Abstract
While the adenosine 5′-diphosphate (ADP) pathway is known to enhance thrombus formation by recruiting platelets and leukocytes to the primary layer of collagen-adhering platelets, its role for the initiation of coagulation has not been revealed. Ex vivo inhibition of the P2Y12 ADP receptor by clopidogrel administration diminished the rapid exposure of tissue factor (TF), the major initiator of coagulation, in conjugates of platelets with leukocytes established by the contact of whole blood with fibrillar collagen. Under in vitro conditions, the P2Y12 and P2Y1 ADP receptors were both found to be implicated in the exposure of TF in collagen-activated whole blood. Immunoelectron-microscopy revealed that collagen elicited the release of TF from its storage pools within the platelets. Functional activation of the intravascular TF was reduced by inhibition of the ADP receptors, partially due to the disruption of the platelet-neutrophil adhesions. Injection of collagen into the venous system of mice increased the number of thrombin-antithrombin complexes, indicative for the formation of thrombin in vivo. In P2Y1-deficient mice, the ability of collagen to enhance the generation of thrombin was impaired. In conclusion, the platelet ADP pathway supports the initiation of intravascular coagulation, which is likely to contribute to the concomitant formation of fibrin at the site of the growing thrombus.
Introduction
In response to endothelial disruption, platelets adhere within seconds to subendothelial matrix molecules, in particular to collagen. Subsequently, additional platelets and leukocytes (neutrophils, monocytes) are recruited to the initial platelet layer, resulting in the formation of a thrombus capable of provisionally occluding the vessel perforation. Platelet recruitment crucially depends on amplification systems provided by autocrine and paracrine factors such as adenosine 5′-diphosphate (ADP) and thromboxane A2. Apart from their adhesive properties, platelets are also involved in the coagulation process, which provides fibrin for the stabilization of the newly formed thrombus.1 Indeed, the generation of thrombin by the prothrombinase complex is mediated predominantly on the surface of the activated platelets. Moreover, the activation of factor IX by factor XI,2 the secretion and generation of the cofactors Va and VIIIa,3 and several further processes implicated in coagulation are promoted by the activated platelets. In particular, changes in the phospholipid distribution occurring in the cell membrane during platelet activation, which result in the externalization of phosphatidylserine and phosphatidylethanolamine,4 efficiently support the function of the prothrombinase complex.
Nevertheless, the principal initial step of coagulation, the formation of a complex between tissue factor (TF) and factor VIIa, is generally thought to take place exclusively in the vascular wall. TF, an integral membrane protein, is indeed known to be constitutively expressed in the plasma membrane of cells resident in the vessel wall, in particular in those of the adventitial layer.5 While earlier studies indicate the presence of TF in the plasma compartment of blood under physiological conditions,6-8 most plasma TF is apparently associated with microparticles.9 Moreover, TF has been shown to be associated with platelets9,10 and with microvesicles released from the activated platelets.9,11 In addition, while monocytes stimulated by lipopolysaccharide (LPS) and other activators are a major location for TF in blood,12 the expression of TF in neutrophils is a matter of debate.13 The amplifying function of ADP on platelet activation led us to evaluate in the present study whether ADP and its platelet receptors participate in the stimulation of the blood-based TF. The platelet-activating effects of ADP are mediated by the ADP receptors P2Y1 and P2Y12,14 which are intracellularly coupled to activation of phospholipase Cβ and inhibition of adenylyl cyclase activity,15-17 respectively. At the site of vascular disruption, the ADP amplification pathway has been shown to be crucial for the aggregation and adhesion of platelets under in vivo conditions.18-21 However, the potential participation of this pathway for the starting process of coagulation has remained undefined so far.
Patients, materials, and methods
Materials
The fluorescein isothiocyanate (FITC)–labeled monoclonal antihuman TF antibody was obtained from American Diagnostica (Greenwich, CT; catalog no. 4508). The unlabeled antihuman monoclonal (VIC7) and the polyclonal TF antibodies directed against the extracellular domain of the human TF9,22 were generously provided by T. Luther (Dresden, Germany). VIC7 recognizes an epitope on the extracellular domain of TF encoded by exon 5 of the TF gene, which is absent from the alternatively spliced form of TF recently described.23 The monoclonal anti-CD15 antibody was from Biotrend (Cologne, Germany). The FITC-labeled immunoglobulin G1 (IgG1) isoantibody was from Becton Dickinson (Heidelberg, Germany). The chromogenic substrate S2222 and Beriplex P/N 500 were from Chromogenix (Mølndal, Sweden) and Dade-Behring (Marburg, Germany). Recombinant TF was obtained from Ortho Diagnostic Systems (Raritan, NJ), and isotonic Ficoll was from Pharmacia Biotech (Freiburg, Germany). The microbeads conjugated with either anti-CD14 or anti-CD15 antibodies and the positive selection column were from Miltenyi Biotec (Bergisch Gladbach, Germany). Collagen (type I) was obtained from Nycomed (Munich, Germany). Anesthetic drugs xylazine and ketamine were from Bayer (Puteaux, France) and Merial (Lyon, France), respectively. MRS-2179 (2′-deoxy-N6-methyladenosine-3′,5′-bisphosphoric acid) was from Sigma-Aldrich (Deisenhofen, Germany). AR-C69931MX was from ASTRA Charnwood (Laighborough, United Kingdom). CTAD (citrate/citric acid, theophylline, adenosine, dipyridamole) anticoagulant was from Becton Dickinson Vacutainer Systems. Clopidogrel was from Sanofi-Synthelabo (Toulouse, France). Monoclonal antibodies directed against human CD42b (glycoprotein Ibα [GPIbα]) (clone SZ2; phycoerythrin [PE] labeled) and human CD62P (P-selectin, clone CLB-thromb/6; FITC labeled) were from Immunotech Coulter (Marseille, France). Antihuman CD45 (clone 30-F11; PE labeled), antihuman CD66b (FITC labeled), and antihuman CD42b (clone HIP1; PE labeled) were from Pharmingen (Meylan, France). Enzygnost TAT was from Behringwerke (Marburg, Germany).
Subjects
To evaluate the contribution of the P2Y12 receptor for the activation of coagulation by the intravascular TF ex vivo, 8 healthy volunteers (4 men, 4 women; age range, 22 to 35 years) received a single dose of clopidogrel (300 mg) on day 1, followed by a daily dose of 75 mg for an additional 3 days. After a washout phase of 3 weeks, the same donors were supplemented with a daily dose of aspirin (100 mg) for a total of 4 days. Blood was drawn immediately before and at day 5 after the first drug intake. The study had been approved by the ethics committee of the Medical Faculty of the Ludwig-Maximilians-Universität Munich, and the experiments were conducted and informed consent provided according to the principles of the Declaration of Helsinki.
Preparation of cells
For the isolation of platelets, venous blood from healthy volunteers (age range, 23 to 32 years) was drawn into sodium citrate (0.38% final concentration) and subsequently centrifuged at 190g for 15 minutes. The upper two thirds of the supernatant representing the platelet-rich plasma (PRP) was supplemented with apyrase (0.2 U/mL). The PRP was centrifuged at 330g for 10 minutes, and the platelet pellet was recovered and resuspended in a buffer composed of 145 mM NaCl, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 5 mM KCl, 1 mM MgCl2, and 5 mM glucose (pH 7.4; resuspension buffer). When iloprost (10 ng/mL) was included into the PRP and the isolated platelets were additionally washed twice with the resuspension buffer, similar results for the platelet-dependent factor Xa formation were obtained as without iloprost and the washing step. The suspensions of the isolated platelets thus obtained contained less than 0.05% of total leukocytes.
Human neutrophils were prepared by incubation of freshly obtained buffy coats with microbeads coupled to anti-CD15 antibodies (Miltenyi Biotec) for 15 minutes at 8°C. The suspensions were thereafter applied onto the positive selection column, and neutrophils were eluted with the antibody buffer (phosphate-buffered saline [PBS] supplemented with 0.13% EDTA [ethylenediaminetetraacetic acid] and 0.15% bovine serum albumin). The purity of the suspensions thus obtained was 92%. Monocytes were isolated by buoyant density centrifugation and subsequent purification with anti-CD14 antibodies, as described earlier.10
Flow cytometry
For the flow cytometric determinations in whole blood, immediately after collection, 70 μL blood anticoagulated with citrate was preincubated for 2 minutes at 37°C without agitation without or with MRS-2179 (1 mM) and AR-C69931MX (10 μM). Blood was stimulated for 10 minutes at 37°C with collagen (10 μg/mL). A 5-μL aliquot of blood was then incubated with 45 μL Tyrode buffer (137 mM NaCl, 2 mM KCl, 12 mM NaHCO3, 0.3 mM NaH2PO4, 2 mM CaCl2, 1 mM MgCl2, 5.5 mM glucose, 5 mM HEPES, pH 7.3) containing the labeled monoclonal antibodies (1:10 final dilution). The samples were incubated at room temperature for 20 minutes, diluted, and fixed with 0.5% (vol/vol) formaldehyde saline before the flow cytometry measurements. All parameters were acquired on a logarithmic scale. The forward scatter and the side scatter parameters were used to first delineate the leukocyte and the isolated platelet populations. To determine the TF exposure associated with the leukocyte fractions, the percentage of TF-positive cells was recorded in the total population of CD45+ cells by using the FITC-labeled anti-TF antibody. Platelet-leukocyte aggregates were determined as the percentage of CD42b+ events in the leukocyte population. The labeled isotype control antibodies were used to delineate the background staining. P-selectin exposure was determined as the percentage of CD62P+ events in the platelet (CD42b+) population. Flow cytometry was performed using a FACSCalibur fluorescence cytometer (Becton Dickinson).
For the detection of platelet-neutrophil conjugate formation, the isolated platelets (107) and neutrophils (105) were incubated in 200 μL resuspension buffer for 5 minutes at 37°C with collagen (12 μg/mL). Subsequently, to 100 μL cell suspension, 1 mL Cell fix (Becton Dickinson) was added, and the suspension was incubated for 30 minutes at room temperature. After centrifugation at 1100g for 5 minutes, the supernatant was discarded, and an aliquot of the pellet was incubated for 15 minutes in the dark with 1 μg anti-CD42b (PE) plus 1 μg of the anti-CD66b antibody (FITC) in the presence of an excess of IgG (50 μg). After the end of the incubation, 500 μL PBS was added. The suspensions were analyzed using a FACScan flow cytometer (Becton Dickinson).
Western blot
Isolated platelets that had been either activated for 15 minutes with collagen (8 μg/mL) and thrombin (0.5 U/mL) or treated with buffer alone were solubilized with 1% Triton X-100. After determining the protein contents of the samples, extracts containing the same amount of protein were loaded onto the gel (sodium dodecyl sulfate–polyacrylamide gel electrophoresis [SDS-PAGE]; with a 12% separating gel). After electroblotting, the membranes were exposed to the monoclonal anti-TF antibody (VIC7) and, subsequently, to a horseradish peroxidase–conjugated antimouse IgG.
TF-induced factor Xa formation
The isolated platelets (107) and neutrophils (106) were incubated for different time intervals in 170 μL resuspension buffer at 37°C; 50 μL portions of the suspensions were added to 100 μL of 0.88 U/mL factor VII present in a coagulation factor concentrate, which also contained factors II, IX, and X (Beriplex P/N 500; Aventis-Behring, Marburg, Germany). Moreover, 50 μL of a 8 mM CaCl2 suspension and 125 μg/mL (final concentrations) of the chromogenic substrate S2222 (Chromogenix) were added. After incubation for 1 minute at room temperature, the increase in the optical density at 405 nm was registered in 5 subsequent 360-second intervals in an enzyme-linked immunosorbent assay (ELISA) reader (Dynatech MR 7000). Each value was determined in triplicate. A standard curve was prepared using dilutions of recombinant TF.
Electron microscopy
For immunoelectronmicroscopy of neutrophils, human blood was drawn into anticoagulant plus fixative solution (3% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2; 50 μL/mL) to prevent activation of the cells. After centrifugation, the platelet-poor plasma was removed, and the cell pellet was covered with the fixative solution and incubated for 30 minutes at 4°C. Then, the upper layer mainly containing leukocytes and platelets was recovered and cut into small blocks. In parallel, platelets were isolated and stimulated for 10 minutes with collagen (12 μg/mL) and thrombin (0.1 U/mL). Thereafter, the activated platelets and their microvesicles were incubated with fixative and equally cut into blocks. After washing, the blocks were suspended in cacodylate buffer (containing 2.3 M sucrose) and plunge-frozen in liquid propane at –180°C (KF80, Reichert-Jung, Vienna, Austria). The frozen samples were dehydrated with ethanol under progressive lowering of the temperature and embedded at –30°C in Lowicryl HM20 (Chemische Werke Lowi, Waldkraiburg, Germany). Ultrathin serial sections of the blood components fixed on Pioloform-coated Ni-grids were incubated for 60 minutes at room temperature with the primary antibody (rabbit polyclonal antihuman TF). After washing, the grids were incubated with a gold-labeled secondary antibody (goat antirabbit 10 nm, Aurion, Wageningen, The Netherlands) and investigated in series with an EM 109 electron microscope (Carl Zeiss, Oberkochen, Germany). Incubations with the secondary antibody alone showed no labeling.
For the detection of fibrin in the pulmonary vasculature of mice, the lungs were fixed by immersion in fixative solution (2.5% glutaraldehyde in 0.1 mM Na+ cacodylate buffer, containing 2% sucrose, pH 7.3) for 60 minutes at room temperature. The tissues were then rinsed, cut into small pieces, and postfixed for 60 minutes at 4°C with 1% osmium tetroxide in cacodylate buffer. Subsequently, they were washed in the same buffer, dehydrated in graded ethanol solutions, and embedded in epon. The resin was allowed to polymerize at 50°C for 2 days. Ultrathin sections (100 nm) were stained with lead citrate and uranyl acetate and examined under a Philips CM 120 BioTwin transmission electron microscope.
Animal model of systemic thromboembolism
Wild-type and P2Y1-deficient mice were produced as described previously,19 and both genotypes were on the same C57BL/6 background. Clopidogrel treatment was performed by oral administration of clopidogrel solubilized in arabic gum, the day before and 2 hours before the experiment. Arabic gum alone was given orally to the control mice group. Male mice weighting 20 to 30 g were anesthetized, and the jugular vein was exposed surgically. Thereafter, collagen (type I; 0.3 mg/kg) plus epinephrine (0.06 mg/kg) was injected within an infusion time frame of 3 to 4 seconds. Because variations from batch to batch have been observed, all thromboembolism experiments were performed with the same batch number of collagen. Blood was drawn from the abdominal aorta 2 minutes after the challenge for platelet count and plasma thrombin-antithrombin (TAT) determinations, as described previously.24
Statistics
The results were subjected to statistical analysis by paired t test or one-way analysis of variance (ANOVA) for multiple comparisons, where appropriate. All mean values are given ± SD.
Results
ADP receptors mediate rapid exposure of intravascular TF
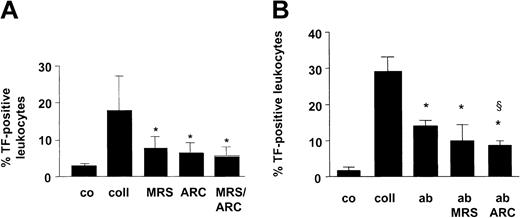
We initially analyzed whether ADP participated in the rapid presentation of TF in human blood, which is promoted by collagen. In blood challenged for 10 minutes with fibrillar collagen (type I), TF was exposed in association with CD14+ and CD15+ leukocytes, in agreement with our earlier results.10 Because the TF exposure was common to different leukocyte fractions, we used a cell surface marker equally expressed in neutrophils and monocytes (CD45) for the detection of the overall TF presentation in blood. Collagen augmented the TF presentation associated with CD45+ cells by 6.1-fold (Figure 1A). In the presence of the selective P2Y1 ADP receptor antagonist MRS-2179 (1 mM), the TF presentation was reduced by 56%. Moreover, AR-C69931MX (10 μM), a specific antagonist of the P2Y12 ADP receptor, decreased the TF presentation by 64% (Figure 1A). Essentially no further inhibition was evoked when the 2 ADP receptor antagonists were added together. In parallel experiments, we found that the collagen-elicited TF presentation was exclusive for neutrophils and monocytes that stained positive for the platelet-specific cell surface marker CD42b 10 (not shown), indicating that TF was exposed in platelet-leukocyte conjugates. To determine whether the effect of the ADP pathway on the TF exposure was dependent on the activation of the integrin GPIIb/IIIa, we performed experiments using the anti-GPIIb/IIIa antibody abciximab, which inhibits platelet aggregation by preventing fibrinogen binding. At the concentration used, abciximab decreased fibrinogen binding by 85% in whole blood stimulated by collagen (10 μg/mL) and reduced by more than 90% collagen-induced aggregation. Abciximab inhibited collagen-induced TF exposure in whole blood by 50% (Figure 1B), indicating that GPIIb/IIIa engagement is needed to obtain maximal TF exposure. The inhibitory effect of abciximab was not further enhanced by MRS-2179. In contrast, AR-C69931MX further reduced the TF exposure compared with the presence of abciximab alone (Figure 1B), suggesting that the P2Y12 receptor was involved in the activation of the TF presentation in the absence of the integrin-mediated outside-in signaling.
ADP receptors and GPIIb/IIIa participate in collagen-induced TF presentation in whole blood. (A) Whole blood was activated for 10 minutes at 37°C with collagen (coll; 10 μg/mL). To the samples with collagen alone, vehicle was added corresponding to the amount of buffer required to suspend the ADP receptor antagonists. In further samples, collagen plus either MRS-2179 (1 mM) or AR-C69931MX (10 μM) was added. Thereafter, the number of TF-positive leukocytes was determined among the total fraction of CD45+ cells. Co indicates control. Means ± SDs; n = 5to8.*P < .05 (versus collagen alone). (B) Effect of abciximab (ab) (20 μg/mL), MRS-2179 (1 mM), and AR-C69931MX (10 μM) on leukocyte TF exposure. Whole blood was stimulated for 10 minutes at 37°C with collagen (10 μg/mL). Means ± SDs; n = 3. *P < .05 (versus collagen alone); §P < .05 (versus abciximab).
ADP receptors and GPIIb/IIIa participate in collagen-induced TF presentation in whole blood. (A) Whole blood was activated for 10 minutes at 37°C with collagen (coll; 10 μg/mL). To the samples with collagen alone, vehicle was added corresponding to the amount of buffer required to suspend the ADP receptor antagonists. In further samples, collagen plus either MRS-2179 (1 mM) or AR-C69931MX (10 μM) was added. Thereafter, the number of TF-positive leukocytes was determined among the total fraction of CD45+ cells. Co indicates control. Means ± SDs; n = 5to8.*P < .05 (versus collagen alone). (B) Effect of abciximab (ab) (20 μg/mL), MRS-2179 (1 mM), and AR-C69931MX (10 μM) on leukocyte TF exposure. Whole blood was stimulated for 10 minutes at 37°C with collagen (10 μg/mL). Means ± SDs; n = 3. *P < .05 (versus collagen alone); §P < .05 (versus abciximab).
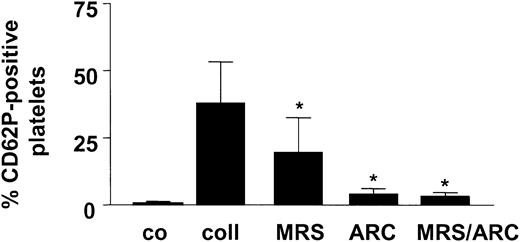
To evaluate whether the secretory response of the platelets was elicited under the same experimental conditions of collagen stimulation of whole blood, α-granule release was estimated by the P-selectin exposure. Collagen caused a substantial increase in the P-selectin exposure, which was reduced by 48% and 90% by blocking the P2Y1 and P2Y12 ADP receptors, respectively (Figure 2). In the presence of both antagonists, the P-selectin appearance was suppressed by 92%. Thus, under the experimental conditions applied, the platelet secretory response was nearly completely suppressed by the ADP receptor antagonists.
Collagen-induced degranulation of platelets is inhibited by the ADP receptor antagonists. Stimulation of whole blood for 10 minutes at 37°C with collagen (coll; 10 μg/mL) elicits the exposure of P-selectin. In additional samples, MRS-2179 (1 mM) and AR-C69931MX (10 μM) plus vehicle were added. Flow cytometric detection using the labeled anti–P-selectin antibody. Co indicates control. Means ± SDs; n = 3. *P < .05 (versus collagen alone).
Collagen-induced degranulation of platelets is inhibited by the ADP receptor antagonists. Stimulation of whole blood for 10 minutes at 37°C with collagen (coll; 10 μg/mL) elicits the exposure of P-selectin. In additional samples, MRS-2179 (1 mM) and AR-C69931MX (10 μM) plus vehicle were added. Flow cytometric detection using the labeled anti–P-selectin antibody. Co indicates control. Means ± SDs; n = 3. *P < .05 (versus collagen alone).
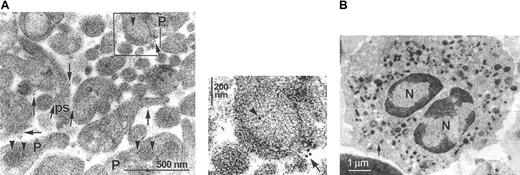
Because platelets were previously shown to store TF in their α-granules,9 we analyzed whether the intraplatelet TF was released by activation with collagen. As visualized by immunoelectronmicroscopy, TF was indeed localized on the plasma membrane of the activated platelets, in particular on the plasmalemmal extensions forming the pseudopodia (Figure 3Ai, arrows). Platelet TF not participating in the surface exposure was predominantly located in the membrane of the α-granules (Figure 3Ai, arrowheads). These results confirm our earlier results obtained with resting platelets, indicating the preferential localization of TF within the α-granules.9 As can be appreciated from Figure 3Aii, degranulation of the α-granules was directly associated with the appearance of TF on the platelet surface. Thus, fusion of the granule membrane with the plasma membrane exposes TF on the surface of the collagen-activated platelets.
Intraplatelet TF is exposed on the surface of activated platelets and microparticles. (A) (i) In thin sections of suspensions of platelets stimulated with collagen and thrombin, TF is visualized by immunoelectronmicroscopy. TF is located on the plasma membrane of the platelets (P) as well as on their pseudopodia (ps; arrows). The identity of the pseudopodia was verified by analyzing a panel of serial thin sections, which indicated their connection with the platelets. In nondegranulating platelets, TF is located within the membrane of the α-granules (arrowheads). (ii) Direct visualization of the degranulation of an α-granule, resulting in the translocation of TF to the cell surface. The panel represents the magnification of the inset marked in the upper panel. Arrows indicate surface TF, whereas arrowheads indicate intraplatelet TF. (B) Virtual absence of TF staining in immunoelectronmicrographs of rapidly prepared neutrophils. The arrow indicates a single gold particle. N indicates nucleus. Note the different diameter scale compared with panel A.
Intraplatelet TF is exposed on the surface of activated platelets and microparticles. (A) (i) In thin sections of suspensions of platelets stimulated with collagen and thrombin, TF is visualized by immunoelectronmicroscopy. TF is located on the plasma membrane of the platelets (P) as well as on their pseudopodia (ps; arrows). The identity of the pseudopodia was verified by analyzing a panel of serial thin sections, which indicated their connection with the platelets. In nondegranulating platelets, TF is located within the membrane of the α-granules (arrowheads). (ii) Direct visualization of the degranulation of an α-granule, resulting in the translocation of TF to the cell surface. The panel represents the magnification of the inset marked in the upper panel. Arrows indicate surface TF, whereas arrowheads indicate intraplatelet TF. (B) Virtual absence of TF staining in immunoelectronmicrographs of rapidly prepared neutrophils. The arrow indicates a single gold particle. N indicates nucleus. Note the different diameter scale compared with panel A.
By Western blotting using a monoclonal antibody that binds to an epitope of the extracellular domain of TF absent from the soluble form (see “Patients, materials, and methods”), we detected the 47-kDa full-length form of TF in the isolated platelets (data not shown). In parallel experiments, we recovered the total leukocyte fraction from the blood and evaluated whether the neutrophils contained TF. This rapid preparation method was preferred over the lengthy isolation of single leukocyte subfractions, because the TF expression might be induced during the isolation procedure. Consistently, the neutrophils included in the total leukocyte preparations were found to exhibit very low intracellular TF labeling (Figure 3B). Moreover, no TF was present on their cell surface.
Functional activation of blood-based TF requires platelet ADP pathway
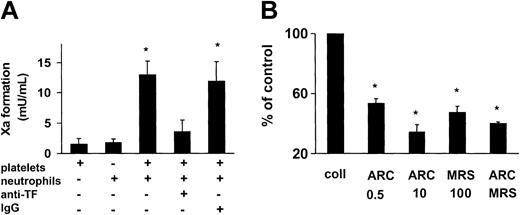
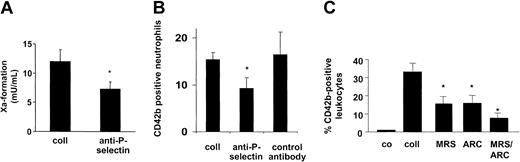
Subsequently, we evaluated whether the platelet ADP receptors were required for the procoagulant competence of the blood-based TF. The TF presented on the platelet surface was functionally silent, because collagen-activated platelets barely supported the formation of the factor Xa (Figure 4A). In view of the association of TF with the platelet-leukocyte conjugates, neutrophils were added to the platelet suspensions. Neutrophils were preferred over monocytes because neutrophils had previously been shown to be devoid of TF after their isolation,10 while, on the other hand, TF synthesis might be induced in the monocytes during the preparation procedure. In the presence of the neutrophils, the factor Xa generation was markedly stimulated when compared with the isolated platelets or isolated neutrophils alone (Figure 4A). The factor Xa formation in the platelet suspensions supplemented with the neutrophils was nearly completely prevented by the monoclonal anti-TF antibody, indicating TF as initiating stimulus. In contrast, the isotype-matched control antibody was ineffective (Figure 4A). In the presence of 0.5 μM and 10 μM of the P2Y12 antagonist AR-C69931MX, the TF activity of the blood cell suspensions was lowered by 46% and 65% (Figure 4B). Moreover, the P2Y1 antagonist MRS-2179 (100 μM) reduced the TF activity by 53%. No further reduction of the TF activity was noted in the simultaneous presence of both inhibitors. Because neutrophils were thus required for the activation of the platelet-exposed TF, we analyzed whether the adhesive interactions between the 2 blood components were necessary for the stimulation of the intravascular TF. An antibody against platelet P-selectin, the major mediator of the initial tethering of platelets to neutrophils, reduced the TF activity of the blood cell suspensions by 40% (Figure 5A). In contrast, the control antibody was ineffective (not shown). Under similar experimental conditions, the anti–P-selectin antibody diminished the number of collagen-elicited platelet-neutrophil conjugates by 39%, the control antibody being ineffective (Figure 5B). Moreover, the anti–P-selectin antibody also suppressed the functional activation of TF in suspensions of activated platelets and monocytes (from 21.8 ± 6.8 [without antibody] to 11.3 ± 3.8 mU/mL [with antibody]; means ± SD, n = 3, P < .05).
ADP receptor antagonism partially prevents intravascular TF activity. (A) Neutrophils promote activation of TF in suspensions of collagen-activated platelets. Isolated platelets (2 × 107) and neutrophils (2 × 106) were suspended in 170 μL resuspension buffer and stimulated with collagen (5 minutes, 37°C). Factor Xa formation was measured by a chromogenic substrate. The monoclonal anti-TF antibody and the isotype control antibody were present at 10 μg/mL. Means ± SDs; n = 4. *P < .05 (versus platelets plus neutrophils). (B) Intravascular TF activity is diminished by ADP receptor antagonists. TF activity in suspensions of collagen-activated platelets and neutrophils was determined as described above. AR-C69931MX and MRS-2179 were present at the indicated concentrations. Means ± SDs; n = 3to5.*P < .05 (versus collagen alone).
ADP receptor antagonism partially prevents intravascular TF activity. (A) Neutrophils promote activation of TF in suspensions of collagen-activated platelets. Isolated platelets (2 × 107) and neutrophils (2 × 106) were suspended in 170 μL resuspension buffer and stimulated with collagen (5 minutes, 37°C). Factor Xa formation was measured by a chromogenic substrate. The monoclonal anti-TF antibody and the isotype control antibody were present at 10 μg/mL. Means ± SDs; n = 4. *P < .05 (versus platelets plus neutrophils). (B) Intravascular TF activity is diminished by ADP receptor antagonists. TF activity in suspensions of collagen-activated platelets and neutrophils was determined as described above. AR-C69931MX and MRS-2179 were present at the indicated concentrations. Means ± SDs; n = 3to5.*P < .05 (versus collagen alone).
ADP pathway contributes to establish platelet-leukocyte adhesions promoting TF function. (A) Platelet-neutrophil adhesions support platelet TF activity. Isolated platelets (2 × 107) and neutrophils (2 × 106) were stimulated with collagen (5 minutes, 37°C) and, subsequently, the factor Xa generation was assessed. Under similar conditions, the anti–P-selectin antibody (10 μg/mL) was added. Means ± SDs; n = 4. *P < .05 (versus collagen alone). (B) Generation of platelet-neutrophil conjugates is reduced by inhibition of P-selectin–mediated adhesion. Conjugate formation was established by determining the number of events being positive for CD42b and CD66b. The anti–P-selectin and isotype control antibody were added at 10 μg/mL. Means ± SDs; n = 4. *P < .05 (versus collagen alone). (C) Formation of platelet-leukocyte conjugates is prevented by inhibition of the ADP receptors. The number of CD42b+ leukocytes among the total fraction of CD45+ cells was determined in collagen-activated whole blood in the absence or presence of MRS-2179 (1 mM) and AR-C69931MX (10 μM). Means ± SEM; n = 5to8.*P < .05 (versus collagen alone).
ADP pathway contributes to establish platelet-leukocyte adhesions promoting TF function. (A) Platelet-neutrophil adhesions support platelet TF activity. Isolated platelets (2 × 107) and neutrophils (2 × 106) were stimulated with collagen (5 minutes, 37°C) and, subsequently, the factor Xa generation was assessed. Under similar conditions, the anti–P-selectin antibody (10 μg/mL) was added. Means ± SDs; n = 4. *P < .05 (versus collagen alone). (B) Generation of platelet-neutrophil conjugates is reduced by inhibition of P-selectin–mediated adhesion. Conjugate formation was established by determining the number of events being positive for CD42b and CD66b. The anti–P-selectin and isotype control antibody were added at 10 μg/mL. Means ± SDs; n = 4. *P < .05 (versus collagen alone). (C) Formation of platelet-leukocyte conjugates is prevented by inhibition of the ADP receptors. The number of CD42b+ leukocytes among the total fraction of CD45+ cells was determined in collagen-activated whole blood in the absence or presence of MRS-2179 (1 mM) and AR-C69931MX (10 μM). Means ± SEM; n = 5to8.*P < .05 (versus collagen alone).
The requirement for platelet-neutrophil adhesions led us to evaluate whether the ADP pathway was implicated in the establishment of platelet-leukocyte conjugates per se. As expected, the number of platelet-leukocyte conjugates in whole blood was markedly enhanced in response to collagen (Figure 5C). Inhibition of the ADP-P2Y1 receptor interaction by MRS-2179 decreased the amount of platelet-leukocyte conjugates by 53%. Moreover, the number of conjugates was lowered to the same extent by AR-C69931MX (Figure 5C). In the presence of both antagonists, the conjugate generation was suppressed by 76%. Taken together, these findings demonstrate that the ADP pathway contributes to the procoagulant function of the intravascular TF, in part by supporting the formation of platelet-neutrophil conjugates.
Contribution of ADP receptors to the in vivo activation of coagulation
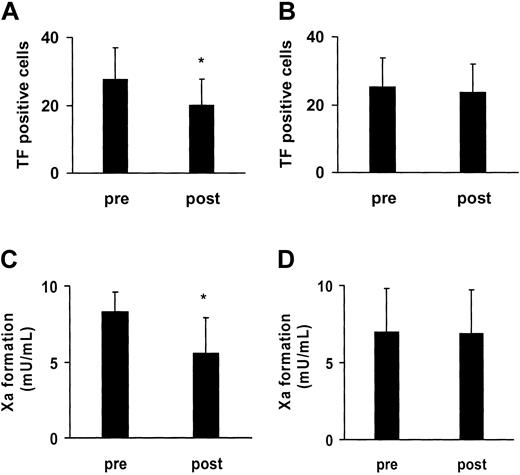
We next evaluated the role of the P2Y12 receptor for the activation of the blood-based TF under ex vivo conditions. To this purpose, 8 healthy volunteers were supplemented with clopidogrel, a selective inhibitor of this receptor. The volunteers received a single dose of 300 mg clopidogrel, followed by a daily dose of 75 mg for 3 further days. At the end of the 4-day supplementation period, the collagen-induced TF presentation induced by collagen in association with the CD14+ cells was reduced by 26% (Figure 6A). The participation of a further amplification system of platelet activation, the thromboxane A2 pathway, was addressed by supplementing the volunteers with aspirin. After a washout period of 3 weeks, the volunteers received a daily dose of aspirin (100 mg) for a total of 4 days. Aspirin did not affect the TF presentation in association with CD14+ cells, as analyzed after the 4-day treatment period (Figure 6B). Experiments with the platelet function analyzer performed in parallel indicated that the 4-day supplementation with aspirin significantly delayed the formation of the platelet thrombus (closure time) in response to collagen and epinephrine (B.E., unpublished data, May 2003). While not completely excluding, this makes it rather unlikely that the donors were nonresponders to this drug. In the same donors, we tested whether the 2 amplifier systems of platelet activation were involved in the functional activation of the platelet TF. After 4 days of treatment with clopidogrel alone, the TF-dependent factor Xa activity of the platelet-neutrophil suspensions was lowered by 33% (Figure 6C). No change in the TF activity was observed following the 4-day treatment with aspirin (Figure 6D). The ex vivo data thus substantiated the results of the in vitro experiments, demonstrating that ADP and its P2Y12 receptor participate in the blood-based initiation of coagulation.
Ex vivo evidence for role of P2Y12 receptor in activation of blood-based TF. Healthy volunteers were supplemented for 4 days with clopidogrel (A,C) and, following a washout phase, for another 4 days with aspirin (B,D). The collagen-elicited TF exposure in association with monocytes (CD14+ cells) in whole blood was determined before (pre) and after (post) the 4-day period (A-B). Under similar conditions, platelets and neutrophils were isolated from the volunteers before (pre) and after (post) treatment with clopidogrel and aspirin, and the TF activity was assessed in suspensions of collagen-activated platelets and neutrophils (C-D). Means ± SDs; n = 8. *P < .05 (versus “pre”).
Ex vivo evidence for role of P2Y12 receptor in activation of blood-based TF. Healthy volunteers were supplemented for 4 days with clopidogrel (A,C) and, following a washout phase, for another 4 days with aspirin (B,D). The collagen-elicited TF exposure in association with monocytes (CD14+ cells) in whole blood was determined before (pre) and after (post) the 4-day period (A-B). Under similar conditions, platelets and neutrophils were isolated from the volunteers before (pre) and after (post) treatment with clopidogrel and aspirin, and the TF activity was assessed in suspensions of collagen-activated platelets and neutrophils (C-D). Means ± SDs; n = 8. *P < .05 (versus “pre”).
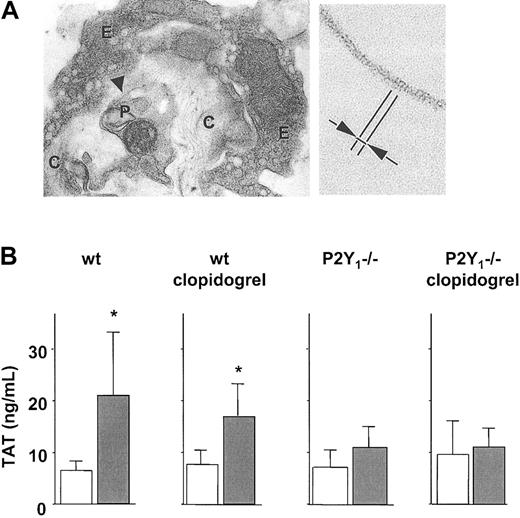
To evaluate the role of ADP and its platelet receptors for the activation of coagulation in vivo, an animal model of thromboembolism was employed. To achieve this, collagen was injected together with epinephrine into the jugular vein of mice, which is known to cause massive pulmonary thrombemboli.25,26 By the use of electron microscopy, we detected fibrin fibers in association with platelet thrombi and adjacent to the injected collagen in several lung vessels (Figure 7A). No fibrin could be observed in the vehicle-treated mice (not shown). The same collagen stimulus augmented the systemic levels of thrombin-antithrombin (TAT) complexes, a quantitative measure for the extent of thrombin generation. Indeed, 2 minutes after injection of collagen into the control mice, the number of TAT complexes in the blood was increased by 3-fold (Figure 7B). Following clopidogrel infusion, the thrombin generation tended to be diminished. In the P2Y1 (–/–) mice, the ability of collagen infusion to generate thrombin was markedly reduced (Figure 7B).
Platelet ADP pathway supports thrombin formation in vivo. (A) Collagen injection elicits intravascular fibrin formation. Transmission electron micrographs of lung from a wild-type (wt) mouse following injection of collagen plus epinephrine. (Left) Thrombus occluding a pulmonary vessel with some rare fibrin fibrils (arrowhead) (E indicates endothelium; C, collagen fibers; and P, platelet). Original magnification, × 14000. (Right) Higher magnification (× 148000) of the fibrin fibrils showing the periodicity of around 20 nm (arrows). (B) Collagen fails to activate thrombin generation in P2Y1 (–/–) mice. Plasma TAT levels following collagen-induced thromboembolism in wt and P2Y1 (–/–) mice, without or with clopidogrel. Empty columns indicate saline injection; filled columns, collagen plus epinephrine. Means ± SDs; n = 7 to 8. *P < .05 compared with same condition without collagen.
Platelet ADP pathway supports thrombin formation in vivo. (A) Collagen injection elicits intravascular fibrin formation. Transmission electron micrographs of lung from a wild-type (wt) mouse following injection of collagen plus epinephrine. (Left) Thrombus occluding a pulmonary vessel with some rare fibrin fibrils (arrowhead) (E indicates endothelium; C, collagen fibers; and P, platelet). Original magnification, × 14000. (Right) Higher magnification (× 148000) of the fibrin fibrils showing the periodicity of around 20 nm (arrows). (B) Collagen fails to activate thrombin generation in P2Y1 (–/–) mice. Plasma TAT levels following collagen-induced thromboembolism in wt and P2Y1 (–/–) mice, without or with clopidogrel. Empty columns indicate saline injection; filled columns, collagen plus epinephrine. Means ± SDs; n = 7 to 8. *P < .05 compared with same condition without collagen.
Discussion
Amplification of platelet activation by ADP is of substantial relevance for the recruitment of platelets and leukocytes to the developing thrombus, required to prevent the loss of blood from the site of vessel wall perforation. Under in vivo conditions, the ADP-mediated amplification system is mainly triggered by the interaction of platelets with the subendothelial collagen. The potential participation of the platelet ADP receptors for the rapid presentation of TF, the major initiator of coagulation, in whole blood was evaluated by using the selective P2Y1 receptor antagonist MRS-2179 and the selective P2Y12 antagonist AR-C69931MX.27,28 Both inhibitors partially prevented the collagen-elicited exposure of the intravascular TF in association with platelet-leukocyte conjugates. In view of the ability of collagen to promote the degranulation of the platelet α-granules, we analyzed whether the same stimulus would enable the exposure of TF on the platelet surface. Earlier results suggest that platelets contain TF,10 which might somehow be released into the extracellular medium upon platelet activation.11,29 By means of immunoelectronmicroscopy, we observed that TF was translocated from the α-granules, its major site of intraplatelet storage,9 to the platelet surface. When combined, these findings suggest that the rapid exposure of the intravascular TF in response to collagen depends in part on the activation of the platelet ADP receptors.
The TF exposed on the surface of the activated platelets themselves is functionally only weakly active,9,29 while the TF released into the extracellular medium appears to exhibit greater functional competence.11,29 In particular, allowing the activated platelets to adhere to the neutrophils enabled the factor X cleavage by the platelet TF/VIIa complex. The functional activation (or decryption) of the platelet TF by the neutrophils could occur by concomitant inactivation of tissue factor pathway inhibitor10,30 (TFPI), the antagonist of the TF pathway. TFPI, which is released by the activated platelets, is indeed efficiently cleaved by neutrophil proteases. In the intercellular microenvironment formed within the platelet-neutrophil conjugates, proteolytic inactivation of TFPI by neutrophil proteases could enhance the ability of the platelet TF to initiate coagulation. Our observations confirm earlier findings demonstrating that neutrophils are capable of supporting the intravascular TF activity and to enhance the fibrin formation in the presence of the activated platelets.9,31-33 When the tethering of platelets to the neutrophil surface was inhibited by the anti–P-selectin antibody, the ability of the intravascular TF to initiate coagulation was suppressed. Ex vivo analyses using specific inhibition of the P2Y12 receptor by clopidogrel confirmed that this ADP receptor partially contributes to the exposure and function of the blood-based TF. The inhibitory effect of clopidogrel on the activation of the blood-based TF was less pronounced in the ex vivo experiments compared with the inhibitory influence of AR-C69931MX in vitro. This is most probably due to the incomplete inhibition of ADP-induced platelet activation elicited with the standard dosage of clopidogrel,34 as also used in the present study. Moreover, the formation of platelet-leukocyte conjugates per se was found to require in part the activation of the platelet ADP receptors, in agreement with earlier work.35,36 Thus, in addition to its participation in the TF exposure, the ADP pathway also contributed to the formation of adhesive contacts between the platelets and neutrophils, necessary for the efficient activation of the intravascular TF. These results extend previous findings reporting about the relevant role of the P2Y12 receptor for the ADP-induced formation of platelet-leukocyte conjugates.37 In our ex vivo analyses, aspirin (100 mg/d) did not inhibit the initiation of coagulation by the platelet-associated TF. In principal agreement with these observations, previous investigations were also unable to assign a role for thromboxane A2 as activator of the platelet-dependent thrombin formation.38,39
The extent of reduction of the TF exposure, of the TF activity, and of platelet-leukocyte conjugate formation was similar for the 2 ADP receptor antagonists analyzed. Moreover, no clear-cut additive effects were observed under the same conditions, when both antagonists were present. Both ADP receptors thus appear to contribute to the activation of the intravascular TF system. Thus, while for the platelet P-selectin exposure a stronger participation of the P2Y12 receptor could be inferred, no such preference was seen in the stimulation of the intravascular TF. The latter system ultimately depends on platelet-leukocyte adhesions, which apart from P-selectin–mediated initial interactions require the firm interactions supported by integrins. The platelet ADP receptors are known to be coupled to different intracellular signaling pathways. While the signaling route initiated by the P2Y1 receptor involves Gq, P2Y12 is linked to Gi, a G protein whose further downstream signaling involves inhibition of the adenylyl cyclase activity.15-17 Engagement of the ADP receptors by ADP is known to cause maximal platelet secretion and signal amplification by activation of the integrin GPIIb/IIIa. Our results suggest that GPIIb/IIIa-related outside-in signaling is required, at least in part, for the intravascular TF presentation elicited by ADP in response to collagen stimulation.
Several earlier investigations underline the participation of ADP and its platelet receptors in thrombus formation and in the efficient closure of vessel wall ruptures. Prolongations in bleeding time and resistance against thrombosis were noted in mice deficient in the P2Y1 receptor as well as in patients lacking the P2Y12 receptor.17-20 Moreover, ADP receptor antagonists are currently widely used as antithrombotic agents for the secondary prevention of cardiovascular diseases. In our study, a role for the P2Y1 ADP receptor in the activation of coagulation could be inferred under in vivo conditions, because collagen failed to stimulate the formation of thrombin in the mice deficient in this ADP receptor. Moreover, preventing the ADP binding to this receptor partially suppressed the ability of the isolated platelets to initiate coagulation in vitro. In the whole blood experiments, higher concentrations of the P2Y1 antagonist were needed to prevent the TF exposure, indicating a reduced efficiency of the antagonist under those conditions.
The blood-based TF apparently enables the initiation of physiologic coagulation within the growing thrombus, allowing the generation of thrombin (and fibrin) at the site, where it is necessary for the efficient sealing of the wound.30 The multilayered thrombus will most likely provide a barrier to the diffusion of coagulation factors previously activated by the vessel wall TF. Because, however, fibrin is essential for the sealing of the developing thrombus, a local fibrin-generating system is apparently required. The intravascular TF system is a likely candidate to fulfill this function. The kinetics of the blood-based TF exposure, which is maximal after 5 to 10 minutes in human blood, also supports the view that the system operates during the growth and the remodeling phase of thrombus development. Under different circumstances, but with principally comparable dynamics, pathological arterial thrombi are known to increase in size by the stepwise enrichment with platelets and leukocytes. Eventually, this will lead to the complete occlusion of the vessel and thereby cause life-threatening ischemic complications. Under those conditions, the platelet ADP pathway is expected to contribute to the activation of coagulation via stimulation of the intravascular TF, thereby augmenting the resistance of the thrombus against the shear stress of the flowing blood and eventually causing irreversible vessel occlusions.
In conclusion, our observations demonstrate that the interactions of ADP with its P2Y1 and P2Y12 receptors participate in the rapid activation of the blood-based TF. Our results suggest that upon interaction of the platelets with collagen, the intraplatelet ADP secreted into the extracellular environment binds to its cell membrane receptors, thereby enhancing the α-granule release. In consequence, prestored TF and P-selectin are likely to be exposed. Concomitantly, GPIIb/IIIa (αIIbβ2 integrin) is activated, which further enhances secretion via outside-in signaling. Adhesive interactions between the activated platelets and the neutrophils are subsequently proposed to enable the functional activation of the rapidly exposed TF. By supporting the rapid intravascular TF presentation and by participating in the formation of platelet-neutrophil conjugates, the ADP receptors thus contribute to sustain the functional competence of the intravascular TF. Accordingly, there appears to be a close temporal and spatial coupling between the platelet recruitment during thrombus development and the initiation of coagulation.
prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-05-1385.
Supported by grants from the Deutsche Forschungsgemeinschaft to B.E.
C.L., M.A., and A.K. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal