Abstract
The adhesion molecules involved in the migration and retention of activated effector CD8 T cells in the lung microcirculation and their recruitment into lung tissue are largely unknown. Here, we have analyzed the role of lymphocyte function–associated antigen-1 (LFA-1) and very late antigen-4 (VLA-4) on adhesion of influenza hemagglutinin (HA)–specific CD8 T-cell clone D4 under shear conditions in an in vitro binding assay and in an in vivo homing assay to the lungs of naive or transgenic Balb/c mice expressing HA (HA-Tg) by a lung-specific promoter. Blocking LFA-1 or intercellular adhesion molecule 1 (ICAM-1) significantly inhibited adhesion of D4 cells to lung vascular endothelium and parenchyma of lung sections. However, blocking VLA-4 or vascular cell adhesion molecule 1 (VCAM-1) had no effect on cell adhesion. Blocking LFA-1 in vivo significantly delayed lethal injury following adoptive transfer of D4 cells into HA-Tg mice as assessed by weight loss and histology. Residence time of adoptively transferred Indium 111 (111In)–labeled D4 cells in lungs of normal and HA-Tg mice as analyzed by dual modality imaging revealed a significantly shorter transit time of 4 hours for the D4 cells upon in vivo blockade of LFA-1. These results demonstrate a crucial role for LFA-1 in retention of activated CD8 T cells in normal mouse lungs and in the progression of lethal injury in HA-Tg mice.
Introduction
Trafficking of leukocytes is largely governed by interaction between adhesion molecules expressed on leukocytes and on the vascular endothelium of postcapillary venules. Selectins mediate the initial rolling and tethering events1,2 following which integrins mediate firm adhesion3 that in turn allows for transmigration of cells into the tissue. This cascade of adhesive interactions occurring between leukocytes and endothelial cells in postcapillary venules is well documented in the systemic microcirculation of many organs.4,5 In the lungs, leukocyte transmigration is believed to occur primarily in alveolar capillaries.6,7 Margination of neutrophils in pulmonary capillaries is largely independent of selectins and the CD18 integrins,8,9 although neutrophil margination in response to formyl methionyl leucyl phenylalanine10 and their prolonged sequestration in response to other mediators is L-selectin–dependent.11-13 Marginated neutrophil numbers in the pulmonary circulation remain unchanged in E-, P-, or L-selectin gene-disrupted mice.11,14 The smaller diameter of the pulmonary capillaries allows close contact between neutrophils and capillary endothelium. Neutrophils have to deform and elongate in order to pass through the capillary segments, which possibly accounts for their delayed transit time through the pulmonary vasculature.15
Recirculation and homing of naive T cells to peripheral lymph nodes requires L-selectin,16 lymphocyte function–associated antigen-1 (LFA-1),17 and CCL19/CCR7 interactions.18 However, the mechanism(s) of lymphocyte trafficking to the lungs is not fully understood. In a murine model of pulmonary inflammation, intercellular adhesion molecule 1 (ICAM-1) has been implicated in lymphocyte recruitment to the lungs.19 α4 integrin and its receptor VCAM-1 have been shown to play an important role in lymphocyte migration to the lungs following intratracheal challenge with sheep red blood cells, which causes a rapid induction of endothelial VCAM-1.20,21 L-selectin and, to a larger extent, ICAM-1 have been shown to be important in lymphocyte migration to the lungs during an allergic inflammatory response in a mouse model of asthma.22 In an adoptive transfer model for alloreactive CD4 T cells, adherence of T cells in the lungs was shown to be partially dependent on LFA-1 and its receptor ICAM-1.23 Bacterial or viral infection has been shown to generate effector and memory CD8 T cells that marginate into nonlymphoid tissues, including the lungs, which suggests that these cells either continuously recirculate through peripheral tissues or reside in them permanently.24 However, the homing mechanisms of cytotoxic CD8 T cells into the lungs have not been investigated so far.
Cytotoxic T cells play a central role in the pathophysiology of many inflammatory lung diseases, wherein they accumulate in the alveolar space and/or in the interstitium25,26 and function primarily in the clearance of virus infection.27 We have previously described transgenic Balb/c mice expressing hemagglutinin (HA-Tg) as a mouse model of CD8 T-cell–mediated lung injury.28 This model allows for selective assessment of the impact of antigen-specific CD8 effector cytotoxic T cells on lung inflammation and injury in the absence of viral infection. Adoptive transfer of histocompatible HA-specific cytotoxic T lymphocyte (CTL) clone D429 into the tail vein of HA-Tg mice (but not in nontransgenic littermates) results in the development of progressive and ultimately lethal pulmonary inflammation and injury over 3 to 5 days, characterized by the accumulation of mononuclear cells in alveolar walls and terminal airways. Pulmonary inflammation and injury develops and progresses without the continued presence of effector CD8 T cells. CD8 T cells were no longer detected in the lungs by 24 to 48 hours after cell transfer. Since the development of CD8 T-cell–mediated injury is antigen specific and likely requires contact between HA-specific CD8 T cells and HA-expressing alveolar type II cells, we wanted to characterize the process of CD8 T-cell localization/retention to the lungs. The process by which these T cells enter the lung parenchyma to initiate the injury process and the role for adhesion molecules in this event are not clear. In this report, we have analyzed the role of integrins LFA-1 and very late antigen-4 (VLA-4) in adhesion assays in vitro and demonstrated a role for LFA-1, but not for VLA-4, in CD8 T-cell adhesion to normal lung tissue. We further analyzed the role of LFA-1 in CD8 T-cell homing and retention in normal and HA-Tg lungs and its impact on lung injury. Our data suggest that LFA-1 is important for retention of CD8 T cells in the lungs and is involved in the lethal injury process.
Materials and methods
Mice
Surfactant protein C hemagglutinin transgenic (SPC-HA-Tg28 ) mice in the H-2d haplotype, expressing the A/Japan/305/57 HA under the transcriptional control of the SPC promoter, or BALB/c mice were used in this study. Animals used were 8 to 10 weeks of age.
Flow cytometry
HA 210-219–specific CD8 T-cell clone D4 cells were stained with the following fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated antibodies (BD Pharmingen, San Diego, CA): anti-CD8 (clone 53-6.7), anti–LFA-1 (clone M17/4), anti–VLA-4 (clone R1-2), anti-CD11b (clone M1/70), anti-CD25 (clone PC61), anti-CD69 (clone H1.2F3), and anti-CD44 antibodies (clone IM7). Flow cytometry was performed on a Becton Dickinson FACScan using Cellquest software (Becton Dickinson, Palo Alto, CA).
Stamper-Woodruff assay
Stamper-Woodruff assay was performed as previously described30 with some modifications. Briefly, 1 × 106 D4 cells were applied to 10-μm-thick frozen lung sections on glass slides in 100 μL Hanks balanced salt solution (HBSS) buffer containing 1 mM CaCl2 and 1 mM MgCl2. Slides were placed on a rotary shaker for 20 minutes at 70 rpm at room temperature. Slides were then gently rinsed in HBSS to remove unbound cells and fixed in 2% glutaraldehyde for 15 minutes, washed once with HBSS, air dried, and stained with Giemsa stain for microscopic observation. Bound cells were counted in 3 random fields of view per section at × 20 magnification. For blocking experiments, D4 cells were incubated with 20 μg/mL of appropriate monoclonal antibody (mAb) in 200 μL HBSS for 30 minutes at 4°C and washed prior to use in the assay.
Adoptive transfer
On day 5 following stimulation with irradiated syngeneic splenocytes that were infected with A/Japan/57 influenza, CD8 T-cell clone, D4 cells were separated from stimulators by density gradient centrifugation. Briefly, cells were layered over FicoLite LM (Atlanta Biologics, Norcross, GA) and spun at 2000 rpm for 10 minutes. Cells at the interface were carefully collected and washed once with Dulbecco modified Eagle medium (DMEM) + 10% fetal bovine serum (FBS), spun, and then washed once with plain DMEM. The washed cells were resuspended in plain DMEM for intravenous injections. The purity of the D4 cells was more than 98% by CD8 and tetramer staining (data not shown). Cells (10 × 106) were injected via tail vein into transgenic mice. To block LFA-1, cells were incubated with 300 μg anti–LFA-1 antibody (TIB-217, American Type Culture Collection, Manassas, VA) for 30 minutes and cells and antibody were injected intravenously. Mice were monitored daily for change in body weight.
Histology of lung tissue
At 48 and 72 hours following transfer of D4 cells, mice were killed and lungs were inflated with air before fixation in 10% neutral buffered formalin. Sections (5 μm) were cut from fixed, paraffin-embedded tissue and stained with hematoxylin and eosin (H&E).
Indium111 labeling and dual modality imaging
D4 cells (5 × 106) were washed in plain RPMI, resuspended gently in 100 μL HBSS containing 50 μg tropolone (Sigma, St Louis, MO) and 200 μCi (7.4 MBq) 111Indium (Perkin Elmer, Boston, MA), and incubated for 15 minutes at room temperature. Cells were washed with RPMI containing 10% FBS twice to remove unbound radioactivity, resuspended in 300 μL plain RPMI, and injected via tail vein in mice following administration of ketamine (125 μg/g body weight; Ketlar, Parke Davis, NJ) and xylazine (12.5 μg/g body weight; Phoenix Scientific, St Joseph, MO). Dual modality whole-body imaging was done at indicated time points following cell transfers using a charge-coupled device–based x-ray detector and a small field-of-view (FOV) gamma camera. The common FOV of the x-ray detector and gamma detectors was approximately 7.5 cm2. The x-ray and gamma-ray detectors have both been designed and built with spatial resolution that is appropriate for small-animal research (0.05-mm and 1.8-mm pixel sizes, respectively). Following image acquisition, the interactive data language imaging software was used to merge the x-ray and gamma-ray images to obtain a single coregistered image containing both structural (x-ray) and functional (gamma ray) information. Quantitative analysis of radioactivity in the lungs was done on the merged images by defining the region of interest using the rib cage as a reference to identify the lungs.
Data analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) on ranks (Kruskal-Wallis) with a multiple pairwise comparison test (Dunns test).
Results
Surface expression of adhesion molecules
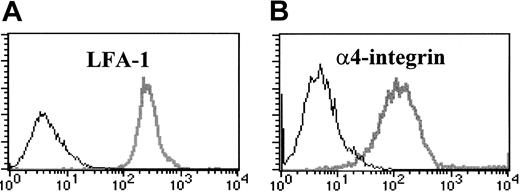
To identify candidate adhesion molecules involved in the development of CD8 T-cell–mediated injury in the lungs of HA-Tg mice, we analyzed cell-surface expression of β2 and α4 integrins on D4 cells at day 5 after stimulation by flow cytometry. As shown in Figure 1, D4 cells express high levels of αLβ2 integrin (LFA-1) and α4β1 integrin (VLA-4) but do not express αMβ2 integrin (Mac-1, not shown). D4 cells show an activated phenotype as confirmed by expression of activation markers CD25, CD69, and CD44 (data not shown).
Activated CD8 T cells express LFA-1 and α4-integrin (VLA-4). HA210-219–specific CD8 T-cell clone D4 cells were incubated with FITC- or PE-conjugated monoclonal antibodies to LFA-1 (A) or VLA-4 (B) (gray lines), or isotype control antibody (black lines).
Activated CD8 T cells express LFA-1 and α4-integrin (VLA-4). HA210-219–specific CD8 T-cell clone D4 cells were incubated with FITC- or PE-conjugated monoclonal antibodies to LFA-1 (A) or VLA-4 (B) (gray lines), or isotype control antibody (black lines).
In vitro binding assay
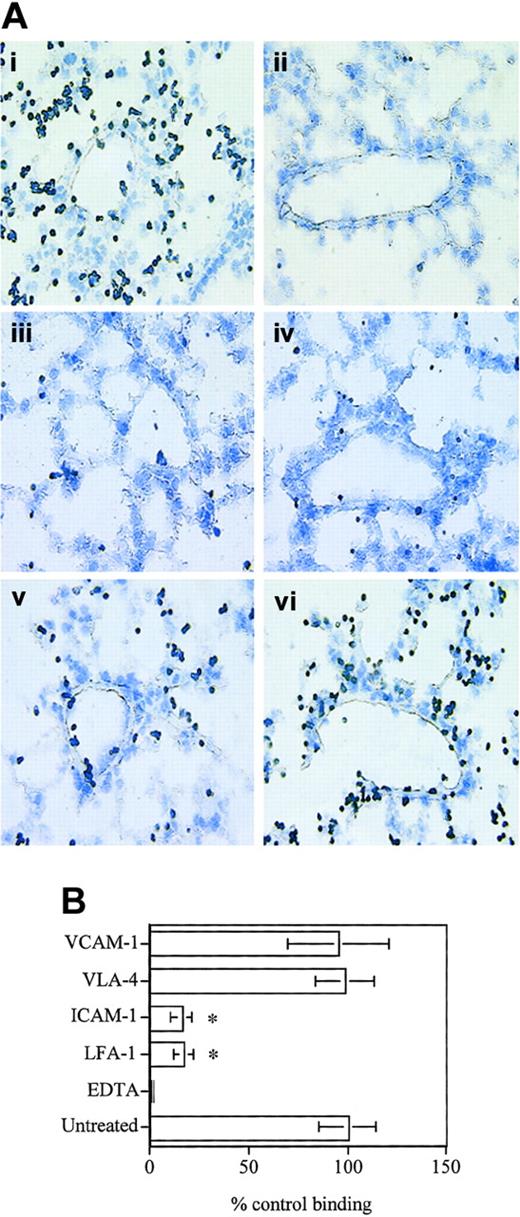
Using the Stamper-Woodruff assay, we next analyzed the role of LFA-1/ICAM-1 and VLA-4/VCAM-1 in D4 binding to frozen lung sections. D4 cells were found to adhere to the lung vascular endothelium as well as the parenchyma (Figure 2A-B) under simulated physiologic shear-stress conditions, and this binding was dependent on divalent cations, as the binding was completely abolished in the presence of EDTA (ethylenediaminetetraacetic acid) (Figure 2). Blocking antibodies to either VLA-4 or VCAM-1 (Figure 2) did not have any effect on T-cell adhesion. On the other hand, blocking LFA-1 or ICAM-1 resulted in a significant inhibition of binding (∼ 80%; Figure 2B), indicating that D4 binding to the lung vascular endothelium as well as the parenchyma is largely LFA-1–dependent. The binding pattern of D4 cells to the lung tissue correlates well with the endothelial and parenchymal expression of ICAM-1 in normal mouse lungs (Dixon et al23 and data not shown) indicating that LFA-1/ICAM-1 interaction could play a major role in CD8 T-cell binding to the lung vasculature and parenchyma.
D4 cells bind to lung vascular endothelium and interstitium. (A) D4 cells (1 × 106) were applied to 10-μM-thick frozen lung sections on glass slides in HBSS buffer containing 1 mM CaCl2 and 1 mM MgCl2 with or without 5 mM EDTA. Slides were placed on a rotary shaker for 20 minutes at 70 rpm at room temperature. Slides were rinsed in HBSS to remove unbound cells, fixed in 2% glutaraldehyde, and stained with Giemsa stain. For blocking experiments, D4 cells or lung sections were incubated with 20 μg/mL of appropriate mAb in 200 μL HBSS for 30 minutes and washed prior to use in the assay. Control antibodies against major histocompatibility complex (MHC) class I or CD3 did not inhibit binding (data not shown). (i) Untreated cells. (ii) EDTA. (iii) LFA-1. (iv) ICAM-1. (v) VLA-4. (vi) VCAM-1. Original magnification, × 40. (B) Data are expressed as cells bound to the lung section per field of view as a percent of cells bound in the untreated .group ± SD. Data pooled from 3 independent experiments are shown. *Significantly different (P < .001) from untreated group.
D4 cells bind to lung vascular endothelium and interstitium. (A) D4 cells (1 × 106) were applied to 10-μM-thick frozen lung sections on glass slides in HBSS buffer containing 1 mM CaCl2 and 1 mM MgCl2 with or without 5 mM EDTA. Slides were placed on a rotary shaker for 20 minutes at 70 rpm at room temperature. Slides were rinsed in HBSS to remove unbound cells, fixed in 2% glutaraldehyde, and stained with Giemsa stain. For blocking experiments, D4 cells or lung sections were incubated with 20 μg/mL of appropriate mAb in 200 μL HBSS for 30 minutes and washed prior to use in the assay. Control antibodies against major histocompatibility complex (MHC) class I or CD3 did not inhibit binding (data not shown). (i) Untreated cells. (ii) EDTA. (iii) LFA-1. (iv) ICAM-1. (v) VLA-4. (vi) VCAM-1. Original magnification, × 40. (B) Data are expressed as cells bound to the lung section per field of view as a percent of cells bound in the untreated .group ± SD. Data pooled from 3 independent experiments are shown. *Significantly different (P < .001) from untreated group.
Adoptive transfer in HA-Tg mice
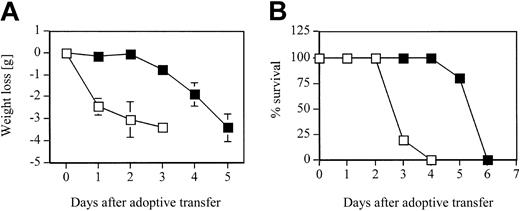
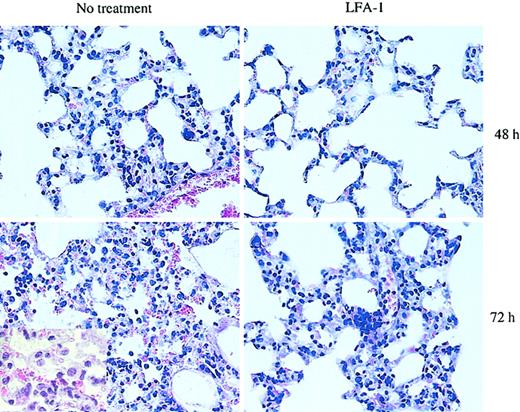
Since blocking LFA-1 significantly reduced the adhesive interaction of D4 cells with pulmonary endothelial cells as well as the interstitium in the in vitro binding assay, we tested the contribution of LFA-1 to the development of CD8 T-cell–mediated lung injury and to the kinetics of pulmonary inflammatory response in the HA-Tg mice. D4 cells were adoptively transferred into HA-Tg mice with or without anti–LFA-1 antibody treatment. There was a significant delay of 48 to 72 hours in the induction of injury as assessed by loss of body weight in the LFA-1–treated group (Figure 3A). In the untreated group, mice began to lose body weight within 24 hours following cell transfer. The loss of body weight correlates with the time to death of the untreated HA-Tg mice that die by day 4 and the LFA-1–treated HA-Tg mice that die by day 6 (Figure 3B). Analysis of lung histology at 48 hours after transfer in the LFA-1–treated group showed mild inflammation characterized by lesser infiltration of alveolar walls by mononuclear and polymorphonuclear cells compared with the untreated group that showed dense infiltration of inflammatory cells (Figure 4). At 72 hours after transfer when the LFA-1–treated mice had begun to lose body weight, the histology appeared to be comparable with that observed at 48 hours for the untreated group. At this time point, further injury progression in the untreated group is evident by vascular damage and cellular infiltration into the alveolar spaces.
Weight loss and mortality. (A) Weight loss and (B) mortality rate in HA-Tg mice after adoptive transfer of 10 × 106 CD8 T-cell clone D4 cells either untreated (□) or treated with 300 μg anti–LFA-1 antibody (▪). n = 5 mice per group. Data are representative of 2 independent experiments. Negative numbers indicate weight loss. Error bars indicate mean ± SD.
Weight loss and mortality. (A) Weight loss and (B) mortality rate in HA-Tg mice after adoptive transfer of 10 × 106 CD8 T-cell clone D4 cells either untreated (□) or treated with 300 μg anti–LFA-1 antibody (▪). n = 5 mice per group. Data are representative of 2 independent experiments. Negative numbers indicate weight loss. Error bars indicate mean ± SD.
Histologic analysis of lung morphology of HA-Tg mice. D4 cells (10 × 106) were injected intravenously with or without anti–LFA-1 antibody. Lungs were harvested at 48 or 72 hours following cell transfer, sectioned, and stained with H&E. The extent of interstitial inflammation is much less in the anti–LFA-1–treated group compared with the untreated group at 48 hours. Lungs harvested from animals receiving untreated D4 cells show more cellular infiltration and a greater and more widespread thickening of the alveolar walls. At 72 hours, significant alveolitis and mononuclear infiltration of the alveolar spaces are evident in the untreated lungs. Original magnification, × 40. Inset, × 100 magnification.
Histologic analysis of lung morphology of HA-Tg mice. D4 cells (10 × 106) were injected intravenously with or without anti–LFA-1 antibody. Lungs were harvested at 48 or 72 hours following cell transfer, sectioned, and stained with H&E. The extent of interstitial inflammation is much less in the anti–LFA-1–treated group compared with the untreated group at 48 hours. Lungs harvested from animals receiving untreated D4 cells show more cellular infiltration and a greater and more widespread thickening of the alveolar walls. At 72 hours, significant alveolitis and mononuclear infiltration of the alveolar spaces are evident in the untreated lungs. Original magnification, × 40. Inset, × 100 magnification.
Retention of CD8 T cells in the lungs is LFA-1–dependent
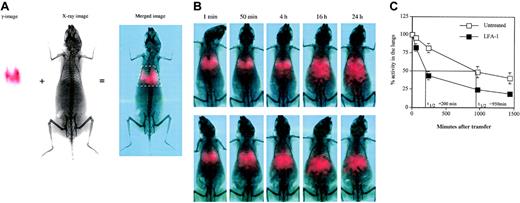
One possible explanation for the observation from adoptive transfer experiments is that blocking LFA-1 results in decreased residence time of the T cells in the lungs. This would be a result of inhibition of LFA-1 interaction with ICAM-1 that is constitutively expressed both on the vascular endothelium and in the interstitium (data not shown and Dixon et al23 ), thereby possibly interfering with the endothelial transmigration process. To test this, we analyzed the transit time of the adoptively transferred 111In-labeled D4 cells using dual modality planar imaging (Figure 5A) that permits temporal analysis of cell movement in vivo. The transit time of D4 cells through the lungs of HA-Tg mice was found to range between 16 and 24 hours (Figure 5B) and was similar to D4 cell transit through lungs of normal BALB/c (data not shown). The pattern of movement of T cells from the lungs predominantly to the liver remained unchanged following LFA-1 blockade. LFA-1 blockade significantly reduced the transit time of D4 cells compared with untreated ones (Figure 5C). The difference in retention time in the lungs was apparent as early as 50 minutes following cell transfer when 20% of the D4 cells had already left the lungs in the LFA-1–treated group. By 4 hours, 65% of the D4 cells had left the lungs in the LFA-1–treated group compared with 15% in the untreated group. As an isotype control (rat immunoglobulin G2a [IgG2a]), we used TIB241 antibody that binds CD44 expressed at high levels on the D4 cells. Blocking CD44 has no effect on the D4 cell transit time compared with the untreated D4 cells and did not alter injury progression or the time to death (data not shown).
Dual modality imaging. (A) Dual modality imaging (γ-image, x-ray image, and merged image) at one minute following adoptive transfer of 5 × 106 111In-labeled D4 cells intravenously is shown. White box in the merged image indicates the defined region of interest used for quantitation of radioactivity in the lungs. In the red-green-blue merged image, the red channel indicates counts from the γ-image. (B) Dual modality imaging of 111In-labeled D4 cells without (top row) or with (bottom row) anti–LFA-1 antibody after transfer intravenously at the indicated time points. Data are representative of 3 independent experiments. (C) Distribution of 111In-labeled D4 cells at the indicated time points after transfer. Data pooled from 3 independent experiments are shown. Data are represented as percentage radioactivity (mean ± SD) in the region of interest defined for the lungs using the rib cage as a reference, with the activity at one minute represented as 100%.
Dual modality imaging. (A) Dual modality imaging (γ-image, x-ray image, and merged image) at one minute following adoptive transfer of 5 × 106 111In-labeled D4 cells intravenously is shown. White box in the merged image indicates the defined region of interest used for quantitation of radioactivity in the lungs. In the red-green-blue merged image, the red channel indicates counts from the γ-image. (B) Dual modality imaging of 111In-labeled D4 cells without (top row) or with (bottom row) anti–LFA-1 antibody after transfer intravenously at the indicated time points. Data are representative of 3 independent experiments. (C) Distribution of 111In-labeled D4 cells at the indicated time points after transfer. Data pooled from 3 independent experiments are shown. Data are represented as percentage radioactivity (mean ± SD) in the region of interest defined for the lungs using the rib cage as a reference, with the activity at one minute represented as 100%.
Discussion
Lymphocyte homing is a tightly regulated process, and our knowledge regarding the role of adhesion molecules and chemokines and their receptors in lymphocyte migration to secondary lymphoid organs16,31 and tertiary sites, such as the gut32-34 and skin,31,35,36 is advancing rapidly. However, despite the availability of T-cell–induced lung injury models,23,37,38 the molecular basis of lymphocyte migration to the lungs is not well understood so far. Since the lung is the portal of entry for airborne pathogens, lymphocytes recirculate through the lungs continuously. Regardless of the infectious agent and the route of infection in the host, antigen-specific effector as well as memory CD8 T cells have been shown to reach the lungs and reside in the lung parenchyma for a prolonged period.24
Our study was designed to address the molecular requirements for CD8 T-cell homing to naive lungs and to lungs of HA-transgenic mice. Surprisingly, we found the same patterns and kinetics of homing, irrespective of whether antigen was present or not. This suggests that normal lung microvessels can constitutively support the retention of activated CD8 T cells. Indeed, ICAM-1, one of the ligands for LFA-1, is expressed in resting lungs under baseline conditions.23
Adoptively transferred HA210-219–specific CD8 T cells in normal HA-Tg mice reside in the lungs for 24 to 48 hours and induce pulmonary injury in a FasL- or perforin-independent manner.39 In order to initiate injury, the transferred T cells have to first cross the vascular endothelium and migrate across the interstitium to reach the type II alveolar epithelial cells that present the cognate antigen. The mechanism by which these effector CD8 T cells get across the pulmonary vasculature and reside in the lungs is not clear. In this study, we demonstrate that (1) using an in vitro binding assay, blocking LFA-1 significantly decreases T-cell adhesion to the lung tissue, which suggests that LFA-1 may be involved in this process; (2) blocking LFA-1 significantly delays onset of lethal injury; and (3) this delay in the onset of lethal injury correlates with accelerated egress of T cells from the lungs following LFA-1 blockade.
LFA-1 is a β2 integrin (CD11a/CD18) that is expressed on all leukocytes and has been shown to be important in firm adhesion of leukocytes to the endothelial cells, which allows for their migration across the endothelium to the site of inflammation.3,17 Following sequestration in the lungs, emigration of neutrophils occurs via 2 pathways: one that is CD11/CD18-dependent and the other that is not. The pathway of choice appears to be stimulus-dependent. Stimuli such as Escherichia coli, E coli lipopolysaccharide (LPS), IgG immune complexes, interleukin-1 (IL-1), and phorbol myristate acetate (PMA) result in CD11/CD18-dependent emigration of neutrophils.40 CD11/CD18-independent neutrophil emigration is induced by stimuli such as C5a, Staphylococcus aureus, and Streptococcus pneumoniae.40 The mechanism for the CD11/CD18-independent neutrophil emigration is not clear. In response to inflammatory stimuli such as LPS, bacteria, or cytokines, the inflamed lung microvasculature can express additional endothelial adhesion molecules beyond ICAM-1,6 which may support adhesion of CD8 T cells under inflammatory conditions, but this was not investigated in the present study.
LFA-1 is involved in migration of all subsets of lymphocytes into the peripheral lymph nodes (pLNs), and LFA-1–/– mice show decreased trafficking of lymphocytes to pLNs.17 Blocking β1 and β2 integrins has been shown to almost completely inhibit the migration of T cells into delayed hypersensitivity lesions.41 Although migration of naive as well as activated T cells has been studied in detail,42,43 there is a paucity of data analyzing the role of adhesion molecules in migration of effector CD8 T cells to normal lungs. In the isolated buffer-perfused rat lung model, LFA-1, but not CD44, has been shown to influence the trapping of lymphocytes in healthy rat lungs.44 One study analyzing the in vivo dissemination of cytotoxic T lymphocyte hybridomas demonstrated the presence of LFA-1, but not VLA-4, on all the hybridomas that migrated to lymphoid and nonlymphoid tissues, suggesting a role for LFA-1 in cell migration.45 In addition, LFA-1 is an important accessory molecule in promoting target cell lysis. Some of the effects of LFA-1 antibody treatment on lethality and weight loss may be due to a reduced ability to lyse target tissue. However, the altered D4 cell trafficking observed after LFA-1 antibody is not due to this effect, since no antigen was present in these experiments.
Owing to the architecture of the pulmonary capillary network in the lungs, it is believed that sequestration of leukocytes in the lungs is due to the constraints imposed on the circulating cells by the smaller diameter of the microcapillaries. Pulmonary capillaries have been shown to be the site for neutrophil sequestration and margination.6,7 The extent to which this model can be applied to lymphocytes is unclear. In this report, we demonstrate a role for the CD18 integrin LFA-1, expressed on activated CD8 T cells, in mediating CD8 T-cell adhesion to noninflamed pulmonary endothelium as well as to the lung interstitium. LFA-1, but not VLA-4, significantly decreases in vitro cell adhesion (Figure 2B). Blocking ICAM-1 resulted in a similar decrease in adhesion as observed with blocking LFA-1, suggesting an important role for the LFA-1/ICAM-1 interaction in this binding. This result is consistent with the expression pattern of ICAM-1. ICAM-1, a ligand for LFA-1, is constitutively expressed on the noninflamed pulmonary endothelium as well as the lung parenchyma, whereas VCAM-1 expression needs to be induced.23
Despite high surface levels of VLA-4 on the activated CD8 T cells, blocking VLA-4 had no effect on in vitro cell adhesion. This is consistent with the data reported for adoptive transfer of Th1 CD4+ cells,23 wherein the neutralizing anti–VLA-4 antibody PS/2 had no effect on the adherence of Th1 cells in the lungs. The VLA-4 ligand VCAM-1 is not expressed constitutively on lung endothelium (Dixon et al23 and data not shown). During an inflammatory response, VCAM-1 is up-regulated on the pulmonary vascular endothelium, and, in the presence of VCAM-1, VLA-4 on T cells has been implicated in T-cell recruitment to the lungs.20 MAb PS/2 has been used previously to inhibit VLA-4–dependent CD8 T-cell rolling on inflamed venular endothelium in vivo in an acute tumor necrosis factor α (TNF-α)– and interferon γ (IFN-γ)–mediated inflammation model.46 Since our study focuses on CD8+ T-cell homing to the resting lung, our data do not exclude the possibility that activated CD8+ T cells might home to inflamed lungs through a VCAM-1/VLA-4–dependent pathway.
CD8 T cells bind lung sections on endothelial cells as well as the parenchyma corresponding to the sites of ICAM-1 expression in the lungs (data not shown). Blocking ICAM-1 resulted in a similar decrease in adhesion as observed with blocking LFA-1, suggesting an important role for the LFA-1/ICAM-1 interaction in this binding. We further investigated the role of LFA-1 in the injury process induced following adoptive transfer of HA-specific CD8 T cells into HA-Tg mice. Disease induction is grossly evident by labored breathing, lethargy, and death by day 3 or 4 after transfer. Histologically, the lungs show extensive infiltration of the alveolar septa and air spaces with mononuclear cells. As disease progresses, the alveolar wall integrity breaks down resulting in intra-alveolar hemorrhage.28 Blocking LFA-1 results in a significant delay in onset of disease progression by 48 to 72 hours (Figure 3A). However, blocking LFA-1 does not confer absolute protection from lethal injury but delays the time to death by about 48 hours. These data suggest that while LFA-1 appears to play an important role in initiating the injury cascade, there is an LFA-1–independent mechanism of pulmonary injury in this model. The delay in the onset of injury analyzed by body weight loss as well as by histology shows far less injury both at 48 hours, when the weight loss is minimal to absent, as well as at 72 hours, when the weight loss becomes detectable in the LFA-1–treated group. It is possible that blocking LFA-1/ICAM-1 interaction allows only a small fraction of the transferred T cells to get across the blood vessel into the interstitium in an LFA-1–independent manner, with a majority of them remaining intravascular. The movement of the transmigrated cells toward the antigen could possibly be further delayed if they use ICAM-1 expressed in the parenchyma as a substrate for moving toward the type II epithelial cells. Alternatively, T cells may not use LFA-1/ICAM-1 interaction for transmigration and movement toward the antigen, and the observed effects could be due to a delay in the infiltration of LFA-1 expressing non-T cells, such as macrophages and neutrophils, following antigen recognition by the transferred T cells.
We analyzed the residence time of the untreated versus the LFA-1–blocked T cells in the lungs using a novel dual modality imaging technology (Williams et al47 and Figure 5A). Dual modality imaging technique is a powerful tool to track dynamic cell migration patterns in vivo in animals over a period of time. This technology surpasses the currently available cumbersome methods that require killing animals at each time point in order to remove organs for counting radioactivity.23,48 Tracking 111In-labeled CD8 T cells clearly reveals a significant difference in the transit time of untreated versus anti–LFA-1–treated effector CD8 T cells through the lungs (half-life [t1/2] = 200 minutes upon LFA-1 blockade compared with 950 minutes in the untreated group, Figure 5B-C). As shown in Figure 5A, 20% of the anti–LFA-1 treated cells leave the lungs as early as 50 minutes following transfer, and by 4 hours 65% of the cells have moved out of the lungs predominantly to the liver. On the other hand, only about 15% of the cells in the untreated group leave the lungs at 4 hours after transfer (Figure 5B). Also, the 24-hour time point reveals a higher percentage of cells remaining in the lungs of the untreated group compared with the LFA-1 group. This result clearly demonstrates that LFA-1 plays a crucial role in retention of activated CD8 T cells in the lungs. As an isotype control (rat IgG2a), we used TIB241 antibody that binds CD44 expressed at high levels on the D4 cells (data not shown). Blocking CD44 has no effect on the D4 cell transit time compared with the untreated D4 cells (data not shown).
It has been shown that the adherence of adoptively transferred alloreactive CD4 Th1 cells23 and CD4 T cells activated in vitro with anti-CD348 in the lungs is partially dependent on LFA-1. Blocking LFA-1 on activated Th1 cells showed significant reduction in residence time in the lungs at the single one-hour time point tested in both these studies. These data and the present study suggest that CD4 and CD8 T cells possibly have similar requirements for migration and residence in the lungs. Interestingly, the kinetics of migration as well as the localization in the lungs of type 1 versus type 2 CD8 effector cells were shown to be different in a pulmonary virus infection model, and the differences were attributed to the differential expression of chemokine receptors in these T-cell subsets.38 The Tc1 cells localized near the infected airway epithelium, whereas the Tc2 cells were found to be present at a distance away from the infected airway epithelium. This suggests an additional level of refinement in receptor expression on different T-cell subsets that would ensure rapid movement of the appropriate effector T cells to the target site.
In the current model, even after LFA-1 blockade, most CD8 T cells leave the lungs only by 4 hours after adoptive transfer. It can be argued that like neutrophils, the activated CD8 T cells following adoptive transfer are “trapped” in the pulmonary microcirculation owing to the smaller pulmonary capillary diameter and/or lack of deformability of activated T cells. However, it is unlikely to be the only explanation for the prolonged transit time of CD8 T cells since blocking LFA-1 had a pronounced effect on the transit time of the transferred T cells.
In conclusion, our data indicate a crucial role for the adhesion molecule LFA-1 in retention of effector CD8 T cells in the lungs and consequently in the generation of lethal lung injury. Although blocking LFA-1 does not completely block CD8 T-cell retention, this is the first evidence that a specific adhesion molecule is important in homing and retention of effector CD8 T cells in the lungs.
Prepublished online as Blood First Edition Paper, March 6, 2003; DOI 10.1182/blood-2002-10-3159.
Supported by National Institutes of Health (NIH) RO1 grants HL54136 (K.L.) and HL33391 (T.J.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank John Sanders for expert assistance with histology and immunostaining.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal