Abstract
Isotype switch commonly follows onset of somatic hypermutation in the germinal center (GC), with activation-induced cytidine deaminase (AID) as a prerequisite. Mantle cell lymphoma (MCL) with t(11;14) includes a subset with unmutated (UM) and a minor subset with mutated (MUT) VH genes. Here, we investigated whether switch events and AID expression occur in MCL. In 4 of 6 UM and 4 of 7 MUT MCLs, alternative tumor-derived Cγ,α,ϵ transcripts were identified. AID transcripts, including a splice variant, were common to both subsets. AID expression correlated with switch in 8 of 8 cases, but in 3 of 5 cases it occurred with switch absent. Circle transcripts (Iγ-Cμ/Iα-Cμ) were identified in 5 of 7 evaluated cases. In 1 of 12 cases, 12% of tumor cells expressed immunoglobulin L-restricted surface IgA. Ongoing switch recombination events appear to be a feature of MCL, likely restricted to a minor tumor subpopulation, with occasional variant sIg expression. UM MCLs implicate origins from pre-GC B cells and reveal switch events at ectopic sites. (Blood. 2004;103:2795-2798)
Introduction
Mantle cell lymphoma (MCL) is characterized by a nodal infiltrate of neoplastic cells with a distinct histology and surface immunoglobulin M (sIgM)(D)++CD5/19/20/79b+CD23- immunophenotype.1 At presentation, disease may involve the spleen, bone marrow, and extranodal sites, with circulating abnormal lymphoma cells. A signature t(11;14) abnormality in the IgH locus readily identifies MCL.1 Molecular mapping indicates ontogeny of the translocation early in B cells at the stage of DH-JH (diversity region of the heavy chain-joining region of the heavy chain) recombination.2 Translocation alone is considered insufficient for neoplastic transformation,3 which may occur subsequently via 2 pathways. One subset of MCL derives from a cell of origin with unmutated (UM) VH genes and the other minor subset derives from a cell with mutated (MUT) genes.4,5,6
Somatic mutation of V genes generally localizes to germinal centers (GCs) in secondary follicles.7 Isotype switch events mainly follow onset of hypermutation in normal B cells and for both mechanisms, the expression of activation-induced cytidine deaminase (AID) is essential.8,9 Isotype class switch recombination (CSR) by a deletional mechanism involves double-strand DNA breaks.9 Transcription of excised switched circular DNA generates circle transcripts (CTs), a hallmark of active CSR.9,10 AID deficiency blocks CSR completely.9 Isotype switch may occur by additional mechanisms.11,12 Expression of AID in vivo normally requires CD40 ligand (CD40L) signals.13 Circulating normal B cells show no evidence for CSR, although they have a potential to switch with appropriate signals.14,15 In contrast, circulating chronic lymphocytic leukemia (CLL) B cells appear to have engaged such signals and constitutively express clonally related switched transcripts, AID and CTs.15 In addition to a possible role in switch, AID expression in CLL associates with mutations in the preswitch Cμ region but not VH,16 suggesting a potential to target non-V gene loci.
In this study, we evaluated the occurrence of isotype switch and AID expression in subsets of MCL cells differing by VH mutational status.
Study design
MCL samples
Eighteen MCL cases with blood lymphocytosis, part of a larger study with confirmed diagnosis of MCL,5 were evaluated (17 of 18 peripheral blood mononuclear cell [PBMNC] samples; case 11, bone marrow [BM]-derived MNCs). In each, the t(11;14) translocation was verified by karyotype (n = 3) or interphase fluorescence in situ hybridization (FISH; n = 15). Tumor load (% nuclei with CCND1-IgH fusion) was greater than 90% in 8 cases and 43% to 88% in the remainder. Immunophenotype showed sIgM(D)++CD5/19/20/79bFMC7+CD10-CD23-/weak expression. Variant sIg expression was assessed by 3-color flow cytometry with allophycocyanin (APC)-conjugated anti-CD19; phycoerythrin (PE)-conjugated (f(ab′)2) anti-κ or anti-λ light chain; and fluorescein isothiocyanate (FITC)-conjugated (f(ab′)2) anti-μ, anti-α, or anti-γ heavy chain antibodies (Beckman-Coulter, Hialeah, FL; Dakopatts, Glostrup, Denmark).
Analysis of VH genes, switch variant, and circle transcripts
PBMNCs (> 5 × 106 ) were extracted for RNA and cDNA synthesized using oligo-dT.17 Tumor-derived VH genes were identified using replicate polymerase chain reaction (PCR) and cloning procedures.5,17 Eight to 12 tumor-derived clones were fully sequenced. Homology to germ line VH of 98% or above denoted UM status. For identification of variant tumor-derived isotype switch transcripts, specific CDR3-based primers were used in a nested reverse transcriptase-PCR (RT-PCR) protocol.17 IH- and CH-exon-specific primers were used to assay CTs as described.15 CT-amplified products of predicted size were directly sequenced.15
Expression of AID
AID expression was analyzed by standard RT-PCR: forward, 5′-ATGGACAGCCTCTTGATGAAC; reverse, 5′-CTCGTAAGTCATCAACCTCATACA.18 The primers distinguish wild-type and splice variants of AID on the basis of size. Positivity was assessed after 30 cycles. PBMNCs from 4 of 4 healthy cases were also analyzed.
Results and discussion
Of the MCL cases, 11 of 18 were UM and 7 of 18 MUT, displaying intraclonal homogeneity, with cohort mutational status reported previously.5
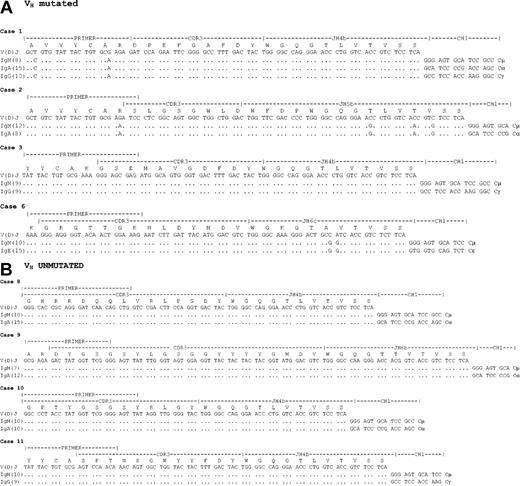
In 4 of 6 UM cases and in 4 of 7 MUT cases, variant tumor-derived isotype switch transcripts were detected using specific CDR3-based primers (Figure 1). Cα,γ transcripts were observed in both subsets, with Cϵ in 1 MUT case (Figure 1 and Table 1). Identification of mature VDJ-Cα,γ,ϵ variant transcripts indicates activation of switch mechanisms in vivo.
Nucleotide and deduced amino acid sequences of the VDJ-Cα,γ,ϵ variant transcripts aligned with tumor VDJ-Cμ sequences in unmutated and mutated VH gene t(11;14) mantle cell lymphoma subsets. Panel A shows the mutated subset and panel B shows the unmutated subset. The number of clones showing identical sequence are indicated in parentheses. The position of the 5′-FR3/CDR3 primer is indicated, and the position of the downstream CH primer (not shown) allowed identification of specific isotype transcripts. Tumor VH sequences have been submitted to the European Molecular Biology Laboratory (EMBL) database (AJ617568-AJ617575).
Nucleotide and deduced amino acid sequences of the VDJ-Cα,γ,ϵ variant transcripts aligned with tumor VDJ-Cμ sequences in unmutated and mutated VH gene t(11;14) mantle cell lymphoma subsets. Panel A shows the mutated subset and panel B shows the unmutated subset. The number of clones showing identical sequence are indicated in parentheses. The position of the 5′-FR3/CDR3 primer is indicated, and the position of the downstream CH primer (not shown) allowed identification of specific isotype transcripts. Tumor VH sequences have been submitted to the European Molecular Biology Laboratory (EMBL) database (AJ617568-AJ617575).
Analysis of isotype switch events and AID expression in MCL
Case . | VH status / % . | CH . | AID, wt . | AID, −ex4 . | CTs . | slg . |
|---|---|---|---|---|---|---|
| 1 | MUT/90.8 | α + γ | + | − | IαCμ | A (12%) |
| 2 | MUT/97.3 | α | + | − | − | − |
| 3 | MUT/97.6 | γ | + | − | IγCμ | − |
| 4 | MUT/96.7 | − | + | − | ND | − |
| 5 | MUT/93.8 | − | + | − | ND | − |
| 6 | MUT/94.6 | ϵ | + | − | ND | − |
| 7 | MUT/95 | − | + | + | ND | − |
| 8 | MUT/98 | α | + | + | IαCμ | − |
| 9 | UM/100 | α | + | + | IαCμ | − |
| 10 | UM/100 | α | + | + | − | − |
| 11 | UM/100 | γ | + | + | IγCμ | − |
| 12* | UM/99.3 | − | − | + | ND | − |
| 13 | UM/100 | − | − | − | ND | ND |
| 14 | UM/98.6 | ND | + | + | ND | ND |
| 15 | UM/100 | ND | + | + | ND | ND |
| 16 | UM/98.3 | ND | + | + | ND | ND |
| 17 | UM/98.6 | ND | + | + | ND | ND |
| 18 | UM/98.3 | ND | + | + | ND | ND |
Case . | VH status / % . | CH . | AID, wt . | AID, −ex4 . | CTs . | slg . |
|---|---|---|---|---|---|---|
| 1 | MUT/90.8 | α + γ | + | − | IαCμ | A (12%) |
| 2 | MUT/97.3 | α | + | − | − | − |
| 3 | MUT/97.6 | γ | + | − | IγCμ | − |
| 4 | MUT/96.7 | − | + | − | ND | − |
| 5 | MUT/93.8 | − | + | − | ND | − |
| 6 | MUT/94.6 | ϵ | + | − | ND | − |
| 7 | MUT/95 | − | + | + | ND | − |
| 8 | MUT/98 | α | + | + | IαCμ | − |
| 9 | UM/100 | α | + | + | IαCμ | − |
| 10 | UM/100 | α | + | + | − | − |
| 11 | UM/100 | γ | + | + | IγCμ | − |
| 12* | UM/99.3 | − | − | + | ND | − |
| 13 | UM/100 | − | − | − | ND | ND |
| 14 | UM/98.6 | ND | + | + | ND | ND |
| 15 | UM/100 | ND | + | + | ND | ND |
| 16 | UM/98.3 | ND | + | + | ND | ND |
| 17 | UM/98.6 | ND | + | + | ND | ND |
| 18 | UM/98.3 | ND | + | + | ND | ND |
Circle transcripts (CTs) were analyzed as described,15 with sequence analysis confirming identity of RT-PCR products at the nucleotide level (data not shown). CT sequences derived from the MCL cases have been deposited in the EMBL databases (AJ617576-AJ617579). For Iγ-Cμ, CTs were generated from alternative splice sites in Sγ. As controls for CT analysis, 2 CLL cases with unmutated VH genes were analyzed under identical conditions,15 and found positive for Iγ-Cμ/Iγ-Cμ (data not shown).
+ indicates present; −, absent; and ND, not determined.
In case no. 12, a role for AID lacking exon 4 in class switch recombination is unclear.
AID mRNA expression is restricted to GC centroblasts or centrocytes in normal B cells.15,19,20 Recently, 3 UM cases of MCL and 4 cases of MCL undefined by VH mutation were separately reported as negative for AID transcripts.20,21 Interestingly, we observed that in both UM and MUT MCL cells, AID expression is a common feature. The primary wild-type (wt) transcript for AID was identified in 9 of 11 UM and 7 of 7 MUT cases (Figure 2 and Table 1). A splice variant transcript lacking exon 4 was identified,20,21 predominantly in UM MCLs (10 of 11 cases; Figure 2 and Table 1), confirmed in each case by sequence analysis (data not shown). No other AID variant transcripts were observed.19,20 AID expression is most likely to be tumor cell related, since normal circulating B cells are devoid of AID expression under assay conditions employed here,15,19,20 as confirmed in 4 of 4 controls (data not shown). Somatic mutation is not ongoing in MUT MCL cells, and AID appears dissociated from VH gene mutational activity. This differs from findings in other non-Hodgkin lymphomas, where AID expression assayed by RT-PCR always correlates with somatically mutated cells20 or with a GC phenotype.21 It also differs from CLL where AID expression associates largely with unmutated VH.19
Correlation of expression of AID and its splice variant with identification of variant tumor-derived isotype switch transcripts and circle transcripts in unmutated and mutated VH gene t(11;14) mantle cell lymphoma. Mutational status of tumor VH genes is shown as % homology to germ line VH gene segment (> 98% homology denotes unmutated status [UM] vs mutated [MUT]). Variant tumor isotype transcripts were identified by amplification with CDR3-specific primers together with downstream isotype-specific CH primer (ND, not determined). Two AID transcripts, wild-type (wt; 656 bp) and variant (loss of exon 4, -ex4; 454 bp), were identified by RT-PCR and amplified bands of predicted size resolved on agarose gels prior to nucleotide sequence verification. AID expression in the lymphoma cell line Ramos served as a positive control. Amplification of VH genes was used to confirm integrity of RNA and loading. Comparable amounts of cDNA were used for VH gene and AID amplification. Weakly amplified bands for variant AID transcripts in 2 cases (MUT case 7 and UM case 12) were confirmed as positive by cloning and sequence analysis. In UM case 11, additional bands in AID amplification were nonspecific as shown by sequence data. In 4 of 4 normal PBMNC preparations, AID expression was not detected under the conditions used (data not shown).
Correlation of expression of AID and its splice variant with identification of variant tumor-derived isotype switch transcripts and circle transcripts in unmutated and mutated VH gene t(11;14) mantle cell lymphoma. Mutational status of tumor VH genes is shown as % homology to germ line VH gene segment (> 98% homology denotes unmutated status [UM] vs mutated [MUT]). Variant tumor isotype transcripts were identified by amplification with CDR3-specific primers together with downstream isotype-specific CH primer (ND, not determined). Two AID transcripts, wild-type (wt; 656 bp) and variant (loss of exon 4, -ex4; 454 bp), were identified by RT-PCR and amplified bands of predicted size resolved on agarose gels prior to nucleotide sequence verification. AID expression in the lymphoma cell line Ramos served as a positive control. Amplification of VH genes was used to confirm integrity of RNA and loading. Comparable amounts of cDNA were used for VH gene and AID amplification. Weakly amplified bands for variant AID transcripts in 2 cases (MUT case 7 and UM case 12) were confirmed as positive by cloning and sequence analysis. In UM case 11, additional bands in AID amplification were nonspecific as shown by sequence data. In 4 of 4 normal PBMNC preparations, AID expression was not detected under the conditions used (data not shown).
AID expression accompanied VDJ-CH switch events in 8 of 8 MCL cases but was observed in 3 cases in the absence of such transcripts (Figure 2 and Table 1), indicating dissociation from switch activity when assessing the whole-tumor population. In those cases displaying variant transcripts, we sought evidence for CSR events by examining CT expression.10,15 In 5 of 7 cases where material was available, we identified Iγ-Cμ and Iα-Cμ CTs, confirmed by sequence analysis (Table 1; data not shown). In normal human circulating B cells, CTs are not detectable in either the naive or memory pool.15 As 6 of 7 of these MCL samples were peripheral mononuclear cells and 1 was BM derived, these CTs are most likely to be tumor associated. AID expression paralleled CTs in 5 of 5 cases (Figure 2 and Table 1). In normal B cells, CD40L and interleukin 4 (IL-4) induce CH germ line transcripts.22 These are also inducible in AID-/- B cells, but IH-CH CTs cannot be generated.9,10 This indicates that specific CTs identified in MCL represent active CSR events. GC-linked CSR is the normal pathway,8,9 and this may explain switching in MUT MCL. However, switch events can occur in extrafollicular sites.23 MCL cells with unmutated VH genes imply origins from pre-GC B cells and appear to have initiated switch events in a non-GC ectopic environment where somatic mutation is silent. For these cells, the potential exists to respond to T-cell-independent antigens.24
When isotype switch to downstream CH occurs by deletional DNA recombination, Cμ is excised as switch circles.9,15,22 In these MCL cases, sIgM(D)++ expression is clearly evident and represents the functional allele, with the other allelic 14q32 site fused to the aberrant t(11;14) translocation.1,2 Fine mapping of both allelic loci by DNA fiber FISH analysis indicates that deletional events in CH appear to occur infrequently in MCL cells.25 Tumor cells, however, retain the potential to generate switched sIg by recombination on the functional allele. In 1 of 12 cases here, a low number (12%) of tumor cells appears to express sIgA (Table 1), suggesting that in most cases, cell numbers bearing variant sIg are below the detection sensitivity of conventional immunophenotyping in MCL. Identification of mature switch variants by nested RT-PCR, together with specific CTs, indicates that CSR events are occurring, most likely in a tumor subpopulation on the functional allele. This level of switch activity in MCL is not readily identifiable when using generic CH probes and primary multiplex RT-PCR,6 a method that lacks the sensitivity of the tumor-specific CDR3-based primers used here.
Our observations suggest that switch events and AID expression may indeed be uncoupled, at least in some cases, and that AID in MCL is a tumor-related activation phenomenon. This raises the specter of aberrant chromosomal events in MCL.
Prepublished online as Blood First Edition Paper, October 9, 2003; DOI 10.1182/blood-2003-05-1632.
Supported by The Leukaemia Research Fund (United Kingdom).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Correlation of expression of AID and its splice variant with identification of variant tumor-derived isotype switch transcripts and circle transcripts in unmutated and mutated VH gene t(11;14) mantle cell lymphoma. Mutational status of tumor VH genes is shown as % homology to germ line VH gene segment (> 98% homology denotes unmutated status [UM] vs mutated [MUT]). Variant tumor isotype transcripts were identified by amplification with CDR3-specific primers together with downstream isotype-specific CH primer (ND, not determined). Two AID transcripts, wild-type (wt; 656 bp) and variant (loss of exon 4, -ex4; 454 bp), were identified by RT-PCR and amplified bands of predicted size resolved on agarose gels prior to nucleotide sequence verification. AID expression in the lymphoma cell line Ramos served as a positive control. Amplification of VH genes was used to confirm integrity of RNA and loading. Comparable amounts of cDNA were used for VH gene and AID amplification. Weakly amplified bands for variant AID transcripts in 2 cases (MUT case 7 and UM case 12) were confirmed as positive by cloning and sequence analysis. In UM case 11, additional bands in AID amplification were nonspecific as shown by sequence data. In 4 of 4 normal PBMNC preparations, AID expression was not detected under the conditions used (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/7/10.1182_blood-2003-05-1632/6/m_zh80070459090002.jpeg?Expires=1763471387&Signature=5B5NvqU5lGpozFt9HqCAPh5S2dfsNDOtNseaVUWIifLjUTTHsOnfoJuD8SA6XIZ3~Rk6by~rCBIf0m7~D8acWF1aOqGL5QMhel7fv7vtKEWNFFpYdnV5cQrEJqx-~O~3s~kIPldxRSRY8obwVQJogur4X1xtQo~vtEpogNiSEbIseQwRJktgz8NhRiQUchXzftCiKUD~VkqlCV4ITCOwZPxiEJN53~LZee~WNNZGNUmr6laURm7WH73H3e841Ii8xdOO4MgDSvjv~pD7EVgoaVJzrxQQ0WCGYBa2EZ4POeiWxt0nYaPqfhLY1GICaSLDx8BfIC3XPrzzSPA8bqif6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal