Abstract
Since endotoxins are potent inducers of stem cell mobilization, we hypothesized that their presence in the gut may play a role in cytokine-induced mobilization. To address this possibility we added ciprofloxacin and polymyxin B to the drinking water of Balb/c mice mobilized with either interleukin-8 (IL-8), granulocyte colony-stimulating factor (G-CSF), or flt3 ligand (FL). The yield of colony-forming units (CFUs) was significantly reduced in all mice treated with these antibiotics when compared with controls (IL-8: 192 ± 61 vs 290 ± 64, P < .05; G-CSF: 1925 ± 1216 vs 3371 ± 1214, P < .05; FL: 562 ± 213 vs 1068 ± 528, P < .05). Treatment with ciprofloxacin eliminated only aerobic Gramnegative bacteria from the feces without effect on mobilization. Polymyxin B treatment did not result in decontamination but significantly reduced the number of mobilized hematopoietic progenitor cells (HPCs) most likely due to the endotoxin binding capacity of polymyxin B. More than 90% of the gastrointestinal flora consists of anaerobic bacteria. Elimination of the anaerobic flora by metronidazol led to a significantly reduced number of mobilized HPCs when compared with controls (IL-8: 55 ± 66 vs 538 ± 216, P < .05). Germ-free OF1 mice showed a significantly reduced mobilization compared with their wild-type controls (IL-8 controls: 378 ± 182, IL-8 germ free: 157 ± 53, P < .05). Finally, we performed reconstitution experiments adding Escherichia coli–derived endotoxins to the drinking water of decontaminated mice. This resulted in partial restoration of the IL-8–induced mobilization (67 ± 28 vs 190 ± 98.1, P <.01). Our results indicate that endotoxins serve as cofactors in cytokine-induced mobilization. Modification of the endotoxin content by antibiotic treatment may affect the yield of cytokine-induced mobilization.
Introduction
To reduce the risk of infections in neutropenic patients a combination of protective isolation and gut decontamination by oral administration of antibiotics is often used.1-4 Since it has been shown that selective elimination of the major potentially pathogenic microorganisms from the digestive tract is as effective or even superior to total decontamination in preventing infections in neutropenic patients,5-8 selective gut decontamination9 or partial antibiotic decontamination10 mainly directed against aerobic Gram-negative rods and fungi is usually applied. The various antibiotics used for selective decontamination can be divided into 2 main groups, those that are absorbed11-13 after oral administration (eg, cotrimoxazole, quinolones) and those that are not absorbed2-4 after oral administration (eg, polymyxin, colistin, aminoglycosides).
Mobilized hematopoietic progenitor cells (HPCs) are increasingly used for hematopoietic recovery instead of bone marrow to allow rapid hematopoietic recovery after autologous and allogeneic transplantation.14-18 An increasing number of different growth factors and chemokines such as granulocyte colony-stimulating factor (G-CSF),19,20 granulocyte-macrophage colony-stimulating factor (GM-CSF),19,21 stem cell factor (SCF),22 flt3 ligand (FL),23 interleukin-1 (IL-1),24 IL-3,25 IL-8,26 and thrombopoietin27 when either administered alone or in combination28 are capable of mobilizing peripheral blood progenitor cells. In the clinical setting, the growth factor G-CSF has become the standard for autologous as well as allogeneic peripheral blood stem cell transplantation.29-31
Although patients receiving selective gut decontamination or systemic antibiotic treatment while concurrently being treated with G-CSF are able to mobilize peripheral blood progenitor cells, little is known of the effect of antibiotics on the efficacy of mobilization.
Endotoxins are ubiquitously present and constitute an important component of the normal intestinal flora and are known to induce HPC mobilization.32-34 We therefore hypothesized that low levels of endotoxins passing through the intestinal mucosa into the blood may contribute to the levels of circulating HPCs. Here, we used a mouse model to study the effects of selective antibiotic decontamination on cytokine-induced stem cell mobilization. We found that the ability to mobilize in response to IL-8, G-CSF, or FL was significantly reduced in animals receiving endotoxin binding antibiotics or antibiotics that modulate the anaerobic flora. The mobilizing capacity was partially restored after adding endotoxins to the drinking water of decontaminated mice. These observations indicate that endotoxins are involved as cofactors in cytokine-induced hematopoietic stem cell mobilization.
Materials and methods
Animals
Male Balb/c mice between 8 to 12 weeks of age were purchased from Broekman BV (Someren, the Netherlands). OF1 mice either specific pathogen free (SPF) or germ free were purchased from Iffa-Credo (Lyon, France). The OF1 mice were kept at the animal facility of the Rega Institue of the University of Leuven (Belgium). Upon arrival, the germ-free animals were transferred to and kept in insulators under strict germ-free conditions. They were fed ad libitum with mixed complete aliment R-03 (Usine d'Alimentation Rationelle, Epinay sur Orge, France), which was irradiated at 45 kGy, and canned sterile water (Sleutels Conserven, Panninge, the Netherlands), which was reautoclaved after transfer into the sterile drinking bottles. Feces of these mice were cultured and no aerobic Gram-negative bacteria were found. All other mice were fed commercially available rodent chow. Control mice received acidified water ad libitum.
Antibiotic treatment
There were 5 different antibiotic regimens consisting of combinations of 3 different antibiotics administered through the drinking water (Table 1). The first regimen consisted of 100 mg/L ciprofloxacin (Bayer Nederland BV, Mijdrecht, the Netherlands) 70 mg/L polymyxin B (Bupha BV, Uitgeest, the Netherlands), and 20 g/L saccharose. The second regimen contained 125 mg/L ciprofloxacin and 20 g/L saccharose. The third regimen consisted of 100 mg/L polymyxin B and 20 g/L saccharose. The fourth regimen consisted of 500 mg/L metronidazol (NPBI, Amstelveen, the Netherlands) and 20 g/L saccharose, and in the fifth regimen the mice received 500 mg/L metronidazol, 125 mg/L ciprofloxacin, and 20 g/L saccharose. All antibiotics were administered during 1 week before cytokine-induced stem cell mobilization and were continued throughout the administration of the different cytokines.
Characteristics of the different antibiotics used for selective decontamination of the gut
. | Effect on aerobic bacteria . | Effect on anaerobic bacteria . | Decontamination* . | Endotoxin binding . |
|---|---|---|---|---|
| Ciprofloxacin35 | + | - | + | - |
| Polymyxin B36-39 | ± | - | - | + |
| Metronidazol40,41 | - | + | - | - |
. | Effect on aerobic bacteria . | Effect on anaerobic bacteria . | Decontamination* . | Endotoxin binding . |
|---|---|---|---|---|
| Ciprofloxacin35 | + | - | + | - |
| Polymyxin B36-39 | ± | - | - | + |
| Metronidazol40,41 | - | + | - | - |
No aerobic Gram-negative bacteria were present in the feces.
Endotoxin treatment
Endotoxin lipopolysaccharide (LPS) derived from Escherichia coli, sero-type 026:B6 (> 10 000 U/ng LPS; Sigma-Aldrich, Zwijndrecht, the Netherlands) was used. LPS was administered in the drinking water for 1 week at a concentration of 33 μg/mL.
Cytokines
Recombinant human granulocyte colony-stimulating factor (rhu-G-CSF, filgrastim [Neupogen]; a kind gift from Amgen BV, Breda, the Netherlands) and FL (a kind gift from Immunex, Seattle, WA) were diluted to the desired concentration in endotoxin-free phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) and administered as an intraperitoneal injection. FL contained 25 pg endotoxin per milligram of protein. Recombinant human IL-8 (a kind gift from the Novartis Forschungsinstitut, Vienna, Austria) was purified from E coli expressing a synthetic gene.42 The concentration of endotoxin was less than 0.05 U/mL as determined by the Limulus polyphemus amoebocyte lysate assay.
Preparation of cell suspensions
Mice were killed by CO2 asphyxiation. Peripheral blood was drawn by cardiac puncture, and white blood cell (WBC) counts were performed on a Sysmex F800 (TOA Medical Electronics, Kobe, Japan). Manual neutrophil counts were performed after May-Grünwald-Giemsa (MGG) staining of the peripheral blood. Blood mononuclear cell (MNC) suspensions were obtained after Ficoll separation as described previously.24 The bone marrow cells were removed from the femur by flushing the femur under sterile conditions with RPMI 1640 containing 500 μg/mL penicillin, 250 μg/mL streptomycin, and 2% fetal bovine serum (FBS; GIBCO, Grand Island, NY), and 6% heparin (400 IU/mL).
FACS analysis
Cells were incubated for 30 minutes at 4°C with anti-CD3e and anti-CD45R/B220 antibodies both phycoerythrin (PE)–conjugated and anti–GR-1 antibody–conjugated to fluorescein isothiocyanate (FITC; PharMingen, San Diego, CA.).
The cells were then washed with PBS containing 0.8 g/L albumin, and incubated at 4°C with Cy-chrome–conjugated anti–Ly-5 antibody (Ly-5–cyanin-5 (Cy 5); PharMingen) for 30 minutes. After washing, the cells were resuspended in PBS containing 0.8 g/L albumin, and fluorescence intensity was analyzed using fluorescence-activated cell sorting (FACS; Becton Dickinson, Mountain View, CA). The GR-1 strong positive, CD3–, B220-PE–negative cells within the total population of Ly5-Cy5–positive cells were then regarded as mature neutrophils.43
Progenitor cell assays
Colony forming units–granulocyte macrophage (CFU-GMs) were cultured as described previously.40 Briefly, peripheral blood MNCs were cultured in 3.5-cm dishes containing 5 × 105 cells per mL in semisolid medium in the presence of recombinant murine GM-CSF (1.25 ng/mL, kindly provided by Dr E. Liehl, Novartis Forschungsinstitut). Bone marrow cells were cultured in concentrations of 1 × 105 per mL. After 6 days of culture at a fully humidified atmosphere of 37°C containing 5% CO2, the number of colonies, defined as an aggregate of more than 20 cells, was scored using an inverted microscope.
CAFC assay
In vitro determination of hematopoietic progenitor cell frequencies was performed by limited dilution analysis (LDA) of cobblestone area–forming cells (CAFCs) in microcultures according to the method previously described.44,45 Briefly, cells were seeded on a pre-established stromal layer of the murine preadipocyte cell line, FBMD-1 (kindly provided by Dr R. E. Ploemacher, Erasmus University, Rotterdam, the Netherlands). At weekly intervals until day 35 after initiation of the cultures, cobblestone areas were scored using an inverted microscope. Cobblestone areas are defined as colonies of immature hematopoietic cells (at least 6 cells per colony) residing within the pre-established stromal layer. The proportion of negative wells at each dilution was used in a Poisson-based LDA calculation to determine the CAFC frequency.44,46
Analysis of mouse feces
Mice were housed in plastic cages on sawdust, both of which were replaced daily. Fresh fecal pellets were collected in 500 μL sterile saline after 1 week of administration of antibiotics. Then, 10-fold serial dilutions were made, the lower limit being 1:1000. Samples (100 μL) of the dilutions were inoculated on blood agar plates (MacConkey III–agar plates; Oxoid, Basingstoke, United Kingdom). The 1:1000 dilution was also inoculated after it had first been incubated for 2 days at 37°C. Of the 1:10 dilution an extra Sabouraud and aesculine plate were inoculated for elective purposes. All plates were incubated at 37°C. After 24 and 48 hours of incubation various colonies were isolated and identified based on morphology and Gram staining. Before starting the mobilization schedule, the feces of all mice were cultured to determine the presence of aerobic Gram-negative rods. After treatment with ciprofloxacin either alone or in combination with polymyxin B, all mice were free of aerobic Gram-negative rods (ie, adequately decontaminated). After treatment with metronidazol or with polymyxin B added to the drinking water in a concentration of 100 mg/L, aerobic Gram-negative rods were still cultured from the feces of these mice.
Serum concentration of endotoxins, IL-6, and TNF-α
Quantitative measurements of endotoxins were carried out using the commercially available endotoxin test QCL-1000 (Bio Whittaker, Boehringer Ingelheim Bioproducts, Verviers, France). The detection limit was 0.06 endotoxin units per milliliter of serum (EU/mL). In the serum of the same group of mice, IL-6 and TNF-α were measured using an enzyme-linked immunosorbent assay (ELISA) technique (Diaclone, CLB, Amsterdam, the Netherlands). All test were used according to the manufacturer's instructions.
Detection of MMP-9 (gelatinase B) activity
Gelatinase B/matrix metalloproteinase 9 (MMP-9) activity in freshly frozen plasma samples was determined by sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) zymography as described earlier.47
Experimental design
Mice were treated with different regimens of antibiotics, endotoxin, or a combination of both for 1 week. Before the injection of cytokines, feces of the mice were collected and cultured. Mobilization was induced in pretreated or untreated control animals by either a single intraperitoneal injection of saline or 30 μg IL-8, or daily intraperitoneal injections of 5 μg G-CSF for 5 days, or daily injections of 10 μg FL for 5 days. At 20 minutes after the injection with saline or IL-8 or 24 hours after the last injection of G-CSF or FL, mice were killed by CO2 asphyxiation, peripheral blood was obtained by cardiac puncture, and femurs were removed. Cell suspensions were prepared according to the described procedures. The numbers of CFU-GMs in the peripheral blood and in the femur of the different animals were determined. An MGG staining of the peripheral blood was performed as well as FACS analysis of the bone marrow. In a number of animals, a CAFC assay was performed of peripheral blood and bone marrow cells. The institutional ethical committees on animal experiments of Leiden University Medical Center and the Rega Institute, Laboratory for Molecular Immunology, approved the experimental protocol.
Statistical analysis
Differences were evaluated using the Student t test. P values of less than .05 were considered statistically significant. To calculate the CAFC frequency, a Poisson-based LDA was used. The 95%, 99%, and 99.9% confidential intervals were calculated, and when the confidential intervals were not overlapping the P value was considered to be less than 0.05%, 0.01%, and 0.001%, respectively.
Results
Effect of antibiotic treatment on the peripheral blood counts and on peripheral blood progenitor cell mobilization
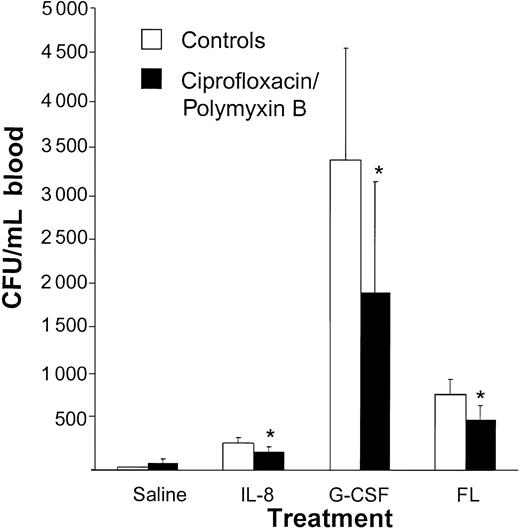
To study the effect of selective antibiotic decontamination (ie, elimination of Gram-negative bacteria) on stem cell mobilization, decontaminated mice were treated with mobilization-inducing cytokines. Stem cell mobilization in response to IL-8, G-CSF, or FL was significantly reduced in mice decontaminated with ciprofloxacin and polymyxin B compared with mice that had not received antibiotic treatment (saline: 26 ± 8 CFUs/mL vs 71 ± 42 CFUs/mL; IL-8: 290 ± 64 CFUs/mL vs 192 ± 61 CFUs/mL, P < .05; G-CSF: 3371 ± 1214 CFUs/mL vs 1925 ± 1216 CFUs/mL, P < .05; FL 818 ± 175 CFUs/mL vs 552 ± 149 CFUs/mL, P < .05; Figure 1).
The cytokine-induced mobilization of progenitor cells is decreased by treatment with ciprofloxacin and polymyxin B. Mice were treated with 100 mg/L ciprofloxacin, 70 mg/L polymyxin B, and 20 mg/L saccharose added to their drinking water for 1 week. While the antibiotic treatment was continued, mice were mobilized with either a single intraperitoneal injection of 30 μg IL-8 (n = 8), or 5 μg G-CSF (n = 15) or 10 μgFL(n = 9), both for 5 days. At 20 minutes after the IL-8 injection and 24 hours after the last injection with G-CSF or Flt 3 ligand, mice were killed and blood was harvested. Results are expressed as means ± SD. *P < .05 compared with the saline pretreated controls (n = 3-10). Results are expressed as means ± SD.
The cytokine-induced mobilization of progenitor cells is decreased by treatment with ciprofloxacin and polymyxin B. Mice were treated with 100 mg/L ciprofloxacin, 70 mg/L polymyxin B, and 20 mg/L saccharose added to their drinking water for 1 week. While the antibiotic treatment was continued, mice were mobilized with either a single intraperitoneal injection of 30 μg IL-8 (n = 8), or 5 μg G-CSF (n = 15) or 10 μgFL(n = 9), both for 5 days. At 20 minutes after the IL-8 injection and 24 hours after the last injection with G-CSF or Flt 3 ligand, mice were killed and blood was harvested. Results are expressed as means ± SD. *P < .05 compared with the saline pretreated controls (n = 3-10). Results are expressed as means ± SD.
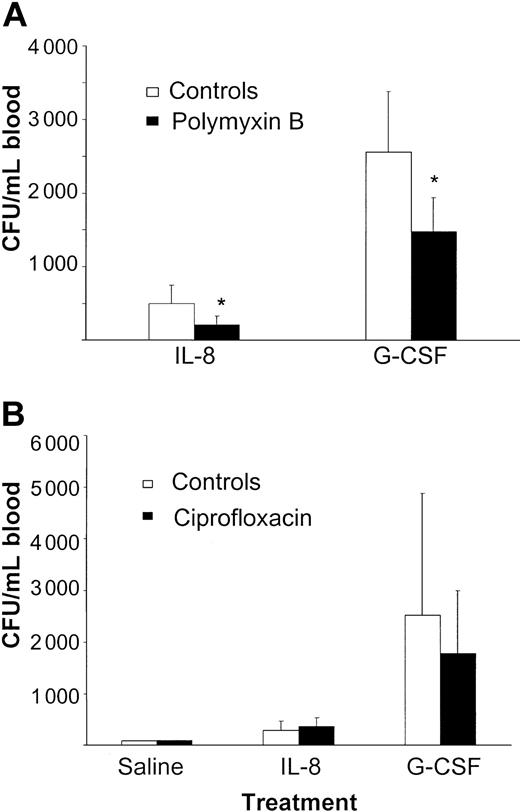
We next studied the influence of each of these 2 antibiotics. Although treatment with only polymyxin B did not result in adequate decontamination of mice, the IL-8– and G-CSF–induced mobilizations were significantly reduced (IL-8: 500 ± 252 CFUs/mL vs 212 ± 115 CFUs/mL, P < .05; G-CSF: 2557 ± 825 CFUs/mL vs 1475 ± 460 CFUs/mL, P < .05; Figure 2A).
Treatment with oral polymyxin B reduces cytokine-induced stem cell mobilization, while treatment with ciprofloxacin has no effect on IL-8– or G-CSF–induced mobilization. (A) Mice were treated with 100 mg/L polymyxin B and 20 mg/L saccharose added to their drinking water for 1 week. Mice were then mobilized with either a single intraperitoneal injection of 30 μg IL-8 (n = 13) or 5 μg G-CSF (n = 15) per day for 5 days. At 20 minutes after the IL-8 injection and 24 hours after the injection with G-CSF, mice were killed and blood was harvested. Results are expressed as means ± SD. *P < .05 compared with the controls (n = 6-8). (B) Mice were treated with 125 mg/L ciprofloxacin and 20 mg/L saccharose added to their drinking water for 1 week. The control mice received normal drinking water. Antibiotic treatment was continued when mice were mobilized with either a single injection of 30 μg IL-8 (controls: n = 6, treatment group: n = 9) or 5 μg G-CSF (controls: n = 4, treatment group: n = 10) per day for 5 days. At 20 minutes after the IL-8 injection and 24 hours after the injection with G-CSF, the mice were killed and blood was harvested. Results are expressed as means ± SD.
Treatment with oral polymyxin B reduces cytokine-induced stem cell mobilization, while treatment with ciprofloxacin has no effect on IL-8– or G-CSF–induced mobilization. (A) Mice were treated with 100 mg/L polymyxin B and 20 mg/L saccharose added to their drinking water for 1 week. Mice were then mobilized with either a single intraperitoneal injection of 30 μg IL-8 (n = 13) or 5 μg G-CSF (n = 15) per day for 5 days. At 20 minutes after the IL-8 injection and 24 hours after the injection with G-CSF, mice were killed and blood was harvested. Results are expressed as means ± SD. *P < .05 compared with the controls (n = 6-8). (B) Mice were treated with 125 mg/L ciprofloxacin and 20 mg/L saccharose added to their drinking water for 1 week. The control mice received normal drinking water. Antibiotic treatment was continued when mice were mobilized with either a single injection of 30 μg IL-8 (controls: n = 6, treatment group: n = 9) or 5 μg G-CSF (controls: n = 4, treatment group: n = 10) per day for 5 days. At 20 minutes after the IL-8 injection and 24 hours after the injection with G-CSF, the mice were killed and blood was harvested. Results are expressed as means ± SD.
In contrast, adequate decontamination was observed after treatment with ciprofloxacin as monotherapy, but without an effect on the mobilizing capacity (saline: 77 CFUs/mL vs 88 CFUs/mL; IL-8: 290 ± 173 CFUs/mL vs 364 ± 171 CFUs/mL, P = .41; G-CSF: 2525 ± 2352 CFUs/mL vs 1784 ± 1211 CFUs/mL, P = .44; Figure 2B). This observation led to the hypothesis that a decrease in mobilization was due to the endotoxin binding capacity of polymyxin B and that ciprofloxacin had no effect on mobilization since the anaerobic Gram-negative endotoxins containing flora were not eliminated.
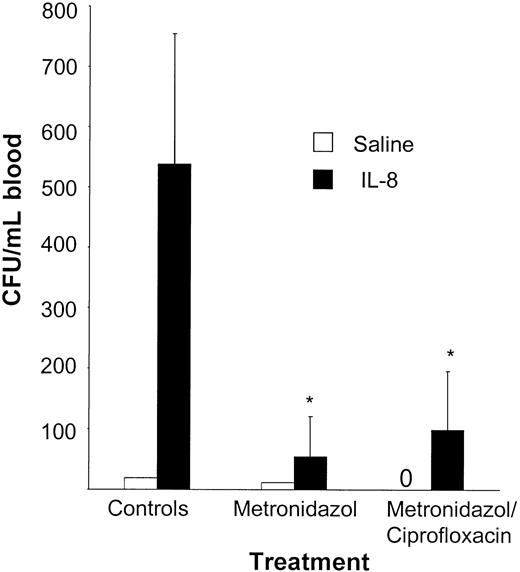
To study the role of anaerobic Gram-negative flora on mobilization, animals were treated with metronidazol alone or in combination with ciprofloxacin. Both treatment schedules resulted in a significant decrease of the IL-8–induced mobilization when compared with the control group (saline + metronidazol: 12 CFUs/mL vs saline + metronidazol + ciprofloxacin: 0 CFUs/mL vs saline control: 19 CFUs/mL; IL-8 + metronidazol: 55 ± 66 CFUs/mL vs IL-8 + metronidazol + ciprofloxacin: 98 ± 165 CFUs/mL vs IL-8 control: 538 ± 216 CFUs/mL, P < .05; Figure 3).
IL-8–induced mobilization is significantly reduced after treatment with metronidazol. Mice were receiving either normal drinking water (n = 5), water treated with 500 mg/L metronidazol and 20 g/L saccharose (n = 5), or water treated with 500 mg/L metronidazol, 100 mg/L ciprofloxacin, and 20 g/L saccharose (n = 5) for 1 week. Mice were mobilized with a single intraperitoneal injection of 30 μg IL-8. At 20 minutes after the IL-8 injection, the mice were killed and blood was harvested. Results are expressed as means ± SD. *P < .05 compared with the controls (n = 2).
IL-8–induced mobilization is significantly reduced after treatment with metronidazol. Mice were receiving either normal drinking water (n = 5), water treated with 500 mg/L metronidazol and 20 g/L saccharose (n = 5), or water treated with 500 mg/L metronidazol, 100 mg/L ciprofloxacin, and 20 g/L saccharose (n = 5) for 1 week. Mice were mobilized with a single intraperitoneal injection of 30 μg IL-8. At 20 minutes after the IL-8 injection, the mice were killed and blood was harvested. Results are expressed as means ± SD. *P < .05 compared with the controls (n = 2).
No effect on the WBC count or the absolute number of neutrophils in blood or bone marrow was seen in animals treated with various antibiotic regimes compared with untreated controls (data not shown).
Peripheral blood cell counts and peripheral blood progenitor cell mobilization in germ-free mice (OF-1)
It was considered that the reduced mobilizing capacity in decontaminated mice was due to a nonspecific effect of the antibiotics used. Mobilization experiments were therefore performed in germ-free OF-1 mice not treated with antibiotics. IL-8–induced mobilization in germ-free mice and in OF-1 mice treated with ciprofloxacin and polymyxin B was significantly reduced compared with the control group not receiving antibiotics (IL-8 controls: 378 ± 182 CFUs/mL, IL-8 antibiotics: 225 ± 109 CFUs/mL, P < .05; IL-8 germ-free: 157 ± 53 CFUs/mL, P < .05; Figure 4). The peripheral blood counts between the 3 groups of mice were not different (data not shown).
In germ-free mice the IL-8–induced mobilization is significantly reduced. There were 3 groups of OF1 mice mobilized with a single intraperitoneal injection of 30 μg IL-8 or saline: normal controls (saline: n = 3, IL-8: n = 7), mice that had been treated with ciprofloxacin and polymyxin B for 1 week (saline: n = 3, IL-8: n = 7), and germ-free OF1 mice (saline: n = 3, IL-8: n = 7) not treated with antibiotics. At 20 minutes after the IL-8 injection, the mice were killed and blood was harvested. Results are expressed as means ± SD. *P < .05 compared with the controls.
In germ-free mice the IL-8–induced mobilization is significantly reduced. There were 3 groups of OF1 mice mobilized with a single intraperitoneal injection of 30 μg IL-8 or saline: normal controls (saline: n = 3, IL-8: n = 7), mice that had been treated with ciprofloxacin and polymyxin B for 1 week (saline: n = 3, IL-8: n = 7), and germ-free OF1 mice (saline: n = 3, IL-8: n = 7) not treated with antibiotics. At 20 minutes after the IL-8 injection, the mice were killed and blood was harvested. Results are expressed as means ± SD. *P < .05 compared with the controls.
Effect of treatment with LPS on the mobilization of HPCs in control and partially decontaminated mice
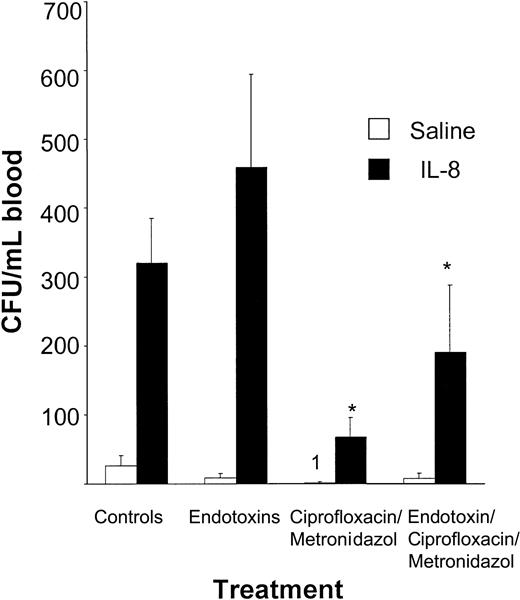
To confirm that the reduced mobilizing capacity in decontaminated mice was due to elimination or inactivation of endotoxin, we performed endotoxin reconstitution experiments in decontaminated mice. The IL-8–induced mobilization in mice, decontaminated by treatment with ciprofloxacin and metronidazol, was significantly increased when LPS was added to the drinking water in comparison with decontaminated controls (67 ± 28 CFUs/mL vs 190 ± 98 CFUs/mL, P < .002; Figure 5), indicating that addition of endotoxin partially restored mobilization.
The IL-8–induced mobilization in decontaminated mice is restored after oral administration of endotoxins. Mice were receiving either normal drinking water (n = 7); drinking water containing endotoxins (33 mg/L) and saccharose (20 mg/L) (n = 8); drinking water containing 125 mg/L ciprofloxacin, 500 mg/L metronidazol, and 20 mg/L saccharose (n = 7); or water containing 125 mg/L ciprofloxacin, 500 mg/L metronidazol, 20 mg/L saccharose, and endotoxins (n = 7). After one week of treatment, mice were mobilized with a single intraperitoneal injection of PBS containing 1% bovine serum albumin or 30 μg IL-8. At 20 minutes after the injection, mice were killed and blood and bone marrow were obtained. Results are expressed as means ± SD. *P < .05 compared with the controls.
The IL-8–induced mobilization in decontaminated mice is restored after oral administration of endotoxins. Mice were receiving either normal drinking water (n = 7); drinking water containing endotoxins (33 mg/L) and saccharose (20 mg/L) (n = 8); drinking water containing 125 mg/L ciprofloxacin, 500 mg/L metronidazol, and 20 mg/L saccharose (n = 7); or water containing 125 mg/L ciprofloxacin, 500 mg/L metronidazol, 20 mg/L saccharose, and endotoxins (n = 7). After one week of treatment, mice were mobilized with a single intraperitoneal injection of PBS containing 1% bovine serum albumin or 30 μg IL-8. At 20 minutes after the injection, mice were killed and blood and bone marrow were obtained. Results are expressed as means ± SD. *P < .05 compared with the controls.
Administration of drinking water containing endotoxins had no effect on circulating steady-state levels of HPCs (endotoxins: 9 ± 6 CFUs/mL vs controls: 26 ± 16 CFUs/mL). However, the IL-8–induced mobilizing capacity in mice receiving LPS was also significantly increased when compared with the controls (459 ± 135 CFUs/mL vs 320 ± 66 CFUs/mL, P < .02; Figure 5). These results indicate a relation between the IL-8–induced mobilization and the concentration of endotoxins in the gastrointestinal tract (ie, a higher concentration of endotoxin leads to an increased mobilizing capacity).
Serum concentration of endotoxins, IL-6, TNF-α, and MMP-9
It was considered that enhancement of mobilization by endotoxins would be mediated by the induction of proinflammatory cytokines (ie, IL-6 and TNF). In all serum samples of the 4 different groups of mice (control mice, mice treated with endotoxins only, mice treated with ciprofloxacin and metronidazole, and mice treated with ciprofloxacin, metronidazole, and endotoxins) the level of endotoxins measured was below the detection level of 0.06 EU/mL (data not shown). Similarly, all serum levels of IL-6 and TNF-α were below the detection limit. Previously, we demonstrated that IL-8–induced mobilization coincides with an increase in proteolytic neutrophil-derived enzymes, in particular MMP-9. MMP-9 levels as determined by zymography showed a similar increase after administration of IL-8 in all 4 groups.
The effect of treatment with antibiotics on the number of progenitor cells in the femur
In Balb/c, OF-1, and germ-free OF-1 mice, the number of colony-forming units per femur was not influenced by any treatment (data not shown). In Balb/c mice the effect of the ciprofloxacin/polymyxin B treatment on the number of neutrophils in the bone marrow was established. The number of neutrophils as determined by FACS analysis was not changed compared with the control group (data not shown).
CAFC assay of peripheral blood and bone marrow
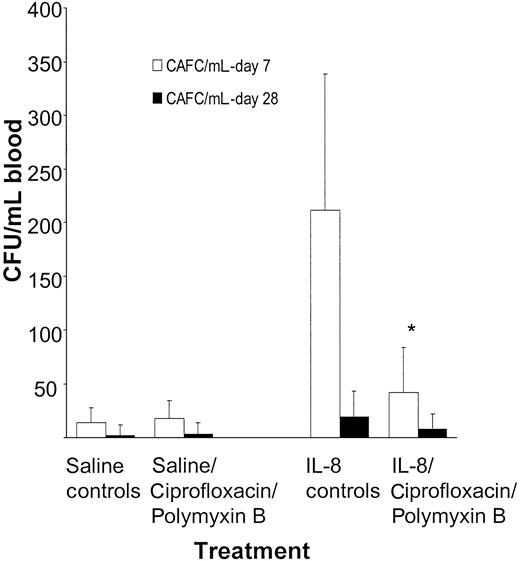
To determine the effect of decontamination on committed progenitor cells and cells with repopulating ability, the number of early (day 7) and late (day 28) CAFCs was assessed in peripheral blood of mice decontaminated with ciprofloxacin and polymyxin B and mobilized with a single injection of 30 μg IL-8. The numbers of circulating early CAFCs were significantly reduced in IL-8–mobilized mice decontaminated with ciprofloxacin and polymyxin B compared with controls not treated with antibiotics (P < .05). Similarly, the numbers of late CAFCs were also reduced. However, the absolute numbers in peripheral blood of late CAFCs were low and the difference did not reach statistical significance (P > .05; Figure 6).
The numbers of CAFCs in peripheral blood are decreased after pretreatment with ciprofloxacin and polymyxin B. Balb/c mice were treated with 100 mg/L ciprofloxacin, 70 mg/L polymyxin B, and 20 mg/L saccharose added to their drinking water. The antibiotic treatment was continued and mice were mobilized with a single intraperitoneal injection of 30 μg IL-8. At 20 minutes after the IL-8 injection, mice were killed and blood was harvested. Results are expressed as means ± SD. *P < .05 compared with the controls.
The numbers of CAFCs in peripheral blood are decreased after pretreatment with ciprofloxacin and polymyxin B. Balb/c mice were treated with 100 mg/L ciprofloxacin, 70 mg/L polymyxin B, and 20 mg/L saccharose added to their drinking water. The antibiotic treatment was continued and mice were mobilized with a single intraperitoneal injection of 30 μg IL-8. At 20 minutes after the IL-8 injection, mice were killed and blood was harvested. Results are expressed as means ± SD. *P < .05 compared with the controls.
Discussion
In the present study we addressed the role of endotoxins in cytokine-induced stem cell mobilization. First we studied the effect of selective antibiotic decontamination, resulting in the elimination of the Gram-negative aerobic intestinal flora, on IL-8–, G-CSF–, and FL-induced mobilization. In mice decontaminated with a combination of ciprofloxacin and polymyxin B we observed a significant reduction in the mobilizing capacity when compared with untreated controls. The numbers of not only committed progenitor cells but also the more primitive progenitor cells were decreased as shown by the CAFC assay; although the differences for CAFC day 28 were not statistically significant. Next we determined the contribution of each of the 2 components of this mobilization-inhibiting antibiotic combination. Following treatment with ciprofloxacin, which is absorbable after oral administration,35 no aerobic Gram-negative bacteria were cultured from the feces of mice, while mobilization in response to IL-8 and G-CSF was not reduced. In contrast, treatment with polymyxin B resulted in a significant inhibition of mobilization without eliminating the aerobic Gram-negative flora.36 These apparently conflicting results can be explained by the endotoxin binding capacity of polymyxin B.37,38 The lack of inhibition of mobilization observed in mice treated with only ciprofloxacin can be explained by residual endotoxins derived from the anaerobic Gram-negative flora that constitute more than 90% of the bacterial flora of the intestines.39,40
In order to eliminate both the aerobic and the anaerobic Gram-negative flora we then used a combination of ciprofloxacin and metronidazol.41 Indeed, a significant reduction in the number of the mobilized progenitor cells was observed. Moreover, treatment with only metronidazol also resulted in a significantly reduced mobilization. The significantly lower mobilization observed in mice treated with either polymyxin B or metronidazol was not due to a reduction in the size of the progenitor cell pool, since the number of CFUs in the bone marrow was comparable with the control mice. In addition we observed no sign of diminished colony growth following addition of the different antibiotics to the culture medium at concentrations that exceeded the expected serum concentrations (data not shown). These data indicate that endotoxins derived from anaerobic Gram-negative bacteria play a major role in cytokine-induced stem cell mobilization.
Finally, to eliminate possible direct effects of antibiotics on mobilization we studied stem cell mobilization in germ-free mice not receiving antibiotic treatment. Germ-free OF-1 mice not treated with antibiotics showed a significantly reduced mobilization compared with control mice that were not decontaminated. The effects seen on mobilization in the germ-free mice were similar to the inhibition observed in control mice that were decontaminated with ciprofloxacin and polymyxin B.
To further support the hypothesis that endotoxins in the gastrointestinal tract serve as cofactors in cytokine-induced peripheral blood stem cell mobilization, we performed endotoxin reconstitution experiments in decontaminated mice. IL-8–induced mobilization was significantly enhanced in decontaminated mice (metronidazol/ciprofloxacin) receiving drinking water also containing endotoxins. In these animals a partial restoration of mobilization was observed following oral administration of endotoxins.
The mechanisms underlying this effect are presently unknown. Endotoxins are also known as potent inducers of mobilization following systemic administration.32,33 Under clinical and experimental conditions the lipopolysaccharide component of the aerobic Gram-negative bacteria wall damages the endothelial cell48 finally leading to the synthesis and the release of proinflammatory mediators (TNF, IL-1, IL-6, and IL-8) from macrophages.49 The endotoxin concentration in the gut is very likely to be decreased by the treatment with polymyxin B or metronidazol, resulting in a diminished production of cytokines by macrophages. We hypothesized that the increase in stem cell mobilization observed after administration of endotoxin was due to the induction of proinflammatory cytokines that could synergize in the induction of stem cell mobilization. The serum concentrations of TNF-α and IL-6 were not increased. These data, however, do not rule out the possibility that these or other cytokines act at a local level in the intestinal tract rather than acting at a systemic level.
Previous studies in our laboratory have shown that neutrophils in the peripheral blood are indispensable for IL-8–induced mobilization,50 whereas neutrophils in the bone marrow were suggested to be important in the G-CSF–induced mobilization.51 The antibiotics used did not alter the number of neutrophils in the peripheral blood or the number of neutrophils in the bone marrow. Pruijt et al showed that IL-8–induced mobilization in rhesus monkeys is dependent on gelatinase B.52 In the present study we found that gelatinase B levels induced by IL-8, measured by zymography, were not affected by the treatment with antibiotics (data not shown). This supports more recent data in mice showing that gelatinase B levels reflect in vivo activation of neutrophils but are not essential for HPC mobilization in the mouse.50
Recent evidence indicates that LPS-activated signaling through toll-like receptor 4 augments chemokine-induced neutrophil migration through modulation of chemokine receptors.53 It is therefore conceivable that LPS-induced modification of neutrophil migration is related to alterations in stem cell mobilizing capacity.
The enhancement of the mobilization may be dependent on the concentration of endotoxins in the gastrointestinal tract. As a consequence, modification of the endotoxin content or binding of endotoxins may affect the levels of cytokine-induced mobilization. Cytokine-induced mobilization was lowest in mice receiving antibiotic decontamination and highest in control mice receiving oral endotoxins. These data suggest that cytokine-induced mobilization is enhanced by endotoxins in a dose-dependent way.
In conclusion, our studies indicate that endotoxins may serve as cofactors in cytokine-induced mobilization of peripheral blood progenitor cells. Our observations could potentially have implications for the antibiotic treatment of patients undergoing treatment with rHu-G-CSF to mobilize peripheral blood progenitor cells for autologous transplantation. Elimination of the anaerobic flora of the gut or the use of antibiotics that bind endotoxins may impair the yield of the peripheral blood stem cell mobilization.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2002-07-2270.
Supported by a grant from the Fund for Scientific Research FWO-Vlaanderen, the “Geconcerteerde Onderzoeks Acties” (GAO), and by the Dutch Cancer Society (NKB-RUL 99-2029).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ilse Vanaelst, Jan Mertens, Marijke Fröhlich, and Jan Didden for technical help.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal