Abstract
Angiogenesis, the development of new blood vessels from existing vasculature, is crucial for the development and metastasis of solid tumors. Here, we show for the first time that a 24–amino acid peptide derived from the amino terminus of the alpha chain of human fibrinogen (termed “alphastatin”) has potent antiangiogenic properties, inhibiting both the migration and tubule formation of human dermal microvascular endothelial cells in response to vascular endothelial growth factor (VEGF) or basic fibroblast growth factor (bFGF) in vitro. Moreover, alphastatin markedly inhibits the growth of tumors in a syngeneic murine model. Tumors from mice receiving daily injections of alphastatin for 12 days exhibited large areas of intravascular disruption and thrombosis with substantial cellular necrosis. Importantly, alphastatin administration had no detectable effect on vessels in such normal tissues as liver, lungs, and kidney. Taken together, these data indicate that alphastatin is a potent new antiangiogenic agent in vitro and antivascular agent in vivo.
Introduction
Angiogenesis, the growth of new capillaries from an existing host vascular bed, involves the migration, proliferation, and differentiation of endothelial cells and is crucial for the growth and metastasis of tumors.1 Hemostatic mechanisms control blood flow by regulating platelet adherence and fibrin deposition, and various hemostatic proteins have also been implicated recently in angiogenesis.2-4
Fibrinogen accumulation, and subsequent clotting to form a fibrin meshwork in the stroma, has long been recognized as a feature of solid tumors and is thought to result from prominent vascular permeability in these tissues.5,6 This has led to speculation that fibrin may be important in initiating and/or maintaining tumor angiogenesis. However, subsequent experiments using fibrinogen-deficient mice have shown that while fibrinogen/fibrin deficiency inhibits the formation of metastasis in mice, it fails to inhibit primary tumor angiogenesis or growth.7
We have previously demonstrated that a 50-kDa fragment containing the central domain of human fibrinogen (fibrinogen E fragment; FgnE), formed when plasmin cleaves the carboxyl termini of the paired α, β, and γ chains of fibrinogen, is a potent antiangiogenic factor. It inhibits vascular endothelial growth factor (VEGF)–induced migration and tubule formation by human dermal microvascular endothelial cells (HuDMECs) in vitro8 and tumor growth in a syngeneic murine model in vivo by selectively disrupting tumor endothelium and causing widespread intravascular thrombosis.9
Fibrinogen can also be cleaved by thrombin, which removes the amino termini of both the α and β chains, termed fibrinopeptide A and B, respectively, to yield fibrin monomers. These in turn are cleaved by plasmin to produce the fragment closely related to FgnE, fibrin E fragment (FnE). This has been shown to be proangiogenic, stimulating the migration and tubule formation of HuDMECs in vitro10 and vessel formation in the chick chorioallantoic membrane (CAM) assay.11 As plasmin cleaves the amino terminus of the β chain between amino acids (aa's) 42 and 43, in both E fragments, the antiangiogenic FgnE differs from FnE only in that the former contains 16 amino acids at the end of each amino terminus of the α chains, fibrinopeptide A (FpA) (Figure 1A). These are not present in FnE. This led us to speculate that the antiangiogenic properties of FgnE may be mediated by FpA. However, when a linear form of FpA was tested it was found to be inactive in vitro.10
A schematic diagram of fibrinogen E-fragment and alphastatin. (A) The central domain of fibrinogen, fibrinogen E fragment, consists of 2 α chains (black), 2 β chains (dark gray), and 2 γ chains (light gray). Alphastatin is located at the amino terminus of each of the α chains, a region which contains fibrinopeptide A (FpA). (B) Alphastatin, the first 24 amino acids of the α chain, consists of a β bend stabilized by a salt bridge between R19 and D7 (arrow). The thrombin cleavage site is denoted by a vertical line and the sequence of FpA is from A-R.
A schematic diagram of fibrinogen E-fragment and alphastatin. (A) The central domain of fibrinogen, fibrinogen E fragment, consists of 2 α chains (black), 2 β chains (dark gray), and 2 γ chains (light gray). Alphastatin is located at the amino terminus of each of the α chains, a region which contains fibrinopeptide A (FpA). (B) Alphastatin, the first 24 amino acids of the α chain, consists of a β bend stabilized by a salt bridge between R19 and D7 (arrow). The thrombin cleavage site is denoted by a vertical line and the sequence of FpA is from A-R.
The crystal structure of fibrinogen has been described12 and nuclear magnetic resonance studies have suggested the presence of a β-bend conformation at the NH2 terminus of each α chain.13 This may be stabilized by a salt bridge between Asp7 and Arg19, and the β bend would place Phe9 in close proximity to the thrombin cleavage site (the Arg16-Gly17 bond).13,14 We proposed, therefore, that each FpA is held in a β-bend conformation at the end of the α chains of FgnE and that this conformation may be essential for the antiangiogenic activity of FgnE (Figure 1B). We synthesized the first 24 amino acids of the alpha chain of fibrinogen (which included the salt bridge between Asp7 and Arg19) and called the resultant peptide “alphastatin” (Figure 1). The present studies demonstrate that alphastatin inhibits the ability of VEGF and basic fibroblast growth factor (bFGF) to stimulate the migration and differentiation of HuDMECs in vitro. Furthermore, daily intraperitoneal injections of alphastatin in a syngeneic tumor model also selectively disrupt tumor endothelium in vivo, causing widespread intravascular thrombosis and retarding tumor growth.
Materials and methods
Cells and cell culture
Adult HuDMECs were obtained commercially (TCS Cell Works, Milton Keynes, United Kingdom) and cultured in microvascular endothelial cell growth medium. This medium contains heparin (10 ng/mL), hydrocortisone, human epidermal growth factor (10 ng/mL), human fibroblast growth factor (10 ng/mL), and dibutyryl cyclic adenosine monophosphate (AMP). The medium was supplemented with 5% heat-inactivated fetal calf serum (FCS), 50 μg/mL gentamicin, and 50 ng/mL amphotericin B (TCS Cell Works). An SV40-transformed murine endothelial cell line, SVEC4-10, was obtained from American Type Culture Collection (ATCC, Manassas, VA). The murine colonic adenocarcinoma CT26 cell line is an N-nitrosos-N-methylurethane–induced Balb/c tumor obtained from Professor Ian Hart, (Imperial Cancer Research Fund [ICRF], London, United Kingdom). These murine cell lines were maintained by in vitro passage in Dulbecco Minimal Eagle Medium (DMEM), 10% FCS, 1% penicillin, and 1% streptomycin. Cells were grown at 37°C in a 100% humidified incubator with a gas phase of 5% CO2 and routinely screened for Mycoplasma.
Proteins and peptides
Recombinant human VEGF165 and bFGF were purchased from R&D Systems (Abingdon, United Kingdom). Alphastatin was synthesized by Dr A. Moir (Molecular Biology and Technology, University of Sheffield, Sheffield, United Kingdom) using standard peptide synthesis techniques and purified to more than 93% using high-performance liquid chromatography (HPLC).
Migration assay
The migration assay, adapted from Malinda et al15 involved the use of a 48-well microchemotaxis chamber (Neuro Probe, Cabin John, MD) with 8-μm pore size polycarbonate membranes (Neuro Probe) coated with 100 μg/mL collagen type IV. VEGF or bFGF alone (10 ng/mL) or with various concentrations of alphastatin (10 nM, 100 nM, 1000 nM) were dissolved in DMEM + 1% FCS and placed in the bottom wells. The membrane was then positioned and 50 μL of 25 × 104 HuDMECs/mL (in DMEM containing 10% FCS) was added to the top chamber. The chambers were incubated at 37°C for 4.5 hours then migrated cells on the bottom surface were fixed and stained with Hema “Gurr” rapid staining kit (Merck, Leicester, United Kingdom) and counted at × 160 magnification in 3 random fields per well in 3 replicate wells and repeated 3 times.
Proliferation assay
The MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was used as described previously16 to assess proliferation of HuDMECs. The cells were seeded into 96-well microtiter plates at 3 × 104 cells/mL in DMEM + 10% FCS in the presence of various concentrations of alphastatin for 24, 48, and 96 hours. At these time points a quarter volume of MTT solution (2 mg MTT/mL phosphate-buffered saline [PBS]) was added to each well, and each plate was incubated for 4 hours at 37°C resulting in an insoluble purple formazan product. The medium was aspirated and the precipitates dissolved in 100 μL of dimethyl sulfoxide (DMSO) buffered at pH 10.5. The absorbance was then read at 540 nm using a Dynex enzyme-linked immunosorbent assay (ELISA) plate reader (Ashford, Middlesex, United Kingdom).
Tubule formation assay
Twenty-four–well plates were coated with 30 μL/well of growth factor–reduced (GF-reduced) Matrigel (Becton Dickinson Labware, Bedford, MA). Endothelial cells on this matrix migrated and formed tubules within 6 hours of plating.17 Cells (HuDMECs and SVEC4-10) were seeded at 4 × 104 cells/mL and incubated for 6 hours in 50 μL DMEM + 1% FCS alone (control) or medium ± 10, 100, 1000 nM alphastatin and with or without 10 ng/mL bFGF or VEGF. Wells were then fixed and stained with hematoxylin and eosin (H&E), visualized (at × 40 magnification), and tubule formation was assessed in 3 randomly selected fields of view per well using the image analysis package, Scion Image (Scion, Frederick, MA), as described by us.8
Cytotoxicity assay
Cells were seeded at a density of 1 × 105 to 2 × 105 cells per well in full growth medium in the absence or presence of alphastatin. After 24 hours, both live cells (following removal by trypsinization) and dead (floating) cells were harvested. The cell viability of all cells present was assessed using propidium iodide staining of 5000 cells in each of triplicate samples per treatment using a FACScan (Becton Dickinson) equipped with a blue laser excitation of 15 mW at 488 nm. The data were collected and analyzed using Cell Quest software (Becton Dickinson).
Tumor inoculation and alphastatin injections
All experiments were performed on 6-week-old immunocompetent Balb/c mice weighing 20 g (N = 13), obtained from Sheffield University Field Laboratories and were approved by the United Kingdom Home Office: Project Licence Number PPL40/1557 (NJB). Animals were anesthetized with an intraperitoneal injection of 1:1 diazepam and hypnorm (Janssen Pharmaceutical, High Wycombe, United Kingdom). Mice were inoculated subcutaneously with 2 × 106/mL viable CT26 cells in 100 μL medium. When the tumors had grown to 100 to 350 mm3 mice were injected intraperitoneally daily for 12 days with alphastatin (0.025 mg/kg/d; 7 mice) or vehicle (PBS; 6 mice) for 12 days. Tumor volumes were calculated daily using calipers.18 Animal weights and well-being were also monitored daily.
Histologic analysis
After 12 days mice were killed and tumors and normal tissues were excised, divided into 2 halves, and fixed in either 10% neutral buffered formalin or zinc-based fixative19 overnight then processed into paraffin wax. Formalin-fixed tissue sections were stained for H&E or martius yellow-brilliant crystal scarlet-soluble blue (MSB; a histologic stain for fibrin).20 Tumor necrosis was assessed using a Chalkley grid method as described previously.21
Vascular density assessment
Zinc-fixed sections were exposed to a rat monoclonal antimurine CD31 (1:100; Pharmingen, San Diego, CA) specific for endothelial cells for 60 minutes at room temperature and immunoreactivity was detected using the avidin-biotin complex (ABC) rat elite kit (Vector Laboratories, Orton Southgate, United Kingdom) and diaminobenzidine. Maximal vascular density was quantified using the Chalkley grid method.22
Statistical analysis
All data shown are means ± SEM, and representative data from one of 3 replicate experiments are shown. Statistical analysis was performed using a Mann-Whitney U test for nonparametric data. Data were considered statistically significant at P < .05.
Results
Effects of alphastatin on HuDMECs in vitro
Initial experiments were performed to determine whether alphastatin affected the 3 main stages in the angiogenesis pathway. These were carried out by establishing the effects of alphastatin on migration, proliferation, and tubule formation by HuDMECs in response to 10 ng/mL VEGF and bFGF in vitro. Exposure to alphastatin significantly (P < .006) inhibited both bFGF- and VEGF-induced migration in the Boyden chamber assay in a dose-dependent manner, achieving significance at 10 nM (Figure 2A). Exposure to alphastatin at doses of between 10 and 1000 nM for up to 48 hours had no significant effect on HuDMEC proliferation (Figure 2D), as assessed using the MTT assay. Tubule formation by HuDMECs on wells coated with growth factor–reduced Matrigel was significantly (P < .01) inhibited by alphastatin in a dose-dependent manner in both the absence and presence of bFGF and VEGF (Figure 2B). As we had previously demonstrated that 1000 nM FgnE is cytotoxic for HuDMECs,8 the cytotoxicity of 10, 100, and 1000 nM alphastatin on HuDMECs was assessed in vitro and found to have no detectable cytotoxic effect at these doses (data not shown). When HuDMECs were pre-exposed to alphastatin (1000 nM) for 2 hours and washed prior to addition to the GF-reduced Matrigel assay, the tubule formation of these HuD-MECs was inhibited to the same extent as cells that were exposed to alphastatin during the course of the assay (Figure 2C).
In vitro effects of alphastatin. (A) HuDMEC migration across a collagen-coated filter in response to medium alone (control) or medium containing 10 ng/mL VEGF or bFGF. (B) Tubule formation by HuDMECs on GF-reduced Matrigel in response to medium alone (control) or medium containing 10 ng/mL VEGF or bFGF, and (D) HuDMEC proliferation over a 48-hour period. (C) Tubule formation by HuDMECs exposed to either alphastatin (1000 nM) for 2 hours prior to the assay and then washed and added to the assay in the absence of alphastatin (but in the presence or absence of bFGF [10 ng/mL] or VEGF [10 ng/mL]) or HuDMECs exposed to alphastatin (1000 nM) during the assay alone (in the presence or absence of bFGF [10 ng/mL] or VEGF [10 ng/mL]). All data shown are means ± SEM. *P < .03 compared with respective “no alphastatin” groups.
In vitro effects of alphastatin. (A) HuDMEC migration across a collagen-coated filter in response to medium alone (control) or medium containing 10 ng/mL VEGF or bFGF. (B) Tubule formation by HuDMECs on GF-reduced Matrigel in response to medium alone (control) or medium containing 10 ng/mL VEGF or bFGF, and (D) HuDMEC proliferation over a 48-hour period. (C) Tubule formation by HuDMECs exposed to either alphastatin (1000 nM) for 2 hours prior to the assay and then washed and added to the assay in the absence of alphastatin (but in the presence or absence of bFGF [10 ng/mL] or VEGF [10 ng/mL]) or HuDMECs exposed to alphastatin (1000 nM) during the assay alone (in the presence or absence of bFGF [10 ng/mL] or VEGF [10 ng/mL]). All data shown are means ± SEM. *P < .03 compared with respective “no alphastatin” groups.
Comparison between the effects of alphastatin and FgnE on HuDMECs in vitro
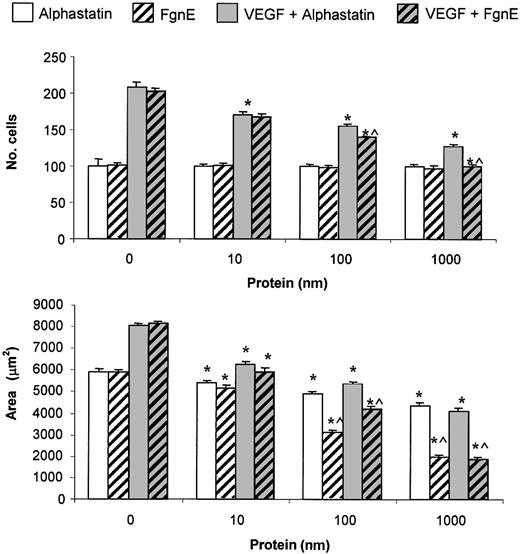
As alphastatin is derived from FgnE, we compared equimolar concentrations of the 2 proteins on the migration and tubule formation of HuDMECs. Although both proteins inhibit migration and tubule formation of HuDMECs in vitro, FgnE was significantly more effective at inhibiting than alphastatin at the highest 2 doses (100 and 1000 nM) in both the presence and absence of VEGF (10 ng/mL; Figure 3).
Comparison of the in vitro effects of alphastatin and FgnE. (A) HuDMEC migration across a collagen-coated filter in response to medium alone or medium containing 10 ng/mL VEGF. (B) Tubule formation by HuDMECs on GF-reduced Matrigel in response to medium alone (control) or medium containing 10 ng/mL VEGF. All data shown are means ± SEM. *P < .02 compared with respective “no protein” groups. ^P < .05 compared with respective alphastatin groups.
Comparison of the in vitro effects of alphastatin and FgnE. (A) HuDMEC migration across a collagen-coated filter in response to medium alone or medium containing 10 ng/mL VEGF. (B) Tubule formation by HuDMECs on GF-reduced Matrigel in response to medium alone (control) or medium containing 10 ng/mL VEGF. All data shown are means ± SEM. *P < .02 compared with respective “no protein” groups. ^P < .05 compared with respective alphastatin groups.
Secondary structure is necessary for alphastatin activity
As alphastatin consists of FpA (aa's 1-16) attached to a further 8 amino acids, we synthesized the short peptide, aa's 17-24. When this and FpA were tested in the GF-reduced Matrigel experiment, neither was seen to inhibit tubule formation (data not shown). This was in direct contrast to alphastatin, which at equimolar concentrations caused a significant inhibition of tubule formation.
Effects of alphastatin on murine cells in vitro
Although fibrinogen is a highly conserved protein cross-species, there are minor differences between rodents and humans.23,24 Alphastatin is derived from the human fibrinogen α-chain sequence, therefore it was necessary to establish that human alphastatin inhibited murine endothelial cell function prior to the evaluation of activity in vivo. Alphastatin was found to inhibit tubule formation by the murine microvascular endothelial cell line, SVEC4-10, both in the absence and presence of the growth factors bFGF and VEGF (data not shown). The effects of alphastatin on the growth and cytotoxicity of the murine tumor cell line investigated in this paper, CT26, were tested in the MTT proliferation assay and propidium iodide staining of cells on the fluorescence-activated cell sorter (FACS). Alphastatin did not alter CT26 cell proliferation over a 96-hour exposure period nor was it cytotoxic for these cells over a 24-hour exposure period at doses of up to 10 μM (data not shown).
Effects of alphastatin on tumor growth
As FgnE has previously been shown by us to inhibit the growth of CT26 tumors grown in Balb/C mice (a syngeneic tumor model),9 the same model was used to determine the effects of alphastatin in vivo. CT26 tumors implanted subcutaneously reached 100 to 350 mm3 in volume between days 14 and 18 following implantation. At this time animals were allocated into either treatment (alphastatin 0.025 mg/kg/d; n = 7) or control (PBS; n = 6) groups. Animals were then injected intraperitoneally every day for 12 days according to the appropriate regimen (PBS or alphastatin in PBS). Tumors in the control group continued to grow steadily over the treatment period, reaching a final tumor volume of 2642 ± 363 mm3. However, animals injected with alphastatin exhibited a significantly (P < .05) reduced growth rate between days 5 and 12, reaching a final tumor volume of only 1212 ± 174 mm3 (Figure 4A). Similar results were obtained in 2 further cohorts of such tumor-bearing mice. Alphastatin administration appeared to be well tolerated in vivo, with no significant effect on body weight or the general well-being of the animals (as determined by the absence of lethargy, intermittent hunching, tremors, or disturbed breathing patterns).
In vivo effects of alphastatin. In vivo effects of alphastatin on (A) the volume and (B) histologic appearance of CT26 tumors grown in Balb/C mice. Data are shown as mean ± SEM. (B) Tumors were excised from control (i,iii) or alphastatin-treated (ii,iv) mice and general morphology/histology was examined at low magnification (i-ii, bar = 100 μm) or stained with an antimurine CD31 antibody and viewed at higher magnification (iii-iv, bar = 50 μm). Cells in control tumors exhibited a compact regular morphology (i) with many patent vessels in the viable regions (white arrows; i) lined with a continuous single layer of endothelial cells (iii). By contrast, alphastatin-treated tumors exhibited an irregular overall morphology with increased levels of necrosis (N; ii,iv) and large distended vessels found in areas of viable tumor as well as in areas of necrosis (as in ii,iv) showing a central thrombosis (T; ii,iv) and lined with patchy incomplete endothelial cells (arrows; iv). (C) No effect of alphastatin injections was evident on the vascular endothelium of nonmalignant murine tissue in vivo. CD31+ cells lining vessels in a range of nonmalignant tissues (arrows); lungs (i,iv), liver (ii,v), and kidneys (iii,vi) from mice were unaffected by alphastatin treatment (iv-vi) and resembled those from control (i-iii) mice. Bar in vi = 50 μm.
In vivo effects of alphastatin. In vivo effects of alphastatin on (A) the volume and (B) histologic appearance of CT26 tumors grown in Balb/C mice. Data are shown as mean ± SEM. (B) Tumors were excised from control (i,iii) or alphastatin-treated (ii,iv) mice and general morphology/histology was examined at low magnification (i-ii, bar = 100 μm) or stained with an antimurine CD31 antibody and viewed at higher magnification (iii-iv, bar = 50 μm). Cells in control tumors exhibited a compact regular morphology (i) with many patent vessels in the viable regions (white arrows; i) lined with a continuous single layer of endothelial cells (iii). By contrast, alphastatin-treated tumors exhibited an irregular overall morphology with increased levels of necrosis (N; ii,iv) and large distended vessels found in areas of viable tumor as well as in areas of necrosis (as in ii,iv) showing a central thrombosis (T; ii,iv) and lined with patchy incomplete endothelial cells (arrows; iv). (C) No effect of alphastatin injections was evident on the vascular endothelium of nonmalignant murine tissue in vivo. CD31+ cells lining vessels in a range of nonmalignant tissues (arrows); lungs (i,iv), liver (ii,v), and kidneys (iii,vi) from mice were unaffected by alphastatin treatment (iv-vi) and resembled those from control (i-iii) mice. Bar in vi = 50 μm.
Effects of alphastatin on tumor histology
Histologic analysis revealed the presence of a central area of necrosis in all CT26 tumors, which was usually surrounded by a viable rim of tumor cells 300 to 600 μm in width. The central necrotic areas were frequently large and confluent and showed loss of cellular detail. Necrosis, assessed as a percentage of tumor section area using the Chalkley grid counting method, was significantly (P < .02) more extensive in the alphastatin-treated group (% necrosis in treated vs control, 43.6 ± 4.3 vs 11.4 ± 3.25). To determine whether the reduced volume of alphastatin-treated tumors was due to an effect of this peptide on the tumor vascular supply, endothelial cells in blood vessels were identified in tumor sections using immunostaining with anti–platelet cell adhesion molecule 1 (PECAM-1; CD31) antibody (Figure 4B) and the Chalkley grid counting method was used to assess the density of microvessels.22 Microvessel density was similar in the outer viable rim of tumor cells (the uniform layer of cells adjacent to the tumor periphery with well-defined nuclei) in control (5.63 ± 0.15) and alphastatin-treated (6.45 ± 0.47) tumors. Microvessel density was significantly (P < .01) higher in the inner, less viable region of tumor cells abutting the necrotic central areas in alphastatin-treated (7 ± 0.52) than in control (4.49 ± 0.26) tumors. Fibrin deposition, as identified by MSB staining, was also increased in and around blood vessels in the inner viable rim and the central necrotic core of alphastatin-treated more so than in control tumors. In the outer viable rim of alphastatin-treated tumors, although the vessel lumen remained patent and contained red blood cells, fibrin deposition was evident around many vessels. Alphastatin was found to have no such effects on the endothelium in the normal tissues examined (lungs, liver, and kidneys; Figure 4C).
Discussion
We have previously shown that a proteolytic cleavage product of fibrinogen, FgnE, inhibits endothelial cell migration and tubule formation in vitro8 and growth of CT26 tumors in vivo.9 The present study shows that a small peptide derived from the amino terminus of the α chain of human fibrinogen mimics these effects of FgnE. The delay in CT26 tumor growth appeared to be largely due to endothelial disruption and increased microvascular thrombosis, an effect restricted to activated tumor endothelium over the treatment period studied.
We originally proposed that the fact that alphastatin was held in a β-bend conformation within the FgnE molecule was essential for its inhibitory effects on activated endothelial cells. This was supported by the fact that neither the FpA portion of alphastatin (aa's 1-16) nor aa's 17-24 were found to mimic the inhibitory effects of alphastatin in vitro.10
Comparing the effects of FgnE and alphastatin, it can be seen that FgnE at an equivalent dose is more effective at inhibiting both migration and tubule formation of endothelial cells. This may be due to further conformational constraints placed on the amino terminal of the α chain when it is bound to the parent FgnE protein (constraints that are missing in alphastatin). Twenty-five percent to 30% of α chains of FgnE are phosphorylated at Ser3 in the bloodstream enabling a salt bridge with Arg23 that further stabilizes the β-bend structure.14,25 This configuration is thought to produce a more efficient enzyme-substrate combination, which may lead, in part, to the increased efficacy of FgnE compared with alphastatin, which does not carry this phosphorylation (due to lack of exposure to the relevant enzymes that cause such phosphorylations). A further difference is seen between the activities of alphastatin and FgnE when the cytotoxic effects on HuDMECs are compared. FgnE was cytotoxic at 1 μM, whereas alphastatin is not cytotoxic even up to 10 μM (data not shown). This suggests that the region of the FgnE molecule responsible for the cytotoxic effect is found elsewhere within the protein and not the alphastatin region. Therefore, it remains a possibility that the reason for FgnE being more efficient at inhibiting endothelial cell function in the in vitro assays used may, in part, be due to the cytotoxic effects of the protein. Although detectable cytotoxicity does not occur at the lower doses of FgnE, this cytotoxic portion of the protein may contribute to the inhibitory effects observed within such assays. Therefore, FgnE may have dual active sites, one being the amino terminus of the α chain and another that has yet to be determined.
The fact that alphastatin inhibits the migration and tubule formation of HuDMECs in response to both VEGF or bFGF suggests that these effects most likely operate at a postreceptor locus common to the VEGF and bFGF signaling pathways in these cells. Reports by Sahni and coworkers26,27 indicate that fibrinogen is capable of binding to both VEGF and bFGF, but neither appears to bind to the alphastatin region of fibrinogen,27,28 so it is unlikely that alphastatin inhibits the action of these growth factors by such an indirect mechanism. Furthermore, exposure of HuDMECs to alphastatin for 2 hours, prior to addition of the cells to the GFR-Matrigel assay in vitro (which was then run in the absence of alphastatin), was sufficient to significantly inhibit both VEGF- and bFGF-induced tubule formation on GFR-Matrigel.
In our syngeneic tumor model, the growth of CT26 tumors continued over the 12-day injection period, whereas mice injected daily with alphastatin demonstrated a significant reduction in tumor volume from the fifth day of administration and this was maintained for the 12 days of injection. Although the dose of alphastatin administered was calculated to reach 100 nM in the murine bloodstream (assuming a total blood volume of 2 mL per 20 g mouse), the administration was via the intraperitoneal route, so it is possible that the concentration of active peptide reaching the tumor vasculature was less than this, due to incomplete absorption from the peritoneum and/or enzymatic degradation in the bloodstream.
Histologic examination of resected control tumors and nonmalignant tissues revealed that the endothelial cells formed an intact, continuous, CD31+ layer lining the blood vessels, indicating that the blood vessels were patent with no evidence of endothelial disruption. However, alphastatin treatment resulted in vessel disruption in all but the thin outer rim of tumors, characterized by a discontinuous endothelial cell lining with many cells clumped and/or fragmented. There was no inflammatory cell accumulation around the disrupted vessels as opposed to unaffected vessels elsewhere in the tumor. In addition, many vessels contained (and were often surrounded by) extensive fibrin deposition, suggesting the formation of extensive thrombosis. This resulted in inadequate blood supply to central areas of tumor cells and the formation of large areas of necrosis. These findings suggest that alphastatin is selectively cytotoxic for the activated endothelial cells lining tumor vessels. However, we found no evidence in our in vitro studies that alphastatin could trigger VEGF-activated endothelial cells to apoptose (data not shown), and analysis of CT26 tumor sections following DAPI (4,6 diamidino-2-phenylindole) staining and/or using morphologic analysis of endothelial cell disruption in tumor cells indicated that apoptosis of the endothelial cells had not occurred in treated tumors, although this requires more conclusive investigation using TUNEL (terminal deoxynucleotidyl transferase [TdT]–mediated deoxyuridine triphosphate [dUTP] nick-end labeling) staining of tumor sections. Alternatively, this peptide may be able to selectively disrupt activated endothelial cells in tumor vessels by some other, as yet undefined, mechanism(s). Both mechanisms could induce the coagulation seen in vessels of alphastatin-treated tumors. Further studies are also warranted to ascertain whether the antiangiogenic effects of alphastatin seen in vitro play any part in the antivascular effects of this peptide in tumor-bearing mice.
A general activation of hemostatic pathways is unlikely as no signs of thrombosis or fibrin deposition were observed in blood vessels in the lungs, liver, or kidneys of alphastatin-treated mice. Indeed, most of the vessels in these normal tissues were packed with erythrocytes, indicating that vessels were not occluded. This important observation indicates that alphastatin binds to activated rather than quiescent endothelial cells and confirms that the receptor(s) for alphastatin may be expressed only on activated endothelium in tumor vessels. This also supports a possible therapeutic role for alphastatin as an agent to specifically target activated endothelium in areas of angiogenesis, rather than endothelial cells in the quiescent vasculature.
It will be noted that the vascular counts were higher in alphastatin-treated animals in the inner, less viable rim of tumor cells abutting the areas of necrosis (when this was present) compared with the same region in control tumors. This was not due to an increase in tumor angiogenesis but a consequence of the dilation of, and thrombosis within, vessels in these tumor areas, causing an increased number of intersections between each vessel and the Chalkley grid.
Although alphastatin treatment resulted in marked signs of vessel coagulation and concomitant necrosis in CT26 tumors, a thin, outer viable rim of these tumors (in which both vessels and tumor cells were largely unaffected) was usually also present. One explanation for this unaffected outer viable rim may be that as subcutaneous CT26 tumors grow they co-opt pre-existing vessels from the surrounding tissue rather than generate new vessels by angiogenesis. This would mean that vessels in the outer viable rim would be unlikely to be actively proliferating and thus may not express the receptor(s) for alphastatin.
As it remained a possibility that alphastatin could mediate its effects in vivo by causing an inhibition in tumor cell growth or induction of apoptosis of CT26 cells, we tested the effects of alphastatin on CT26 cells in vitro. Lengthy exposures to alphastatin in vitro had no effect on CT26 viability or proliferation, suggesting that alphastatin does not target tumor cells directly in this murine tumor model. Taken together, these in vitro and in vivo observations suggest that alphastatin targets blood vessels in tumors, causing endothelial cell damage and triggering a hemostatic response (ie, fibrin deposition and thrombosis). This is likely to have gradually occluded affected vessels, causing inadequate perfusion of surrounding areas and increased necrosis, which in turn reduced tumor growth in vivo.
Neutrophils are known to play a role within tumor angiogenesis29 and it has been demonstrated that the adhesion molecule CD11c/CD18 on neutrophils interacts with amino acids 17-19 (Gly-Pro-Arg) on the α chain of fibrinogen.30 This sequence of amino acids is also contained within the alphastatin molecule, therefore it is possible that side effects may be induced in vivo, including enhanced susceptibility to bacterial infection. However, it is unclear whether such binding occurs when the alphastatin sequence is no longer part of the whole fibrinogen molecule.
Human rather than murine alphastatin was used in these studies to allow a direct comparison with the data generated by us using the parent molecule, human FgnE.9 However, this means that the possibility exists that the effects on the tumor vasculature and growth observed in the current studies were due, at least in part, to the potential immunogenic effects of human alphastatin in mice. However, this is an unlikely explanation for the observed effects as there was no visible microscopic evidence of a peritoneal cellular reaction to alphastatin injections, and histologic analysis of lungs, liver, and kidneys from alphastatin-treated animals showed no cellular or structural abnormalities. Moreover, a similar disruption to tumor vessels and delay in tumor growth has recently been demonstrated in human prostate xenografts (PC3; A. Fowles, personal oral communication, July 2002) and in human breast xenografts (HT29; S. Stribbling, personal oral communication, September 2003) grown subcutaneously in immunocompromised (nude) mice where no such immune reaction could occur.
Following prolonged application of human alphastatin in mice, a loss of antivascular efficacy may occur due to a humoral reaction to the injected protein, which could mean that the therapeutic efficacy of long-term administration of human alphastatin might be greater in humans than in the mice used here. The effects of alphastatin shown here were obtained using a relatively low dose of alphastatin and a short period of administration (12 days). However, a similar inhibition of tumor growth was also seen when the dose of alphastatin was increased 10-fold to 0.25 mg/kg/day and the injection period extended by a further 5 days (data not shown).
In conclusion, the data presented demonstrate for the first time that a small peptide derived from the amino terminus of the α chain of fibrinogen (alphastatin) inhibits both endothelial cell migration and tubule formation in vitro and the growth of experimental murine tumors in vivo. This latter effect was due at least in part to disruption of activated endothelial cells leading to intravascular thrombosis and widespread tumor necrosis. This peptide, or a smaller/cyclatised peptide derivative, may have utility in the treatment of cancer and other angiogenesis-dependent diseases.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-07-2192.
Two of the authors (C.E.L. and N.J.B.) have declared a financial interest in BioActa Ltd, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 2. In vitro effects of alphastatin. (A) HuDMEC migration across a collagen-coated filter in response to medium alone (control) or medium containing 10 ng/mL VEGF or bFGF. (B) Tubule formation by HuDMECs on GF-reduced Matrigel in response to medium alone (control) or medium containing 10 ng/mL VEGF or bFGF, and (D) HuDMEC proliferation over a 48-hour period. (C) Tubule formation by HuDMECs exposed to either alphastatin (1000 nM) for 2 hours prior to the assay and then washed and added to the assay in the absence of alphastatin (but in the presence or absence of bFGF [10 ng/mL] or VEGF [10 ng/mL]) or HuDMECs exposed to alphastatin (1000 nM) during the assay alone (in the presence or absence of bFGF [10 ng/mL] or VEGF [10 ng/mL]). All data shown are means ± SEM. *P < .03 compared with respective “no alphastatin” groups.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/2/10.1182_blood-2003-07-2192/6/m_h80245542002.jpeg?Expires=1767816456&Signature=PiR11nXsNTE4BqEDPVblJkJLEJcRanSUfgbiENHcESbuAwN4UI9zcumVAkhkcuiEIoFl6TsXKODbH6b2m3Pm9zL810t8kqMt9-tYcEQ~p5GT~63hdg1AL0MZjO8gXebYgeb1JJ3a7vPXpGQbM3pixMlbzlyMkLaMfrwhCv3Ic21ZchW~KUYOfKjnJiohlvb21NvY577CQ32Aa5VpavL24hS5DR1MSpRotBycRkqzBpPdyt2AW3DgVmDl8Cum2KE8Sld7Egdz0aBQJR2TTKmgpIgN0d~7i0FyizwTtVOJ5tzQ4swV8IGFC4rzWKFbLI-IfcQqwIhlI4Ifq-B7dlDIJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal