c-kit receptor (CD117) is expressed by erythroid, megakaryocytic, and myeloid precursors and mature mast cells.1 Imatinib mesylate (STI571), a well-established inhibitor of bcr-abl protein tyrosine kinase and currently used for the treatment of chronic myeloid leukemia,2 also inhibits c-kit receptor kinase activity.3
A previous study in Blood reported that c-kit is expressed in most cases of anaplastic large cell lymphoma (ALCL) and approximately half of Hodgkin disease (HD) tumors, but not in other low- or high-grade non-Hodgkin lymphomas.4 c-kit has also been reported to be expressed in most HD cell lines.5 In view of the possible use of STI571 as an experimental treatment for these lymphomas, we assessed c-kit expression at the RNA and protein levels in 5 known classical HD cell lines (HD-MYZ, HDLM2, L-428, KM-H2, and L-1236; purchased from DSMZ, Braunschweig, Germany) and 5 ALK-positive ALCL cell lines, including Karpas 299, SR-786, and SU-DHL-1 (DSMZ) and JB-6 and TS-G1 (a gift from Dr. D. Jones, Houston, TX). The gastrointestinal stromal tumor cell line ST-882 (a gift from Dr J. Trent, Houston, TX) and the megakaryoblastic leukemia cell line MO7e (a gift from Dr M. Andreeff, Houston, TX) served as positive controls for c-kit expression. Using standard reverse transcriptase-polymerase chain reaction (RT-PCR), flow cytometry (monoclonal antibody 104D2; BD Biosciences Pharmingen, San Diego, CA), and Western blot analysis (polyclonal antibodies from DAKO, Carpinteria, CA, and Santa Cruz Biotechnology, Santa Cruz, CA), no evidence of c-kit RNA or protein was detected in any of the HD and ALCL cell lines (Figure 1A). These results were further confirmed by immunohistochemistry using formalin-fixed, paraffin-embedded cell blocks prepared from these cell lines.
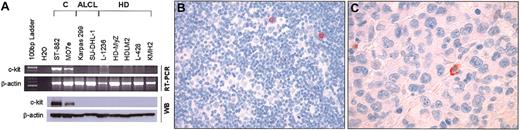
Absence of c-kit expression in HD and ALK-positive ALCL cell lines and tumors. (A) Top: Expression of c-kit mRNA in HD and ALCL cell lines as detected by RT-PCR as described elsewhere5 using the following primers: 5′-ACACCCTGTTCACTCCTTTGCTGA-3′ (forward), and 5′-GACTCCTTTGAATGCAGAAGA-3′ (reverse). The ST-882 and MO7e cell lines served as positive controls. Bottom: Immunoblots showing expression of c-kit protein in HD and ALCL cell lines. All HD and ALCL cell lines tested were negative for c-kit. A 145-kDa band corresponding to c-kit receptor was detected only in the ST-882 and MO7e control cell lines. β-actin served as a control for the amount of protein loaded in each well. (B,C) Representative cases of classical HD (B) and ALK-positive ALCL (C) showing no c-kit immunoreactivity in tumor cells. Note that occasional plasma cells are positive for c-kit. Original magnifications: B, × 200; C, × 400. Stained with immunoperoxidase with hematoxylin counterstain.
Absence of c-kit expression in HD and ALK-positive ALCL cell lines and tumors. (A) Top: Expression of c-kit mRNA in HD and ALCL cell lines as detected by RT-PCR as described elsewhere5 using the following primers: 5′-ACACCCTGTTCACTCCTTTGCTGA-3′ (forward), and 5′-GACTCCTTTGAATGCAGAAGA-3′ (reverse). The ST-882 and MO7e cell lines served as positive controls. Bottom: Immunoblots showing expression of c-kit protein in HD and ALCL cell lines. All HD and ALCL cell lines tested were negative for c-kit. A 145-kDa band corresponding to c-kit receptor was detected only in the ST-882 and MO7e control cell lines. β-actin served as a control for the amount of protein loaded in each well. (B,C) Representative cases of classical HD (B) and ALK-positive ALCL (C) showing no c-kit immunoreactivity in tumor cells. Note that occasional plasma cells are positive for c-kit. Original magnifications: B, × 200; C, × 400. Stained with immunoperoxidase with hematoxylin counterstain.
c-kit expression was also immunohistochemically assessed in 52 HD (47 classical HD, 5 lymphocyte-predominant HD) and 22 ALK-positive ALCL tumors using methods described elsewhere6 and a polyclonal antibody (DAKO) reactive with c-kit that is reported to yield optimal immunohistochemical results.7 All HD and ALK-positive ALCL tumors were negative for c-kit(Figure 1B). Our data are in agreement with 2 recent reports that demonstrated no evidence of c-kit immunoreactivity in a total of 32 HD tumors.8,9 These results and our data are contrary to those of Pinto and coworkers,4 published in Blood, who found c-kit expression in fresh-frozen sections of 11 of 16 ALCL and 11 of 21 HD tumors. Subsequently, the same group reported that 5 of 6 HD cell lines expressed a functional c-kit receptor.5 In both studies, the authors used the 17F11 monoclonal antibody that recognizes an extracellular domain of c-kit. The 17F11 antibody was raised by immunization of a Balb/c mouse with leukemic blasts from a patient with acute nonlymphocytic leukemia and is reported to react positively with most leukemic blasts of myeloid, but not lymphoid, lineage or peripheral blood cells10 Since the 17F11 antibody is no longer commercially available, we are not able to test its immunoreactivity in this study.
The explanation for the remarkable discrepancy among previous studies regarding c-kit expression in HD and ALCL is uncertain. However, the results obtained using the 17F11 antibody have not been confirmed using other commercially available antibodies.8,9
We conclude that c-kit RNA and protein are consistently not expressed in HD and ALK-positive ALCL cell lines and tumors. These findings suggest that c-kit tyrosine kinase is probably not an appropriate target for therapeutic agents such as STI571 in the treatment of patients with HD and ALCL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal