis induction.
Inhibition of NPM/ALK signaling promotes Bcl-XL/Bad heterodimerization and disruption of mitochondrial homeostasis. (A) K-299 cells were incubated with vehicle alone (DMSO), herbimycin A (10 μM), LY294002 (25 μM), or left untreated (NT) for 8 hours. Mitochondrial extracts (left panels) and total lysates (right panels) were immunoprecipitated by using an anti-Bcl-XL polyclonal antibody. Supernatant (IP:sup) depleted of Bcl-XL complexes and immunoprecipitates (IP: Bcl-XL) were analyzed by Western blot with anti-Bcl-XL and Bad antibodies. (B) Disruption of mitochondrial transmembrane potential (ΔΦm) was assessed after an 8-hour incubation with LY294002 (25 μM) or the equivalent amount of DMSO by FACS analysis of JC-1 dye-stained cells. Apoptotic cells fall into the R5 region (right panel) showing a reduced red FL2-H-fluorescence: R5 = 2.1%, in the control sample (upper right panel) and 47.6% in the LY294002-treated sample (lower right panel). The left panels show cells plotted as forward scatter (FSC) against side scatter (SSC) showing no substantial alteration in cell morphology at this early stage of apoptosis induction.
These data indicate that NPM/ALK blocks the onset of apoptosis by preventing Bcl-XL/Bad heterodimerization and associated preapoptotic mitochondrial perturbations. Therefore, NPM/ALK can deliver its antiapoptotic signal through modulation of the expression levels of Bcl-XL and of its active/inactive conformational states via PI-3kinase/AKT/Bad signaling pathway.
Inhibition of Bcl-XL induces apoptosis in NPM/ALK-transformed lymphoid cells in vitro
The above reported results provide evidence for the hypothesis that NPM/ALK expression protects cells from cell death through the Bcl-XL pathway.
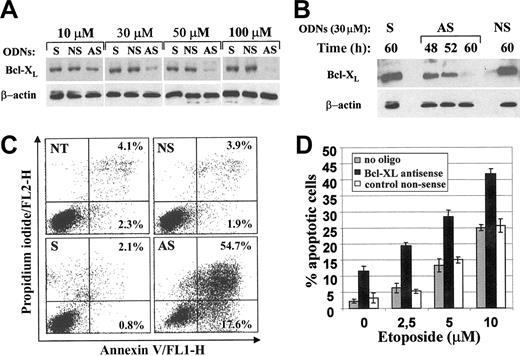
Since antisense ODNs represent a potent approach to downmodulate the expression of specific genes,38 we used antisense strategies targeted to Bcl-XL in order to biologically validate this death regulator as a potential therapeutic target in NPM/ALK+ malignancies. Ba/F3-NPM/ALK+ cells were treated for 72 hours with increasing concentrations of antisense Bcl-XL ODNs corresponding to the AUG-containing region of Bcl-XL mRNA. A dose-dependent inhibition of Bcl-XL protein levels was observed with an almost complete inhibition at 100 μM AS-ODN (Figure 4A). There was no effect of control ODNs representing sense (S) or nonsense scrambled (NS, 4 base mismatched) ODNs on Bcl-XL expression. The 2 control oligonucleotides had some nonspecific effects on cell survival at 50 μM and 100 μM (data not shown). To avoid such nonspecific toxic effects, all subsequent experiments have been performed with 30 μM ODN. A time course experiment showed down-regulation of Bcl-XL protein expression after 60 hours of incubation with Bcl-XL antisense ODNs (Figure 4B). This slightly preceded the massive cell death induction in Ba/F3-NPM/ALK+ cells at 96 hours (Figure 4C). A considerable fraction of NPM/ALK-expressing cells incubated with Bcl-XL antisense ODNs was found in either early (17.6%) or late (54.7%) stages of apoptosis, whereas control ODNs failed to modify the baseline proportion of apoptotic cells compared with the untreated cultures (NT). Figure 4D shows that treatment with Bcl-XL antisense makes cells more sensitive to killing by etoposide, an antineoplastic drug currently used in the treatment of ALCL. When Ba/F3-N/A+ cells incubated with a nonsense oligonucleotide or no oligonucleotide were treated with etoposide, a dose-dependent induction of apoptosis occurred with 25% dead cells at 10 μM drug concentration. In contrast, the combination of the Bcl-XL antisense oligonucleotide and 10 μM etoposide resulted in more than 40% cell death.
Specific antisense oligonucleotide-mediated down-regulation of Bcl-XL increased apoptosis of NPM/ALK+ cells in vitro. (A) Ba/F3-NPM/ALK+ cells were incubated for 72 hours with increasing concentrations of either sense (S), nonsense scrambled (NS), or antisense (AS) phosphorothioated oligonucleotides (ODNs) targeting the Bcl-XL AUG region. Fresh oligonucleotides were added at 0, 16, 24, 32, 48, and 60 hours. Western blot analysis was performed 72 hours after treatment. Blots were reprobed with an anti-β-actin specific antibody as control for protein loading. (B) Total cell lysates from Ba/F3-NPM/ALK+ cells treated with 30 μM Bcl-XL AS-ODN for 48, 52, and 60 hours were compared with those of cultures incubated with sense (S) and nonsense scrambled (NS) ODNs for 60 hours for Bcl-XL expression by immunoblotting. (C) The fraction of apoptotic cells was evaluated by annexin V-propidium iodide staining and flow cytometric analysis after 96 hours of treatment with ODNs or in the untreated control (NT). The values indicate the percentages of cells in early (lower right quadrant) and late (upper right quadrant) apoptosis. (D) Ba/F3-N/A+ cells treated for 48 hours with Bcl-XL antisense, control nonsense scrambled, or no oligodeoxynucleotides (oligo) were incubated with increasing concentration of etoposide for additional 18 hours. Apoptosis was evaluated by AO staining at the end of drug incubation; the data are represented as the mean ± standard deviation (SD) of 3 independent experiments.
Specific antisense oligonucleotide-mediated down-regulation of Bcl-XL increased apoptosis of NPM/ALK+ cells in vitro. (A) Ba/F3-NPM/ALK+ cells were incubated for 72 hours with increasing concentrations of either sense (S), nonsense scrambled (NS), or antisense (AS) phosphorothioated oligonucleotides (ODNs) targeting the Bcl-XL AUG region. Fresh oligonucleotides were added at 0, 16, 24, 32, 48, and 60 hours. Western blot analysis was performed 72 hours after treatment. Blots were reprobed with an anti-β-actin specific antibody as control for protein loading. (B) Total cell lysates from Ba/F3-NPM/ALK+ cells treated with 30 μM Bcl-XL AS-ODN for 48, 52, and 60 hours were compared with those of cultures incubated with sense (S) and nonsense scrambled (NS) ODNs for 60 hours for Bcl-XL expression by immunoblotting. (C) The fraction of apoptotic cells was evaluated by annexin V-propidium iodide staining and flow cytometric analysis after 96 hours of treatment with ODNs or in the untreated control (NT). The values indicate the percentages of cells in early (lower right quadrant) and late (upper right quadrant) apoptosis. (D) Ba/F3-N/A+ cells treated for 48 hours with Bcl-XL antisense, control nonsense scrambled, or no oligodeoxynucleotides (oligo) were incubated with increasing concentration of etoposide for additional 18 hours. Apoptosis was evaluated by AO staining at the end of drug incubation; the data are represented as the mean ± standard deviation (SD) of 3 independent experiments.
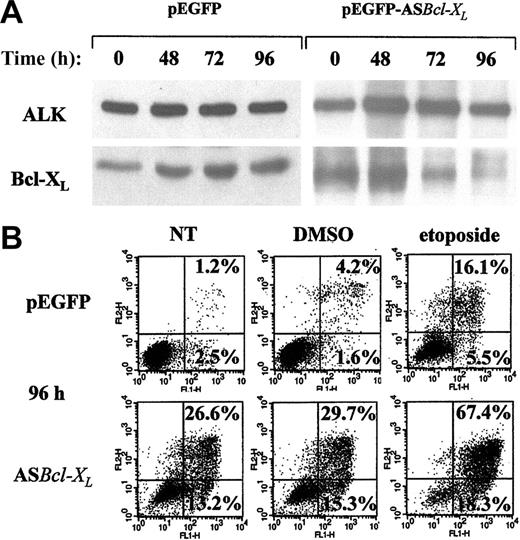
To determine the effect of Bcl-XL down-regulation also in ALK+ ALCL cells, K-299 cells were transiently transfected with a plasmid encoding full-length Bcl-XL antisense cDNA (ASBcl-XL). Figure 5A shows a time-dependent inhibition of Bcl-XL expression with a maximal effect at 96 hours after transfection. The percentage of apoptotic cells was analyzed by annexin V-propidium iodide staining. Figure 5B shows that down-regulation of Bcl-XL caused approximately 40% of the cell population (13.2% + 26.6%) to undergo cell death (Figure 5B, left panels).
Effect of Bcl-XLL down-regulation on cell survival in K-299 cells. K-299 cells were transiently transfected with a pEGFP control vector (left panels) or ASBcl-XL-GFP plasmid (right panels) encoding full-length Bcl-XL antisense cDNA. Cultures containing at least 60% of transfected cells were used for further analysis by Western blot and apoptosis determination. (A) Western blot analysis of total lysates for the expression of Bcl-XL was performed at the indicated time points after transfection. NPM/ALK levels are presented as a protein loading control. (B) At 80 hours after transfection, cultures were treated for 16 hours with DMSO (middle panels), 10 μM etoposide (right panels), or left untreated (left panels). Apoptosis induction was assessed using an annexin V binding assay and flow cytometric analysis.
Effect of Bcl-XLL down-regulation on cell survival in K-299 cells. K-299 cells were transiently transfected with a pEGFP control vector (left panels) or ASBcl-XL-GFP plasmid (right panels) encoding full-length Bcl-XL antisense cDNA. Cultures containing at least 60% of transfected cells were used for further analysis by Western blot and apoptosis determination. (A) Western blot analysis of total lysates for the expression of Bcl-XL was performed at the indicated time points after transfection. NPM/ALK levels are presented as a protein loading control. (B) At 80 hours after transfection, cultures were treated for 16 hours with DMSO (middle panels), 10 μM etoposide (right panels), or left untreated (left panels). Apoptosis induction was assessed using an annexin V binding assay and flow cytometric analysis.
Furthermore, Bcl-XL down-regulation was also associated with an increased susceptibility of cells to the genotoxic compound etoposide (Figure 5B, right panels), while vehicle alone had little effect (Figure 5B, middle panels), suggesting a synergistic effect of DNA damage and Bcl-XL down-regulation on cell death induction.
Conditional suppression of Bcl-XL expression impairs the tumorigenic activity of NPM/ALK in vivo
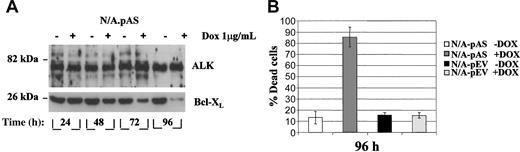
The doxycycline (Dox)-inducible reverse tetracycline transactivator (rtTA) is frequently used for manipulations of transcription levels in a temporally regulated fashion in vivo.39 Using a “pTet-ON” antisense system, murine Ba/F3-NPM/ALK+ cells were stably transfected with a plasmid encoding a Bcl-XL antisense sequence under the control of a doxycycline (Dox)-responsive promoter (Ba/F3-N/A.pAS). After 96 hours of Dox treatment (1 μg/mL) in vitro, Ba/F3-N/A.pAS (N/A-pAS) cells were found to have less than 10% of the initial steady-state level of Bcl-XL (Figure 6A), while the NPM/ALK expression levels were unaffected by the presence of Dox. At the same time, cell viability was significantly decreased (approximately 90% apoptotic cells) as indicated by acridine orange staining, while Ba/F3-NPM/ALK+ cells transfected with an empty vector control (N/A-pEV) did not show any cell death induction in the presence of Dox (Figure 6B).
Dox-inducible down-regulation of Bcl-XL in stable pTet-ON transfectants in vitro. (A) Western blot analysis of Bcl-XL expression in a representative Ba/F3-NPM/ALK+ clone (N/A-pAS) cultured in absence/presence of a single dose of doxycycline (Dox 1 μg/mL) for the indicated times. (B) A Ba/F3-N/A.ASBcl-XL antisense-inducible clone (N/A-pAS) and a Ba/F3-NPM/ALK+ clone bearing an empty vector (N/A-pEV) were grown in triplicate in the absence (-Dox) or presence (+Dox) of doxycycline for 4 days. A single dose of doxycycline (1 μg/mL) was added in the culture medium at the beginning of the experiment and replaced after 2 days. Apoptosis was evaluated by AO staining as reported in Figure 4D. Error bars indicate SD.
Dox-inducible down-regulation of Bcl-XL in stable pTet-ON transfectants in vitro. (A) Western blot analysis of Bcl-XL expression in a representative Ba/F3-NPM/ALK+ clone (N/A-pAS) cultured in absence/presence of a single dose of doxycycline (Dox 1 μg/mL) for the indicated times. (B) A Ba/F3-N/A.ASBcl-XL antisense-inducible clone (N/A-pAS) and a Ba/F3-NPM/ALK+ clone bearing an empty vector (N/A-pEV) were grown in triplicate in the absence (-Dox) or presence (+Dox) of doxycycline for 4 days. A single dose of doxycycline (1 μg/mL) was added in the culture medium at the beginning of the experiment and replaced after 2 days. Apoptosis was evaluated by AO staining as reported in Figure 4D. Error bars indicate SD.
Based on these in vitro results, we examined the ability of Dox-driven Bcl-XL antisense transcription to inhibit the tumorigenic potential of NPM/ALK-transformed Ba/F3 cells in vivo. Ba/F3-NPM/ALK cells (10 × 106) bearing a Dox-inducible empty vector (N/A-pEV) or a Bcl-XL antisense (N/A-pAS) transcript were inoculated intravenously into nude mice (Figure 7A). Half of the mice from each group was maintained under continuous administration of Dox (0.2 mg/mL) in the drinking water. The N/A-pEV and N/A-pAS cell lines induced a fatal disease in 7 of 8 (Figure 7, white squares) and in 15 of 15 (Figure 7, black squares) control animals not receiving Dox, respectively, within a similar time frame (4-8 weeks). The animals showed weight loss, poor fur status, and inguinal/axillary adenopathies. Macroscopic examination of dead animals showed massive para-aortic adenopathies and splenomegaly (data not shown). Doxycycline administration prevented the occurrence of this wasting syndrome in 15 of 15 mice injected with N/A-pAS cells (Figure 7, black circles), while it did not affect the appearance and growth of NPM/ALK+ tumors in the control group (N/A-pEV) (Figure 7, white circles). The inhibitory effect of Dox lasted throughout the 2 months of its administration and was also stable after withdrawal of Dox for a further 2 months. Cells (N/A-pEV and N/A-pAS) were also injected subcutaneously, producing a local growth within 8 days (data not shown). In Table 1, Dox administration for 21 days (starting 24 hours after tumor cell injections) prevented the appearance of tumors in 9 of 12 mice inoculated with N/A-pAS cells, with no effect on tumor growth in mice injected with N/A-pEV cells. To further document the in vivo down-regulation of Bcl-XL by Dox, animals bearing measurable subcutaneous N/A-pEV and N/A-pAS tumors were also treated with Dox. Figure 7B shows that Dox administration for 3 days reduced the expression of Bcl-XL in 2 N/A-pAS regressing tumors without effects in control (N/A-pEV)-treated animals. NPM/ALK protein levels are used as loading controls and did not show substantial variations.
Impaired growth of NPM/ALK-expressing cells in vivo after Bcl-XL down-regulation.(A) Ba/F3-NPM/ALK+ cells (10 × 106) bearing an empty vector control (N/A-pEV, □) and a Dox-inducible ASBcl-XL antisense transgene (N/A-pAS, ○) were injected intravenously into nude mice. Half of the mice from each group (N/A-pEV, ▪; N/A-pAS, •) was maintained under continuous administration of doxycycline (0.2 mg/mL) in the drinking water. Survival of the animals was monitored every other day. (B) Animals injected with N/A-pEV and N/A-pAS cells and bearing measurable nodules (mean tumor weight was 235 mg; 95% confidence interval [CI] = 200-320 mg) were treated with Dox (0.2 mg/mL) in the drinking water. After 3 days of Dox treatment, mice were killed and tumors extracted and analyzed in Western blot (WB) with anti-Bcl-XL and anti-ALK antibodies.
Impaired growth of NPM/ALK-expressing cells in vivo after Bcl-XL down-regulation.(A) Ba/F3-NPM/ALK+ cells (10 × 106) bearing an empty vector control (N/A-pEV, □) and a Dox-inducible ASBcl-XL antisense transgene (N/A-pAS, ○) were injected intravenously into nude mice. Half of the mice from each group (N/A-pEV, ▪; N/A-pAS, •) was maintained under continuous administration of doxycycline (0.2 mg/mL) in the drinking water. Survival of the animals was monitored every other day. (B) Animals injected with N/A-pEV and N/A-pAS cells and bearing measurable nodules (mean tumor weight was 235 mg; 95% confidence interval [CI] = 200-320 mg) were treated with Dox (0.2 mg/mL) in the drinking water. After 3 days of Dox treatment, mice were killed and tumors extracted and analyzed in Western blot (WB) with anti-Bcl-XL and anti-ALK antibodies.
Down-regulation of Bcl-XL prevents growth of subcutaneous ALK+ tumor xenografts
. | . | . | Tumorigenicity in nude mice . | . | . | ||
|---|---|---|---|---|---|---|---|
| . | Growth properties in vitro . | . | Tumors/no. of injections . | . | Dox treatment, d . | ||
| Cells . | −DOX . | +DOX . | −DOX . | +DOX . | . | ||
| N/A.pEV | + | + | 12/12 | 12/12 | 21 | ||
| N/A.pAS | + | − | 12/12 | 3/12 | 21 | ||
. | . | . | Tumorigenicity in nude mice . | . | . | ||
|---|---|---|---|---|---|---|---|
| . | Growth properties in vitro . | . | Tumors/no. of injections . | . | Dox treatment, d . | ||
| Cells . | −DOX . | +DOX . | −DOX . | +DOX . | . | ||
| N/A.pEV | + | + | 12/12 | 12/12 | 21 | ||
| N/A.pAS | + | − | 12/12 | 3/12 | 21 | ||
In vitro growth and in vivo tumorigenic potential of N/A.pEV and N/A.pAS cells. Cells (5 × 105) were cultured for 4 days in absence/presence of 1 μg/mL Dox. Cell viability was evaluated by acridine orange staining as indicated in Figure 6B. N/A.pEV and N/A.pAS cells (20 × 106) were injected subcutaneously in nude mice. At 24 hours after injection, half of each group was maintained in the presence of Dox (0.2 mg/mL) in the drinking water and treatment lasted for 21 days. Mice were monitored daily for the appearance of tumors.
These findings reveal a crucial role for Bcl-XL in mediating the in vivo transforming potential of NPM/ALK.
Discussion
Protection from cell death after cytokine withdrawal or exposure to conventional anticancer drugs has been reported in human NPM/ALK+ ALCLs.9,31 In particular, NPM/ALK exerts its antiapoptotic effect delaying cytosolic accumulation of cytocrome c and activation of caspase-3.31,32 These findings support a mitochondrial control of the apoptogenic proteolytic cascade, although the involvement of Bcl-2 family members in ALK+ ALCL remains unclear.
In the present report we provide direct evidence of a functional link between Bcl-XL and the survival potential of NPM/ALK. Several antisense strategies have been used to directly validate Bcl-XL as a crucial cell fate regulator associated to the transforming ability of NPM/ALK in vitro and in vivo. Tyrosine kinase activity of NPM/ALK is required to up-regulate Bcl-XL protein levels in pro-B murine Ba/F3 cells as well as in human ALK+ ALCL-derived cells, apparently without effects on Bcl-2 expression. The immunohistochemically undetectable Bcl-2 expression in human ALK-positive ALCLs has been recently correlated with the favorable clinical outcome of these lymphomas compared with ALK-negative ALCLs.40,41 However, the expression of Bcl-XL13,15 and Mcl-1 antiapoptotic protein,42 another Bcl-2-like member, could represent important prognostic factors for those ALK+ ALCL patients refractory to the current highly toxic conventional therapy.43 Our findings in human ALK+ ALCL-derived cell lines indicate that Bcl-XL may provide alternative mechanisms resulting in prolonged tumor cell survival. The involvement of Bcl-XL in protecting human K-299 cells from apoptosis-inducing agents was investigated in vitro showing that Bcl-XL down-regulation significantly increased their susceptibility to etoposide. In addition, up-regulation of Bcl-XL in murine Ba/F3 cells transformed by several hybrid oncogenes, including NPM/ALK, has been recently proposed to work in concert with the overexpression of RAD51 to delay the G2/M phase transition of the cell cycle, thereby promoting drug resistance.44 Therefore, these findings support the possibility that inappropriate Bcl-XL expression or its functional modulation may represent an important indicator of the outcome of disease treatment and also has important therapeutic implications in NPM/ALK-mediated oncogenesis. In fact, a few resistant NPM/ALK-expressing cells may be selected during remission by a cytotoxic drug, since a third of “ALKoma” patients experience multiple relapses.45,46
Bcl-XL has been identified to be involved in the neoplastic transformation caused by several oncogenes such as BCR/ABL.47,48 Increased expression of Bcl-XL has been reported in acute myeloid leukemia (AML) and multiple myeloma patients with poor clinical response.49,50 The Jak/Stat and PI-3kinase/AKT activation pathways have been involved in Bcl-XL regulation in multiple hematopoietic cell lineages.51,52 There are 2 recent reports using cell lines derived from ALK+ ALCLs that have shown that Bcl-XL, but not Mcl-1 or survivin, can be downmodulated by treatment with the Jak/Stat inhibitor AG490.13,15 In particular, the Bcl-XL expression rate seems to be strictly related to the NPM/ALK-induced high level of Stat3 protein as well as its constitutive activation in ALK+ ALCLs.13
We also investigated the possibility that posttranslational mechanisms, other than transcriptional induction of Bcl-XL, might influence the balance of competing death/life of ALK+ ALCLs toward survival.
In our experiments, we were unable to detect any coimmunoprecipitation with NPM/ALK and phosphorylated form of Bcl-XL (data not shown), as instead previously described for Bcl-2.53 In contrast, the Bcl-XL antagonist Bad has been found to be primarily regulated by phosphorylation. A PI-3kinase/AKT-mediated phosphorylation of Bad on serine 136 is required for coupling NPM/ALK-delivered survival signals to the mitochondrial death machinery. In fact, LY294002-induced Bad dephosphorylation was able to promote association of Bcl-XL/Bad heterodimers triggering the onset of commitment to apoptosis as indicated by the sudden mitochondrial membrane depolarization. Other than AKT serine/threonine kinase,23,26 the reversible phosphorylation of Bad can be modulated by survival-promoting kinases including Ras-induced mitogen-activated protein (MAP) kinases,22 p21-activated kinase 1,54 protein kinase A (PKA)55 as well as phosphatases, such as protein phosphatase 1 (PP1)56 and a PP2a-like enzyme.57 This could explain the slower mobility form of Bad, which does not appear to be influenced by inhibition of PI-3/AKT kinases in ALK+ ALCL cells. However, further studies are warranted to clarify the biologic implications of these pathways in terms of NPM/ALK-enhanced survival and regulation of Bcl-2 family proteins.
The significant in vitro and in vivo apoptotic effects of Bcl-XL down-regulation in different cell types including human ALK+ ALCL-derived cell lines highlight another important point: the selection of therapeutic targets downstream of known oncogenes. It is known that NPM/ALK activates several signal transduction pathways, therefore it could be hypothesized that the down-regulation of a single protein (or its inhibition) could be easily bypassed by activation of parallel pathways. The data presented here indicate that in the context of NPM/ALK-mediated oncogenicity the presence of Bcl-XL remains crucial and accounts for most of the survival potential of neoplastic cells. This point is of general importance because primary oncogenes are not always easy targets for therapies, especially when they do not code for an enzyme.
It is important to note in this regard that there is presently considerable interest in discovery of small-molecule inhibitors targeted at the BH3-binding hydrophobic groove of Bcl-XL, which can antagonize its pore-forming activity58,59 and protein-protein interactions.60 These attempts are based on the hypothesis that Bcl-XL down-regulation in normal cells will not result in significant toxicity, an important aspect that was not investigated here and will require further analysis.
In conclusion, our results provide evidence that Bcl-XL is positively regulated by NPM/ALK in human lymphoid cells and could represent a relevant target for a new generation of therapeutics for the treatment of ALK+ ALCLs. By altering the ratio of prosurvival and proapoptotic proteins through Bcl-XL down-regulation or inhibition, it may still be possible to antagonize the pleiotropic antiapoptotic cascades activated by NPM/ALK in lymphoma cells.
Prepublished online as Blood First Edition Paper, December 4, 2003; DOI 10.1182/blood-2003-09-3144.
Supported by the Italian Association for Cancer Research (AIRC), CNR, EU (Prokinase Research), José Carreras, and Antonio Castelnuovo Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Stephan W. Morris (St Jude Research Hospital, Memphis, TN) for the NPM/ALK-transformed Ba/F3 cells as well as for NPM/ALK kinase-defective expression construct. We thank Dr Karen Pulford (John Radcliffe Hospital, Oxford, United Kingdom) for the monoclonal anti-ALK1 antibody and Dr Fabrice Gouilleux (ICGM-INSERM, Aimes, France) for kindly providing the pRSV-Bcl-XL cDNA expression vector.

![Figure 7. Impaired growth of NPM/ALK-expressing cells in vivo after Bcl-XL down-regulation.(A) Ba/F3-NPM/ALK+ cells (10 × 106) bearing an empty vector control (N/A-pEV, □) and a Dox-inducible ASBcl-XL antisense transgene (N/A-pAS, ○) were injected intravenously into nude mice. Half of the mice from each group (N/A-pEV, ▪; N/A-pAS, •) was maintained under continuous administration of doxycycline (0.2 mg/mL) in the drinking water. Survival of the animals was monitored every other day. (B) Animals injected with N/A-pEV and N/A-pAS cells and bearing measurable nodules (mean tumor weight was 235 mg; 95% confidence interval [CI] = 200-320 mg) were treated with Dox (0.2 mg/mL) in the drinking water. After 3 days of Dox treatment, mice were killed and tumors extracted and analyzed in Western blot (WB) with anti-Bcl-XL and anti-ALK antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/7/10.1182_blood-2003-09-3144/6/m_zh80070459170007.jpeg?Expires=1763462246&Signature=kqxTVb9episBNgDPEZLWyMWuylWJEbKe1CCKougUF8M5vFSFMvV-ASKrwUQdC207Q4ACX~XahIOkB4jHkXfQc48zKfL6T~CPWUL7S2z4M55L8lemPFsFjK0iTZCHgZG8bC7MnvwuZn~s1uFkY~lpd4jWJqYCcRIYsBsk5DVWGBsR-79zJvzkypuC3vjgxdGutBuxJ2YT~1BkBKCuuFXLv7uVW1JF~pQNYu7IK55PlFjlZiSTmT0fpQBynnbHKnhqEMsOQfZOwjKq53XKDxq5QfXW9I3V8usl0xqCrjZkkeo73mQVbn2TNZuAPcTIkler51lm6TqQGluReteQMCBtGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal