Abstract
The FLT3 receptor tyrosine kinase is highly expressed in most acute leukemias and frequently mutated in acute myeloid leukemia (AML). The mutated form of the receptor is constitutively activated and known to play an important role in AML, but the activation state of the overexpressed wild-type (wt) receptor is, at present, unknown. In this study, we examined the activation state of the wild-type receptor in AML. We found that the wild-type receptor was constitutively phosphorylated/activated in 8 of 12 primary AML samples and 4 of 13 leukemia cell lines. To explain why wtFLT3 is often activated, we investigated the expression of its ligand, FL, by these same cells. Coexpression of FL with FLT3 was a universal finding in both primary AML samples and leukemic-derived cell lines. To further prove that autocrine signaling was accounting for the activation, we showed that conditioned media but not fresh media was able to activate FLT3. In addition, an antibody that blocks binding of ligand to the receptor blocks FLT3 activation. Finally, depletion of FL from conditioned media is able to block the activation of FLT3. Taken together, these findings represent strong evidence that wtFLT3 is often constitutively activated in AML and thus, like its mutated form, might contribute to the altered signaling that characterizes leukemogenesis.
Introduction
FLT3 ligand (FL) is a hematopoietic growth factor that plays roles in the proliferation and differentiation of a number of blood cell lineages.1-3 It is expressed in membrane-bound and soluble forms and is produced by bone marrow stromal cells, T cells, and endothelial cells.1,2,4 Its receptor, FLT3, is structurally related to the c-kit (KIT), c-fms (FMS), and platelet-derived growth factor (PDGF) receptors, members of the class III receptor tyrosine kinase family.5-7 FL synergizes with other cytokines, including interleukin-3 (IL-3), IL-6, stem cell factor (SCF), and granulocytemacrophage colony-stimulating factor (GM-CSF) to enhance the expansion of hematopoietic stem/progenitor cells.1,2,8,9 By itself, it causes a large stimulation of dendritic cell numbers.10-12
FL expression has been examined in a large panel of human leukemia/lymphoma cell lines representing all major hematopoietic malignancies, and all cell lines were positive, at least at the level of mRNA.13-15 FL RNA expression was also identified in a number of organs including spleen, ovary, testis, intestine, and kidney.1,2 Expression of FL by primary leukemia cells has not been examined prior to this report.
Incubation of leukemic blasts with added FL results in enhanced proliferation in some, but not all, cases of acute leukemia as well as a decreased rate of spontaneous apoptosis.13,16-20 Chronic overexpression of FL by retroviral transfection of hematopoietic progenitors has been shown to induce hematologic malignancies after a long latency period.21
Interaction of the FLT3 receptor with FL causes receptor dimerization, leading to the activation of its tyrosine kinase domain and receptor autophosphorylation.3 Activated FLT3 transduces signals through association with or phosphorylation of a number of cytoplasmic proteins, including phospholipase C; the p85 subunit of phosphatidylinositol 3-kinase (PI3); VAV; casitas B-lineage lymphoma (CBL); Src homology 2 domain-containing phosphotyrosine phosphatase 2 (SHP2); Src homology 2 domain–containing inositol 5-phosphatase (SHIP); signal transducer and activator of transcription 5 (STAT 5), ras guanosine triphosphatase (GTPase)–activating protein; and Src family tyrosine kinases.22-27 FLT3 is expressed on most acute leukemias of myeloid and B-lymphoid origin.13,28,29 It is also expressed at very high levels by leukemias having myeloid-lymphoid leukemia (MLL) translocations and partial tandem duplications.30,31 Somatic mutations of FLT3 involving in-frame internal tandem duplications (ITDs) in the juxtamembrane domain or D835 point mutations in the activation loop occur in 17% to 34% and 7% of patients with AML, respectively.32-36 Both of these types of mutations lead to constitutive activation of FLT3 in a ligand-independent manner. In addition, the FLT3/ITD mutations are associated with poor prognosis in AML.37-41 Constitutive activation of FLT3 leads to autonomous, cytokine-independent growth with subsequent transformation of hematopoietic cell lines.27,35,42 However, one report found that the presence of ITD mutations does not always result in phosphorylation of FLT3.42
In this study, we explored the possibility that FL and FLT3 could be coexpressed in primary AML blasts, and that this could lead to the constitutive activation of FLT3 signaling. Our findings suggest that autocrine, intracrine, or paracrine stimulatory loop mechanisms of FLT3 activation may possibly contribute to the pathogenesis of AML.
Materials and methods
Reagents
Recombinant human FL and IMC-NC7 antibody were kindly provided by ImClone Systems (New York, NY). Rat monoclonal anti-FL antibody (Mab M5, IgG2a) was kindly provided by Immunex (Seattle, WA). Rat IgG2a isotype control and goat antirat phycoerythrin (PE)–conjugated secondary antibody were purchased from BD PharMingen (San Jose, CA). Rabbit antihuman FLT3 antibody was obtained from SantaCruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antiphosphotyrosine antibody 4G10 and recombinant protein A–agarose were purchased from Upstate Biotechnology (Lake Placid, NY). Horseradish peroxidase–conjugated secondary antibody and enhanced chemiluminescence (ECL) detection system were from Amersham (Arlington Heights, IL).
Primary AML samples
Bone marrow samples were obtained under institutional review board–approved protocols from patients with AML at the Sidney Kimmel Cancer Center at Johns Hopkins University (Baltimore, MD), MD Anderson Cancer Center (Houston, TX), and Hospital Saint-Antoine (Paris, France) prior to therapy. Leukemic blast cells were separated by Ficoll-Hypaque (Amersham, Piscataway, NJ) density gradient centrifugation as described previously.43
Cell lines
Human leukemia/lymphoma cell lines (EM2, EM3, LAZ221, REH, U937, HL-60, KG-1a, WSU-NHL, MOLM-1, BV173, K562, MV4-11, HEL, EOL-1, and ML-1) were cultured in complete medium, which is RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS) and antibiotics (penicillin and streptomycin), at 37°C with 5% CO2.44
Reverse transcription–polymerase chain reaction (RT-PCR)
Total cellular RNA was extracted from cultured cell lines and patients' blast cells using RNeasy columns (Qiagen, Valencia, CA). RNA (50 ng-100 ng) was reverse transcribed and amplified using the One-Step RT-PCR kit (Invitrogen, Carlsbad, CA). PCR was performed in the presence of the following primer pairs: 5′-AGCCCAACAACCTATCTCCT-3′ and 5′-GTCTGGACGAAGCGAAGACA-3′, specific for FL and amplifies a 352–base pair (bp) sequence; 5′-TGTCGAGCAGTACTCTAAACA-3′ and 5′-ATCCTAGTACCTTCCCAAACTC-3′, specific for the juxtamembrane region of FLT3 where FLT3/ITDs occur and amplifies a 366-bp sequence32 ; 5′-TTCACAGAGACCTGGCCG-3′ and 5′-TTGCCCCTGACAACATAG-3′, specific for the region of FLT3 where D835 point mutations occur and amplifies a 118-bp sequence; 5′-GCTCGTCGTCGACAACGGCTC-3′ and 5′-CAAACATGATCTGGGTCATCTTCTC-3′, specific for β-actin and amplifies a 353-bp sequence. Reverse transcription was performed at 50°C for 30 minutes. Thirty-five cycles of amplification were then performed at 94°C for 30 seconds for denaturation, at 55°C for 30 seconds for annealing, and finally at 72°C for 1 minute for extension (DNA iCycler; Bio-Rad, Hercules, CA). PCR products were resolved on a 2.5% agarose gel in the presence of ethidium bromide and photographs were taken under UV transillumination. If an FLT3/ITD mutation is present, a slower migrating band than wild-type (wt) FLT3 is visualized. To detect mutations at D835, EcoRV digestion of the PCR-amplified product which includes D835 and I836 codons was carried out prior to electrophoresis.35
Immunoprecipitation and Western blot analysis
Cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed for 30 minutes in ice-cold NP-40 lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 100 mM NaF, 10% glycerol, 1% NP-40, and 10 mM EDTA [ethylenediaminetetraacetic acid]) containing protease and phosphatase inhibitors (2 mM sodium orthovanadate, 50 μg/mL antipain, 5 μg/mL aprotinin, 1 μg/mL leupeptin, and 10 μg/mL phenylmethylsulfonyl fluoride; Sigma, St Louis, MO). Clarified lysates (500 μg) were incubated with rabbit polyclonal antibody to human FLT3 followed by incubation with protein A–agarose at 4°C. The immunoprecipitates were washed 3 times with ice-cold TBS-T (10 mM Tris-HCl, pH 7.4, 100 mM NaCl, 0.1% Tween-20) resuspended in sodium dodecyl sulfate (SDS) sample buffer, heated, and separated by SDS–polyacrylamide gel electrophoresis (PAGE) in 8% gels. Gels were blotted onto polyvinylidene fluoride microporous membrane (Millipore, Bedford, MA) and stained with the indicated antibody. Antibody binding was detected by incubation with a horseradish peroxidase–conjugated secondary antibody, followed by chemiluminescence detection.
Immunostaining of cells
Aliquots of cells (1 × 106) were transferred into 1 mL ice-cold fixative solution (PBS with 4% paraformaldehyde and 1 mM sodium vanadate). After fixation for 10 minutes, cells were collected by centrifugation and washed twice with 1 mL Tris (0.1 M, pH 8.0). Cells were permeabilized and stained by incubation for 2 hours at 25°C in 0.1 mL permeabilization buffer (PBS with 2% bovine serum albumin [BSA], 2% fetal bovine serum, 1 mM sodium vanadate, 1 mM EDTA, 0.2% Tween 20, and 0.01% SDS) supplemented with anti-FL antibody or rat IgG2a isotype control. Cells were washed once with 1 mL permeabilization buffer and incubated for 1 hour at 25°C with goat antirat PE-conjugated secondary antibody, diluted in permeabilization buffer. The stained cells were washed with 0.5 mL PBS and analyzed by flow cytometry (FACSort; Becton Dickinson, San Jose, CA) using CellQuest software.
Results
FLT3 is constitutively phosphorylated in some leukemia/lymphoma-derived cell lines and leukemic blasts of some patients with AML
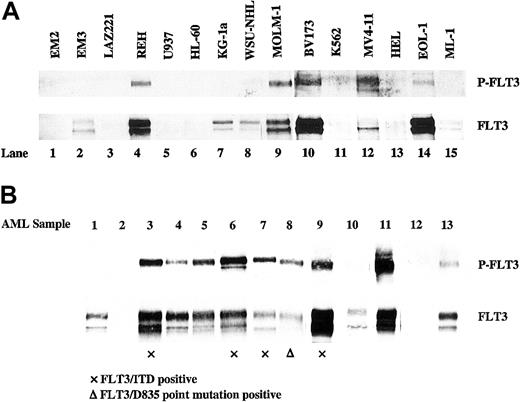
Constitutively activating FLT3 mutations clearly play a role in up to one-third of AML cases. The high level of expression of wtFLT3 that has been previously demonstrated in an additional half of AML cases raises the question of whether wtFLT3 signaling could also play a role in leukemogenesis.28,29 However, expression itself is insufficient to support such a role. More important is the question of whether or not FLT3 is activated in these cells. To begin to answer this question, 15 human leukemia/lymphoma-derived cell lines were analyzed for FLT3 expression and state of activation by immunoprecipitation and Western blotting with antiphosphotyrosine antibody. Nine of the 15 cell lines expressed detectable FLT3 protein (ML-1, MOLM-1, KG1a, WSU-NHL, EM3, EOL-1, MV4-11, REH, and BV173), and 5 of these showed constitutive phosphorylation of FLT3 (MOLM-1, EOL-1, MV4-11, REH, and BV173) in the absence of added ligand (Figure 1A; Table 1). Of note, autophosphorylation of FLT3 tended to correlate with the cell lines expressing the highest levels of FLT3 protein. The possible exception was MV4-11 cells, which contain an FLT3/ITD mutation (Figure 2A).45
FLT3 is expressed and autophosphorylated in a number of human leukemia/lymphoma cell lines and primary AML blasts. Total cellular protein extracts derived from 1 × 107 cells were immunoprecipitated with anti-FLT3 antibody. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same blot was stripped and reprobed with anti-FLT3 antibody (bottom panel). (A) Human leukemia/lymphoma cell lines. (B) Primary AML blast samples (prior to protein preparation, newly thawed leukemic blast cells were incubated in complete medium for 12 hours).
FLT3 is expressed and autophosphorylated in a number of human leukemia/lymphoma cell lines and primary AML blasts. Total cellular protein extracts derived from 1 × 107 cells were immunoprecipitated with anti-FLT3 antibody. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same blot was stripped and reprobed with anti-FLT3 antibody (bottom panel). (A) Human leukemia/lymphoma cell lines. (B) Primary AML blast samples (prior to protein preparation, newly thawed leukemic blast cells were incubated in complete medium for 12 hours).
Expression of FLT3 and FL in leukemia/lymphoma-derived cell lines
. | FLT3 . | . | . | . | . | FL . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell line . | Normal FLT3 mRNA . | ITD . | D835 . | FLT3 protein . | P-FLT3 . | FL mRNA . | MFI of FL by FACS . | |||||
| HL-60 | + | - | - | - | - | + | 12.0 | |||||
| ML-1 | + | - | - | + | - | + | 46.2 | |||||
| MOLM-1 | + | - | - | +++ | + | + | 40.8 | |||||
| K562 | - | - | - | - | - | + | 53.1 | |||||
| KG1a | + | - | - | + | - | + | 19.4 | |||||
| EM2 | + | - | - | - | - | + | 24.1 | |||||
| HEL | ± | - | - | - | - | + | 31.3 | |||||
| WSU-NHL | + | - | - | + | - | + | 68.2 | |||||
| U937 | + | - | - | - | - | + | 43.8 | |||||
| EM3 | + | - | - | ++ | - | + | 17.7 | |||||
| EOL-1 | + | - | - | ++++ | + | + | 37.6 | |||||
| LAZ221 | + | - | - | - | - | + | 14.1 | |||||
| MV4-11 | - | + | - | ++ | + | + | 13.1 | |||||
| REH | + | - | - | ++++ | + | + | 36.7 | |||||
| BV173 | + | - | - | ++++ | + | + | 25.2 | |||||
. | FLT3 . | . | . | . | . | FL . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell line . | Normal FLT3 mRNA . | ITD . | D835 . | FLT3 protein . | P-FLT3 . | FL mRNA . | MFI of FL by FACS . | |||||
| HL-60 | + | - | - | - | - | + | 12.0 | |||||
| ML-1 | + | - | - | + | - | + | 46.2 | |||||
| MOLM-1 | + | - | - | +++ | + | + | 40.8 | |||||
| K562 | - | - | - | - | - | + | 53.1 | |||||
| KG1a | + | - | - | + | - | + | 19.4 | |||||
| EM2 | + | - | - | - | - | + | 24.1 | |||||
| HEL | ± | - | - | - | - | + | 31.3 | |||||
| WSU-NHL | + | - | - | + | - | + | 68.2 | |||||
| U937 | + | - | - | - | - | + | 43.8 | |||||
| EM3 | + | - | - | ++ | - | + | 17.7 | |||||
| EOL-1 | + | - | - | ++++ | + | + | 37.6 | |||||
| LAZ221 | + | - | - | - | - | + | 14.1 | |||||
| MV4-11 | - | + | - | ++ | + | + | 13.1 | |||||
| REH | + | - | - | ++++ | + | + | 36.7 | |||||
| BV173 | + | - | - | ++++ | + | + | 25.2 | |||||
ITD indicates FLT3/ITD mutation; D835, FLT3/D835 mutation; MFI, mean fluorescence intensity; P-FLT3, phosphorylated FLT3; FACS, fluorescence-activated cell sorting.
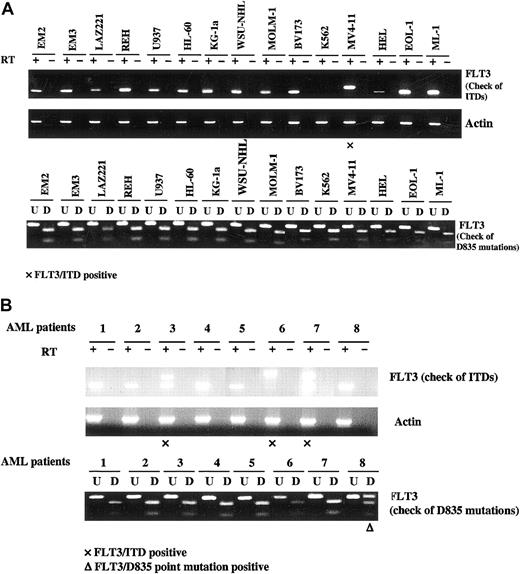
FLT3 transcripts are expressed by human leukemia/lymphoma-derived cell lines and primary AML blasts. Total cellular RNA was extracted from leukemia/lymphoma cell lines and patients' blast cells. RNA (50 ng-100 ng) was reverse transcribed and amplified using primer pairs for FLT3 and actin. PCR products were resolved on 2.5% agarose gel in the presence of ethidium bromide, and photographs were taken under UV transillumination. If an FLT3/ITD mutation is present, a higher molecular weight band than wtFLT3 will be visualized. To detect mutations at D835, EcoRV digestion of the PCR-amplified region including the D835 and I836 codons was used prior to electrophoresis. These codons are encoded by the sequence GATATC, which forms the EcoRV restriction site. If this site is mutated, the PCR product will be resistant to digestion. (U, undigested; D, digested.) (A) Human leukemia/lymphoma derived cell lines. (B) Primary AML blasts. Patients 3, 6, and 7 expressed FLT3/ITD mutations. Patient 8 expressed a D835 mutation.
FLT3 transcripts are expressed by human leukemia/lymphoma-derived cell lines and primary AML blasts. Total cellular RNA was extracted from leukemia/lymphoma cell lines and patients' blast cells. RNA (50 ng-100 ng) was reverse transcribed and amplified using primer pairs for FLT3 and actin. PCR products were resolved on 2.5% agarose gel in the presence of ethidium bromide, and photographs were taken under UV transillumination. If an FLT3/ITD mutation is present, a higher molecular weight band than wtFLT3 will be visualized. To detect mutations at D835, EcoRV digestion of the PCR-amplified region including the D835 and I836 codons was used prior to electrophoresis. These codons are encoded by the sequence GATATC, which forms the EcoRV restriction site. If this site is mutated, the PCR product will be resistant to digestion. (U, undigested; D, digested.) (A) Human leukemia/lymphoma derived cell lines. (B) Primary AML blasts. Patients 3, 6, and 7 expressed FLT3/ITD mutations. Patient 8 expressed a D835 mutation.
The activation state of FLT3 in primary AML cells was examined next. FLT3 protein was immunoprecipitated from leukemic blasts isolated from diagnostic specimens of bone marrow from patients with AML. Prior to protein preparation, all frozen primary samples included in this study were incubated for 12 hours after they were thawed. In 20 samples assessed, 18 samples expressed detectable FLT3, and in 15 of these, FLT3 was phosphorylated in the absence of exogenously added FL (Table 2; 13 of these samples are shown in Figure 1B; 6 of these samples are shown in Figure 5, lanes 7-9 and 16-18).
Expression of FLT3 and FL by 33 primary AML blasts examined by Western blotting and FACS analysis
. | FLT3 . | . | . | . | . | FL . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Normal FLT3 mRNA . | ITD . | D835 . | FLT3 protein . | P-FLT3 . | FL mRNA . | MFI of FL by FACS . | |||||
| 1 | + | - | - | + | - | NA | NA | |||||
| 2 | + | - | - | - | - | NA | NA | |||||
| 3 | + | + | - | + | + | NA | NA | |||||
| 4 | + | - | - | + | + | NA | NA | |||||
| 5 | + | - | - | + | + | + | NA | |||||
| 6 | + | + | - | + | + | NA | NA | |||||
| 7 | + | + | - | + | + | + | NA | |||||
| 8 | + | - | + | + | + | + | NA | |||||
| 9 | + | + | - | + | + | + | 56.4 | |||||
| 10 | + | - | - | + | + | + | 12.1 | |||||
| 11 | + | - | - | + | + | + | 290.4 | |||||
| 12 | + | - | - | - | - | + | 19.1 | |||||
| 13 | + | - | - | + | + | + | 37.1 | |||||
| 14 | + | - | - | + | + | + | 58.6 | |||||
| 15 | + | - | - | + | + | NA | 224.4 | |||||
| 16 | + | - | - | ± | - | NA | 14.9 | |||||
| 17 | + | + | - | + | + | NA | 21.1 | |||||
| 18 | + | - | - | + | + | NA | 68.6 | |||||
| 19 | + | + | - | + | - | NA | 29.5 | |||||
| 20 | + | + | - | + | + | NA | 16.07 | |||||
| 21 | + | - | - | NA | NA | + | 26.6 | |||||
| 22 | + | - | + | NA | NA | + | 1.6 | |||||
| 23 | + | + | - | NA | NA | + | 28.7 | |||||
| 24 | + | + | - | NA | NA | + | 56.2 | |||||
| 25 | + | + | - | NA | NA | + | 52.8 | |||||
| 26 | + | - | - | NA | NA | + | 36.3 | |||||
| 27 | + | - | - | NA | NA | + | 56.9 | |||||
| 28 | + | - | - | NA | NA | + | 43.8 | |||||
| 29 | + | - | - | NA | NA | + | 90.6 | |||||
| 30 | + | - | - | NA | NA | + | 5.8 | |||||
| 31 | + | - | - | NA | NA | + | 17.3 | |||||
| 32 | + | + | - | NA | NA | + | 34.7 | |||||
| 33 | + | + | - | NA | NA | + | 18.4 | |||||
. | FLT3 . | . | . | . | . | FL . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. . | Normal FLT3 mRNA . | ITD . | D835 . | FLT3 protein . | P-FLT3 . | FL mRNA . | MFI of FL by FACS . | |||||
| 1 | + | - | - | + | - | NA | NA | |||||
| 2 | + | - | - | - | - | NA | NA | |||||
| 3 | + | + | - | + | + | NA | NA | |||||
| 4 | + | - | - | + | + | NA | NA | |||||
| 5 | + | - | - | + | + | + | NA | |||||
| 6 | + | + | - | + | + | NA | NA | |||||
| 7 | + | + | - | + | + | + | NA | |||||
| 8 | + | - | + | + | + | + | NA | |||||
| 9 | + | + | - | + | + | + | 56.4 | |||||
| 10 | + | - | - | + | + | + | 12.1 | |||||
| 11 | + | - | - | + | + | + | 290.4 | |||||
| 12 | + | - | - | - | - | + | 19.1 | |||||
| 13 | + | - | - | + | + | + | 37.1 | |||||
| 14 | + | - | - | + | + | + | 58.6 | |||||
| 15 | + | - | - | + | + | NA | 224.4 | |||||
| 16 | + | - | - | ± | - | NA | 14.9 | |||||
| 17 | + | + | - | + | + | NA | 21.1 | |||||
| 18 | + | - | - | + | + | NA | 68.6 | |||||
| 19 | + | + | - | + | - | NA | 29.5 | |||||
| 20 | + | + | - | + | + | NA | 16.07 | |||||
| 21 | + | - | - | NA | NA | + | 26.6 | |||||
| 22 | + | - | + | NA | NA | + | 1.6 | |||||
| 23 | + | + | - | NA | NA | + | 28.7 | |||||
| 24 | + | + | - | NA | NA | + | 56.2 | |||||
| 25 | + | + | - | NA | NA | + | 52.8 | |||||
| 26 | + | - | - | NA | NA | + | 36.3 | |||||
| 27 | + | - | - | NA | NA | + | 56.9 | |||||
| 28 | + | - | - | NA | NA | + | 43.8 | |||||
| 29 | + | - | - | NA | NA | + | 90.6 | |||||
| 30 | + | - | - | NA | NA | + | 5.8 | |||||
| 31 | + | - | - | NA | NA | + | 17.3 | |||||
| 32 | + | + | - | NA | NA | + | 34.7 | |||||
| 33 | + | + | - | NA | NA | + | 18.4 | |||||
ITD indicates FLT3/ITD mutation; D835, FLT3/D835 mutation; MFI, mean fluorescence intensity; P-FLT3, phosphorylated FLT3; FACS, fluorescence-activated cell sorting; NA, not available.
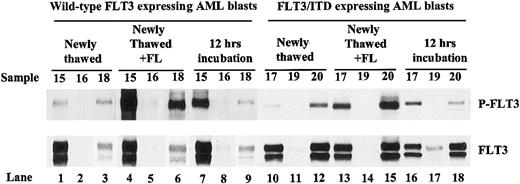
Incubation of leukemic blast cells in complete medium for 12 hours increases the FLT3 autophosphorylation in some primary AML specimens. Protein lysates were prepared from washed, newly thawed AML blast cells with or without FL (100 ng/mL) stimulation or after incubation for 12 hours in complete medium. Total cellular protein was immunoprecipitated with anti-FLT3 antibody. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same blot was stripped and reprobed with anti-FLT3 antibody (bottom panel).
Incubation of leukemic blast cells in complete medium for 12 hours increases the FLT3 autophosphorylation in some primary AML specimens. Protein lysates were prepared from washed, newly thawed AML blast cells with or without FL (100 ng/mL) stimulation or after incubation for 12 hours in complete medium. Total cellular protein was immunoprecipitated with anti-FLT3 antibody. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same blot was stripped and reprobed with anti-FLT3 antibody (bottom panel).
Constitutive phosphorylation of FLT3 is seen without ITD or D835 point mutations in some human leukemia/lymphoma-derived cell lines and primary AML blast specimens
ITD and D835 mutations of FLT3 result in constitutive phosphorylation of FLT3.35,46 The presence of these mutations could account for the constitutively phosphorylated FLT3 seen in leukemia/lymphoma-derived cell lines and primary AML blasts shown in Figure 1. To investigate this possibility, cell lines and patient samples were assessed for the presence of FLT3 activating mutations. The parts of FLT3 amplified correspond to the juxtamembrane domain, where ITD mutations occur, and the kinase domain, where D835 and I836 point mutations occur. Wild-type FLT3 transcripts were expressed in 13 of 15 cell lines while 1 (MV4-11) expressed only an FLT3/ITD mutation and no cell lines expressed D835 mutations (Figure 2A; Table 1). This result is consistent with previous reports from our group and other groups.45,47 In 101 primary AML samples tested in this study, 61 samples expressed only wtFLT3 while 30 samples expressed FLT3/ITD mutations and 10 samples expressed D835 mutations. Among 30 FLT3/ITD-positive samples, 3 expressed only FLT3/ITD transcript while the others also expressed wtFLT3 transcript. All 10 D835 point mutation positive samples expressed both mutant and wild-type FLT3 (Figure 2B, Table 2). Of note, of the 5 leukemia/lymphomaderived cell lines and 10 cases of primary AML showing constitutive phosphorylation of FLT3 in Figure 1B, the phosphorylation of only one cell line and 5 primary AML samples was accounted for by ITD or D835 mutations (Figure 2; Table 1; Table 2). This strongly suggests that other nonmutant mechanisms of constitutive receptor activation may be operative in many cases of AML blasts. One possible mechanism would be FLT3 activation by endogenous FL production by the cells themselves.
FL is coexpressed with FLT3 by human leukemia/lymphoma-derived cell lines and primary AML blasts
FL and FLT3 mRNA expression by primary AML blasts and leukemia/lymphoma cell lines was investigated by RT-PCR. Coexpression of FL by 14 FLT3-expressing leukemia/lymphoma cell lines (Figure 2A; Figure 3A; Table 1) and 90 primary AML samples (55 samples containing only wtFLT3, 25 samples containing FLT3/ITD, and 10 samples containing D835 mutation) was a universal finding (Figure 2B; Figure 3B; Table 2). However, the presence of a transcript detected by RT-PCR does not necessarily translate into expression of protein. We thus examined FL protein expression in the cell lines and primary blasts.
FL transcripts are expressed by human leukemia/lymphoma-derived cell lines and primary AML blasts. Total cellular RNA was extracted from cell lines and patients' blast cells. RNA (50 ng-100 ng) was reverse transcribed and amplified using primer pairs for FL and actin. PCR products were resolved on 2.5% agarose gel in the presence of ethidium bromide, and photographs were taken under UV transillumination. (A) Leukemia/lymphoma cell lines. (B) Primary AML blast samples.
FL transcripts are expressed by human leukemia/lymphoma-derived cell lines and primary AML blasts. Total cellular RNA was extracted from cell lines and patients' blast cells. RNA (50 ng-100 ng) was reverse transcribed and amplified using primer pairs for FL and actin. PCR products were resolved on 2.5% agarose gel in the presence of ethidium bromide, and photographs were taken under UV transillumination. (A) Leukemia/lymphoma cell lines. (B) Primary AML blast samples.
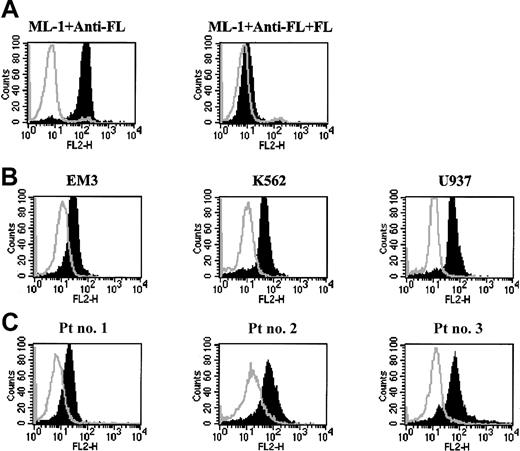
In order to investigate FL protein expression, staining was performed by fixing and permeabilizing the cells followed by fluorescence-activated cell sorting (FACS) analysis with an anti-FL monoclonal antibody.48 Consistent positivity for FL was observed in the ML-1 cell line (Figure 4A, left panel). To confirm that this was due to the specific binding of antibody to FL protein, the antibody was preincubated with recombinant human FL prior to immunostaining of the cells. Specificity for FL was confirmed as the histogram shifted back toward that obtained with isotypematched control antibody (Figure 4A, right panel). The production of FL protein by 15 human leukemia/lymphoma derived cell lines and 25 primary AML samples were subsequently investigated. It was found that all of them expressed detectable FL, though the mean fluorescence intensity (MFI) was variable (Figure 4; Table 1; Table 2). Cell-surface–restricted staining showed no detectable FL expressed on the cell surface of leukemia/lymphoma cell lines except MOLM-1 (data not shown). Because FL was universally expressed by leukemia cells while FLT3 was constitutively phosphorylated in only some cell lines and AML samples, it was possible the expression level of FL might correlate with FLT3 phosphorylation status. However, there was no obvious correlation of the level of FL expression quantitated by MFI with the constitutive phosphorylation of FLT3 in these leukemia/lymphoma cell lines or primary AML blasts (Tables 1-2).
FL protein is expressed by human leukemia/lymphoma cell lines and primary AML blasts. Cells (1 × 106) were fixed, permeabilized, and stained with either anti-FL or isotype control antibody followed by staining with PE-conjugated secondary antibody. The stained cells were then analyzed by flow cytometry. The open histogram represents staining with isotype control. The shaded histogram represents staining with anti-FL antibody. (A) Left panel shows the expression of FL in the ML-1 cell line. Right panel shows the results of staining the same cells with anti-FL antibody that had been preincubated with FL for 16 hours. (B) FL expression by several leukemia cell lines. (C) FL expression by several primary AML samples.
FL protein is expressed by human leukemia/lymphoma cell lines and primary AML blasts. Cells (1 × 106) were fixed, permeabilized, and stained with either anti-FL or isotype control antibody followed by staining with PE-conjugated secondary antibody. The stained cells were then analyzed by flow cytometry. The open histogram represents staining with isotype control. The shaded histogram represents staining with anti-FL antibody. (A) Left panel shows the expression of FL in the ML-1 cell line. Right panel shows the results of staining the same cells with anti-FL antibody that had been preincubated with FL for 16 hours. (B) FL expression by several leukemia cell lines. (C) FL expression by several primary AML samples.
Incubation of leukemic blast cells in complete medium for 12 hours increases the FLT3 autophosphorylation in some primary AML specimens
FL is known to be expressed in both transmembrane and soluble forms.49 Because only low amounts of cell membrane FL was seen, it is likely that the secreted soluble form of FL plays a role in the cells where constitutive activation of wtFLT3 was observed. If leukemic blasts are capable of producing soluble FL, an increase of FLT3 autophosphorylation would be expected after these cells are incubated for a time in medium after being thawed, in comparison to washed, newly thawed blast cells. FLT3 activation was assessed by Western blotting in newly thawed AML blasts from 6 patients with AML (3 expressing wtFLT3 and 3 expressing FLT3/ITD mutations) before and after incubation for 12 hours in complete medium. An increase in the phosphorylation of FLT3 was observed in 2 cases after incubation (Figure 5). One case expressed wtFLT3 (Figure 5, upper panel, sample 1, lane 7 vs lane 1), and the other expressed FLT3/ITD (Figure 5, sample 4, lane 16 vs lane 10). Response to exogenous FL addition was seen in wtFLT3 expressing leukemic blast cells (Figure 5, lanes 4 and 6 vs lanes 1 and 3) and in leukemic blast cells harboring FLT3/ITD mutations (Figure 5, lanes 13 and 15 vs lanes 10 and 12). The finding in the FLT3/ITD leukemic cells in response to addition of FL is likely the result of activation of the wtFLT3 receptor that is also expressed by these cells.
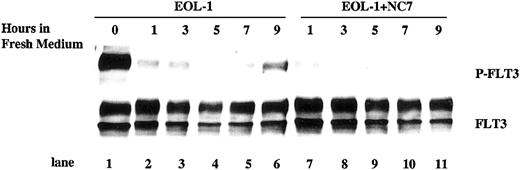
Autophosphorylation of FLT3 in EOL-1 cells and leukemic blast cells shows a time-dependent pattern after the conditioned medium is replaced with fresh medium
In log-phase growth, a high level of autophosphorylation of FLT3 is present in EOL-1 cells, which express wtFLT3 (Figure 6, lane 1). If this activation is a result of stimulation by soluble FL produced by the EOL-1 cells, washing the cells should remove the activating FL, resulting in decreased FLT3 phosphorylation. This FLT3 phosphorylation would then be expected to recover as FL is again produced. To test this hypothesis, EOL-1 cells growing in log phase were washed twice with PBS and then cultured in fresh medium. After 1, 3, 5, 7, and 9 hours, EOL-1 cells were harvested and the states of FLT3 protein expression and phosphorylation were assessed. Consistent with the hypothesis, phosphorylation of FLT3 decreased after the cells were incubated in fresh medium (Figure 6, lanes 2, 3, and 4 vs lane 1). Over time, phosphorylation started to recover and became significant by 9 hours of culture (Figure 6, lane 6). Importantly, these changes in phosphorylation occur without significant changes in FLT3 protein expression (Figure 6, lower panel). The decrease and recovery of FLT3 phosphorylation was also observed in the REH cell line after conditioned medium was replaced with fresh medium (data not shown). Thus, it appeared that EOL-1 cells are able to produce adequate soluble FL to activate the FLT3 receptor. In order to confirm that autocrine FL production is the explanation for the activation of FLT3 in EOL-1 cells, IMC-NC7, an anti-FLT3 antibody that blocks FL binding to the receptor, was utilized. After being washed twice, EOL-1 cells were cultured in fresh medium containing IMC-NC7 antibody (10 μg/mL). The recovery of phosphorylation of FLT3, which could be detected after 7 hours in cells cultured in fresh medium alone, was not observed in cells incubated with fresh medium containing IMC-NC7 antibody (Figure 6, lanes 10 and 11 vs lanes 5 and 6). An isotype control antibody did not interfere with FLT3 phosphorylation (data not shown).
Autophosphorylation of FLT3 in EOL-1 cells shows a time-dependent pattern after conditioned medium is replaced with fresh medium. EOL-1 cells (4 × 106 cells/mL) were washed twice and then cultured in fresh medium with or without IMC-NC7 antibody (10 μg/mL). Cells were collected at 0, 1, 3, 5, 7, and 9 hours and lysed in protein lysis buffer. Total cellular protein was immunoprecipitated with anti-FLT3 antibody. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same blot was stripped and reprobed with anti-FLT3 antibody (bottom panel).
Autophosphorylation of FLT3 in EOL-1 cells shows a time-dependent pattern after conditioned medium is replaced with fresh medium. EOL-1 cells (4 × 106 cells/mL) were washed twice and then cultured in fresh medium with or without IMC-NC7 antibody (10 μg/mL). Cells were collected at 0, 1, 3, 5, 7, and 9 hours and lysed in protein lysis buffer. Total cellular protein was immunoprecipitated with anti-FLT3 antibody. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same blot was stripped and reprobed with anti-FLT3 antibody (bottom panel).
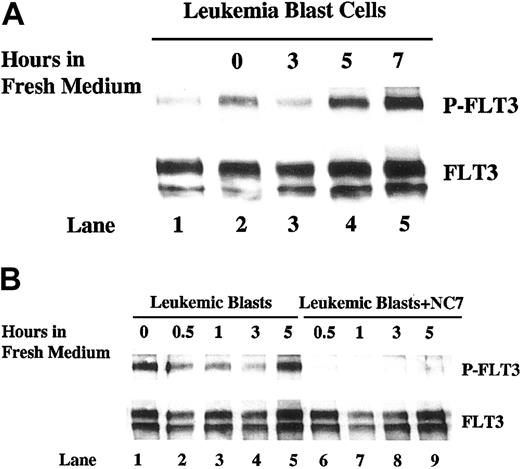
Supporting evidence was also obtained from a primary infant leukemia sample with an MLL translocation. Constitutively phosphorylated FLT3 was detected in the frozen blast cells that were studied immediately after being thawed and washed. However, autophosphorylation of FLT3 increased if these cells were cultured in the medium for 12 hours after being thawed (Figure 7A, lane 2 vs lane 1). Similar to EOL-1 cells, replacing the conditioned medium with fresh medium resulted in decreased phosphorylation of FLT3 by 3 hours in the primary blasts (Figure 7A, lane 3). Autophosphorylation of FLT3 recovered by 5 hours (Figure 7A, lanes 4 and 5). Addition of IMC-NC7 antibody was able to prevent the recovery of FLT3 phosphorylation (Figure 7B). Again, the changes in phosphorylation occurred without significant changes in FLT3 protein expression (Figure 7, lower panels).
Autophosphorylation of FLT3 in leukemia blast cells shows a time-dependent pattern after conditioned medium is replaced with fresh medium. (A) Frozen leukemia blast cells were thawed, washed, and cultured in fresh medium for 12 hours. Cells (4 × 106 cells/mL) were then washed twice and incubated in fresh medium. Cells were collected at 0, 3, 5, and 7 hours (lanes 2-5) and lysed. Another 10 × 106 cells were lysed right after being thawed and washed (lane 1). Total cellular protein was immunoprecipitated with anti-FLT3 antibody. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same blot was stripped and reprobed with anti-FLT3 antibody (bottom panel). (B) Frozen leukemia blast cells were thawed, washed, and cultured in fresh medium for 12 hours. Cells were then washed twice and incubated with fresh medium with or without IMC-NC7 antibody (10 μg/mL) at a density of 4 × 106 cells/mL. Cells were collected at 0, 0.5, 1, 3, and 5 hours and lysed in protein lysis buffer. Lysates were then analyzed as in panel A.
Autophosphorylation of FLT3 in leukemia blast cells shows a time-dependent pattern after conditioned medium is replaced with fresh medium. (A) Frozen leukemia blast cells were thawed, washed, and cultured in fresh medium for 12 hours. Cells (4 × 106 cells/mL) were then washed twice and incubated in fresh medium. Cells were collected at 0, 3, 5, and 7 hours (lanes 2-5) and lysed. Another 10 × 106 cells were lysed right after being thawed and washed (lane 1). Total cellular protein was immunoprecipitated with anti-FLT3 antibody. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same blot was stripped and reprobed with anti-FLT3 antibody (bottom panel). (B) Frozen leukemia blast cells were thawed, washed, and cultured in fresh medium for 12 hours. Cells were then washed twice and incubated with fresh medium with or without IMC-NC7 antibody (10 μg/mL) at a density of 4 × 106 cells/mL. Cells were collected at 0, 0.5, 1, 3, and 5 hours and lysed in protein lysis buffer. Lysates were then analyzed as in panel A.
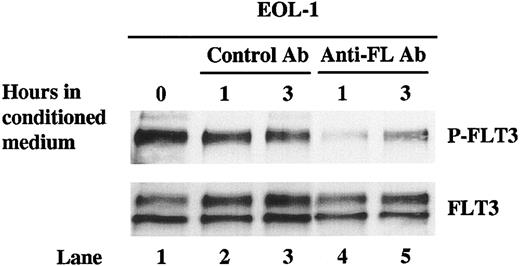
Depleting conditioned medium of FL decreases the phosphorylation of FLT3 in EOL-1 cells
If leukemic cells produce soluble FL, which results in the autocrine activation of the FLT3 receptor, depleting the conditioned medium of FL should interfere with this activation. To test this hypothesis, an anti-FL antibody was used to immunoprecipitate FL from the conditioned medium of EOL-1 cells. The same conditioned medium incubated with an isotype control antibody was used as the control. EOL-1 cells that were washed and then cultured with the conditioned medium showed a good level of FLT3 phosphorylation (Figure 8, upper panel, lanes 2 and 3 vs lane 1). In contrast, incubating the same washed cells with conditioned medium depleted of FL resulted in decreased FLT3 phosphorylation at 1 hour with some recovery by 3 hours (Figure 8, upper panel, lanes 4 and 5 vs lane 1).
Depleting conditioned medium of FL decreases the phosphorylation of FLT3 in EOL-1 cells. Conditioned medium of EOL-1 cells were first incubated with anti-FL monoclonal antibody followed by incubation with Protein G–agarose. The immunoprecipitates were then separated from the medium by centrifuging. Conditioned medium treated with an isotype control antibody was used as the control. EOL-1 cells were washed twice and cultured in the anti-FL– or isotype control antibody–treated conditioned medium for 1 and 3 hours. Total cellular protein was immunoprecipitated with anti-FLT3 antibody. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same blot was stripped and reprobed with anti-FLT3 antibody (bottom panel).
Depleting conditioned medium of FL decreases the phosphorylation of FLT3 in EOL-1 cells. Conditioned medium of EOL-1 cells were first incubated with anti-FL monoclonal antibody followed by incubation with Protein G–agarose. The immunoprecipitates were then separated from the medium by centrifuging. Conditioned medium treated with an isotype control antibody was used as the control. EOL-1 cells were washed twice and cultured in the anti-FL– or isotype control antibody–treated conditioned medium for 1 and 3 hours. Total cellular protein was immunoprecipitated with anti-FLT3 antibody. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same blot was stripped and reprobed with anti-FLT3 antibody (bottom panel).
Discussion
Hematopoiesis is a complex process in which survival, proliferation, differentiation, and other functions are at least partially regulated through a network of growth factors.50 FLT3 is a member of the type III RTK family, which is expressed on hematopoietic stem/progenitor cells.5-7 Its ligand, FL, is a hematopoietic stem/progenitor cell growth factor which appears to cooperate with other cytokines to promote clonal expansion and development along several different hematopoietic lineages.1,2,8,9
FL expression appears to be a ubiquitous finding in human leukemia/lymphoma cell lines when assessed by Northern blot, though not all cell lines had detectable protein expressed on the cell surface.14,15 In this study, expression of FL by primary AML blast cells was measured by both RT-PCR and immunostaining for FL both on the cell surface and inside the cell. FL mRNA was detectable in all 90 AML blast samples assessed in this study. Among those samples, 19 were further examined by immunostaining and all showed detectable FL protein expression. Fifteen human leukemia/lymphoma-derived cell lines were also analyzed for FL expression. In agreement with the primary AML sample result, all of them were positive for FL transcripts and protein. Additionally, FLT3 transcript was detected in all the AML primary samples and the majority of leukemia/lymphoma-derived cell lines. FLT3 protein was also detected in 18 of 20 primary AML samples and 9 of 15 cell lines by Western blot. Thus, coexpression of FLT3 and FL occurs in the majority of AML cells.
Almost all hematopoietic cell lines express FL mRNA and protein, but not all of them have detectable ligand expression on the cell surface. This could be explained by most cells expressing the secreted form of FL and also by the possibility of coexpressed FLT3 binding FL and blocking its detection. The observation on coexpression of receptor and ligand by the same cell suggests possible autocrine, intracrine, or paracrine stimulatory loops. Similar mechanisms of autostimulated growth have been described for leukemia cells with regard to GM-CSF, SCF, and vascular endothelial growth factor (VEGF), among others.51-54
FL shows synergistic stimulation of normal hematopoietic stem/progenitor cells when combined with other growth factors. Stimulation of AML cells by FL alone or in combination with other cytokines has been observed with G-CSF, GM-CSF, IL-3, and SCF.16,18-20 Thus, our finding of universal expression of FL by primary AML cells suggests FL/FLT3 signaling might well be contributing to leukemogenesis.
Constitutive activation of FLT3 through activating mutations leads to autonomous, cytokine-independent growth with subsequent transformation of cells.27,42 In this work, we demonstrated constitutive activation/autophosphorylation of wtFLT3 in leukemic blasts of some AML samples and leukemia/lymphoma cell lines. Expression of FL may be necessary for the constitutively activated FLT3 observed in these cases. However, FL expression alone may not be sufficient to activate FLT3 as it was also found in some cases that do not show evidence of activation. The relative level of FL expression did not correlate as well as the level of FLT3 protein with the level of FLT3 phosphorylation. The role of autocrine FL in constitutive FLT3 activation is strongly suggested by experiments utilizing EOL-1 cells and leukemia blast cells. When these cells were washed and cultured in fresh medium, the phosphorylation of FLT3 decreased and then recovered over a number of hours. It is likely that this time period is required for enough soluble FL to be secreted from the cells to reach the threshold that is required to activate the FLT3 receptor. Further confirming this possibility, an antibody that blocks the binding of FL to the FLT3 receptor prevented the recovery of FLT3 phosphorylation in both EOL-1 cells and leukemia blast cells. In addition, depleting the conditioned medium of EOL-1 cells using immunoprecipitation with an anti-FL antibody resulted in a decrease in FLT3 phosphorylation. Thus, an autocrine loop appears to be responsible for the autophosphorylation of FLT3 observed in these cells.
FLT3 autophosphorylation in primary AML samples has been reported previously with 12 (8 of 12 containing FLT3/ITD) of 23 and 3 (1 of 3 containing FLT3/ITD) of 27 samples reported as positive in these studies, respectively.42,55 In our study, 15 (6 of 15 containing FLT3/ITD, 1 of 15 containing D835 mutation, and 8 of 15 containing only wtFLT3) of 20 primary AML samples revealed FLT3 autophosphorylation. The variation in the rate of FLT3 autophosphorylation observed by different groups may result from differences in the percentage of samples expressing FLT3 mutations and from different ways of processing blast cells. In this study, leukemic blast cells were incubated for 12 hours in medium prior to protein preparation. This may give blast cells a time period to produce FL which subsequently activates the FLT3 receptor. This hypothesis is supported by the increase of FLT3 autophosphorylation observed in blast cells after incubation in medium for 12 hours in comparison to newly thawed blast cells (Figure 1B; Figure 6A).
In conclusion, coexpression of FLT3 receptor and its ligand, FL, occurs in a high percentage of primary AML cases and in one MLL case that were examined. The subsequent autocrine stimulatory loop may result in wtFLT3 signaling contributing to the pathogenesis of AML and MLL leukemia. Therefore, exploring the effects of inhibiting wtFLT3 signaling in AML and in MLL leukemia31 is warranted.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-06-1969.
Supported by grants to D.S. from the National Cancer Institute (CA91177 and CA90668), the Leukemia and Lymphoma Society, and the Children's Cancer Foundation, and by a National Institutes of Health T32CA60441 training grant (B.R.B.).
Several of the authors (Z.Z., D.L., D.H., L.W., Y.L.) are employees of ImClone Systems, Inc, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
D.S. is the Douglas Kroll Research Foundation Translational Researcher of The Leukemia and Lymphoma Society. We would like to thank Stewart Lyman of Immunex for generously providing the anti-FL antibody, and Dr Hans G. Drexler for providing several of the cell lines (EM2, EM3, EOL-1, LAZ221, MOLM-1, and WSU-NHL) used in this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal