Abstract
The effects of telomerase inhibition with an oligonucleotide N3′ → P5′ thiophosphoramidate (GRN163) complementary to the telomerase template region were examined on human multiple myeloma (MM) and non-Hodgkin lymphoma (NHL) cell lines, primary MM cells, and tumor xenografts. GRN163 treatment reduced telomerase levels in all cells and induced more rapid telomeric shortening. Continuous GRN163 treatment for 7 to 14 days resulted in proliferative arrest, morphologic changes, and apoptosis characteristic of cell crisis in tumor cell lines with short (1.7-5.4 kb) but not long (9-11 kb) telomeres. Intratumoral administration of GRN163 also inhibited the growth of MM and NHL xenografts established from cell lines with short telomeres (Hs602 lymphoma, 2.7 kb; CAG myeloma, 2.7 kb) and increased tumor apoptosis. However, GRN163 therapy of NHL xenografts established from cells with long telomeres (11.0 kb) had equivocal effects on tumor growth and did not induce apoptosis during this time frame. Systemic daily intraperitoneal administration of GRN163 in myeloma xenografts with short telomere lengths also decreased tumor telomerase levels and reduced tumor volumes. These data demonstrate that telomerase is important for the replication of mature B-cell neoplasia by stabilizing short telomeres, and they suggest that telomerase inhibition represents a novel therapeutic approach to MM and NHL.
Introduction
The preservation of telomeres, DNA-protein structures located at the ends of eukaryotic chromosomes, is essential to the immortalization process. In most normal somatic cells, telomeric DNA is progressively lost with each cell division until a critically short telomere length is obtained, and telomeres lose the ability to protect linear chromosomal ends. The resultant telomeric dysfunction induces cell “crisis” with chromosomal instability, end-to-end chromosome fusions, activation of DNA checkpoint responses, apoptosis, and cell death.1 Human telomerase is a complex ribonucleoprotein reverse transcriptase composed of an RNA template (hTR) and a catalytic protein subunit (hTERT), which is responsible for synthesizing d-(TTAGGG)n telomeric repeats at chromosomal ends. Stabilizing telomeric DNA through telomerase up-regulation or activating alternative mechanisms of telomere maintenance is essential if cells are to survive and proliferate indefinitely.2 Telomerase activity (TA) is not found in most normal somatic cells, which lack hTERT, but it has been detected in high levels in 85% to 90% of human cancers.1 Although telomerase is not an oncogene,3 transfecting hTERT into normal epithelial or endothelial cells transformed with the simian virus 40 large T-antigen and the N-ras oncogene allows cells to bypass crisis and ultimately to achieve malignancy, confirming the role of telomerase in cellular immortalization and tumorigenesis.1 Telomerase inhibition by numerous strategies, including dominant-negative telomerase mutants,4,5 small–molecular-weight compounds,6 reverse-transcriptase inhibitors,7 G-quadruplex interactive agents,8,9 and antisense oligonucleotides complementary for hTR10-12 has been shown to limit the growth of immortalized tumor cell lines by inducing progressive telomere shortening and cell death.
Telomerase activation plays a unique role in the biology of normal and malignant B lymphocytes.13-15 Unlike most normal human somatic cells that express telomerase only during early development, B lymphocytes express telomerase during development and retain the ability to up-regulate telomerase and to elongate telomere length after maturation in response to antigens or to activation through the B-cell antigen receptor.16 Germinal center B cells and tonsillar B cells in hyperplastic follicles have high levels of telomerase. It has been proposed that B-cell neoplasms arise in part from normal mature B-cell clones, which reactivate or “reinduce” high telomerase levels during the process of malignant transformation.17 Telomerase activation has been implicated in the evolution of monoclonal gammopathy of undetermined significance (MGUS) to multiple myeloma and plasma cell leukemia.18,19 High levels of TA have been found in half the patients with B-cell non-Hodgkin lymphoma (NHL) compared with benign lymphoid tissue and have been correlated with apoptosis deregulation, higher grade lymphoma, and chemotherapy-refractory disease.20-22 In a study of 16 NHL patient samples, 6 had high telomerase levels and shorter telomere lengths at disease relapse than at diagnosis.21 Patients with B-cell chronic lymphocytic leukemia (B-CLL) and multiple myeloma (MM) whose tumors expressed high levels of telomerase had shorter overall survival times and were more likely to have advanced disease and other poor prognostic clinical features.23,24 Inducing TA by interleukin-6 (IL-6) or insulin-like growth factor (IGF) in MM cell lines has been associated with enhanced growth, survival, and drug resistance.25,26
Telomerase may function to preserve telomeric shortening either by steric protection of critically shortened telomeric DNA through telomerase “capping”27 or by stabilization of telomeres when telomere length becomes a trigger for cell senescence or apoptosis.28,29 Our analysis of telomeric DNAdistribution in primary MM specimens showed preferential stabilization of shorter telomeres, particularly when the mean telomere restriction fragment (TRF), a method of assessing telomere length by Southern blot analysis, fell below 5.5 kb. Longer telomeres (longer than 5.5 kb) in tumors continued to progressively erode.24 Although these data support the concept of preferential telomerase stabilization of short telomeres in mature B-cell neoplasia, until now there have been few data on the direct effects of telomerase inhibition in MM or NHL.
In this study, we evaluated the effect of telomerase inhibition with an oligonucleotide N3′ → P5′ thiophosphoramidate compound complementary to the RNA template region of human telomerase, GRN163, on the growth of human MM and NHL cell lines in vitro and in xenograft mouse models.30 We found that GRN163 decreased TA in all cell lines and enhanced telomeric shortening in a lymphoma cell line (telomere length, 4.5 kb) after 33 days. Continuous GRN163 exposure of malignant cell lines with short telomeres (1.7-5.4 kb) resulted in the rapid dose-dependent induction of proliferative arrest and cell death, whereas up to 40 days of GRN163 treatment of cells with long telomere lengths did not influence proliferation. MM and NHL cells with short telomeres treated with GRN163 underwent proliferative arrest, morphologic changes, increased apoptosis, and eventual cell death characteristic of cell crisis as early as 1 to 2 weeks after exposure (2-15 population doublings). Furthermore, in vivo GRN163 treatment of MM and NHL xenografts established from cell lines with short telomeres (Hs602 lymphoma, 2.7 kb; CAG myeloma, 2.7 kb) inhibited tumor growth by 40% to 50% compared with saline- and mismatch–oligonucleotide-treated controls. Tumor tissue from GRN163-treated animals exhibited marked apoptosis. In contrast, GRN163 therapy for lymphoma xenografts established from cells with long telomeres (11 kb) had equivocal effects on tumor growth with no increased apoptosis after 3 weeks of treatment. These findings confirm that telomerase plays an important role in the biology of NHL and MM and suggest that telomerase inhibition may represent an effective therapeutic approach for a substantial subset of B-cell malignancies.
Patients, materials, and methods
Reagents
GRN163 is a 13-mer 2′-deoxyoligonucleotide N3′→P5′ thiophosphoramidate oligonucleotide complementary to the template region of the RNA component (hTR) in the active site of telomerase (5′-TAGGGTTAGACAA).30 GRN137227, abbreviated 227, is a mismatch 13-mer oligonucleotide compound in which 2 dinucleotide pairs in the GRN163 sequence are flipped, disrupting the G-triplet (5′TAGGTGTGAACAA); 227 was used as a negative control. Oligonucleotides were dissolved in phosphate-buffered saline (PBS) and stored at –20°C.
Cell lines and patient cells
All human cell lines have been previously described.31-36 Informed consent was obtained from MM patients on an institutional review board (IRB)–approved protocol in accordance with the tenets of the Declaration of Helsinki. Patient MM cells were purified from bone marrow samples by Ficoll-Hypaque density-gradient centrifugation followed by CD138+ cell selection using CD138 magnetic beads (Miltenyi Biotec, Auburn, CA). Cells were cultured in RPMI 1640 medium (10% fetal bovine serum, 2 mM l-glutamine, 4.5 g/L glucose, 1.5 g sodium bicarbonate, 1 U/mL penicillin/streptomycin, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], and 1 nmol sodium pyruvate) and were maintained at 37°C with 5% CO2.
Fluorescence studies
Cells were incubated with 3′-fluorescein–tagged GRN163 oligonucleotide (10 μM; Geron, Menlo Park, CA) without additional cationic lipids or permeabilizing agents. At timed intervals, cells were removed, washed with PBS, and analyzed using flow cytometry.
In vitro experiments
Cells (1 × 105) were prepared in 6-well plates with PBS, GRN163 (1-10 μM), or mismatch oligonucleotide (10 μM 227). Cells were harvested, counted, and replated in the presence of fresh drug every 3 to 5 days. For proliferation experiments, viable cells were enumerated 3 times by trypan blue dye exclusion using a hematocytometer and a phase-contrast microscope. Population doublings (PDLs) were calculated as follows: Pn = Pn–1 + [(ln [total number of cells]) – (ln [number of cells plated])/ln2]. For TRF measurements and TA assays, harvested cells were pelleted at 6000 rpm for 6 minutes, washed in cold PBS, and stored at –80°C.
Measuring telomere restriction fragment length by Southern blot analysis
TRF lengths were determined as described previously.37 In brief, high-molecular–weight DNA was extracted from 5 × 105 to 1 × 106 cells using the Nucleon BACC2 DNA extraction kit (RPN 8502; Amersham Life Science, Buckinghamshire, United Kingdom). Mean TRF length was measured using the TeloTAGGG telomere length assay kit (Roche Molecular Biochemical, Indianapolis, IN). Two micrograms purified DNA was digested with HinfI/RsaI mixture (10 U/μg DNA) for 2.5 hours, separated by 0.8% agarose gel electrophoresis in 1 × Tris-acetate-EDTA (ethylenediaminetetraacetic acid), and transferred onto a nylon membrane (Hybond N+; Amersham Life Science). After a rinse in 2 × SSC and neutralizing solution, the filter was hybridized to a digoxigenin-labeled probe specific for telomeric repeats for 3 hours at 42°C. The filter was then washed twice in 2 × SSC 0.1% sodium dodecyl sulfate (SDS) for 5 minutes, each wash at room temperature, and was placed in a 0.2 × SSC 0.1% SDS solution at 50°C for 15 minutes with 1 repeat. To determine TRF length, the hybridized probe was incubated with a digoxigenin-specific antibody covalently coupled to alkaline phosphatase. The immobilized probe was visualized by a highly sensitive chemiluminescent substrate for alkaline phosphatase (CDP-Star; Applied Biosystems, Foster City, CA), which detected the TRF DNA on Hyperfilm (Amersham Life Science). Mean TRF length was defined as described previously.37 The amount of telomeric DNA was calculated by integrating the volume of each smear using MacBas V2.5 software (Fuji, Stamford, CT).
Measuring telomerase activity by TRAP assay
Amodified version of the telomeric repeat amplification protocol (TRAP; Geron) was used. In brief, 2 × 105 cells were extracted in 200 μL lysis buffer, or cellular protein was extracted from snap-frozen tumor tissues stored at –80°C. When preparing extracts from tumor tissues, RNase inhibitor (Roche Molecular Biochemical) was added to the lysis buffer before extraction at a final concentration of 100 U/mL. Two microliters total cellular lysate or 0.1 μg protein extract was used for each reaction. Samples were exposed to 30 minutes of telomerase extension at 30°C and were subjected to polymerase chain reaction (PCR) for 28 cycles in the presence of a CY5-5′ end-labeled TS primer followed by electrophoresis on a 12.5% polyacrylamide gel. Gels were scanned in a Molecular Dynamics Storm (Amersham Bioscience) using the red fluorescence mode and were quantified using Image Quant software (IQMac v1.2; Molecular Dynamics, Sunnyvale, CA). A standard batch of SK-N-SH neuroblastoma (NB) cell line was used as a positive control for each run. Negative controls were obtained from heat inactivation of the RNA template for each cell extract, and lysis buffer was used as PCR control. Background activity was subtracted, and relative TAwas measured as the ratio of the telomerase-specific ladder to that of a 36-bp internal standard. All values were then expressed as a percentage of TA over positive NB SK-N-SH cell line controls.
Flow cytometric analysis of GRN163-treated tumor cell lines
Apoptosis and cell necrosis were evaluated by dual-color staining with annexin V-phycoerythrin (PE) and propidium iodide (PI) according to the manufacturer's instructions (PharMingen, San Diego, CA) and were quantified by fluorescence-activated cell sorting (FACScan).5 Cell-cycle analysis was evaluated by ethidium bromide staining for DNA content of extracted nuclei.
Xenograft experiments
Nonobese diabetic/severe combined immunodeficient (NOD/LTSZPrko/J [NOD/SCID]) mice (Jackson Laboratories, Bar Harbor, ME) were maintained in core animal facilities under an institute-approved animal protocol in accordance with the Principles of Laboratory Animal Care (National Institutes of Health publication no. 85-23; revised 1985). Mice were sublethally irradiated with 3 cGy from a gamma source and were inoculated with 10 × 106 tumor cells by way of a subcutaneous route. When tumor volumes approached 100 mm3, mice were divided into experimental groups of 5 to 8 mice each. Intratumoral injections of PBS (diluent), GRN163 (10 nmol), or 227 (10 nmol) were given in 20 μL total volume 5 days per week. Intraperitoneal injections of PBS or GRN163 (50 mg/kg) were given daily. Intraperitoneal doxorubicin was given twice weekly at 3 mg/kg for 6 doses. Animal weight and tumor measurements were obtained 3 times weekly. Tumor volume was calculated using the formula mm3 = 4/3πr3, where r = (l + w)/4 using the 2 largest perpendicular axes of the tumor (l indicates length; w, width). Animals were killed when moribund or when 1-dimensional tumor diameter exceeded 2.0 cm (according to the guidelines of the animal core facilities). Xenograft experiments were repeated at least twice. Statistical evaluation of tumor volumes was performed using 2-tailed student t test analysis with unequal variance.
Immunohistochemical staining of tumor xenograft tissues
Apoptosis in xenografts was assessed using TdT-mediated dUTP-biotin nick-end labeling (TUNEL). Tissues from killed animals were fixed, deparaffinized, rehydrated, and digested in 20 μg/mL proteinase K solution for 15 minutes at room temperature. Slides were then washed in PBS and refixed in 4% formalin, washed again in PBS, and equilibrated before biotinylated nucleotide mix and TdT enzyme were added for 90 minutes at 37°C. Slides were washed in PBS, blocked in 2% PBS/bovine serum albumin (BSA), and incubated in avidin-biotin complex (ABC) vector reagent for 1 hour at room temperature. Tissues were stained with 3,3′-diaminobenzidine and were counterstained with Harris hematoxylin before dehydration and mounting. Ki67 immunohistochemical staining was performed as previously detailed.20
Results
GRN163 is a prototype of a new class of 13-mer 2′-deoxy N3′→P5′ thiophosphoramidate oligonucleotides designed to complement the template domain of the RNA component in the active site of telomerase (hTR).38 The hTR contains a crucial 11-nucleotide–long template region, part of which serves as the basis for telomere elongation by TTAGGG repeats. GRN163 forms a high-affinity sequence-specific duplex with the complementary RNA strand that is further stabilized through interactions between the oligonucleotide backbone and hTERT. GRN163 is resistant to hydrolysis by cellular nucleases and exhibits high thermodynamic stability.30 Earlier biochemical assays demonstrated that GRN163 inhibits human telomerase in a sequence-specific manner, with IC50 values in the sub-nanomolar range and IC50 values of 0.5 μM against solid tumor cell lines in the absence of uptake enhancers.30
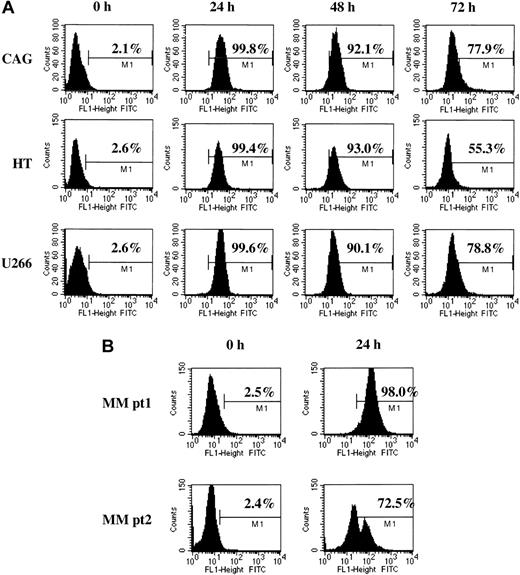
In the absence of exogenous uptake enhancers, 3′-fluorescein–labeled GRN163 oligonucleotide was added to cultured cells. Fluorescence was measured using flow cytometry. GRN163-fluorescein isothiocyanate (FITC) was detected in cell lines for more than 96 hours and in CD138+ primary MM cells for more than 24 hours. Representative results of GRN163-FITC uptake were as follows: CAG MM: 0 hour, 2.1%; 24 hours, 99.8%; 48 hours, 92.1%; and 72 hours, 77.9%; U266 MM: 0 hour, 2.6%; 24 hours, 99.6%; 48 hours, 90.1%; and 72 hours, 78.8%; and HT NHL: 0 hour, 2.6%; 24 hours, 99.4%; 48 hours, 93.0%; and 72 hours, 55.3%. Twenty-four–hour uptake rates of fluorescein-labeled GRN163 in CD138+ cells from MM patients 1 and 2 were 98.0% and 72.5%, respectively (Figure 1).
Cellular uptake of fluorescein-labeled GRN163 oligonucleotide into lymphoma and myeloma cell lines. Fluorescein-tagged GRN163 oligonucleotide (10 μM) was added to cultured cells in the absence of additional cationic lipids or permeabilizing agents. Cells were removed at various time intervals, washed with PBS to remove unbound oligonucleotide, and analyzed by flow cytometry. Shown are representative cellular uptake levels in 3 cell lines (CAG, HT, U266; panel A) and CD138+ MM cells from 2 patients (B).
Cellular uptake of fluorescein-labeled GRN163 oligonucleotide into lymphoma and myeloma cell lines. Fluorescein-tagged GRN163 oligonucleotide (10 μM) was added to cultured cells in the absence of additional cationic lipids or permeabilizing agents. Cells were removed at various time intervals, washed with PBS to remove unbound oligonucleotide, and analyzed by flow cytometry. Shown are representative cellular uptake levels in 3 cell lines (CAG, HT, U266; panel A) and CD138+ MM cells from 2 patients (B).
GRN163 reduced telomerase activity and induced progressive telomere shortening in neoplastic B cells. Among human myeloma and lymphoma lines, mean TRF varied between 1.7 and 11 kb with universally high TA (Tables 1, 2).
Mean telomere length measured in human lymphoma and myeloma cell lines
Cell line (tumor) . | Characteristics . | Mean TRF, kb . |
|---|---|---|
| CAG (myeloma) | CD38+EBV-CD19- IgG κ chain | 2.7 |
| SK0-007 subclone (myeloma) | IgE λ light chain EBV+ | 1.7 |
| U266 (myeloma) | CD38+ IgE λ light chain | 9.0 |
| Hs602 (lymphoma) | CD19+CD20+CD45+CD25-CD3- | 2.7 |
| HT (lymphoma) | CD19+CD20+CD21+CD22+CD25- p53 mutant | 11.0 |
| RL (lymphoma) | CD19+CD20+CD21+CD22+bcl-2+ t(14;18), p53 mutant | 5.4 |
| SKI-DLBCL1 (lymphoma) | CD19+CD20+CD45+CD3-CD5-CD10- CD23-EBV- | 4.9 |
Cell line (tumor) . | Characteristics . | Mean TRF, kb . |
|---|---|---|
| CAG (myeloma) | CD38+EBV-CD19- IgG κ chain | 2.7 |
| SK0-007 subclone (myeloma) | IgE λ light chain EBV+ | 1.7 |
| U266 (myeloma) | CD38+ IgE λ light chain | 9.0 |
| Hs602 (lymphoma) | CD19+CD20+CD45+CD25-CD3- | 2.7 |
| HT (lymphoma) | CD19+CD20+CD21+CD22+CD25- p53 mutant | 11.0 |
| RL (lymphoma) | CD19+CD20+CD21+CD22+bcl-2+ t(14;18), p53 mutant | 5.4 |
| SKI-DLBCL1 (lymphoma) | CD19+CD20+CD45+CD3-CD5-CD10- CD23-EBV- | 4.9 |
TRF measurement performed as described in “Patients, materials, and methods.” Ig indicates immunoglobulin.
GRN163 treatment decreased telomerase activity in human myeloma and lymphoma cell lines
Treatment . | CAG (%) . | SKO-007 (%) . | U266 (%) . | Hs602 (%) . | HT (%) . | RL (%) . | SKI-DLBCL (%) . |
|---|---|---|---|---|---|---|---|
| PBS | 166 (100) | 255 (100) | 68 (100) | 203 (100) | 248 (100) | 94 (NA) | 50 (100) |
| GRN163 1 μM | 100 (60) | 232 (91) | 38 (56) | 69 (34) | 114 (45) | 9 (10) | 10 (20) |
| GRN163 3 μM | 86 (52) | 196 (77) | 27 (40) | 29 (14) | 111 (45) | 18 (20) | NA |
| GRN163 10 μM | NA | NA | 27 (40) | NA | NA | NA | NA |
| 227 10 μM | 116 (70) | NA | 92 (100) | 170 (84) | NA | NA | NA |
Treatment . | CAG (%) . | SKO-007 (%) . | U266 (%) . | Hs602 (%) . | HT (%) . | RL (%) . | SKI-DLBCL (%) . |
|---|---|---|---|---|---|---|---|
| PBS | 166 (100) | 255 (100) | 68 (100) | 203 (100) | 248 (100) | 94 (NA) | 50 (100) |
| GRN163 1 μM | 100 (60) | 232 (91) | 38 (56) | 69 (34) | 114 (45) | 9 (10) | 10 (20) |
| GRN163 3 μM | 86 (52) | 196 (77) | 27 (40) | 29 (14) | 111 (45) | 18 (20) | NA |
| GRN163 10 μM | NA | NA | 27 (40) | NA | NA | NA | NA |
| 227 10 μM | 116 (70) | NA | 92 (100) | 170 (84) | NA | NA | NA |
Telomerase activity was measured after 14 days of GRN163 treatment. Levels are as quantified in percentage R8/NB in TRAP assay (“Patients, materials, and methods”) and are expressed as percentage of PBS-treated controls.
NA indicates not available.
Two-week treatment with GRN163 (1-10 μM) resulted in dose-dependent reductions in TA in all lines independent of telomere length. Representative results of GRN163 (3-10 μM) on TA in 2 myeloma cell lines (CAG TRF, 2.7 kb; U266 TRF, 9.0 kb) and CD138+ cells from 2 MM patients are shown with summarizations of additional cell line data (Figure 2; Table 2). TRAP assays of CAG, HT, and U266 cells incubated with GRN163 (10 μM) for 24 to 96 hours demonstrated that telomerase inhibition (3%-23% of controls) persisted for at least 4 days in cell lines cultured in GRN163-containing media (data not shown).
GRN163 reduced telomerase activity in neoplastic B-cell lines and primary MM cells. GRN163 treatment for 14 days decreased telomerase activity in 2 representative MM cell lines (CAG TRF, 2.7 kb; U266 TRF, 9.0 kb) in a dose-dependent and telomere-independent manner compared with vehicle (PBS) and 227 (mismatch oligonucleotide) controls. GRN163 treatment (10 μM) for 24 hours also decreased telomerase activity in CD138+ cells from 2 MM patients.
GRN163 reduced telomerase activity in neoplastic B-cell lines and primary MM cells. GRN163 treatment for 14 days decreased telomerase activity in 2 representative MM cell lines (CAG TRF, 2.7 kb; U266 TRF, 9.0 kb) in a dose-dependent and telomere-independent manner compared with vehicle (PBS) and 227 (mismatch oligonucleotide) controls. GRN163 treatment (10 μM) for 24 hours also decreased telomerase activity in CD138+ cells from 2 MM patients.
Continuous GRN163 treatment induced more rapid telomeric shortening than PBS or 227 in MM and NHL cell lines. Representative results from 3 cell lines with different initial TRF lengths are shown (CAG, 2.7 kb; HT, 11 kb; U266, 9.0 kb). For example, mean telomere lengths of CAG cells after PBS treatment were longer (day 4, 2.8 kb; day 12, 3.0 kb; day 17, 2.6 kb) than they were for GRN163 (10 μM)–treated cells (day 4, 2.8 kb; day 12, 2.5 kb; day 17, 1.8 kb) (Figure 3).
GRN163 induced progressive telomeric shortening in myeloma and lymphoma cells for 40 days of continuous treatment. Mean telomere lengths were evaluated in 3 cell lines (CAG [panel A], HT [panel B], and U266 [panel C]) with varying initial telomere lengths at intervals of 4 to 7 days during continuous PBS or GRN163 (10 μM) treatment. Results from all 3 cell lines demonstrated that GRN163 treatment (10 μM) consistently resulted in more rapid telomere shortening than PBS-treated controls in all cell lines. Representative TRF blots of CAG MM (initial TRF, 2.7 kb), HT NHL (initial TRF, 11 kb), and U266 MM cells (initial TRF, 9.0 kb) are shown.
GRN163 induced progressive telomeric shortening in myeloma and lymphoma cells for 40 days of continuous treatment. Mean telomere lengths were evaluated in 3 cell lines (CAG [panel A], HT [panel B], and U266 [panel C]) with varying initial telomere lengths at intervals of 4 to 7 days during continuous PBS or GRN163 (10 μM) treatment. Results from all 3 cell lines demonstrated that GRN163 treatment (10 μM) consistently resulted in more rapid telomere shortening than PBS-treated controls in all cell lines. Representative TRF blots of CAG MM (initial TRF, 2.7 kb), HT NHL (initial TRF, 11 kb), and U266 MM cells (initial TRF, 9.0 kb) are shown.
GRN163 treatment rapidly induced growth arrest, apoptosis, and cell death in myeloma and lymphoma cell lines with short telomeres after 2 weeks. NHL and MM cell lines with variable telomere lengths were continuously exposed to PBS, GRN163 (1-10 μM), or 227 (10 μM) in vitro for up to 40 days. GRN163 treatment induced growth arrest in a dose-dependent fashion (10 μM > 3 μM > 1 μM). Although they were not influenced by tumor type, the effects of GRN163 on proliferation appeared to be dictated by the telomere lengths of target cells with more rapid onsets of growth arrest in cells with short telomeres (1.7-5.4 kb) after 7 to 14 days of treatment, equivalent to 2 to 15 population doublings (PDs). GRN163 (1-10 μM) treatment of cell lines with long telomeres (9-11 kb) had no effect on growth during 40 days of treatment, equivalent to 17 to 30 PDs. Proliferation of 227 (10 μM)–treated cells was similar to that of PBS-treated cells in all tested lines. Evaluating cell lines after the completion of treatment (days 14-40) demonstrated that 60% to 100% of cells with short telomeres were apoptotic or necrotic compared with 3% to 7% of cells with long telomeres, as determined by annexin/PI dual staining (Figure 4).
Growth arrest induced in NHL and MM cell lines with short telomeres. Multiple myeloma (A,B,F) and non-Hodgkin lymphoma (C,D,E,G) cell lines with different mean telomere lengths were plated in 6-well plates in the presence of PBS, GRN163 (1-10 μM), or a mismatch oligonucleotide control 227 (10 μM) for up to 40 days. Cells were harvested, counted, and replated in the presence of fresh drug or PBS every 3 to 5 days. Viable cells were enumerated 3 times by trypan blue dye exclusion using a hematocytometer and a phase-contrast microscope. Average cell numbers were obtained, and PDs were calculated using the formula Pn = Pn–1 + [([ln (total number of cells]) – (ln [number of cells plated])/ln2]. Data are shown as calculated PDs over days of treatment. Percentages of apoptotic or dead cells at the end of treatment were measured as the percentage of annexin/PI dual-staining cells by flow cytometry.
Growth arrest induced in NHL and MM cell lines with short telomeres. Multiple myeloma (A,B,F) and non-Hodgkin lymphoma (C,D,E,G) cell lines with different mean telomere lengths were plated in 6-well plates in the presence of PBS, GRN163 (1-10 μM), or a mismatch oligonucleotide control 227 (10 μM) for up to 40 days. Cells were harvested, counted, and replated in the presence of fresh drug or PBS every 3 to 5 days. Viable cells were enumerated 3 times by trypan blue dye exclusion using a hematocytometer and a phase-contrast microscope. Average cell numbers were obtained, and PDs were calculated using the formula Pn = Pn–1 + [([ln (total number of cells]) – (ln [number of cells plated])/ln2]. Data are shown as calculated PDs over days of treatment. Percentages of apoptotic or dead cells at the end of treatment were measured as the percentage of annexin/PI dual-staining cells by flow cytometry.
CAG cells (TRF, 2.7 kb) treated with GRN163 (10 μM) for 7 days exhibited morphologic changes (increased cell size, cytoplasmic vacuolization, and nuclear aberrations) and enhanced annexin/PI dual staining indicative of apoptosis/necrosis, whereas PBS- or 227 (10 μM)–treated cells had normal morphology with low levels of annexin/PI dual staining (Figure 5). GRN163 treatment did not affect cell cycling (data not shown).
Morphologic changes, apoptosis, and cell death induced in CAG myeloma cells (TRF, 2.7 kb) after 7 days of in vitro GRN163 (10 μM) treatment. CAG myeloma cells (1 × 105) were prepared in 6-well plates with PBS, GRN163 (1-10 μM), or a mismatch oligonucleotide control 227 (10 μM). Cells were harvested, counted, and replated in the presence of fresh drug on day 4. On day 7 of treatment, cells were collected and (A) viewed under × 60 light microscopy for morphologic changes. (B) Dual-color staining of cells for annexin/PI uptake indicative of cell apoptosis and necrosis was also performed.
Morphologic changes, apoptosis, and cell death induced in CAG myeloma cells (TRF, 2.7 kb) after 7 days of in vitro GRN163 (10 μM) treatment. CAG myeloma cells (1 × 105) were prepared in 6-well plates with PBS, GRN163 (1-10 μM), or a mismatch oligonucleotide control 227 (10 μM). Cells were harvested, counted, and replated in the presence of fresh drug on day 4. On day 7 of treatment, cells were collected and (A) viewed under × 60 light microscopy for morphologic changes. (B) Dual-color staining of cells for annexin/PI uptake indicative of cell apoptosis and necrosis was also performed.
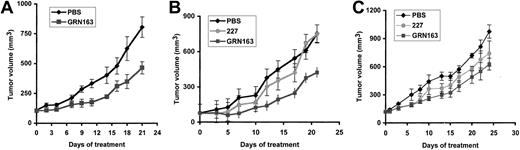
Intratumoral GRN163 treatment reduces xenograft growth in tumor cells with short telomeres by increasing apoptosis. Previous testing of telomerase inhibitors in murine xenograft models with rapidly proliferating or bulky tumors has been limited by the death of the host animal resulting from malignant disease before the onset of growth inhibition.7 To further study the activity of GRN163 on tumor growth in vivo, we established xenografts of MM and NHL cell lines with variable telomere lengths in sublethally irradiated NOD/SCID mice and treated them with PBS, GRN163 (10 nmol), or 227 (10 nmol) injected directly into the flank tumors 5 times a week for 3 to 4 weeks. We found that GRN163 treatment significantly reduced tumor growth in xenografts established from cell lines with short telomeres (CAG myeloma, 2.7 kb; Hs602 lymphoma, 2.7 kb). For example, CAG xenografts treated with PBS for 21 days had a mean tumor volume (TV) of 756 ± 89 mm3 compared with GRN163-treated TV of 466 ± 38 mm3 (P ≤ .007) after 21 days (Figure 6A). Hs602 xenografts treated with GRN163 also demonstrated inhibition of tumor growth compared with controls (day 21, PBS TV, 753 ± 101 mm3; 227 TV, 750 ± 283 mm3; GRN163 TV, 424 ± 126 mm3) (day 21, PBS vs GRN163, P ≤ .034; PBS/227 vs GRN163, P ≤ .031) (Figure 6B). In contrast, GRN163 treatment of HT xenografts (TRF, 11 kb) had equivocal effects (TV, 596 ± 71 mm3) when compared with PBS (mean TV, 973 ± 70 mm3) and 227-treated controls (TV, 738 ± 134 mm3) (Figure 6C). Statistical analysis of tumor volumes on day 22 demonstrated borderline significance for HT xenografts treated with PBS compared with GRN163 (P ≤ .048) but no significance for 227 compared with GRN163 (P ≤ .36) or PBS/227 compared with GRN163 treatment (P ≤ .10). TA in tumors treated with intratumoral GRN163 (TA, 84 ± 3.9) was 50% lower than in PBS-treated tumors (TA, 164 ± 8.5) (data not shown).
Tumor volume growth curves of lymphoma and myeloma xenografts in NOD/SCID mice treated with intratumoral injections of PBS, 10 nmol GRN163, or 10 nmol 227 (missense oligonucleotide). NOD/SCID mice were inoculated with 10 × 106 tumor cells by way of a subcutaneous route. When tumor volumes approached 100 mm3, mice were divided into groups of 5 to 8 animals and were treated with intratumoral PBS (♦), 227 (10 nmol) (•), or GRN163 (10 nmol) (▪) 5 days per week for 3 weeks. Mean tumor volumes ± SE (bars) are shown for the following xenografts: (A) CAG myeloma (TRF, 2.7 kb) (PBS vs GRN163, P ≤ .007 on day 21), (B) Hs602 lymphoma (TRF, 2.7 kb) (PBS vs GRN163, P ≤ .034 on day 21), (C) HT lymphoma (TRF, 11 kb) (PBS vs GRN163, P ≤ .048 on day 22; 227 vs GRN163, P ≤ .36 on day 22). Additional 2-tailed t test analysis on the log (TV) at day 22 in HT xenografts demonstrated PBS versus GRN163 (P ≤ .041) and 227 versus GRN163 (P ≤ .24).
Tumor volume growth curves of lymphoma and myeloma xenografts in NOD/SCID mice treated with intratumoral injections of PBS, 10 nmol GRN163, or 10 nmol 227 (missense oligonucleotide). NOD/SCID mice were inoculated with 10 × 106 tumor cells by way of a subcutaneous route. When tumor volumes approached 100 mm3, mice were divided into groups of 5 to 8 animals and were treated with intratumoral PBS (♦), 227 (10 nmol) (•), or GRN163 (10 nmol) (▪) 5 days per week for 3 weeks. Mean tumor volumes ± SE (bars) are shown for the following xenografts: (A) CAG myeloma (TRF, 2.7 kb) (PBS vs GRN163, P ≤ .007 on day 21), (B) Hs602 lymphoma (TRF, 2.7 kb) (PBS vs GRN163, P ≤ .034 on day 21), (C) HT lymphoma (TRF, 11 kb) (PBS vs GRN163, P ≤ .048 on day 22; 227 vs GRN163, P ≤ .36 on day 22). Additional 2-tailed t test analysis on the log (TV) at day 22 in HT xenografts demonstrated PBS versus GRN163 (P ≤ .041) and 227 versus GRN163 (P ≤ .24).
CAG xenografts treated with GRN163 (10 nmol) demonstrated increased TUNEL staining compared with controls (Figure 7A-C). In contrast, HT xenografts demonstrated no differences in TUNEL staining after PBS, GRN163, or 227 treatment (Figure 7D-F). No differences in proliferation rates were seen using Ki67 staining (data not shown).
Apoptosis in tumor xenografts as measured by TUNEL staining. Tumors harvested from mice after 21 days of treatment were fixed in formaldehyde, deparaffinized, and subjected to immunohistochemical analysis. Shown are TUNEL stains of CAG myeloma xenografts with short telomeres after intratumoral treatment with (A) PBS, (B) 227 (10 nmol), and (C) GRN163 (10 nmol) 5 times a week for 3 weeks. These are compared with TUNEL stains of HT tumor xenografts with long telomeres after intratumoral treatment with (D) PBS, (E) 227, or (F) GRN163 on the same schedule. Apoptotic cells are indicated in brown (arrows). Original magnification, × 40.
Apoptosis in tumor xenografts as measured by TUNEL staining. Tumors harvested from mice after 21 days of treatment were fixed in formaldehyde, deparaffinized, and subjected to immunohistochemical analysis. Shown are TUNEL stains of CAG myeloma xenografts with short telomeres after intratumoral treatment with (A) PBS, (B) 227 (10 nmol), and (C) GRN163 (10 nmol) 5 times a week for 3 weeks. These are compared with TUNEL stains of HT tumor xenografts with long telomeres after intratumoral treatment with (D) PBS, (E) 227, or (F) GRN163 on the same schedule. Apoptotic cells are indicated in brown (arrows). Original magnification, × 40.
Inhibiting xenograft growth with GRN163 was equivalent to doxorubicin chemotherapy. Intratumoral GRN163 treatment (10 nmol 5 times a week) compared favorably with the maximally tolerated dose of intraperitoneal doxorubicin (3 mg/kg 2 times a week for 6 doses) (P < .05) (Table 3).39
Inhibition of xenograft growth by GRN163 telomerase template antagonist is equivalent to doxorubicin chemotherapy
Agent . | Dose . | Average tumor diameter (mm ± SE) . | Average TV (mm3 ± SE) . | P . | No. animals treated . |
|---|---|---|---|---|---|
| PBS | — | 11.4 ± 0.4 | 794 ± 89 | — | 16 |
| GRN163 | 10 nmol, 5 ×/wk | 9.5 ± 0.2 | 461 ± 38 | ≤ .002 | 14 |
| Doxorubicin | 3 mg/kg, 6 doses | 9.7 ± 0.4 | 487 ± 57 | ≤ .008 | 8 |
Agent . | Dose . | Average tumor diameter (mm ± SE) . | Average TV (mm3 ± SE) . | P . | No. animals treated . |
|---|---|---|---|---|---|
| PBS | — | 11.4 ± 0.4 | 794 ± 89 | — | 16 |
| GRN163 | 10 nmol, 5 ×/wk | 9.5 ± 0.2 | 461 ± 38 | ≤ .002 | 14 |
| Doxorubicin | 3 mg/kg, 6 doses | 9.7 ± 0.4 | 487 ± 57 | ≤ .008 | 8 |
NOD/SCID mice were sublethally irradiated and inoculated with 10 × 106 CAG myeloma cells by way of a subcutaneous route. When tumor volumes approached 100 mm3, mice were divided into 3 groups of 4 to 5 mice per group. Animals were treated with PBS or GRN163 (10 nmol) injected locally 5 days per week for 3 weeks or with doxorubicin (3 mg/kg) injected through an intraperitoneal route twice a week for 3 weeks. Tumors were measured by calipers, and tumor volume was calculated using the formula mm3 = 4/3 πr3, where r = (1 + w)/4. Shown are tumor measurements on day 21 of treatment. P values are for TV PBS versus treatment.
—indicates not applicable.
Systemic GRN163 administration decreased tumor telomerase levels and tumor growth with minimal toxicity. Mice bearing subcutaneous CAG myeloma xenografts were treated with daily intraperitoneal injections of PBS or GRN163 (50 mg/kg daily) for 3 weeks. Systemic GRN163 treatment (50 mg/kg) decreased tumor TA by 60% to 90% compared with controls and markedly reduced xenograft growth (Figure 8). For example, on day 23 of treatment, the mean tumor volume of GRN163-treated xenografts was 387 ± 105 mm3 compared with a mean TV for controls of 803 ± 254 mm3 (P ≤ .19). Mice treated with systemic GRN163 exhibited no adverse effects (weight loss, clinical signs, peripheral blood counts, or survival) compared with controls (data not shown).
Systemic GRN163 treatment decreased tumor telomerase levels and inhibited the growth of myeloma xenografts in mouse models. NOD/SCID mice were inoculated with 10 × 106 CAG myeloma cells by way of a subcutaneous route. When tumor volumes approached 100 mm3, mice were divided into 2 groups of 5 to 8 mice per group. (A) Mean tumor volumes ± SE (bars) in animals treated with daily intraperitoneal PBS (♦) or GRN163 (▪) (50 mg/kg per day) for 3 weeks (PBS vs GRN163 on day 23; P ≤ .19) are shown. (B) Representative telomerase activity in tumor xenografts harvested from animals after 14 and 21 treatment days, respectively, is also displayed.
Systemic GRN163 treatment decreased tumor telomerase levels and inhibited the growth of myeloma xenografts in mouse models. NOD/SCID mice were inoculated with 10 × 106 CAG myeloma cells by way of a subcutaneous route. When tumor volumes approached 100 mm3, mice were divided into 2 groups of 5 to 8 mice per group. (A) Mean tumor volumes ± SE (bars) in animals treated with daily intraperitoneal PBS (♦) or GRN163 (▪) (50 mg/kg per day) for 3 weeks (PBS vs GRN163 on day 23; P ≤ .19) are shown. (B) Representative telomerase activity in tumor xenografts harvested from animals after 14 and 21 treatment days, respectively, is also displayed.
Discussion
These results confirm the crucial role of human telomerase in maintaining the replication potential of mature B-cell neoplasia and suggest that telomerase inhibition may represent a novel therapeutic strategy for MM and NHL tumors with short telomeres. GRN163 is a prototype of a new class of 13-mer 2′-deoxy N3′→P5′ thiophosphoramidate oligonucleotides designed to complement the template domain of the RNA component (hTR) in the active site of telomerase.38 The GRN163 compound forms a high-affinity, sequence-specific duplex with the complementary RNA strand that is further stabilized through interactions between the oligonucleotide backbone and hTERT, is resistant to hydrolysis by cellular nucleases, and exhibits high thermodynamic stability.30 The effects of GRN163 were evaluated on the growth of human MM and NHL cell lines, primary MM cells, and tumor xenografts. GRN163 treatment reduced telomerase levels in all cells and induced progressive telomeric shortening in cell lines. Short-term GRN163 treatment limited the growth of lymphoma and myeloma cell lines in vitro with the onset of proliferative arrest correlating with initial telomere length. Tumor cells with short telomeres treated with GRN163 for as few as 7 days underwent growth arrest, morphologic changes, increased apoptosis, and cell death characteristic of crisis. GRN163 treatment of MM and NHL xenografts established from cell lines with short telomeres (2.7 kb) reduced the growth of tumor xenografts by 40% to 50%.
There may be alternative explanations for the apparent relative resistance of the cell lines with long telomeres to the induction of crisis or apoptosis by GRN163 treatment. It has been demonstrated that substantial telomerase inhibition is required to induce growth arrest and apoptosis.40 Using a hammerhead ribozyme that cleaved hTERT mRNA, 50% to 75% inhibition of TA resulted in minimal effects on the growth of transformed cells, whereas more than 75% inhibition resulted in growth arrest and apoptosis.40 The potency of oligonucleotide telomerase inhibitors is at least partially a function of bioavailability (ie, cellular uptake) and may vary significantly in cultured cell lines. For example, the IC50 for telomerase inhibition by GRN163 in prostate cancer cell lines in the absence of cellular uptake enhancers varied more than 5-fold between different cell lines (averaging between 1.4 μM and 7.57 μM). It is possible that the relative resistance to apoptosis in the U266 cell line (TRF, 9.0 kb) compared with the CAG cell line (TRF, 2.7 kb) was caused by insufficient telomerase inhibition resulting from a less than IC50 dose in the U266 line rather than longer telomere length. However, our experiments using fluorescently labeled GRN163 (Figure 1) revealed no significant differences in cellular uptake between HT (TRF, 11 kb) and U266 cells (TRF, 9.0 kb) and CAG cells (TRF, 2.7 kb) to account for the differences in growth responses to GRN163 treatment (Figure 4). In a similar example, GRN163 (3 μM) treatment of the SKO-007 cell line (TRF, 1.7 kb) inhibited TA by only 23% but resulted in higher levels of apoptosis and growth arrest than identically treated U266 and HT cells during the same time period (Figure 4; Table 2; data not shown).
These data support the hypothesis that telomerase functions in mature B-cell neoplasms by preferentially stabilizing short telomeres, thereby preventing the activation of cellular crisis in malignant cells with chromosomal telomere loss.28,29 We have recently shown that elevated TA correlated with shorter telomere lengths, poorer overall survival, and increased cytogenetic abnormalities in tumor samples from 193 MM patients. Our analysis of telomeric DNA distribution in these MM samples showed preferential stabilization of short telomeres with progressive erosion of longer telomeres, particularly when mean TRF decreased to less than 5.5 kb.24 The current study extends and confirms these earlier data by demonstrating that inhibiting telomerase in MM and NHL cells with a mean TRF shorter than 5.5 kb resulted in apoptosis and cell death within 2 to 15 PDs. In contrast, GRN163 treatment of MM and NHL cells with TRF longer than 5.5 kb decreased telomerase levels and shortened telomeres but did not influence growth within 30 PDs. It would be expected that much longer treatment regimens would be required to reduce telomeric DNA to critically short lengths. Other groups have proposed mechanisms for telomerase independent of telomere length in MM and NHL. Increased TA in MM in the presence of IL-6 or IGF has been found to correlate with enhanced survival and drug resistance of MM cells by a phosphatidylinositol 3 kinase (PI3K)/Akt/nuclear factor (NF)–κB-dependent pathway.25 Tumor necrosis factor treatment resulted in the translocation of the hTERT protein from the cytoplasm to the nucleus in an MM cell line and was inhibited in the presence of specific NF-κB inhibitors.26 Although these studies suggest that cytokines may regulate telomerase activation and may promote the malignant phenotype of MM cells, we found that the primary result of telomerase inhibition with GRN163 was rapid induction of cell crisis correlating with reductions in TA and telomere length. We did not observe changes in proliferation rates in cultured cells or xenografts treated with GRN163, nor did we note changes in the underlying TA of CAG myeloma cells incubated in vitro with IL-6 or IGF (data not shown). These results imply that the preeminent role of telomerase in B-cell neoplasia is maintaining and stabilizing critically short telomeric DNA.
To date, the significance of telomerase-independent mechanisms of replication, such as alternative lengthening of telomeres, in MM and NHL is unknown.41-43 We have previously shown that only 7 of 115 MM patient samples had excessively long telomeres, and only 5 of these 7 had low telomerase levels, suggesting that alternative lengthening of telomeres is present in only a fraction of MM patients.24 Similar data in primary NHL patients are inconclusive.15 In this study, we observed no evidence of alternative lengthening of telomeres in vitro. Other groups have not reported any apparent activation of alternative lengthening of telomeres in other cultured cells with elevated TA undergoing treatment with small molecular inhibitors.4,7,11 Although telomerase inhibition with dominant-negative hTERT mutants has resulted in escaping clones, this phenomenon occurred only in cultured cell lines, after prolonged continuous culture (longer than 290 days in one study) and is of uncertain relevance to MM and NHL.5,44
Telomerase has long been considered an unusually challenging target for drug discovery, largely because early studies of other telomerase inhibitors indicated that a long lag period was required before growth arrest in immortalized solid tumor cell lines.4,11 It has long been speculated that, unlike chemotherapy during which immediate antiproliferative effects are apparent after 1 to 2 doses, telomerase inhibitors dependent on mechanisms of telomere attrition would necessitate continuous long-term administration for weeks to months to produce antitumor effects. It may be that the effects of telomerase inhibition on tumor growth differ between different cancers. Our results indicate that NHL and MM cells with short telomeres may be highly dependent on telomerase to prevent cellular crisis caused by critically short and dysfunctional telomeres. The rapid induction of cell death in malignant B-cell lines as a result of telomerase inhibition with GRN163 occurred within 2 to 15 PDs, suggesting that inducing cell crisis in these cells did not require significant additional telomeric erosion to trigger cell death. Similarly, the growth of tumor xenografts with short telomeres treated with GRN163 through intratumoral or systemic routes (Figures 6, 8) started to diverge from controls after less than 1 week of therapy and inhibited tumor growth by 40% to 50% after 3 weeks of continuous treatment. That the onset of responses did not correlate precisely with mean TRF is consistent with the hypothesis that the shortest telomere length, and not the mean telomere length, determines the degree of telomeric dysfunction and dictates the onset of growth arrest induced by telomerase inhibition.45
Telomerase is an ideal target for oligonucleotide therapy because the critical RNA template of the ribonucleoprotein essential for telomeric elongation is exposed and accessible to competitive binding by nucleic acids.12,46 Previous testing of a reverse transcriptase telomerase inhibitor in murine xenograft models with rapidly proliferating or bulky tumors has been limited by the death of the host animal from malignant disease before the onset of growth inhibition. However, in those experiments, the telomerase inhibitor (BIBR1532) failed to induce apoptosis or cell death in vitro even after prolonged treatment.7 In the current study, intratumoral GRN163 treatment of myeloma and lymphoma xenografts with short telomeres reduced tumor volumes by 40% to 50% compared with controls and was as effective as doxorubicin monotherapy in xenograft models. Although a potential limitation of oligonucleotide therapies is bioavailability to tumor tissues,46 our results demonstrated that daily intraperitoneal GRN163 treatment decreased telomerase levels in flank xenografts (by up to 92% of control tumors) and reduced myeloma tumor growth (Figure 8). In addition, sublethally irradiated NOD/SCID mice with tumor xenografts treated with systemic daily intraperitoneal GRN163 (up to 50 mg/kg per day) exhibited no significant adverse effects compared with PBS-treated mice. Specifically, no differences in weight loss, clinical symptoms, or peripheral blood cell counts were observed between GRN163- and control-treated mice (data not shown).
Theoretically, telomerase inhibitors such as GRN163 may damage normal stem cells, a valid consideration for patients with MM and NHL who have undergone prior myeloablative therapies. However, the longer telomere lengths of stem cells relative to tumor cells may offer some measure of protection. We have previously shown that the mean telomere length of MM plasma cells was markedly shorter than that of normal peripheral blood granulocytes and lymphocytes from the same patients.24 Telomere lengths in peripheral blood cells after allogeneic stem cell transplantation are only transiently shortened.47 Additional studies of the direct effects of GRN163 on human hematopoietic stem cell function are under way.
Previous evaluations of NHL and MM patients indicate that significant subsets of patients with these diseases may benefit from antitelomerase strategies. Clinically aggressive NHL tumors express high telomerase levels and have shorter telomere lengths at disease progression or relapse than at initial diagnosis.21,22 The mean telomere length of malignant plasma cells in MM patients is half that of normal plasma cells, and more than 25% of MM tumors are characterized by telomere lengths shorter than 4 kb.24 If additional studies confirm that tumor cells with long telomeres show relative resistance to the induction of apoptosis by telomerase inhibitors, it may be necessary to screen patients for tumors characterized by high telomerase levels and relatively short telomeres to determine which diseases would be most likely to respond to antitelomerase therapies.
Telomerase inhibitors are most likely to be clinically beneficial in combination with conventional cytotoxic chemotherapy or after surgery or high-dose chemotherapy, when the tumor burden is minimal. Telomerase inhibition and telomeric dysfunction have been shown to enhance the chemosensitivity of tumor cells to DNA-damaging agents48-50 and to affect tumor angiogenesis.51 Alternatively, combination regimens of telomerase inhibitors and cytotoxic chemotherapy may overcome the relative resistance to inducing apoptosis in tumor cells with long telomeres.41 Approaches to enhance the efficacy of GRN163 by combining it with other antitumor modalities are being explored in these NHL and MM cell lines and tumor xenograft models. Our current data indicate that disrupting telomeric maintenance in mature B-cell malignancies inhibits tumor growth. The ability to target the replicative potential of lymphoma and myeloma cells with antitelomerase template antagonists may represent a novel therapeutic approach for B-cell neoplasia.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-02-0546.
Supported in part by National Institutes of Health grant CA-09207-24 (E.S.W.); ASCO Young Investigator Award (E.S.W.); Memorial Sloan-Kettering Cancer Center Experimental Therapeutics Program (M.A.S.M., E.S.W.); National Cancer Institute (NCI) grants 5 U19 CA67842 (A.C.C., M.A.S.M.) and PO1CA 55819 (M.A.S.M.); a Leukemia and Lymphoma Society of America Specialized Center of Research grant (S.C.-K., M.A.S.M.); and the Charles H. Revson Foundation.
A.C.C., K.P., and S.G. are employed by Geron Corporation, whose potential product was studied in this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Kang Zhang, Sheik Baksh, Diane Domingo, and the staff at the Molecular Cytology Core Facility (Memorial Sloan-Kettering Cancer Center [MSKCC]) for their excellent technical assistance. Cell lines were provided by A. D. Zelenetz and R. K. Chaganti (MSKCC) and J. Epstein (University of Arkansas). Patient MM cells were provided through ongoing collaboration with Weill Medical College, Cornell University. We thank C. B. Harley, K. Y. Chung, D. B. Karpf, and M. S. Halfon for helpful feedback.


![Figure 3. GRN163 induced progressive telomeric shortening in myeloma and lymphoma cells for 40 days of continuous treatment. Mean telomere lengths were evaluated in 3 cell lines (CAG [panel A], HT [panel B], and U266 [panel C]) with varying initial telomere lengths at intervals of 4 to 7 days during continuous PBS or GRN163 (10 μM) treatment. Results from all 3 cell lines demonstrated that GRN163 treatment (10 μM) consistently resulted in more rapid telomere shortening than PBS-treated controls in all cell lines. Representative TRF blots of CAG MM (initial TRF, 2.7 kb), HT NHL (initial TRF, 11 kb), and U266 MM cells (initial TRF, 9.0 kb) are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-02-0546/6/m_h80145467003.jpeg?Expires=1769085440&Signature=sRCZo4vad8JhhCmE5DBQVyL2oe5nNQv25G~G7HchSwMmbsVUTqoAqocZPkV-LtsjmAFiUxxOlFHxh-R413Seu~xdXl3Se5PRsXZGrl29hPZ2lzjA28Aeyoj-rTYRGNIoj9kONbbiclvn3XWhmzTn9MBItW4vuU51CBPhhSOqoc~d8BGf8L13J~csdntCAAUVS~22Q1FhBxOXBkh3twn1b5iE6sxxEmMMXC6YdLqnDQo3RQW47UtphUC-y1Zv1yXHWwa0xjBgsPFEjDaSvBH0AHeY8JhrMf5IkdlkeqmQDpYBjPqe1~oOWvvfgpvK2v~ebhZZRLMIKvmToOSoxf~HYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Growth arrest induced in NHL and MM cell lines with short telomeres. Multiple myeloma (A,B,F) and non-Hodgkin lymphoma (C,D,E,G) cell lines with different mean telomere lengths were plated in 6-well plates in the presence of PBS, GRN163 (1-10 μM), or a mismatch oligonucleotide control 227 (10 μM) for up to 40 days. Cells were harvested, counted, and replated in the presence of fresh drug or PBS every 3 to 5 days. Viable cells were enumerated 3 times by trypan blue dye exclusion using a hematocytometer and a phase-contrast microscope. Average cell numbers were obtained, and PDs were calculated using the formula Pn = Pn–1 + [([ln (total number of cells]) – (ln [number of cells plated])/ln2]. Data are shown as calculated PDs over days of treatment. Percentages of apoptotic or dead cells at the end of treatment were measured as the percentage of annexin/PI dual-staining cells by flow cytometry.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-02-0546/6/m_h80145467004.jpeg?Expires=1769085440&Signature=parQ--rRa4OLZ~zrHr5~8sRFQM-k0idRh~xjwpwXdjw63Kqb0dHobLDUJ6wQRhCu29QW3bdWhfQKt2v-tmldCvQvEHK5hQbm-VrCYe1Vq1gxs4RXDvnkkHimbufuu373I5-cWaAs5Ulwx0RJRmFe0RWnlAtdgZm0WbLnYSEvxHebm7AgfO5DzYcx9fJd1jg7qxnQWfNPViuI8ofKbrrC5HP9Du1ZVsI3Tj-zv923-1ChiHm-wLOjp9rSBQbrALdWe0OHzs2F-5ce1J9O3ED634~ACuk7oIgJvTtb0aPGsj0ExuKMU46y7CHOuukv5dIfQBGxbGBAW2AjrG5PeYgwWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal