Abstract
Interactions between the protein kinase C (PKC) and Chk1 inhibitor UCN-01 and the heat shock protein 90 (Hsp90) antagonist 17-AAG have been examined in human leukemia cells in relation to effects on signal transduction pathways and apoptosis. Simultaneous exposure (30 hours) of U937 monocytic leukemia cells to minimally toxic concentrations of 17-AAG (eg, 400 nM) and UCN-01 (eg, 75 nM) triggered a pronounced increase in mitochondrial injury (ie, loss of mitochondrial membrane potential [Δψm]; cytosolic release of cytochrome c), caspase activation, and apoptosis. Synergistic induction of apoptosis was also observed in other human leukemia cell types (eg, Jurkat, NB4). Coexposure of human leukemia cells to 17-AAG and the PKC inhibitor bisindolylmaleimide (GFX) did not result in enhanced lethality, arguing against the possibility that the PKC inhibitory actions of UCN-01 are responsible for synergistic interactions. The enhanced cytotoxicity of this combination was associated with diminished Akt activation and marked down-regulation of Raf-1, MEK1/2, and mitogen-activated protein kinase (MAPK). Coadministration of 17-AAG and UCN-01 did not modify expression of Hsp90, Hsp27, phospho-JNK, or phospho-p38 MAPK, but was associated with further p34cdc2 dephosphorylation and diminished expression of Bcl-2, Mcl-1, and XIAP. In addition, inducible expression of both a constitutively active MEK1/2 or myristolated Akt construct, which overcame inhibition of ERK and Akt activation, respectively, significantly attenuated 17-AAG/UCN-01–mediated lethality. Together, these findings indicate that the Hsp90 antagonist 17-AAG potentiates UCN-01 cytotoxicity in a variety of human leukemia cell types and suggest that interference with both the Akt and Raf-1/MEK/MAP kinase cytoprotective signaling pathways contribute to this phenomenon.
Introduction
17-Allyl-aminogeldanamycin (17-AAG) is a derivative of the ansamycin geldanamycin that is currently undergoing clinical development in humans.1 17-AAG interferes with the function of heat shock proteins (Hsp's), particularly Hsp90.2 Heat shock proteins such as Hsp90 serve as chaperones and are involved in regulation of the intracellular disposition of diverse cellular proteins.3 Inhibition of Hsp90 disrupts the folding of these proteins, thereby increasing their susceptibility to ubiquitinization and proteasomal degradation.4 Exposure of cells to 17-AAG or geldanamycin has been shown to down-regulate expression of diverse signal transduction and cell cycle regulatory proteins, including Raf-1, cyclin D, ErbB, and Akt, among others.5 Of these, considerable attention has recently focused on Akt, a serine threonine kinase that lies downstream of phosphatidylinositol 3-kinase (PI3K) and that is frequently dysregulated in human cancers.6,7 Akt has a large number of downstream targets implicated in survival and cell cycle regulation, including Bad,8 caspase-9,9 m-TOR,10 GSK,11 forkhead transcription factors,12 among others.13 Although the mechanism by which heat shock protein antagonists such as 17-AAG kills cells is uncertain, and may vary with the cell type, recent evidence suggests that down-regulation of Akt may play an important role in this process.14
UCN-01 (7-hydroxystaurosporine) is a staurosporine derivative that was developed as a specific inhibitor of protein kinase C15 (PKC) and that is currently undergoing phase 1 and 2 evaluation in humans.16 UCN-01 is also a cyclin-dependent kinase (CDK) inhibitor that arrests cells in G1.17 Recently, however, UCN-01 has been identified as an inhibitor of Chk1, which regulates the phosphorylation and proteasomal degradation of the Cdc25C phosphatase.18 By interfering with Cdc25C degradation, UCN-01 opposes inhibitory phosphorylation of p34cdc2 on Thr14 and Tyr15 sites, and in so doing, abrogates checkpoint control in cells exposed to various DNA-damaging agents.19-21 UCN-01 also triggers activation/phosphorylation of the mitogen-activated protein kinase (MAPK; ERK, extracellular-regulated kinase) in several malignant hematopoietic and nonhematopoietic cell types; moreover, interference with this process, that is, by pharmacologic inhibitors of MEK1/2 (MAPK kinase 1/2) results in a pronounced increase in mitochondrial damage and apoptosis.22-24 More recently, UCN-01 has been found to interfere with Akt activation in in vitro systems.25 In view of the ability of agents that interfere with the PI3K/Akt signal transduction pathway (eg, LY294002) to potentiate the lethal effects of cytotoxic drugs,26,27 it is conceivable that the latter action might contribute to synergistic interactions involving UCN-01.
The role of the PI3K/Akt pathway in promoting tumor cell survival is well documented.28 Because down-regulation of Akt has been associated with administration of heat shock protein antagonists,29 and in light of recent evidence suggesting that UCN-01 may disrupt this signaling cascade,25 the possibility arose that 17-AAG and UCN-01 might cooperate to antagonize tumor cell survival by inhibiting this pathway. To test this possibility, we have examined interactions between 17-AAG and UCN-01 in human leukemia cells, emphasizing effects on signaling pathways and induction of apoptosis. Here we report that these agents interact in a highly synergistic manner to induce mitochondrial damage, caspase activation, and apoptosis in several human leukemia cell types. Moreover, evidence is presented suggesting that in addition to disrupting Akt signaling, interference with the Raf-1/MEK/MAPK pathway plays a significant functional role in synergistic interactions between UCN-01 and 17-AAG in these cells.
Materials and methods
Cells
U937 and HL60 human leukemia cells were purchased from American Type Culture Collection (Rockville, MD). Jurkat and NB4 cells were purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). All were cultured in RPMI 1640 supplemented with sodium pyruvate, minimal essential medium (MEM) essential vitamins, l-glutamate, penicillin, streptomycin, and 10% heat-inactivated fetal calf serum (FCS; Hyclone, Logan, UT). They were maintained in a 37°C, 5% CO2, fully humidified incubator, passed twice weekly, and prepared for experimental procedures when in log-phase growth (cell density 4 × 105 cells/mL).
Reagents
17-Allyl-aminogeldanamycin (17-AAG) and 7-hydroxystaurosporine (UCN-01) were kindly provided by the Developmental Therapeutics Program, National Cancer Institute (Bethesda, MD), formulated in dimethyl sulfoxide (DMSO; Sigma Chemical, St Louis, MO) as 10–2 M stock solutions, and stored at –20°C. DiOC6, N-tosyl-l-phenylalanine chloromethyl ketone (TPCK) and cycloheximide (CHX) were purchased from Sigma Chemical, dissolved in DMSO, and stored at –20°C. The PKC inhibitor bisindoylmaleimide I (GF109203X) and the proteasome inhibitor MG 132 (Calbiochem, La Jolla, CA) were formulated in DMSO, added 0.5 hour before the addition of 17-AAG. The pan-caspase inhibitor BOC-d-fmk was purchased from Enzyme System Products (Livermore, CA) and dissolved in DMSO.
Experimental format
Logarithmically growing cells were placed in sterile plastic T flasks (Corning Inc, Corning, NY) to which were added the designated drugs and the flasks replaced in the incubator for intervals. At the end of the incubation period, cells were transferred to sterile centrifuge tubes, pelleted by centrifugation at 400g at room temperature (RT) for 10 minutes, and prepared for analysis.
Assessment of apoptosis
After drug exposures, cytocentrifuge preparations were stained with Wright-Giemsa and viewed by light microscopy to evaluate the extent of apoptosis (ie, cell shrinkage, nuclear condensation, formation of apoptotic bodies, etc) as described previously.30 For these studies, the percentage of apoptotic cells was determined by evaluating 500 cells/condition in triplicate. To confirm the results of morphologic analysis, annexin V/phosphatidylinositol (PI; BD PharMingen, San Diego, CA) staining was used as per the manufacturer's instructions. For these experiments 2 × 105 cells/condition were harvested, and analysis carried out using a Becton Dickinson FACScan cytofluorometer (Mansfield, MA).
Determination of MMP (Δψm)
Mitochondrial membrane potential (MMP) (Δψm) was monitored using DiOC6.30 For each condition, 4 × 105 cells were incubated in 1 mL 40 nM DiOC6 at 37°C in for 15 minutes and subsequently analyzed using a Becton Dickinson FACScan cytofluorometer with excitation and emission settings of 488 and 525 nm, respectively. Control experiments documenting the loss of MMP (Δψm) were performed by exposing cells to 5 μM carbamoyl cyanide m-chlorophenylhydrazone (Sigma Chemical; 15 minutes, 37°C), an uncoupling agent that abolishes the MMP.
Preparation of S-100 fractions and assessment of cytochrome c release
U937 cells were harvested after drug treatment as described previously29 by centrifugation at 600g for 10 minutes at 4°C and washed in phosphate-buffered saline (PBS). Cells (4 × 106) were lysed by incubating for 3 minutes in 100 μL lysis buffer (75 mM NaCl, 8 mM Na2HPO4, 1 mM NaH2PO4, 1 mM EDTA [ethylenediaminetetraacetic acid], and 350 μg/mL digitonin). The lysates were centrifuged at 12 000g for 5 minutes, and the supernatant was collected and added to an equal volume of 2 × Laemmli buffer. The protein samples were quantified and separated by 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
Immunoblot analysis
Immunoblotting was performed as described previously.30 In brief, drug-treated cells were pelleted by centrifugation and lysed immediately in Laemmli buffer (1× = 30 mM Tris [tris(hydroxymethyl)aminomethane] base, pH 6.8, 2% SDS, 2.88 mM β-mercaptoethanol, and 10% glycerol), and briefly sonicated. Homogenates were quantified using Coomassie protein assay reagent (Pierce, Rockford, IL). Equal amounts of protein were boiled for 10 minutes, separated by SDS-PAGE, and transferred to nitrocellulose membrane. After blocking in triethanolamine-buffered saline–Tween (TBS-T; 0.05%) and 5% milk at RT for 1 hour, the blots were incubated in fresh blocking solution with an appropriate dilution of primary antibody at 4°C overnight. The sources of antibodies were as follows: Bcl-2, mouse monoclonal, Dako (Carpinteria, CA); Bcl-xL, Bid, Bak, and Bax, rabbit polyclonal, Santa Cruz Biotechnology (Santa Cruz, CA); XIAP, rabbit polyclonal, Cell Signaling Technology (Beverly, MA); Mcl-1, mouse monoclonal, PharMingen (San Diego, CA); Raf-1, mouse monoclonal, Santa Cruz Biotechnology; MEK and phospho-MEK, Cell Signaling Technology; ERK1/2 and phospho-ERK1/2 (Thr202/Tyr204), rabbit polyclonal, Cell Signaling Technology; phospho-JNK, mouse monoclonal, Santa Cruz Biotechnology; phospho-p38 MAPK, rabbit polyclonal, Cell Signaling Technology; cdc2, and phospho-cdc2, rabbit polyclonal, Cell Signaling Technology; Akt, and phospho-Akt, rabbit polyclonal, Cell Signaling Technology; phospho-GSK-3α/β, Cell Signaling Technology; cytochrome c, mouse monoclonal, PharMingen; Smac/DIABLO, Upstate Biotechnology (Lake Placid, NY); AIF, mouse monoclonal, Santa Cruz Biotechnology; Hsp90, rabbit polyclonal, Santa Cruz Biotechnology; Hsp70, mouse monoclonal, Santa Cruz Biotechnology; Hsp27, mouse monoclonal, Cell Signaling Technology; poly(ADP-ribose)polymerase (PARP), mouse monoclonal, Calbiochem; caspase-3, mouse monoclonal, BD Transduction Laboratories, (Lexington, KY); caspase-8 and caspase-9, rabbit polyclonal, PharMingen; α-tubulin, mouse monoclonal, Calbiochem; and actin, rabbit polyclonal, Sigma Chemical. Membranes were washed 3 × 15 minutes in TBS-T and then incubated with a 1:2000 dilution of horseradish peroxidase–conjugated secondary antibody (Bio-Rad Laboratories, Hercules, CA) at RT for 1 hour. Blots were subsequently washed 3 × 15 minutes in TBS-T and then developed by enhanced chemiluminescence (Pierce).
Generation of stably transfected cell lines
A caspase-8 dominant-negative (C8-DN) construct subcloned into pcDNA vector 3.1 (Invitrogen, Carsbad, CA) was transfected in U937 leukemia cells by electroporation as described previously.31 Transfectant U937 cells stably overexpressing Bcl-2 or Bcl-xL were obtained as reported previously.32 These cells, designated as U937/Bcl-2 or Bcl-xL cells, were generated along with their empty vector counterparts (ie, U937/pCEP4 or U937/pcDNA 3.1; Invitrogen). U937 transfectant cell lines were maintained as described in the presence of hygromycin B (200 μg/mL) or geneticin (400 μg/mL). Transfectant cell lines were transferred to selection-free media 24 hours before experimentation. All experiments were performed on cells in logarithmic phase.
“Tet-on” inducible Jurkat cell lines
Stable Jurkat clones inducibly expressing myristoylated Akt (myr-Akt) and MEK (Upstate Biotechnology), which are constitutively active, were generated as follows. Myc-tagged myr-Akt and HA-tagged-MEK (HA-MEK) were separately subcloned into the pTRE2-hyg expression vectors (Clontech Laboratories, Palo Alto, CA) by standard techniques. Jurkat “Tet-on” cells that stably express reverse tetracycline transactivator (rtTA) regulator protein (Clontech) were transfected with myr-Akt-pTRE2-hyg and HA-MEK-pTRE2-hyg by electroporation (600 V, 60 ms) using 0.4-μM cuvettes. Stable clones derived from a single cell were selected in RPMI 1640 media supplemented with 10% of Tet-System-approved FBS (Clontech) in the presence of 400 μg/mL hygromycin. To test for the induced expression of the myr-Akt and HA-MEK, stable clones were left untreated or treated for 24 hours with 2 μg/mL doxycycline (Sigma Chemical), harvested, and analyzed for expression of the appropriate protein by Western blot as described.
Statistical analysis
The significance of differences between experimental conditions was determined using the 2-tailed Student t test. To characterize synergistic or antagonistic interactions between agents, median dose effect analysis33 was used with a commercially available software program (Calcusyn; Biosoft, Ferguson, MO).
Results
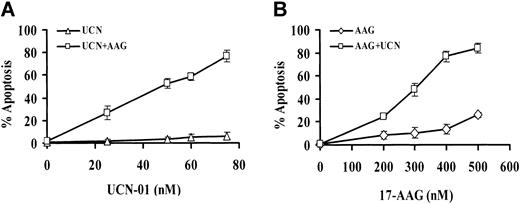
To assess the interaction between UCN-01 and 17-AAG, U937 cells were exposed to each of these agents for 30 hours, alone and in combination, after which the percentage of cells displaying the morphologic features of apoptosis was determined. As shown in Figure 1A, UCN-01 concentrations of 75 nM or less were essentially nontoxic, as was 400 nM 17-AAG administered alone. However, in cells exposed to both agents, a sharp increase in apoptosis was noted at UCN-01 concentrations of 50 nM or greater, which approached 80% of cells at the 75-nM UCN-01 level. Comparable results were obtained when other measures of cell death (eg, flow cytometric analysis of annexin V/PI-stained cells) were used (data not shown). Similarly, 17-AAG concentrations of 400 nM or less were minimally toxic to U937 cells, and a 500-nM concentration only slightly more so (Figure 1B). However, when 17-AAG concentrations of 200 nM or greater were combined with a nontoxic concentration of UCN-01 (ie, 75 nM), apoptosis increased markedly, particularly at higher 17-AAG levels.
Exposure of U937 cells to 17-AAG and UCN-01. (A) U937 cells were exposed for 30 hours to 400 nM 17-AAG (AAG) in the presence or absence of the designated concentration of UCN-01 (UCN). At the end of the incubation interval, the percentage of apoptotic cells was determined by examining Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” For each determination, at least 10 randomly selected fields containing more than 500 cells were evaluated. (B) U937 cells were exposed to 75 nM UCN-01 for 30 hours in the presence or absence of the designated concentration of 17-AAG, after which the percentage of apoptotic cells was determined as described. For both panels, values represent the means ± SD for 3 separate experiments performed in triplicate.
Exposure of U937 cells to 17-AAG and UCN-01. (A) U937 cells were exposed for 30 hours to 400 nM 17-AAG (AAG) in the presence or absence of the designated concentration of UCN-01 (UCN). At the end of the incubation interval, the percentage of apoptotic cells was determined by examining Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” For each determination, at least 10 randomly selected fields containing more than 500 cells were evaluated. (B) U937 cells were exposed to 75 nM UCN-01 for 30 hours in the presence or absence of the designated concentration of 17-AAG, after which the percentage of apoptotic cells was determined as described. For both panels, values represent the means ± SD for 3 separate experiments performed in triplicate.
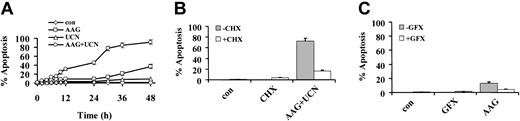
A time-course study of U937 cells exposed to 400 nM 17-AAG with or without 75 nM UCN-01 revealed that combined treatment resulted in a clear increase in cell death by 12 to 24 hours, which become more pronounced at exposure intervals of 30 hours or more (Figure 2A). As noted previously, 75 nM UCN-01 and 17-AAG were minimally toxic over the initial 30 hours, although a modest increase in cell death was noted in cells exposed to 400 nM 17-AAG alone for 48 hours.
Time-course study of U937 cells exposed to 17-AAG and UCN-01. (A) U937 cells were exposed to 400 nM 17-AAG with or without 75 nM UCN-01 for the designated interval, after which the percentage of apoptotic cells was determined as described in “Materials and methods.” (B) U937 cells were exposed to 75 nM UCN-01 plus 400 nM 17-AAG for 30 hours in the presence or absence of 1 μM cycloheximide (CHX), after which the extent of apoptosis was evaluated as described. (C) U937 cells were exposed for 30 hours to 400 nM 17-AAG in the presence or absence of 1 μM bisindolylmaleimide (GFX), after which apoptosis was evaluated as above. In each case, values represent the means ± SD for 3 separate experiments performed in triplicate.
Time-course study of U937 cells exposed to 17-AAG and UCN-01. (A) U937 cells were exposed to 400 nM 17-AAG with or without 75 nM UCN-01 for the designated interval, after which the percentage of apoptotic cells was determined as described in “Materials and methods.” (B) U937 cells were exposed to 75 nM UCN-01 plus 400 nM 17-AAG for 30 hours in the presence or absence of 1 μM cycloheximide (CHX), after which the extent of apoptosis was evaluated as described. (C) U937 cells were exposed for 30 hours to 400 nM 17-AAG in the presence or absence of 1 μM bisindolylmaleimide (GFX), after which apoptosis was evaluated as above. In each case, values represent the means ± SD for 3 separate experiments performed in triplicate.
When U937 cells were exposed to 400 nM 17-AAG plus 75 nM UCN-01 for 30 hours in the presence of CHX (1 μM), the increase in apoptosis was essentially abrogated (Figure 2B), suggesting that an increase in protein synthesis is required for potentiation of lethality. Because UCN-01 is a potent inhibitor of PKC,15 parallel studies were performed using bisindolylmaleimide (GFX; 1 μM), a specific PKC inhibitor. In contrast to results obtained with UCN-01, combined exposure to 17-AAG plus GFX was associated with only a minimal increase in cell death, arguing against the possibility that the PKC inhibitory actions of UCN-01 were primarily responsible for synergistic interactions with 17-AAG (Figure 2C).
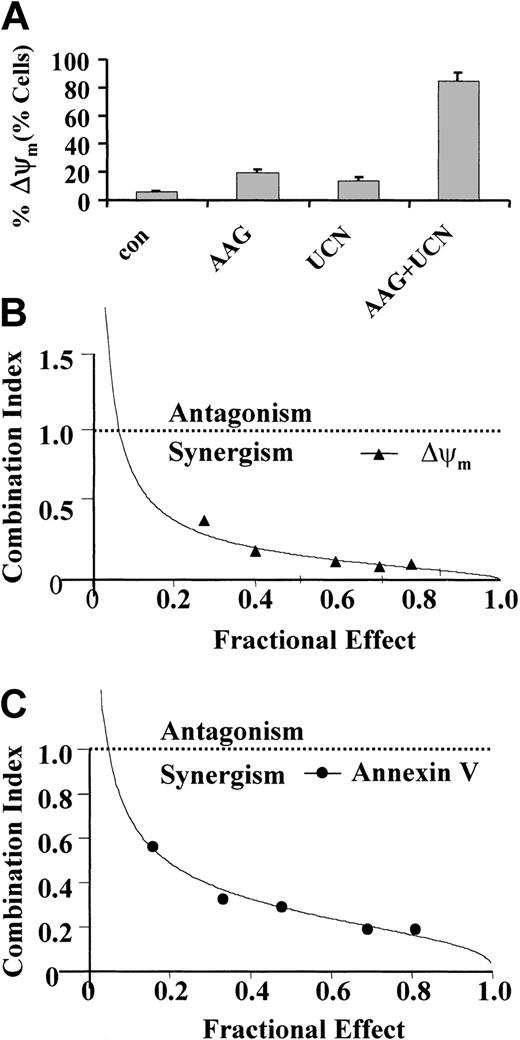
To assess the effects of combined drug treatment on leukemic cell mitochondrial function, loss of MMP (Δψm) was assessed by monitoring uptake of DiOC6 by flow cytometry (Figure 3A). Whereas 400 nM 17-AAG or 75 nM UCN-01 (30 hours) individually had only a modest effect on loss of Δψm, combined treatment resulted in a substantial increase in mitochondrial injury. To characterize interactions between UCN-01 and 17-AAG more rigorously and over a range of drug concentrations, median dose effect analysis was used. When apoptosis by loss of Δψm by DiOC6 uptake (Figure 3B) or annexin V/PI staining (Figure 3C) was monitored, combination index (CI) values considerably less than 1.0 were obtained, corresponding to highly synergistic interactions.
Loss of MMP. (A) U937 cells were exposed to 400 nM 17-AAG with or without 75 nM UCN-01 for 30 hours, after which the loss of MMP (Δψm) was determined by monitoring uptake of DiOC6 by flow cytometry as described in “Materials and methods.” Values represent the means ± SD for 3 separate experiments performed in triplicate. (B-C) U937 cells were exposed to varying concentration of UCN-01 and 17-AAG at a fixed ratio (1:5) for 30 hours, after which loss of MMP (Δψm) and the extent of apoptosis determined by monitoring DiOC6 uptake and annexin V/PI staining, respectively. Combination index values for each fraction affected were determined using commercially available software (Calcusyn; Biosoft). Combination index values less than 1.0 correspond to synergistic interactions.
Loss of MMP. (A) U937 cells were exposed to 400 nM 17-AAG with or without 75 nM UCN-01 for 30 hours, after which the loss of MMP (Δψm) was determined by monitoring uptake of DiOC6 by flow cytometry as described in “Materials and methods.” Values represent the means ± SD for 3 separate experiments performed in triplicate. (B-C) U937 cells were exposed to varying concentration of UCN-01 and 17-AAG at a fixed ratio (1:5) for 30 hours, after which loss of MMP (Δψm) and the extent of apoptosis determined by monitoring DiOC6 uptake and annexin V/PI staining, respectively. Combination index values for each fraction affected were determined using commercially available software (Calcusyn; Biosoft). Combination index values less than 1.0 correspond to synergistic interactions.
Attempts were then made to extend these findings to other human leukemia cell types. As shown in Figure 4A, treatment of NB4 promyelocytic leukemia cells with 300 nM 17-AAG or 50 nM UCN-01 alone was essentially nontoxic, whereas combined treatment (24 hours) resulted in a very substantial increase in cell death. Interestingly, Jurkat lymphoblastic leukemia cells were quite resistant to 17-AAG, experiencing virtually no lethality following a 48-hour exposure to a drug concentration of 1.5 μM. However, when 17-AAG was combined with a minimally toxic concentration of UCN-01 (eg, 75 nM), the extent of apoptosis increased dramatically (Figure 4B). Parallel findings were obtained in the case of HL-60 promyelocytic leukemia cells (data not shown) Thus, combined treatment with UCN-01 and 17-AAG interacted synergistically to promote cell death in several human leukemia cell types.
Exposure of human leukemia cell lines to 17-AAG and UCN-01. Human leukemia cell lines were exposed to 17-AAG with or without UCN-01 for 24 or 48 hours, after which the extent of apoptosis was determined by morphologic analysis of Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” (A) NB4 promyelocytic leukemia cells were exposed to 50 nM UCN-01 with or without 300 nM 17-AAG for 24 hours; (B) Jurkat lymphoblastic leukemia cells were exposed to 75 nM UCN-01 with or without 1.5 μM 17-AAG for 48 hours. In each case, values represent the means ± SD for 3 separate experiments performed in triplicate.
Exposure of human leukemia cell lines to 17-AAG and UCN-01. Human leukemia cell lines were exposed to 17-AAG with or without UCN-01 for 24 or 48 hours, after which the extent of apoptosis was determined by morphologic analysis of Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” (A) NB4 promyelocytic leukemia cells were exposed to 50 nM UCN-01 with or without 300 nM 17-AAG for 24 hours; (B) Jurkat lymphoblastic leukemia cells were exposed to 75 nM UCN-01 with or without 1.5 μM 17-AAG for 48 hours. In each case, values represent the means ± SD for 3 separate experiments performed in triplicate.
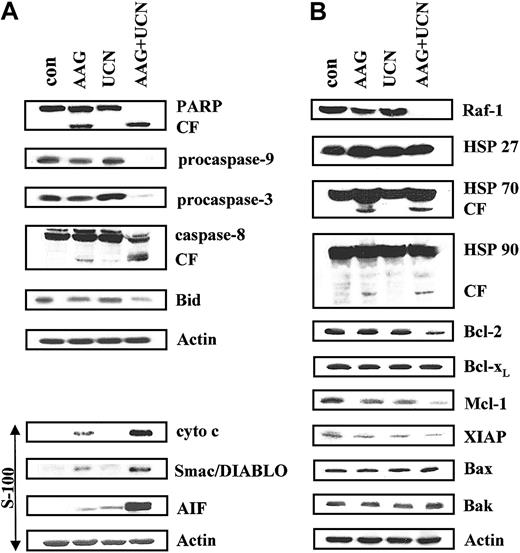
The effects of combined exposure of U937 cells to UCN-01 and 17-AAG were then examined in relation to expression and intracellular disposition of various apoptotic regulatory proteins (Figure 5A). Whereas 75 nM UCN-01 had essentially no effect on PARP after 24 hours, 400 nM 17-AAG resulted in some degradation, consistent with the modest degree of apoptosis observed previously (Figure 1B). However, combined exposure to both agents resulted in virtually complete degradation of full-length (115 kDa) PARP. Coexposure of cells to UCN-01 or 17-AAG individually resulted in minimal cleavage (activation) of procaspase-3 and -9, and little evidence of cleavage of caspase-8 or Bid, events associated with activation of the extrinsic, receptor-related apoptotic pathway.34 In contrast, combined treatment with UCN-01 plus 17-AAG resulted in marked degradation/activation of each of these proteins. In addition, UCN-01 and 17-AAG, administered alone, had little or only modest effects on release of the proapoptotic mitochondrial proteins cytochrome c, Smac/DIABLO, or AIF into the cytosolic S-100 cell fraction. Consistent with effects on apoptosis, combined treatment with UCN-01 plus 17-AAG resulted in a substantial increase in cytosolic release of these proteins, most notably in the case of apoptosis-inducing factor (AIF). Thus, combined treatment with UCN-01 and 17-AAG was associated with a marked increase in release of mitochondrial proapoptotic proteins and activation of both the intrinsic and extrinsic caspase cascades.
Effects on various apoptotic regulatory proteins. (A) U937 cells were exposed to 75 nM UCN-01 with or without 400 nM 17-AAG for 30 hours, after which cells were lysed, proteins separated by SDS-PAGE, and Western analysis performed to monitor expression of various proteins. Whole cell lysates were probed for antibodies to PARP, full-length procaspase-9, procaspase-8, and Bid. In addition, cytosolic S-100 fractions were obtained as described in “Materials and methods,” and Western analysis used to monitor cytosolic release of cytochrome c, Smac/DIABLO, and AIF. (B) Cells were treated as described, after which Western analysis was performed to monitor expression of Hsp27, Hsp70, Hsp90, as well as the apoptotic regulatory proteins Bcl-2, Bcl-xL, Mcl-1, XIAP, Bax, and Bak. For each condition, lanes were loaded with 25 μg protein; blots were subsequently stripped and reprobed with antibodies to actin to ensure equivalent loading and transfer. The results of a representative experiment are shown; an additional study yielded equivalent results. CF indicates cleavage fragment.
Effects on various apoptotic regulatory proteins. (A) U937 cells were exposed to 75 nM UCN-01 with or without 400 nM 17-AAG for 30 hours, after which cells were lysed, proteins separated by SDS-PAGE, and Western analysis performed to monitor expression of various proteins. Whole cell lysates were probed for antibodies to PARP, full-length procaspase-9, procaspase-8, and Bid. In addition, cytosolic S-100 fractions were obtained as described in “Materials and methods,” and Western analysis used to monitor cytosolic release of cytochrome c, Smac/DIABLO, and AIF. (B) Cells were treated as described, after which Western analysis was performed to monitor expression of Hsp27, Hsp70, Hsp90, as well as the apoptotic regulatory proteins Bcl-2, Bcl-xL, Mcl-1, XIAP, Bax, and Bak. For each condition, lanes were loaded with 25 μg protein; blots were subsequently stripped and reprobed with antibodies to actin to ensure equivalent loading and transfer. The results of a representative experiment are shown; an additional study yielded equivalent results. CF indicates cleavage fragment.
Exposure of U937 cells to UCN-01 and 17-AAG for 30 hours, alone or in combination, exerted little effect on Hsp27 and Hsp90 protein expression (Figure 5B). On the other hand, a slight increase in Hsp70 protein levels was noted in cells treated with 17-AAG, consistent with the results of earlier reports.35 However, no further change in Hsp70 expression occurred when 17-AAG was combined with UCN-01, although the presence of a cleavage fragment could be discerned. Treatment of cells with 400 nM 17-AAG alone for 30 hours resulted in a very modest decline in levels of Raf-1, a protein whose expression is regulated by Hsp90.36 Unexpectedly, combined treatment of cells with 17-AAG and UCN-01 resulted in the virtual disappearance of Raf-1 protein. These findings raise the possibility that UCN-01 may enhance the ability of 17-AAG to down-regulate Raf-1 expression.
Effects of these agents, alone and in combination, were then examined in relation to expression of various proapoptotic and antiapoptotic proteins (Figure 5B). A 30-hour exposure to 17-AAG or UCN-01 alone exerted little effect on Bcl-2 expression, whereas combined treatment resulted in a modest reduction in protein expression. Bcl-xL levels remained unchanged with any treatment. Individual exposure to 17-AAG or UCN-01 resulted in small declines in expression of Mcl-1 and XIAP, whereas down-regulation of these proteins was pronounced following combined drug exposure. Lastly, little change in expression of Bax or Bak was noted for any condition. Thus, combined exposure to UCN-01 plus 17-AAG resulted in enhanced down-regulation of the antiapoptotic proteins Bcl-2, Mcl-1, and XIAP.
Consistent with evidence linking 17-AAG/UCN-01–mediated lethality to mitochondrial injury (Figures 3 and 5A), U937 cells ectopically expressing Bcl-2 or Bcl-xL exhibited significant although partial protection from the lethal effects of this drug combination (Figure 6). Although protection was somewhat less pronounced in cells ectopically expressing dominant-negative caspase-8, it was nevertheless significant (Figure 6). The latter findings are consistent with the observation that the UCN-01/17-AAG regimen induced caspase-8 and Bid cleavage (Figure 5A), as well as reports that activation of the extrinsic apoptotic pathway can amplify drug-induced activation of the intrinsic, mitochondrial cascade.37
Percentages of apoptotic cells. U937 cells ectopically expressing Bcl-2, Bcl-xL, or dominant-negative caspase-8 (C8-DN), as well as their respective empty vector controls (pCEP4 for Bcl-2; pcDNA 3.1 for Bcl-xL, C8-DN) were exposed to 400 nM 17-AAG and 75 nM UCN-01 for 30 hours, after which the percentage of apoptotic cells was determined as described in “Materials and methods.” *Significantly less than values for empty vector controls; P > .05; **P < .005. Values represent the means ± SDs for 3 separate experiments performed in triplicate.
Percentages of apoptotic cells. U937 cells ectopically expressing Bcl-2, Bcl-xL, or dominant-negative caspase-8 (C8-DN), as well as their respective empty vector controls (pCEP4 for Bcl-2; pcDNA 3.1 for Bcl-xL, C8-DN) were exposed to 400 nM 17-AAG and 75 nM UCN-01 for 30 hours, after which the percentage of apoptotic cells was determined as described in “Materials and methods.” *Significantly less than values for empty vector controls; P > .05; **P < .005. Values represent the means ± SDs for 3 separate experiments performed in triplicate.
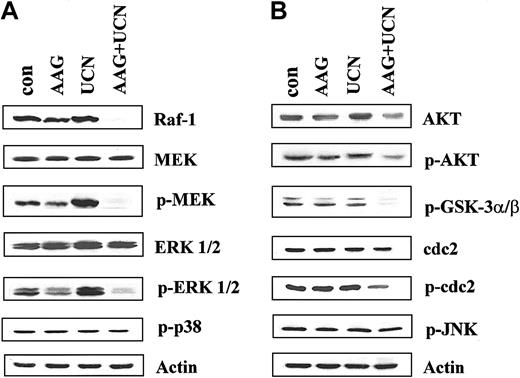
To gain further insights into perturbations in signaling pathways that might contribute to enhanced apoptosis in cells treated with 17-AAG/UCN-01, studies were performed on cells exposed to these agents for 10 hours, that is, at a time point immediately prior to the initial increase in cell death (Figure 2). Interestingly, whereas the agents administered individually had little effect on Raf-1 expression, combined treatment resulted in essentially the complete disappearance of this protein, even at this early exposure interval (Figure 7A). Consistent with these findings, combined treatment essentially abrogated phosphorylation (activation) of MEK, a major Raf-1 substrate.38 Total MEK expression remained unchanged with any treatment. Similarly, phosphorylation of ERK1/2, the primary MEK substrate,39 was largely blocked by UCN-01 plus 17-AAG, but not by either agent administered individually (Figure 7A). In fact, exposure of cells to UCN-01 alone increased ERK1/2 activation, consistent with our earlier findings.21 Total ERK1/2 expression, like that of MEK, remained unperturbed under all conditions.
Apoptosis after exposure to 17-AAG and UCN-01 for 10 hours. U937 cells were exposed to 400 nM 17-AAG with or without 75 nM UCN-01 for 10 hours, after which cells were lysed, proteins separated by SDS-PAGE, and Western analysis used to monitor expression of (A) Raf-1, total MEK, phospho-MEK, total ERK, phospho-ERK, and phospho-p38 MAPK, or (B) total Akt, phospho-Akt, phospho-GSK3α/β, p34cdc2, phospho-p34cdc2, and phospho-JNK. For each condition, lanes were loaded with 25 μg protein; blots were subsequently stripped and reprobed with antibodies to actin to ensure equivalent loading and transfer. The results of a representative experiment are shown; an additional study yielded equivalent results.
Apoptosis after exposure to 17-AAG and UCN-01 for 10 hours. U937 cells were exposed to 400 nM 17-AAG with or without 75 nM UCN-01 for 10 hours, after which cells were lysed, proteins separated by SDS-PAGE, and Western analysis used to monitor expression of (A) Raf-1, total MEK, phospho-MEK, total ERK, phospho-ERK, and phospho-p38 MAPK, or (B) total Akt, phospho-Akt, phospho-GSK3α/β, p34cdc2, phospho-p34cdc2, and phospho-JNK. For each condition, lanes were loaded with 25 μg protein; blots were subsequently stripped and reprobed with antibodies to actin to ensure equivalent loading and transfer. The results of a representative experiment are shown; an additional study yielded equivalent results.
When administered alone UCN-01 or 17-AAG exerted little effect on total Akt levels, although a small decline was noted in cells treated with both agents, possibly reflecting diminished stability of this protein (Figure 7B). Furthermore, whereas 17-AAG alone was relatively ineffective in blocking phosphorylation of Akt, combined treatment resulted in a modest but discernible reduction in phospho-Akt levels. This action was less pronounced, however, than the marked reduction in Raf-1 expression or inhibition of MEK and ERK phosphorylation observed in UCN-01/17-AAG–treated cells. In addition, combined treatment with UCN-01 and 17-AAG resulted in a clear reduction in phospho-GSK, a well-established Akt substrate.40 Coadministration of 17-AAG with UCN-01 also resulted in a modest but unequivocal increase in p34cdc2 activation (dephosphorylation), whereas total protein levels remained constant. In contrast to these findings, simultaneous treatment of cells with UCN-01 and 17-AAG did not modify the phosphorylation status of p38 MAPK or JNK. Thus, coadministration of UCN-01 and 17-AAG resulted in a striking inhibition of the Raf-1/MEK/ERK pathway and a less pronounced but clearly discernible inhibition of Akt and activation of p34cdc2.
In separate time-course studies, effects of the UCN-01/17-AAG regimen in U937 cells were examined at earlier intervals (ie, 4, 6, and 8 hours) in relation to Raf-1 expression and phosphorylation of MEK1/2 and ERK1/2. Although no changes were noted after a 4-hour exposure, by 6 hours a clear reduction in expression of Raf-1 and phospho-MEK1/2 and phosph-ERK1/2 was observed, which became very pronounced by 8 hours (data not shown). These findings indicate that down-regulation of the Raf-1/MEK/ERK axis represents an early consequence of combined treatment of cells with the UCN-01/17-AAG regimen.
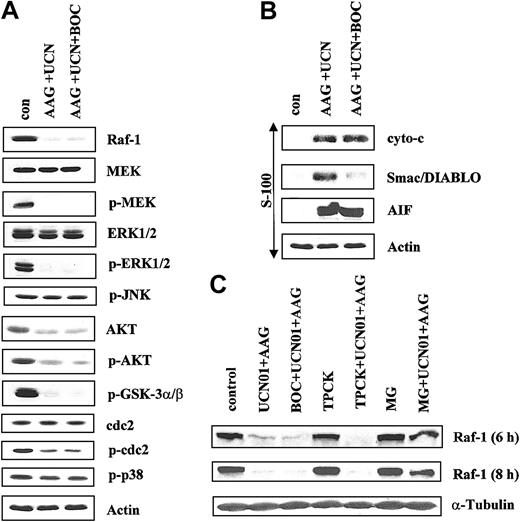
Because proteins involved in signal transduction can themselves be substrates for apoptotic caspases,41 an attempt was made to characterize the hierarchy of events accompanying treatment of cells with UCN-01 and 17-AAG. To this end, U937 cells were exposed for 10 hours to 400 nM 17-AAG plus 75 nM UCN-01 in the presence or absence of the pan-caspase inhibitor BOC-fmk (25 μM), after which the status of various signaling proteins was monitored. As shown in Figure 8A, administration of BOC-fmk failed to block the down-regulation of Raf-1 or of phospho-MEK1/2 or phospho-ERK1/2 in cells exposed to UCN-01 and 17-AAG. Similarly, caspase inhibition did not attenuate the reduction in phospho-Akt and phospho-GSK, or in total Akt levels in UCN-01/17-AAG-treated cells (Figure 8A). Dephosphorylation (activation) of p34cdc2 was also unperturbed by BOC-fmk (Figure 8A). Finally, caspase inhibition failed to modify expression of phospho-p38 MAPK and phospho-JNK in UCN-01/17-AAG–treated cells. Together, these findings indicate that UCN-01/17-AAG–mediated inhibition of the Raf-1/MEK/ERK and Akt pathways, and activation of p34cdc2 do not simply represent a consequence of engagement of the caspase cascade.
Exposure of U937 cells with or without BOC-D-fmk. U937 cells were exposed to 400 nM 17-AAG plus 75 nM UCN-01 for 10 hours in the presence or absence of 25 μM BOC-D-fmk, after which whole cell pellets were lysed, the proteins separated by SDS-PAGE, and Western analysis performed to monitor expression of Raf-1, total MEK, phospho-MEK, total ERK1/2, phospho-ERK1/2, phospho-JNK (A) or total Akt, phospho-Akt, phospho-GSKα/β, p34cdc2, phospho-p34cdc2, and phospho-p38 MAPK (B). Alternatively, cytosolic S-100 fractions were obtained as described in “Materials and methods,” the proteins separated as above, and expression of cytochrome c (cyto c), Smac/DIABLO, and AIF monitored by Western analysis. (C) Cells were treated with UCN-01 plus 17-AAG for 6 or 8 hours in the presence or absence of either BOC-D-fmk (25 μM), TPCK (20 μM), or MG-132 (500 nM), after which Western analysis was performed to monitor expression of Raf-1. For each condition, lanes were loaded with 25 μg protein; blots were subsequently stripped and reprobed with antibodies to actin to ensure equivalent loading and transfer. The results of a representative experiment are shown; 2 additional studies yielded equivalent results.
Exposure of U937 cells with or without BOC-D-fmk. U937 cells were exposed to 400 nM 17-AAG plus 75 nM UCN-01 for 10 hours in the presence or absence of 25 μM BOC-D-fmk, after which whole cell pellets were lysed, the proteins separated by SDS-PAGE, and Western analysis performed to monitor expression of Raf-1, total MEK, phospho-MEK, total ERK1/2, phospho-ERK1/2, phospho-JNK (A) or total Akt, phospho-Akt, phospho-GSKα/β, p34cdc2, phospho-p34cdc2, and phospho-p38 MAPK (B). Alternatively, cytosolic S-100 fractions were obtained as described in “Materials and methods,” the proteins separated as above, and expression of cytochrome c (cyto c), Smac/DIABLO, and AIF monitored by Western analysis. (C) Cells were treated with UCN-01 plus 17-AAG for 6 or 8 hours in the presence or absence of either BOC-D-fmk (25 μM), TPCK (20 μM), or MG-132 (500 nM), after which Western analysis was performed to monitor expression of Raf-1. For each condition, lanes were loaded with 25 μg protein; blots were subsequently stripped and reprobed with antibodies to actin to ensure equivalent loading and transfer. The results of a representative experiment are shown; 2 additional studies yielded equivalent results.
Parallel studies were performed to assess the role of caspases in UCN-01/17-AAG–mediated mitochondrial injury. As shown in Figure 8B, coadministration BOC-fmk failed to block UCN-01/17-AAG–induced cytosolic release of cytochrome c or AIF. In marked contrast, caspase inhibition substantially blocked release of Smac/DIABLO. These observations indicate that mitochondrial release of cytochrome c and AIF in UCN-01/17-AAG–treated cells occur upstream of caspase activation in U937 cells, whereas Smac/DIABLO redistribution represents a secondary, caspase-dependent event.
To investigate further the mechanism underlying reduced expression of Raf-1, U937 cells were exposed for 6 or 8 hours to UCN-01 plus 17-AAG in the presence or absence of BOC-fmk, the serine protease inhibitor TPCK (20 μM), or the proteasome inhibitor MG-132 (500 nM; Figure 8C). Consistent with results noted at 10 hours, BOC-fmk failed to block the reduction in Raf-1 expression in UCN-01/17-AAG–treated cell at either interval. Similarly, diminished expression of Raf-1 continued to be observed in cells treated with TPCK. However, in marked contrast, coadministration of the proteasome inhibitor MG-132 largely reversed Raf-1 down-regulation in UCN-01/17-AAG–treated cells, suggesting that this phenomenon is primarily mediated through proteasomal degradation.
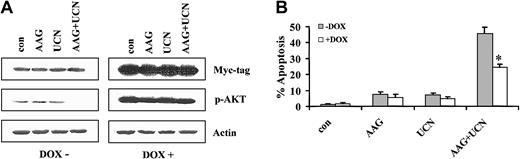
To gain insights into the functional role of the Akt and MEK/ERK pathways in UCN-01/17-AAG–mediated lethality, Jurkat cells inducibly expressing either constitutively active (myristolated) Akt (Jurkat Akt/28) or constitutively active MEK (Jurkat Mek/30) were used. As shown in Figure 9A, cells cultured in the absence of doxycycline displayed minimal expression of a c-Myc tag and modest basal expression of phospho-Akt. Coexposure of these cells to 17-AAG plus UCN-01 (48 hours) diminished Akt phosphorylation. However, when cells were cultured in the presence of doxycycline, a very pronounced increase in expression of the c-Myc tag was noted, along with substantial increases in expression of both total and phospho-Akt. Significantly, in the presence of doxycycline, phospho-Akt was clearly increased in cells exposed to the UCN-01/17-AAG regimen. To define the functional role of enforced activation of Akt in regulating UCN-01/17-AAG–mediated cell death, the extent of apoptosis was monitored in cells exposed to these agents in the presence or absence of doxycycline (Figure 9B). Enforced expression of constitutively active Akt significantly, albeit partially, protected cells from UCN-01/17-AAG–mediated lethality at 24 hours (P < .05; data not shown), and this effect was even more pronounced at 48 hours (Figure 9B; P < .02). Comparable results were obtained with a second Akt-inducible clone (Jurkat Akt/29; data not shown). Collectively, these findings support the notion that down-regulation of Akt plays a functional role in enhanced lethality of the 17-AAG/UCN-01 regimen.
Extent of apoptosis after exposure to doxycycline. (A) Jurkat cells inducibly expressing a constitutively active (myristolated), c-Myc–tagged Akt vector under the control of a tetracycline-responsive promoter were exposed for 48 hours to 5 μM 17-AAG with or without 200 nM UCN-01 in the presence or absence of 2 μg/mL doxycycline. At the end of the incubation period, cell pellets were obtained, the cells lysed, and proteins separated by SDS-PAGE as described in “Materials and methods.” Western analysis was used to monitor expression of the c-Myc tag and phospho-Akt. For each condition, lanes were loaded with 25 μg protein; blots were subsequently stripped and reprobed with antibodies to actin to ensure equivalent loading and transfer. The results of a representative experiment are shown; an additional study yielded equivalent results. (B) Following exposure to 17-AAG with or without UCN-01 for 48 hours in the presence or absence of doxycycline, the percentage of apoptotic cells was determined by morphologic assessment of Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” Values represent the means ± SDs for 3 separate experiments performed in triplicate. *Significantly less than values obtained for cells exposed to UCN-01 plus 17-AAG in the absence of doxycycline; P < .02.
Extent of apoptosis after exposure to doxycycline. (A) Jurkat cells inducibly expressing a constitutively active (myristolated), c-Myc–tagged Akt vector under the control of a tetracycline-responsive promoter were exposed for 48 hours to 5 μM 17-AAG with or without 200 nM UCN-01 in the presence or absence of 2 μg/mL doxycycline. At the end of the incubation period, cell pellets were obtained, the cells lysed, and proteins separated by SDS-PAGE as described in “Materials and methods.” Western analysis was used to monitor expression of the c-Myc tag and phospho-Akt. For each condition, lanes were loaded with 25 μg protein; blots were subsequently stripped and reprobed with antibodies to actin to ensure equivalent loading and transfer. The results of a representative experiment are shown; an additional study yielded equivalent results. (B) Following exposure to 17-AAG with or without UCN-01 for 48 hours in the presence or absence of doxycycline, the percentage of apoptotic cells was determined by morphologic assessment of Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” Values represent the means ± SDs for 3 separate experiments performed in triplicate. *Significantly less than values obtained for cells exposed to UCN-01 plus 17-AAG in the absence of doxycycline; P < .02.
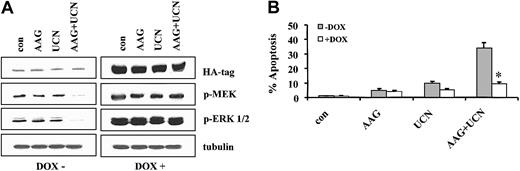
Parallel studies were performed using Jurkat cells stably transfected with a tetracycline-inducible constitutively active MEK1/2 construct (Jurkat Mek/30). Analogous to results obtained with the inducible Akt system, in the absence of doxycycline, cells expressed low basal levels of phospho-MEK1/2 and ERK1/2 (Figure 10A). Moreover, 48 hours of treatment of these cells with UCN-01 plus 17-AAG (but not with drugs administered individually) resulted in a marked decline in phospho-MEK and phospho-ERK. When cells were cultured in the presence of doxycycline, pronounced increases in expression of total MEK, phospho-MEK, and phospho-ERK were observed (Figure 10B). Significantly, enforced expression of constitutively active MEK circumvented the blockade of ERK activation by UCN-01/17-AAG exposure. Notably, enforced activation of MEK and ERK significantly attenuated UCN-01/17-AAG–induced apoptosis at 24 hours (P < .05; data not shown), and these effects were even more pronounced at 48 hours (eg, P < .005). In fact, the protective effects of enforced ERK activation in UCN-01/17-AAG–treated cells were at least as great if not greater than those of Akt activation. Similar results were obtained with a second MEK-inducible clone (Jurkat Mek/17; data not shown). Taken in conjunction with the preceding findings, these observations suggest that interruption of both the Akt and Raf/MEK/ERK pathways by the UCN-01/17-AAG regimen play important functional roles in the synergistic induction of apoptosis by this drug combination.
Expression of HA tag, phospho-MEK, and phospho-ERK1/2. (A) Jurkat cells inducibly expressing a constitutively active, HA-tagged MEK vector under the control of a tetracycline-responsive promoter were exposed for 48 hours to 5 μM 17-AAG with or without 200 nM UCN-01 in the presence or absence of 2 μg/mL doxycycline. At the end of the incubation period, cell pellets were obtained, the cells lysed, and proteins separated by SDS-PAGE as described in “Materials and methods.” Western analysis was used to monitor expression of the HA tag, phospho-MEK, and phospho-ERK1/2. For each condition, lanes were loaded with 25 μg protein; blots were subsequently stripped and reprobed with antibodies to tubulin to ensure equivalent loading and transfer. The results of a representative experiment are shown; an additional study yielded equivalent results. (B) Following exposure to 17-AAG with or without UCN-01 for 48 hours in the presence or absence of doxycycline, the percentage of apoptotic cells was determined by morphologic assessment of Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” Values represent the means ± SD for 3 separate experiments performed in triplicate. **Significantly less than values obtained for cells exposed to UCN-01 plus 17-AAG in the absence of doxycycline; P < .005.
Expression of HA tag, phospho-MEK, and phospho-ERK1/2. (A) Jurkat cells inducibly expressing a constitutively active, HA-tagged MEK vector under the control of a tetracycline-responsive promoter were exposed for 48 hours to 5 μM 17-AAG with or without 200 nM UCN-01 in the presence or absence of 2 μg/mL doxycycline. At the end of the incubation period, cell pellets were obtained, the cells lysed, and proteins separated by SDS-PAGE as described in “Materials and methods.” Western analysis was used to monitor expression of the HA tag, phospho-MEK, and phospho-ERK1/2. For each condition, lanes were loaded with 25 μg protein; blots were subsequently stripped and reprobed with antibodies to tubulin to ensure equivalent loading and transfer. The results of a representative experiment are shown; an additional study yielded equivalent results. (B) Following exposure to 17-AAG with or without UCN-01 for 48 hours in the presence or absence of doxycycline, the percentage of apoptotic cells was determined by morphologic assessment of Wright-Giemsa–stained cytospin preparations as described in “Materials and methods.” Values represent the means ± SD for 3 separate experiments performed in triplicate. **Significantly less than values obtained for cells exposed to UCN-01 plus 17-AAG in the absence of doxycycline; P < .005.
Discussion
The present results indicate that the PKC and Chk1 inhibitor UCN-01 interacts in a highly synergistic manner with the Hsp90 antagonist 17-AAG to trigger mitochondrial injury and cell death in a variety of human leukemia cell types. Recently, considerable attention has focused on the use of signal transduction modulators to enhance the lethal effects of conventional cytotoxic agents. For example, UCN-01 has been shown to potentiate the lethal actions of camptothecin and cytosine arabinoside (ara-C),21,42 and synergistic interactions between geldanamycin and paclitaxel have also been reported.43 However, there is accumulating evidence that simultaneous interruption of 2 cytoprotective signaling pathways represents a particularly potent apoptotic stimulus in neoplastic cells. In the case of UCN-01, coadministration of pharmacologic MEK inhibitors (eg, PD98059 or U0126) has been shown to induce a dramatic increase in cell death in human leukemia and myeloma cells,22,23 as well as in malignant cells of epithelial origin.24 Analogously, the Bcr/Abl kinase inhibitor imatinib mesylate interacts synergistically with pharmacologic MEK1/2 inhibitors,44 the PI3K inhibitor LY294002,45 and farnesyltransferase inhibitors46 in Bcr/Abl+ leukemia cells. Taken together, these findings suggest that neoplastic cells may be ill equipped to escape the lethal consequences of interruption of more than one survival-related signaling pathway. The observation that in human leukemia cells, UCN-01, an inhibitor of PKC, Chk1, and cyclin-dependent kinases15,17,18 interacts synergistically with 17-AAG, an agent known to disrupt multiple signal transduction and cell cycle regulatory pathways,5 provides additional support for this notion.
Based on the present findings, it appears likely that interference with Akt signaling contributes, at least in part, to the marked potentiation of apoptosis observed in leukemia cells exposed to UCN-01 and 17-AAG. Abundant evidence indicates that activation of the Akt pathway exerts antiapoptotic effects, although the mechanisms by which this phenomenon occurs are not entirely understood and may vary with cell type. These include modulation of the activity of other signaling pathways, for example, those related to m-target of rapamycin (m-TOR), nuclear factor κB (NF-κB), glycogen synthase kinase (GSK), p70RSK7, or phosphorylation of apoptotic regulatory proteins such as Bad8 or procaspase-9.9 In this context, 17-AAG has recently been shown to induce degradation of Akt in prostate cancer cells,47 and activation of the PI3K/Akt pathway (ie, by transfection with a constitutively active P110 construct) has been found to protect breast cancer cells from 17-AAG–mediated lethality.14 In this context, Sato and coworkers recently reported that UCN-01 blocked Akt phosphorylation through a PDK1-dependent mechanism.25 Consistent with these results, coadministration of 17-AAG and UCN-01 resulted in a pronounced inhibition of Akt phosphorylation in U937 cells, accompanied by a marked inhibition of GSK phosphorylation. Importantly, enforced activation of Akt provided significant, albeit partial, protection from UCN-01/17-AAG–mediated lethality, indicating that interruption of Akt signaling plays a functional role in the synergistic induction of cell death by this drug combination. Whether the failure of enforced Akt activation to protect cells fully from the UCN-01/17-AAG regimen reflects interference with PDK1-dependent, Akt-independent pathways7,48 or disruption of alternative signaling cascades remains to be determined.
The observation that coadministration of UCN-01 and 17-AAG profoundly down-regulated the Raf-1/MEK/ERK signaling pathway is noteworthy, and this phenomenon appears to be at least as important a contributor to the synergistic lethality of the UCN-01/17-AAG regimen as interference with the Akt cascade. It has long been known that Hsp90 is involved in regulation of Raf-1 intracellular trafficking and homeostasis, and Hsp90 antagonists such as geldanamycin and 17-AAG potently down-regulate expression of this protein.49 However, when administered at the sublethal concentrations used in the current study, 17-AAG minimally reduced Raf-1 expression. Interestingly, coadministration of UCN-01, which has recently been shown to stimulate ERK activation in leukemia and myeloma cells,22,23 markedly potentiated 17-AAG–mediated Raf-1 down-regulation. This process occurred very early (eg, within 4-6 hours), was virtually complete by 24 hours, and proceeded in a caspase-independent manner. The mechanism by which UCN-01 potentiates 17-AAG–mediated Raf-1 down-regulation is unclear, although interactions between Akt- and Raf-related pathways may be pertinent.50 However, the ability of the proteasome inhibitor MG-132 to antagonize UCN-01/17-AAG–mediated Raf-1 down-regulation argues strongly that UCN-01 potentiates the proteasomal degradation of this protein accompanying Hsp90 disruption by 17-AAG. The pronounced down-regulation of Raf-1 was also associated with a marked reduction in phosphorylation of MEK1, a major Raf-1 substrate,51 as well as that of ERK, the primary MEK target.52 Previously, we demonstrated that exposure of multiple malignant hematopoietic and nonhematopoietic cells to UCN-01 induces, through an as yet to be defined mechanism, activation of the MEK/ERK cascade.22-24 Furthermore, interruption of these events (eg, by pharmacologic MEK inhibitors) resulted in a marked increase in mitochondrial injury and apoptosis.22-24 Thus, 17-AAG appears to mimic the actions of pharmacologic MEK inhibitors such as PD98059 and U0126 in blocking UCN-01–mediated ERK activation in these cells and in promoting cell death. Consequently, it is tempting to speculate that one or more of the actions of UCN-01 results in a cytoprotective ERK response and that interference with this process, either upstream, at the level of Raf-1, or more proximally, at the level of MEK1/2, dramatically potentiates cell death. The observation that coadministration of MEK inhibitors22-24 or 17-AAG (this study) resulted in enhanced activation of p34cdc2 may also be relevant because unscheduled activation of this cyclin-dependent kinase is a potent apoptotic stimulus.53 Consistent with this model, enforced expression of constitutively active MEK overcame the blockade of ERK activation and substantially protected cells from UCN-01/17-AAG lethality. In fact, the protective effects of enforced ERK activation were, if anything, greater than those associated with constitutively active Akt. However, given the pleiotropic nature of 17-AAG actions,5 the possibility that other downstream targets of this agent contribute to lethality cannot be excluded.
The present results suggest that the lethal consequences of the 17-AAG/UCN-01 regimen involve activation of both the intrinsic mitochondrial and the extrinsic receptor-related apoptotic pathways. For example, coadministration of UCN-01 and 17-AAG, though relatively ineffective individually, markedly increased release of the proapoptotic mitochondrial proteins cytochrome c, Smac/DIABLO, and AIF. However, whereas redistribution of cytochrome c and AIF were caspase independent, release of Smac/DIABLO was caspase dependent and presumably a secondary event. This finding is consistent with recent reports that release of mitochondrial proteins may be highly stimulus dependent.54 The ability of ectopic expression of Bcl-2 and Bcl-xL, which primarily act to inhibit release of mitochondrial proteins,55 to attenuate 17-AAG/UCN-01–mediated lethality supports an important role for the intrinsic pathway in the lethality of this drug combination. However, 17-AAG/UCN-01 administration also resulted in caspase-8 and Bid cleavage; moreover, the lethality of this combination was attenuated in part by ectopic expression of dominant-negative caspase-8. It is therefore likely that activation of the extrinsic pathway by UCN-01/17-AAG plays a role in amplifying the apoptotic process, as has been shown in the case of conventional cytotoxic agents such as VP-16.37 In any case, these findings are consistent with the results of earlier reports indicating that the cytoprotective Raf/ERK and Akt pathways can block apoptosis proceeding through either the intrinsic mitochondrial or the extrinsic receptor-related pathways.50,56
Although attention has focused on the ability of UCN-01 to block Chk1,18 and, more recently, Akt,25 it is also a potent inhibitor of PKC.14 Because PKC inhibitors are known to be effective inducers of apoptosis,57 the possibility arises that interruption of the PKC pathway may be responsible for, or contribute to, synergistic interactions with 17-AAG. However, GFX, a highly specific PKC inhibitor,58 did not substantially enhance 17-AAG lethality, suggesting that the PKC inhibitory properties of UCN-01 do not play a significant role in synergistic interactions with Hsp90 antagonists. Such findings may be analogous to previous observations that the lethal actions of UCN-01 in lymphoblastic leukemia cells were unrelated to PKC inhibition.59
In summary, the present findings indicate that UCN-01 and the Hsp90 antagonist 17-AAG interact in a highly synergistic manner in human leukemia cells to induce mitochondrial damage and apoptosis. They also suggest that interruption of the Akt and particularly the Raf-1/MEK/ERK cytoprotective signaling pathways play important functional roles in these events. Given the recent introduction of Hsp90 antagonists such as 17-AAG into clinical trials,1 and demonstration of the feasibility of achieving plasma UCN-01 concentrations in excess of those used in the present study,60 the findings reported here could have translational implications for the development of novel antileukemic strategies. In a broader sense, such findings provide further support for the evolving concept that simultaneous interruption of multiple signal transduction/cell cycle regulatory pathways represents a highly potent apoptotic stimulus in neoplastic cells. It will be of considerable interest to determine whether and to what extent this strategy can be extended to other neoplastic cell types, particularly those of epithelial origin, as well as primary tumor cells. Accordingly, such studies are currently under way.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2002-12-3785.
Supported by awards CA 63753 and CA 93738 from the National Institutes of Health and award 6630 from the Leukemia and Lymphoma Society of America. W.J. and C.Y. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal