Abstract
Significant engraftment variability occurs among patients following nonmyeloablative hematopoietic cell transplantation. We analyzed the impact of multiple factors on donor myeloid and T-cell engraftment in 36 patients with metastatic tumors undergoing cyclophosphamide/fludarabine-based conditioning. Higher CD34+ doses facilitated donor myeloid engraftment, while prior chemotherapy exposure facilitated both donor myeloid and T-cell engraftment. At day 30, median donor T-cell and myeloid chimerism was 98% and 76%, respectively, in those patients with prior chemotherapy versus 88% (P = .008) and 26% (P < .0001) in chemotherapy-naive patients. Donor myeloid chimerism at day 45 was predicted by prior chemotherapy exposure and the log10 of the CD34+ dose (adjusted coefficient of determination [R2] = .47; P < .0001), while chemotherapy alone impacted donor T-cell engraftment. Patients with prior chemotherapy were more likely to develop acute grades II to IV graft-versus-host disease (GVHD; 8/18) compared with chemotherapy-naive patients (2/18; P = .031). Thus, tailoring the intensity of nonmyeloablative conditioning based on prior chemotherapy exposure is an important consideration in trial design.
Introduction
Lympho-hematopoietic engraftment following nonmyeloablative hematopoietic cell transplantation (HCT) is influenced by the intensity and type of conditioning.1-11 Although donor T-cell engraftment precedes myeloid engraftment following cyclophosphamide/fludarabine-based conditioning, significant variability among patients in the rate of donor engraftment occurs. Since chimerism patterns may predict some transplantation events (graft-versus-host disease [GVHD], rejection, etc),6,12,13 we investigated the effects of multiple patient and transplantation-related factors on the kinetics of donor myeloid and T-cell engraftment in patients undergoing nonmyeloablative HCT.
Study design
Between May 1999 and July 2002, 36 sequential patients with metastatic solid tumors were treated according to National Heart, Lung, and Blood Institute (NHLBI) institutional review board–approved protocols investigating donor immune-mediated graft-versus-tumor effects after allogeneic HCT. Nonmyeloablative conditioning with cyclophosphamide (120 mg/kg) and fludarabine (125 mg/m2) was followed by infusion of a granulocyte colony-stimulating factor (G-CSF)–mobilized hematopoietic cell allograft from a human leukocyte antigen (HLA)–identical sibling. Cyclosporine (CSA) alone (n = 10) or combined with mycophenolate mofetil (MMF; 1 g twice a day; n = 22) or methotrexate (MTX; 5 mg/m2 intravenously days 1, 3, 6; n = 4) was used as GVHD prophylaxis.
To quantify donor and recipient engraftment after transplantation, a quantitative polymerase chain reaction (PCR)–based analysis of short tandem repeats (STRs) was used to measure chimerism.13,14 DNA was extracted from donor and pretransplantation patient blood samples, and PCR using fluorescent primer sets flanking 8 different STRs and Amelogenin was performed using manufacturer's conditions (Powerplex 1.2; Promega, Madison, WI) to identify an informative allele. Ficoll-hypaque–fractionated mononuclear cells were collected on posttransplantation days +15, +30, +45, +60, and +100 and sorted into CD14+/CD15+ myeloid and CD3+ T cells using immunomagnetic beads (Dynal AS, Oslo, Norway). DNA was extracted and lineage-specific chimerism was performed by PCR using fluorescent primers flanking a single STR previously identified as polymorphic between the patient and donor. Predetermined mixtures of pretransplantation patient and donor DNA were run in conjunction with test samples as quantitative controls (sensitivity for minor populations 2%-3%). The PCR products were analyzed with a 310 ABI PRISM sequencer with the chimerism percentage determined by comparing the ratio of donor and patient bands (Genescan software; both from PE Applied Biosystems, Foster City, CA).
Factors influencing chimerism considered in a multiple regression analysis included patient age, sex, history of prior chemotherapy (any or none), CD34+ and CD3+ dose, patient pretransplantation absolute lymphocyte count (ALC), GVHD prophylactic regimen, and donor-patient sex mismatch. Step-down multiple linear regression15 was used to predict percent donor chimerism on day 45 in the 2 lineages. Variables considered as potential predictors of CD14+/15+ (myeloid) and CD3+ (T-cell) chimerism on day 45 included the following: CD34+ dose or its logarithm (base 10); CD3 dose or its logarithm (base 10); pretransplantation ALC or its logarithm (base 10); age, sex, female-to-male mismatch or any sex mismatch; and whether or not the patient had received prior chemotherapy. For the model, fit was assessed using the overall F-statistic, the overall coefficient of determination (R2), and plots of residuals and the Cook distance. Data analysis was undertaken using Statistical Analysis Package (SAS) version 8.2 (SAS Institute, Cary, NC). Time to acute GVHD was estimated using the Kaplan-Meier method with comparisons using the log-rank test.15
Results and discussion
All 36 patients had progressive, treatment-refractory metastatic solid tumors failing conventional therapy (Table 1). The median age was 48.5 years (range, 33-66 years) and the median ALC (pretransplantation) was 1.24 × 109 cells/L (1240 cells/μL) (range, 0.55 × 109 cells/L to 4.39 × 109 cells/L [range, 550 cells/μL to 4390 cells/μL]). Three patients had prior autologous transplantations and 18 patients (50%) had never received any chemotherapy before undergoing nonmyeloablative conditioning. Among the 18 patients given prior chemotherapy, 16 received at least 1 combination chemotherapy regimen; the median number of prior chemotherapy regimens was 2 (range, 1-4) and the median number of prior chemotherapeutic agents was 3.5 (range, 1-8). Seventeen patients received a sex-mismatched transplant including 4 male-into-female allografts. The median CD34+ and CD3+ doses were 7.6 × 106 cells (range, 1.9 × 106 cells to 16.0 × 106 cells) and 370 × 106 cells (range, 90 × 106 cells to 590 × 106 cells) per kg recipient weight, respectively.
Patient characteristics
Patient no. . | Age, y . | Sex . | Diagnosis . | Prior chemotherapy . | GVHD prophylaxis . | Prior autologous transplantation . | CD34 dose × 106/kg . | CD3 dose × 108/kg . | Pretransplantation lymphocyte count, μL . | Donor/recipient sex matched . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | M | RCC | No | CSA | No | 6 | 1.6 | 1512 | Yes |
| 2 | 60 | M | Melanoma | Yes | CSA | No | 6.9 | 4.5 | 1110 | No (F → M) |
| 3 | 37 | M | RCC | No | CSA | No | 9.8 | 4.4 | 1361 | Yes |
| 4 | 66 | M | Basal cell carcinoma | Yes | CSA | No | 3.2 | 3.8 | 1065 | Yes |
| 5 | 56 | F | RCC | No | CSA | No | 2.7 | 4.8 | 1311 | Yes |
| 6 | 44 | M | RCC | No | CSA | No | 2.2 | 4.1 | 554 | No (F → M) |
| 7 | 65 | M | RCC | Yes | CSA | No | 13.8 | 3.6 | 820 | Yes |
| 8 | 50 | M | RCC | No | CSA | No | 9 | 5.9 | 2864 | No (F → M) |
| 9 | 60 | M | RCC | No | CSA | No | 4.5 | 4 | 1501 | No (F → M) |
| 10 | 42 | M | RCC | No | CSA | No | 9.8 | 2.7 | 1829 | Yes |
| 11 | 36 | M | Adenocarcinoma* | Yes | CSA + MMF | No | 16 | 3.4 | 1202 | No (F → M) |
| 12 | 36 | F | Esophageal cancer | Yes | CSA + MMF | No | 11.9 | 4 | 862 | Yes |
| 13 | 49 | M | Melanoma | Yes | CSA + MMF | No | 9.4 | 4.1 | 1057 | Yes |
| 14 | 52 | M | RCC | No | CSA + MMF | No | 1.9 | 2.7 | 1917 | No (F → M) |
| 15 | 46 | M | Colon cancer | Yes | CSA + MMF | No | 3.9 | 4 | 1482 | Yes |
| 16 | 62 | M | RCC | No | CSA + MMF | No | 5.1 | 2.2 | 1170 | No (F → M) |
| 17 | 51 | M | RCC | No | CSA + MMF | No | 5.6 | 0.9 | 3368 | No (F → M) |
| 18 | 42 | F | Breast cancer | Yes | CSA + MMF | No | 9.7 | 4.7 | 1050 | Yes |
| 19 | 42 | F | RCC | No | CSA + MMF | No | 6.1 | 5 | 1046 | Yes |
| 20 | 56 | M | RCC | No | CSA + MMF | No | 5.5 | 4.2 | 4390 | No (F → M) |
| 21 | 48 | F | RCC | No | CSA + MMF | No | 4 | 2.4 | 922 | No (F → M) |
| 22 | 43 | F | Breast cancer | Yes | CSA + MMF | Yes | 14 | 2.3 | 628 | No (M → F) |
| 23 | 46 | M | Sarcoma | Yes | CSA + MMF | Yes | 10 | 3.9 | 2202 | Yes |
| 24 | 33 | M | RCC | No | CSA + MMF | No | 3.9 | 2.3 | 1536 | No (F → M) |
| 25 | 64 | M | Prostate cancer | No | CSA + MMF | No | 2.1 | 2.7 | 953 | No (F → M) |
| 26 | 48 | F | RCC | Yes | CSA + MMF | No | 4 | 3.5 | 1173 | Yes |
| 27 | 54 | M | RCC | No | CSA + MTX | No | 9 | 3.9 | 1509 | Yes |
| 28 | 48 | M | RCC | No | CSA + MTX | No | 9.2 | 3.7 | 1929 | Yes |
| 29 | 53 | M | RCC | No | CSA + MTX | No | 4.8 | 2 | 1277 | No (F → M) |
| 30 | 53 | M | Colon cancer | Yes | CSA + MMF | No | 4 | 3.2 | 1372 | Yes |
| 31 | 51 | F | RCC | Yes | CSA + MTX | No | 14.4 | 4.8 | 2114 | Yes |
| 32 | 65 | F | Cholangiocarcinoma | Yes | CSA + MMF | No | 8.4 | 3.5 | 1063 | No (M → F) |
| 33 | 46 | F | Adrenocortical carcinoma | Yes | CSA + MMF | No | 10.8 | 2 | 550 | No (M → F) |
| 34 | 51 | F | Hepatocellular carcinoma | Yes | CSA + MMF | No | 11.2 | 3.1 | 689 | No (M → F) |
| 35 | 45 | F | Breast cancer | Yes | CSA + MMF | Yes | 14.4 | 2.5 | 1335 | Yes |
| 36 | 39 | F | Ovarian cancer | Yes | CSA + MMF | No | 12.4 | 5.5 | 800 | Yes |
Patient no. . | Age, y . | Sex . | Diagnosis . | Prior chemotherapy . | GVHD prophylaxis . | Prior autologous transplantation . | CD34 dose × 106/kg . | CD3 dose × 108/kg . | Pretransplantation lymphocyte count, μL . | Donor/recipient sex matched . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | M | RCC | No | CSA | No | 6 | 1.6 | 1512 | Yes |
| 2 | 60 | M | Melanoma | Yes | CSA | No | 6.9 | 4.5 | 1110 | No (F → M) |
| 3 | 37 | M | RCC | No | CSA | No | 9.8 | 4.4 | 1361 | Yes |
| 4 | 66 | M | Basal cell carcinoma | Yes | CSA | No | 3.2 | 3.8 | 1065 | Yes |
| 5 | 56 | F | RCC | No | CSA | No | 2.7 | 4.8 | 1311 | Yes |
| 6 | 44 | M | RCC | No | CSA | No | 2.2 | 4.1 | 554 | No (F → M) |
| 7 | 65 | M | RCC | Yes | CSA | No | 13.8 | 3.6 | 820 | Yes |
| 8 | 50 | M | RCC | No | CSA | No | 9 | 5.9 | 2864 | No (F → M) |
| 9 | 60 | M | RCC | No | CSA | No | 4.5 | 4 | 1501 | No (F → M) |
| 10 | 42 | M | RCC | No | CSA | No | 9.8 | 2.7 | 1829 | Yes |
| 11 | 36 | M | Adenocarcinoma* | Yes | CSA + MMF | No | 16 | 3.4 | 1202 | No (F → M) |
| 12 | 36 | F | Esophageal cancer | Yes | CSA + MMF | No | 11.9 | 4 | 862 | Yes |
| 13 | 49 | M | Melanoma | Yes | CSA + MMF | No | 9.4 | 4.1 | 1057 | Yes |
| 14 | 52 | M | RCC | No | CSA + MMF | No | 1.9 | 2.7 | 1917 | No (F → M) |
| 15 | 46 | M | Colon cancer | Yes | CSA + MMF | No | 3.9 | 4 | 1482 | Yes |
| 16 | 62 | M | RCC | No | CSA + MMF | No | 5.1 | 2.2 | 1170 | No (F → M) |
| 17 | 51 | M | RCC | No | CSA + MMF | No | 5.6 | 0.9 | 3368 | No (F → M) |
| 18 | 42 | F | Breast cancer | Yes | CSA + MMF | No | 9.7 | 4.7 | 1050 | Yes |
| 19 | 42 | F | RCC | No | CSA + MMF | No | 6.1 | 5 | 1046 | Yes |
| 20 | 56 | M | RCC | No | CSA + MMF | No | 5.5 | 4.2 | 4390 | No (F → M) |
| 21 | 48 | F | RCC | No | CSA + MMF | No | 4 | 2.4 | 922 | No (F → M) |
| 22 | 43 | F | Breast cancer | Yes | CSA + MMF | Yes | 14 | 2.3 | 628 | No (M → F) |
| 23 | 46 | M | Sarcoma | Yes | CSA + MMF | Yes | 10 | 3.9 | 2202 | Yes |
| 24 | 33 | M | RCC | No | CSA + MMF | No | 3.9 | 2.3 | 1536 | No (F → M) |
| 25 | 64 | M | Prostate cancer | No | CSA + MMF | No | 2.1 | 2.7 | 953 | No (F → M) |
| 26 | 48 | F | RCC | Yes | CSA + MMF | No | 4 | 3.5 | 1173 | Yes |
| 27 | 54 | M | RCC | No | CSA + MTX | No | 9 | 3.9 | 1509 | Yes |
| 28 | 48 | M | RCC | No | CSA + MTX | No | 9.2 | 3.7 | 1929 | Yes |
| 29 | 53 | M | RCC | No | CSA + MTX | No | 4.8 | 2 | 1277 | No (F → M) |
| 30 | 53 | M | Colon cancer | Yes | CSA + MMF | No | 4 | 3.2 | 1372 | Yes |
| 31 | 51 | F | RCC | Yes | CSA + MTX | No | 14.4 | 4.8 | 2114 | Yes |
| 32 | 65 | F | Cholangiocarcinoma | Yes | CSA + MMF | No | 8.4 | 3.5 | 1063 | No (M → F) |
| 33 | 46 | F | Adrenocortical carcinoma | Yes | CSA + MMF | No | 10.8 | 2 | 550 | No (M → F) |
| 34 | 51 | F | Hepatocellular carcinoma | Yes | CSA + MMF | No | 11.2 | 3.1 | 689 | No (M → F) |
| 35 | 45 | F | Breast cancer | Yes | CSA + MMF | Yes | 14.4 | 2.5 | 1335 | Yes |
| 36 | 39 | F | Ovarian cancer | Yes | CSA + MMF | No | 12.4 | 5.5 | 800 | Yes |
Prior chemotherapy indicates treatment with any chemotherapy prior to allogeneic hematopoietic cell transplantation; Pretransplantation lymphocyte count, the number of lymphocytes per micro liter in patients immediately prior to conditioning; RCC, renal cell carcinoma; CSA, cyclosporine; F → M, a female donor into male recipient; MMF, mycophenolic acid; M → F indicates a male donor into a female recipient. and MTX, methotrexate.
Adenocarcinoma of unknown primary
The median time to neutrophil (> 0.5 × 109/L) and platelet (> 20 × 109/L) recovery was 11 days (range, 8-16 days) and 9 days (range, 0-10 days), respectively. Twenty-eight patients never dropped their platelet count below 20 × 109/L. All patients had mixed (both patient and donor cells detectable) myeloid and/or T-cell chimerism on day 15, although T cells were predominantly donor (median, 95%; range, 55%-100%) in origin; median donor T-cell chimerism was 92%, 95%, 98%, and 100% on days 30, 45, 60, and 100, respectively. In contrast, the minority of myeloid cells on day 15 was donor (median, 30%; range, 1%-94%); median donor myeloid chimerism was 38%, 30%, 35%, and 84% on days 30, 45, 60, and 100, respectively. All patients had sustained donor engraftment with no cases of delayed graft rejection.
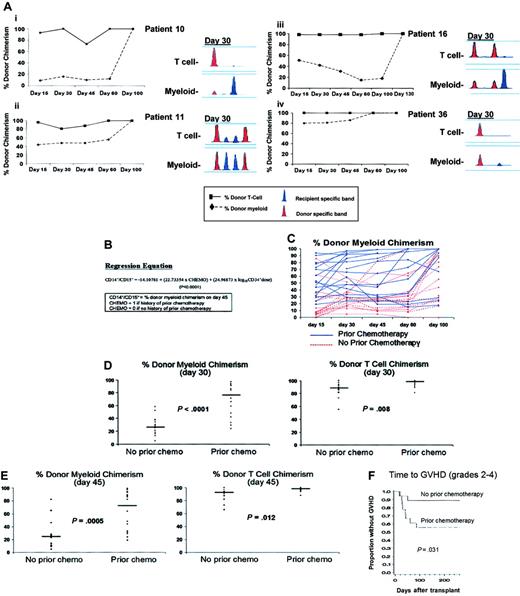
Despite using a single conditioning regimen, the rate and pattern of donor engraftment in both myeloid and T-cell lineages varied considerably among patients (Figure 1A). In a multiple regression analysis, donor myeloid chimerism at day 45 was predicted by prior chemotherapy exposure and the log of the CD34 dose (adjusted R2 = .47; P < .0001), whereas only prior chemotherapy impacted donor T-cell engraftment (Figure 1B-C). Patients receiving chemotherapy prior to transplant conditioning engrafted quicker in both myeloid and T cells at multiple posttransplantation time points compared with chemotherapy-naive patients. On days 30 and 45, median myeloid donor chimerism was 76% (range, 24%-97%) and 73% (range, 19%-99%) in patients with a history of prior chemotherapy and 26% (range, 5%-58%; P < .0001) and 25% (range, 5%-82%; P = .0005) in those without (Table 2).
Variability in engraftment profiles and impact of prior chemotherapy on chimerism and GVHD. (A) The engraftment kinetics of donor T cells (▪) and myeloid cells (♦) are shown in patients 10, 11, 16, and 36. The PCR products of a single informative STR in T-cell and myeloid lineages from day-30 blood samples in the same patients are shown. The informative donor band(s) is shown in red and the informative patient band(s) is shown in blue. (B) Regression equation predicting donor myeloid chimerism on day-45 after HCT. CD14+/15+ is the % donor myeloid chimerism on day 45. CHEMO = 0 if no prior chemotherapy; and CHEMO = 1 if prior chemotherapy and log10 CD34+ dose is the logarithm (base 10) of the CD34+ dose. The interaction between the 2 independent variables was tested and found not to be significant (P > .5). The F value for the model was 14.59, 2, and 29 degrees of freedom; P < .0001. (C) The percentage donor myeloid chimerism on days 15, 30, 45, 60, and 100 in individual patients who had received prior chemotherapy (blue lines) versus those who were chemotherapy naive (red lines). On day 30 (D) and day 45 (E), patients who had received prior chemotherapy had more rapid donor myeloid and T-cell engraftment compared with patients who were chemotherapy naive. Individual (•) and median (—) values of the 18 patients in each cohort are shown. (F) Time to acute grades II to IV GVHD in patients who had received prior chemotherapy compared with chemotherapy naive patients.
Variability in engraftment profiles and impact of prior chemotherapy on chimerism and GVHD. (A) The engraftment kinetics of donor T cells (▪) and myeloid cells (♦) are shown in patients 10, 11, 16, and 36. The PCR products of a single informative STR in T-cell and myeloid lineages from day-30 blood samples in the same patients are shown. The informative donor band(s) is shown in red and the informative patient band(s) is shown in blue. (B) Regression equation predicting donor myeloid chimerism on day-45 after HCT. CD14+/15+ is the % donor myeloid chimerism on day 45. CHEMO = 0 if no prior chemotherapy; and CHEMO = 1 if prior chemotherapy and log10 CD34+ dose is the logarithm (base 10) of the CD34+ dose. The interaction between the 2 independent variables was tested and found not to be significant (P > .5). The F value for the model was 14.59, 2, and 29 degrees of freedom; P < .0001. (C) The percentage donor myeloid chimerism on days 15, 30, 45, 60, and 100 in individual patients who had received prior chemotherapy (blue lines) versus those who were chemotherapy naive (red lines). On day 30 (D) and day 45 (E), patients who had received prior chemotherapy had more rapid donor myeloid and T-cell engraftment compared with patients who were chemotherapy naive. Individual (•) and median (—) values of the 18 patients in each cohort are shown. (F) Time to acute grades II to IV GVHD in patients who had received prior chemotherapy compared with chemotherapy naive patients.
Comparison of donor myeloid and T-cell chimerism in patients with or without a history of prior chemotherapy exposure
Lineage and day after transplantation . | Prior chemotherapy, n = 18; % donor chimerism median (range) . | No prior chemotherapy, n = 18; % donor chimerism median (range) . | P, Wilcoxon rank sum test . |
|---|---|---|---|
| Myeloid day 15 | 52 (19-94) | 8 (1-86) | .0003 |
| Myeloid day 30 | 76 (24-97) | 26 (5-58) | < .0001 |
| Myeloid day 45 | 73 (19-99) | 25 (5-82) | .0005 |
| Myeloid day 60 | 84 (15-100) | 24 (0-100) | .005 |
| Myeloid day 100 | 100 (18-100) | 50 (0-100) | .053 |
| T-cell day 15 | 96 (55-100) | 93 (71-100) | .25 |
| T-cell day 30 | 98 (81-100) | 88 (55-100) | .008 |
| T-cell day 45 | 98 (88-100) | 93 (66-100) | .012 |
| T-cell day 60 | 100 (89-100) | 95 (85-100) | .042 |
| T-cell day 100 | 100 (85-100) | 98 (75-100) | .045 |
Lineage and day after transplantation . | Prior chemotherapy, n = 18; % donor chimerism median (range) . | No prior chemotherapy, n = 18; % donor chimerism median (range) . | P, Wilcoxon rank sum test . |
|---|---|---|---|
| Myeloid day 15 | 52 (19-94) | 8 (1-86) | .0003 |
| Myeloid day 30 | 76 (24-97) | 26 (5-58) | < .0001 |
| Myeloid day 45 | 73 (19-99) | 25 (5-82) | .0005 |
| Myeloid day 60 | 84 (15-100) | 24 (0-100) | .005 |
| Myeloid day 100 | 100 (18-100) | 50 (0-100) | .053 |
| T-cell day 15 | 96 (55-100) | 93 (71-100) | .25 |
| T-cell day 30 | 98 (81-100) | 88 (55-100) | .008 |
| T-cell day 45 | 98 (88-100) | 93 (66-100) | .012 |
| T-cell day 60 | 100 (89-100) | 95 (85-100) | .042 |
| T-cell day 100 | 100 (85-100) | 98 (75-100) | .045 |
Although the absolute differences were smaller, donor T-cell chimerism was also higher in chemotherapy-treated compared with chemotherapy-naive patients on days 30 (median, 98% versus 88%; P = .008) and 45 (median, 98% versus 93%; P = .012) after transplantation (Figure 1D-E). With more rapid donor engraftment, patients who received prior chemotherapy were also more likely to develop acute grades II to IV GVHD (8/18) compared with those who were chemotherapy naive (2/18; P = .031; Figure 1F).
Those with prior chemotherapy had lower pretransplantation ALC (median, 1064 cells/μL vs 1505 cell/μL; P = .02) and received a higher CD34 dose (median, 10.42 × 106 CD34+cells/kg vs 5.36 × 106 CD34+cells/kg; P = .0024) and an equivalent CD3 dose (median, 369 × 106 CD3+ cells/kg vs 324 × 106 CD3+ cells/kg). The addition of MMF or MTX to CSA and other individual variables had no significant impact on engraftment.
We identified factors not related to the conditioning regimen that impact engraftment following nonmyeloablative HCT. In this multiple regression analysis, higher CD34+ cell doses and prior chemotherapy expedited donor myeloid engraftment, while T-cell engraftment was facilitated by chemotherapy exposure alone. Prior chemotherapy exposure likely facilitated donor engraftment by inhibiting the recipient's ability to mount host-versus-graft effects. Decreased autologous hematopoietic recovery, a consequence of chemotherapy compromising recipient hematopoietic “stem cell” reserve, may also have contributed to this effect. Our study included a relatively small cohort of patients who had received a wide range of chemotherapeutic agents, precluding a meaningful analysis of the effect of a single agent or combinations of agents on engraftment. A number of different nonmyeloablative transplant regimens are currently under investigation and therefore our findings may be unique to this particular transplant approach. Other factors not analyzed in this study (eg, pretransplantation CD4+ T-cell count and type of malignancy) could also influence donor engraftment. Our data suggest that patients with a prior history of chemotherapy would require less intensive conditioning to achieve sustained donor engraftment compared with chemotherapy-naive patients. Therefore, the development of investigational trials that tailor the intensity of nonmyeloablative conditioning based on prior chemotherapy exposure should be considered.
Prepublished online as Blood First Edition Paper, October 9, 2003; DOI 10.1182/blood-2003-04-1170.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank John Barrett and the NHLBI transplant physicians, nurses, and support staff. We also wish to thank Jodie Keary and Meghan Shipman for their technical support and the critical assistance of Martha Marquesen and Rose Goodwin to these studies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal