Abstract
Although chemokines are well known to function in chemotaxis, additional roles for these molecules in the immune system are not well understood. Dendritic cells (DCs) developmentally regulate the expression of chemokine receptors to facilitate their migration from the peripheral tissues to regional lymph nodes. Expressions of CCR1 and CCR5 on immature DCs are down-regulated on maturation, whereas CCR7 is selectively expressed on mature DCs. In the present study, we examined the effects of CCL19 and CCL21, 2 CCR7 ligands, on endocytosis of fluorescein isothiocyanate (FITC)–dextran by murine DCs. Both CCL19 and CCL21 markedly induced rapid uptake of FITC-dextran by mature DCs but not immature DCs. In contrast, CCL3, a ligand of CCR1 and CCR5, induced rapid uptake of FITC-dextran by immature DCs but not mature DCs. CCL19-induced endocytosis could be completely blocked by Clostridium difficile toxin B, which inhibits the Rho guanosine triphosphatase proteins, Rho, Rac, and Cdc42. This process was not abrogated by Y-27632, a specific inhibitor of Rho-associated kinase. In addition, CCL19 rapidly enhanced Cdc42 and Rac activity in mature DCs. These findings demonstrate that certain chemokines induce rapid endocytosis in each relevant DC population. It is suggested that CCR7 ligands activate Cdc42 and Rac, thereby inducing the endocytosis in mature DCs.
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) that play a major role in the regulation of immune responses to a variety of antigens.1-3 DCs first capture antigens via constitutive endocytosis, then present these antigens in the context of major histocompatibility complex (MHC) molecules at the cell surface.4,5 The ability of DCs to take up antigens is developmentally regulated and closely related to their endocytic capability.4,5 Immature DCs expressing modest amounts of MHC and costimulatory molecules efficiently capture antigens via constitutive endocytosis. In contrast, mature DCs expressing high levels of MHC and costimulatory molecules capture antigens poorly after down-regulating endocytosis. Thus, it has been considered that mature DCs present antigens incorporated during the immature stage to T cells. These antigens are presented concurrently with high levels of costimulatory molecules to potently activate T cells. It has remained unclear, however, whether mature DCs are not capable of efficiently capturing antigens or these cells potentially capture antigens under a certain condition.
DCs developmentally regulate the expression of chemokine receptors to facilitate their migration from the peripheral tissues to regional lymph nodes (LNs).6,7 High densities of CCR7, the receptor for CCL19 (Epstein-Barr virus–induced receptor ligand chemokine [ELC]) and CCL21 (secondary lymphoid tissue chemokine [SLC]), are expressed on mature, but not immature, DCs.6,7 CCL19 and CCL21, small proteins with molecular weights of approximately 10 kDa, bind to the G protein–coupled CCR7 receptor containing 7 transmembrane domains.8 Both CCL19 and CCL21, constitutively expressed at high levels in the LNs and spleen,9,10 induce the migration of mature DCs into these lymphoid tissues.6,7 Mature DCs fail to migrate into the regional LNs in CCR7-KO mice, correlating with abolished adaptive immune responses.11 Thus, the interaction between CCR7 and its ligands, CCL19 and CCL21, is an essential process controlling the migration of mature DCs into the regional LNs.
Recently, we have demonstrated that CCL19 induces not only chemotaxis but also the extension of dendrites by mature DCs.12 It is unclear, however, whether these chemokines play additional roles in the regulation of the functional properties of mature DCs. We examined the effects of CCL19 and CCL21 on endocytosis in murine DCs. We demonstrate that these chemokines rapidly (within a few minutes) induce endocytosis by mature DCs. We also demonstrate involvement of the Rho family of guanosine triphosphatase (GTPase) proteins, which likely control the actin cytoskeletal organization,13 in the CCL19-induced endocytosis of mature DCs.
Materials and methods
Reagents
Recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) was purchased from Peprotech (London, United Kingdom). Recombinant murine CCL19 was obtained from Genzyme (Cambridge, MA). Recombinant murine CCL3 and CCL21 were obtained from R & D Systems (Minneapolis, MN). Fluorescein isothiocyanate (FITC)–dextran was acquired from Molecular Probes (Eugene, OR). Mannan (Saccharomyces cerevisiae), FITC-albumin (bovine), pertussis toxin (PTX), and lipopolysaccharide (LPS) were purchased from Sigma Chemical (St Louis, MO). Clostridium difficile toxin B and Y-27632 were purchased from Calbiochem (La Jolla, CA). Iscove modified Dulbecco medium (IMDM) was purchased from Sigma Chemical.
Culture media
Culture medium used in the present study was IMDM supplemented with 100 IU/mL penicillin, 100 μg/mL streptomycin, 600 μg/mL l-glutamine, and 50 μM 2-mercaptoethanol. Fibroblast supernatants from NIH/3T3 cells were collected from confluent cultures with IMDM containing 10% heat-inactivated fetal calf serum (FCS).
DC culture
The DC line (BC1) was generated from BALB/c mouse spleen as previously described.14,15 BC1 cells were cultured and expanded in R1 medium, IMDM containing 10% FCS, 30% NIH/3T3 cultured supernatant, and 10 ng/mL mouse recombinant GM-CSF.14,15 BC1 cells are positive for CD11c, MHC class I, MHC class II, CD80, and CD86.14 Although BC1 cells exhibit an immature phenotype, various activating factors including tumor necrosis factor α (TNF-α) and LPS promote maturation of this precursor line. Mature BC1 cells express high levels of MHC and costimulatory molecules and demonstrate potent allostimulatory capability compared with immature BC1 cells.14 In the present study, BC1 cells (3 × 105 cells/mL) treated with 5 μg/mL LPS for 24 hours were considered to be mature cells.
Spleen-derived DCs (SDDCs) were generated from C57BL/6 (B6) splenocytes as described.12 B6 female mice were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan). Single-cell suspensions of the spleens from B6 mice (8 weeks of age) were prepared by passing the organ through a stainless steel mesh. Erythrocytes were lysed by ammonium chloride treatment. The remaining unfractionated cell population was plated at a density of 0.5 to 1 × 106 cells/mL in R1 medium. Fresh R1 medium was added after 4 or 5 days. Cultures were then fed every 3 to 4 days with fresh R1 medium. On day 14 of the culture, weakly adherent and suspension cells (DC-enriched fraction) were collected by treatment with 3 mM EDTA (ethylenediaminetetraacetic acid). DCs were positively selected from this population using anti-CD11c (N418) MicroBeads and MACS column (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. The purified cells were more than 95% CD11c+ cells in purity; these purified populations were used as SDDCs. SDDCs expressed moderate levels of MHC class II and CD86, the expression of which was markedly enhanced by treatment with LPS (Y.Y. et al, unpublished data, June 2001). Treatment with 5 μg/mL LPS (3 × 105 cells/mL) for 24 hours produced mature SDDCs.
Splenic DCs were freshly isolated from B6 mouse splenocytes. The spleens of 9 female B6 mice, aged 8 weeks, were minced and resuspended in 10 mL serum-free IMDM containing 2 mg/mL collagenase D (Boehringer Mannheim, Mannheim, Germany) and 200 μg/mL DNase I (Boehringer Mannheim). The suspension was constantly mixed at room temperature for 15 minutes. Following the addition of 1 mL 30 mM EDTA, the suspension was frequently mixed for an additional 5 minutes. Undigested fibrous material was removed by filtration through a stainless steel sieve. All subsequent steps were performed at 0 to 4°C in phosphate-buffered saline (PBS) containing 2 mM EDTA (EDTA-PBS). Following recovery by centrifugation at 1200 rpm, the cells were suspended in 10 mL 55% Percoll/PBS containing 1 mM EDTA. The cell suspension was overlaid with 1 mL EDTA-PBS, then centrifuged at 1700g for 30 minutes. The cells residing in the low-density fraction at the interface were collected and washed with EDTA-PBS. DCs were then positively selected by MACS separation as described. Cells were then passed through a MACS MS column twice. Separated cells, more than 95% CD11c+ cells in purity, were used as splenic DCs. Splenic DCs treated for 20 hours with LPS (5 μg/mL) in 10% FCS IMDM were used as mature splenic DCs.
Endocytosis
BC1 cells (1 × 105) were incubated in R1 medium buffered with 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) at 37°C. SDDCs and splenic DCs (0.5-1 × 105) were incubated in 10% FCS IMDM buffered with 20 mM HEPES. The endocytic tracer and chemokine were added concurrently, prior to a 0.5- to 60-minute incubation. FITC-dextran or FITC-albumin was added to a final concentration of 0.2 to 1 mg/mL or 2 mg/mL, respectively. CCL19 or CCL21 was added to a final concentration of 20 ng/mL. CCL3 was added to a final concentration of 100 ng/mL. Endocytosis of the tracer was halted at the indicated time points by rapid cooling of the cells on ice. The cells were then washed with ice-cold PBS containing 1% FCS and 0.1% NaN3 and stained with propidium iodide to exclude dead cells. The fluorescence intensity of the cells was analyzed by flow cytometry on EPICS XL (Coulter, Miami, FL). Incubation of cells with the endocytic tracer on ice was used as a background control. The mean fluorescence intensity (MFI) resulting from the subtraction of background control from each experimental sample represented the amount of incorporated tracer. Excluding the cells similar in MFI to the background levels determined the proportion of tracer-positive cells (tracer-internalizing cells). To examine the effect of inhibitors on endocytosis, cells were pretreated for 3 hours with C difficile toxin B (100 ng/mL), PTX (100 ng/mL), or Y-27632 (10 μM) at 37°C. After inhibitor pretreatment, the cells were incubated with the endocytic tracer for 2 minutes at 37°C in the presence of the inhibitor.
To morphologically analyze endocytosis, cells (2 × 106) were incubated with FITC-dextran (1 mg/mL) at 37°C in the presence or absence of CCL19 for 2 minutes. Cells were washed with ice-cold PBS containing 1% FCS and 0.1% NaN3, then fixed in 4% paraformaldehyde for 10 minutes on ice. Following washing, the cells were resuspended in PBS containing 1% FCS and 0.1% NaN3. The cells were applied to a glass slide and subsequently covered by a coverslip. Cell morphology and fluorescence intensity were analyzed by confocal microscopy (BX61-2CHAr-HeNeGSP, FV500, Ver3.1, OLYMPUS, Tokyo, Japan).
Cdc42 and Rac activities
Cdc42 and Rac activities were assessed using a commercially available Cdc42 and Rac activation assay kit (Upstate Biotechnology, Lake Placid, NY), according to the manufacturer's protocol. Cells (5 × 106) cultured in R1 medium buffered with 10 mM HEPES at 37°C were given CCL19 at a final concentration of 20 ng/mL, then incubated for an additional 0.5 to 5 minutes. In some experiments, cells were pretreated with PTX (100 ng/mL) for 3 hours at 37°C, then incubated with CCL19 in the presence of PTX (100 ng/mL). The reaction was stopped by adding an equal volume of 2 × lysis buffer (50 mM HEPES, pH 7.5, 300 mM NaCl, 2% lgepal CA-630, 20 mM MgCl2, 2 mM EDTA, and 20% glycerol), immediately followed by vortexing and cooling on ice. Cell lysates were clarified by centrifugation at 4°C. The GTPase-binding domain of the p21-activated kinase-1 (5 μg), conjugated to agarose beads, was added to the lysate. The reaction mixture was gently rocked for 1 hour at 4°C. The bead pellet was washed with 1 × lysis buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% lgepal CA-630, 10 mM MgCl2, 1 mM EDTA, and 10% glycerol). The bead pellet was resuspended in 35 μL sodium dodecyl sulfate (SDS) sample buffer (125 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 6.8, 4% SDS, 20% glycerol, 100 mM dithiothreitol, 0.2% bromphenol blue) and boiled for 5 minutes. Samples were separated by 15% SDS-polyacrylamide gel electrophoresis, and transferred onto a polyvinylidene difluoride membrane. The membrane was probed with either anti-Cdc42 or Rac monoclonal antibody and developed with horseradish peroxidase (HRP)–conjugated secondary monoclonal antibody by enhanced chemiluminescence. To assess the total amounts of Cdc42 and Rac, the total cell lysate was subjected to the above immunoblotting procedure.
Results
Chemokine-induced endocytosis in a DC line (BC1)
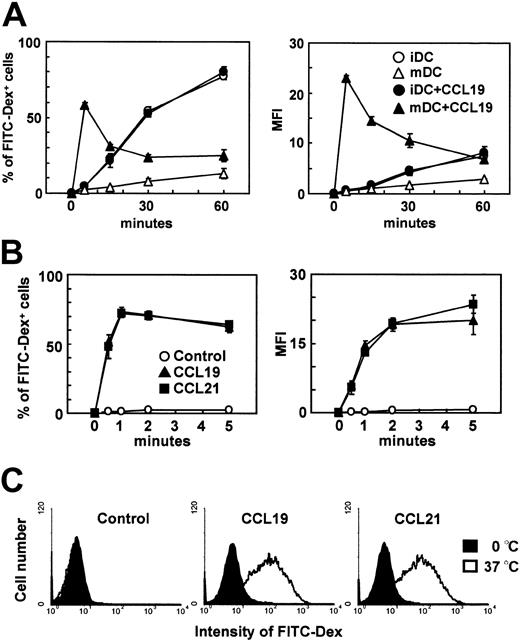
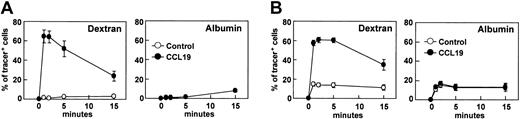
Immature DCs efficiently internalize antigens. This ability, however, is decreased during the maturational process4,5 as the mature DCs acquire potent APC functions. We previously established a murine DC line, BC1 cells, from BALB/c splenocytes.14 Unstimulated BC1 cells are physiologically immature.14 Following treatment with LPS for 24 hours, BC1 cells exhibit a mature DC phenotype (Y.Y., unpublished data, June 2001). We used these immature and mature BC1 cells to examine the effects of the CCR7 ligands, CCL19 and CCL21, on endocytosis of FITC-conjugated dextran. Immature DCs (unstimulated BC1) or mature DCs were incubated with FITC-dextran in the presence or absence of CCL19. Immature DCs internalized FITC-dextran; approximately 80% of the cells became FITC-dextran positive and MFI of the cells incorporating FITC-dextran was 7.6 after 1 hour of incubation (Figure 1A). Mature DCs, however, scarcely internalized FITC-dextran at 1 hour; the proportion of FITC+ cells was less than 15% of the total cells and MFI of the cells was 2.8. CCL19 did not influence FITC-dextran uptake by immature DCs, but significantly increased uptake by mature DCs. In the presence of CCL19, the proportion of mature DCs positive for FITC-dextran was 58.2% after 5 minutes of incubation and MFI of the mature DCs was 23.1. The proportion of FITC+ cells and MFI of the cells decreased thereafter to levels similar to those seen in the absence of CCL19. Endocytosis of FITC-dextran by mature DCs increased with added CCL19 in a dose-dependent manner, peaking at 20 ng/mL after 5 minutes of treatment (Y.Y., unpublished data, July 2002). CCL21 exerted the same effect on mature DCs with a similar dose dependency to CCL19 (Y.Y., unpublished data, July 2002).
The effects of CCR7 ligands on the endocytosis of FITC-dextran by immature or mature DCs. BC1 cells were incubated with FITC-dextran (Dex) at 1 mg/mL in the presence or absence of CCL19 or CCL21 for the indicated times. Unstimulated BC1 cells were used as immature DCs (iDC). BC1 cells treated with LPS for 24 hours were used as mature DCs (mDC). (A) Time course of endocytosis by immature or mature DCs; each symbol represents the mean ± SE of 3 independent experiments. (B) Time course of endocytosis by mature DCs within 5 minutes; each symbol represents the mean ± SE of 3 independent experiments. (C) Amount of FITC-Dex internalized by mature DCs at 1 minute; data are representative of 3 independent experiments.
The effects of CCR7 ligands on the endocytosis of FITC-dextran by immature or mature DCs. BC1 cells were incubated with FITC-dextran (Dex) at 1 mg/mL in the presence or absence of CCL19 or CCL21 for the indicated times. Unstimulated BC1 cells were used as immature DCs (iDC). BC1 cells treated with LPS for 24 hours were used as mature DCs (mDC). (A) Time course of endocytosis by immature or mature DCs; each symbol represents the mean ± SE of 3 independent experiments. (B) Time course of endocytosis by mature DCs within 5 minutes; each symbol represents the mean ± SE of 3 independent experiments. (C) Amount of FITC-Dex internalized by mature DCs at 1 minute; data are representative of 3 independent experiments.
Because CCR7 ligand–induced endocytosis was seen at a very early period, we analyzed endocytosis by mature DCs at earlier time points, ranging from 30 seconds to 5 minutes. Both CCL19 and CCL21 increased endocytosis of FITC-dextran by mature DCs within seconds of the addition of ligand (Figure 1B). After 30 seconds, approximately 50% of mature DCs were positive for FITC-dextran. In the absence of chemokines, mature DCs exhibited negligible endocytosis. The proportion of cells internalizing FITC-dextran peaked at 1 minute after ligand addition (approximately 70% positive), decreasing slightly at 2 and 5 minutes. MFI of mature DCs incorporating FITC-dextran increased significantly from 30 seconds to 2 minutes in the presence of either CCL19 or CCL21. No considerable differences were observed in the time courses of endocytic activity between CCL19- and CCL21-treated cultures. Histogram analysis also indicated significant levels of FITC-dextran uptake by mature DCs as early as 1 minute after treatment with CCL19 and CCL21 (Figure 1C).
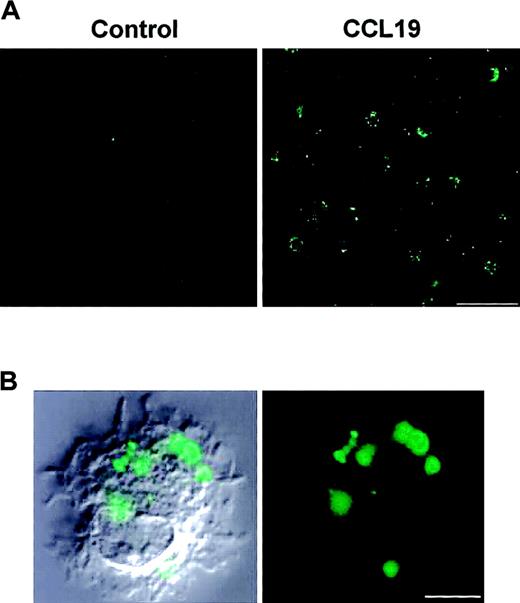
Following a 2-minute incubation with FITC-dextran in the presence or absence of CCL19, we analyzed the morphology of CCL19-induced endocytosis using confocal microscopy (Figure 2). A large number of FITC+ spots, corresponding to FITC-dextran–incorporating endosomes, were observed in CCL19-treated cells, whereas almost no spots were detected in untreated (control) cells.
Morphologic evidence for CCL19-induced endocytosis. Mature DCs that are BC1 cells treated with LPS for 24 hours were incubated with FITC-dextran at 1 mg/mL in the presence or absence of CCL19 for 2 minutes. Following fixation in 4% paraformaldehyde, the fluorescence intensity of the cells was analyzed by confocal microscopy. (A) Endocytosis in the presence or absence (control) of CCL19. Bar represents 50 μm; original magnification, × 60. (B) Immunofluorescence image (right panel) and merged image of CCL19-treated mature DCs with differential interference contrast of the same cell (left panel). Bar represents 5 μm; original magnification, × 360.
Morphologic evidence for CCL19-induced endocytosis. Mature DCs that are BC1 cells treated with LPS for 24 hours were incubated with FITC-dextran at 1 mg/mL in the presence or absence of CCL19 for 2 minutes. Following fixation in 4% paraformaldehyde, the fluorescence intensity of the cells was analyzed by confocal microscopy. (A) Endocytosis in the presence or absence (control) of CCL19. Bar represents 50 μm; original magnification, × 60. (B) Immunofluorescence image (right panel) and merged image of CCL19-treated mature DCs with differential interference contrast of the same cell (left panel). Bar represents 5 μm; original magnification, × 360.
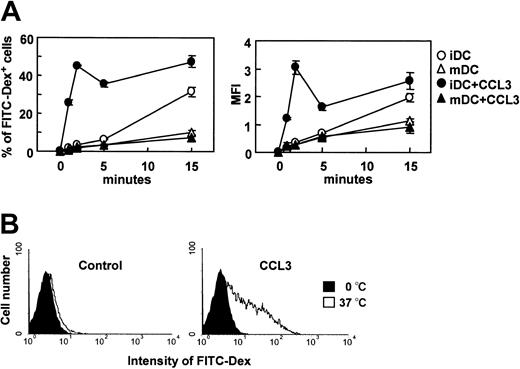
It has been demonstrated that CCR7 ligands are active on mature DCs but not immature DCs, whereas CCL3 (macrophage inflammatory protein 1α), a ligand for CCR1 and CCR5, is active on immature DCs but not mature DCs.16,17 We then analyzed the effect of CCL3 on endocytosis of FITC-dextran by immature and mature DCs. Unlike CCR7 ligands, CCL3 significantly increased FITC-dextran uptake by immature DCs but not mature DCs within a few minutes (Figure 3A). The CCL3-induced uptake of FITC-dextran reached a peak at 2 minutes and decreased to levels comparable to those seen in the absence of CCL3 at 15 minutes. Histogram analysis also indicated a significant level of FITC-dextran uptake by immature DCs at 2 minutes after treatment with CCL3 (Figure 3B).
The effect of CCL3 on the endocytosis of FITC-dextran by immature or mature DCs. BC1 cells were incubated with FITC-dextran (Dex) at 1 mg/mL in the presence or absence of CCL3 for the indicated times. Unstimulated BC1 cells were used as immature DCs (iDC). BC1 cells treated with LPS for 24 hours were used as mature DCs (mDC). (A) Time course of endocytosis by immature DCs (iDC) or mature DCs (mDC); each symbol represents the mean ± SE of 3 independent experiments. (B) Amount of FITC-Dex internalized by immature DCs at 2 minutes; data are representative of 3 independent experiments.
The effect of CCL3 on the endocytosis of FITC-dextran by immature or mature DCs. BC1 cells were incubated with FITC-dextran (Dex) at 1 mg/mL in the presence or absence of CCL3 for the indicated times. Unstimulated BC1 cells were used as immature DCs (iDC). BC1 cells treated with LPS for 24 hours were used as mature DCs (mDC). (A) Time course of endocytosis by immature DCs (iDC) or mature DCs (mDC); each symbol represents the mean ± SE of 3 independent experiments. (B) Amount of FITC-Dex internalized by immature DCs at 2 minutes; data are representative of 3 independent experiments.
CCL19-induced endocytosis in SDDCs and splenic DCs
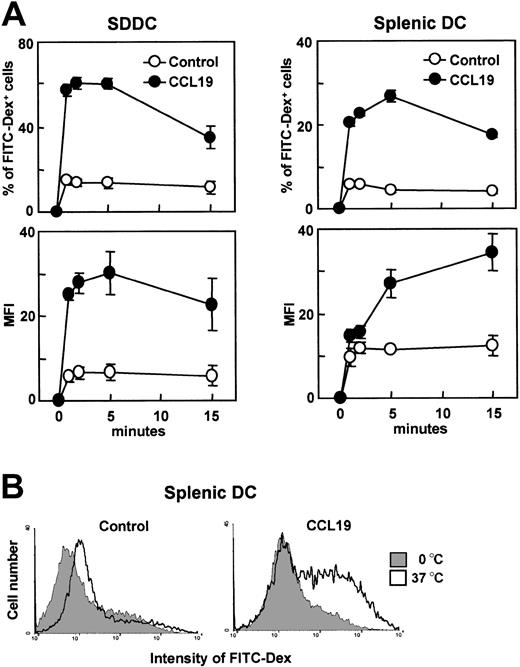
We then examined the effect of CCR7 ligands on the endocytic activity of 2 additional DC culture systems, SDDCs and freshly isolated splenic DCs. SDDCs were generated by culturing mouse splenocytes with GM-CSF and fibroblast supernatant for 14 days.12 SDDCs (CD11c+ > 95%) and freshly isolated splenic DCs (CD11c+ > 95%) were treated with LPS for 24 or 20 hours, respectively, to produce mature DCs. CCL19 significantly increased the uptake of FITC-dextran in both types of mature DCs (Figure 4A). In the presence of CCL19, the proportion of FITC-dextran–positive mature DCs markedly increased within a few minutes, decreasing after 15 minutes, and MFI of the mature DCs still showed an increase at this time point. CCL21 also significantly increased FITC-dextran uptake in mature SDDCs (data not shown). Histogram analysis indicated a significant level of FITC-dextran uptake by mature splenic DCs at 2 minutes after treatment with CCL3 (Figure 4B).
The effect of CCL19 on the endocytosis of FITC-dextran by SDDCs and splenic DCs. SDDCs were generated by culturing splenocytes with fibroblast supernatant and GM-CSF for 14 days. Splenic DCs were freshly isolated from B6 mice. SDDCs and splenic DCs were treated with LPS for 24 or 20 hours, respectively, for use as mature DCs. Mature SDDCs or splenic DCs were incubated with FITC-dextran at 1 mg/mL in the presence or absence of CCL19. (A) Time course of endocytosis; each symbol represents the mean ± SE of 3 independent experiments. (B) Amount of FITC-Dex internalized by mature splenic DCs at 5 minutes; data are representative of 3 independent experiments.
The effect of CCL19 on the endocytosis of FITC-dextran by SDDCs and splenic DCs. SDDCs were generated by culturing splenocytes with fibroblast supernatant and GM-CSF for 14 days. Splenic DCs were freshly isolated from B6 mice. SDDCs and splenic DCs were treated with LPS for 24 or 20 hours, respectively, for use as mature DCs. Mature SDDCs or splenic DCs were incubated with FITC-dextran at 1 mg/mL in the presence or absence of CCL19. (A) Time course of endocytosis; each symbol represents the mean ± SE of 3 independent experiments. (B) Amount of FITC-Dex internalized by mature splenic DCs at 5 minutes; data are representative of 3 independent experiments.
Effect of CCL19 on receptor-independent endocytosis of albumin
Dextrans are thought to be internalized via a receptor-mediated pathway, whereas albumin is internalized via receptor-independent macropinocytosis in DCs.4 We then compared the effect of CCL19 on FITC-albumin macropinocytosis with that on FITC-dextran uptake (Figure 5). In the absence of chemokines, mature BC1 cells poorly internalized both fluorescein-conjugated substrate. Yet although CCL19 increased FITC-dextran uptake by mature BC1 cells, this chemokine did not exert a significant influence on FITC-albumin uptake. Similar results were obtained with SDDCs; CCL19 had no significant effect on the FITC-albumin uptake by mature SDDCs, but markedly increased the FITC-dextran uptake (Figure 5). These findings demonstrate that CCL19 is not involved in receptor-independent macropinocytosis in mature DCs.
The effect of CCL19 on the endocytosis of FITC-albumin by mature DCs. BC1 cells (A) and SDDCs (B) were treated with LPS for 24 hours for use as mature DCs. Mature DCs were incubated with FITC-dextran (1 mg/mL) or FITC-albumin (2 mg/mL) in the presence or absence of CCL19 for the indicated times. Each symbol represents the mean ± SE of 3 independent experiments.
The effect of CCL19 on the endocytosis of FITC-albumin by mature DCs. BC1 cells (A) and SDDCs (B) were treated with LPS for 24 hours for use as mature DCs. Mature DCs were incubated with FITC-dextran (1 mg/mL) or FITC-albumin (2 mg/mL) in the presence or absence of CCL19 for the indicated times. Each symbol represents the mean ± SE of 3 independent experiments.
Effect of mannan on CCL19-induced endocytosis of FITC-dextran
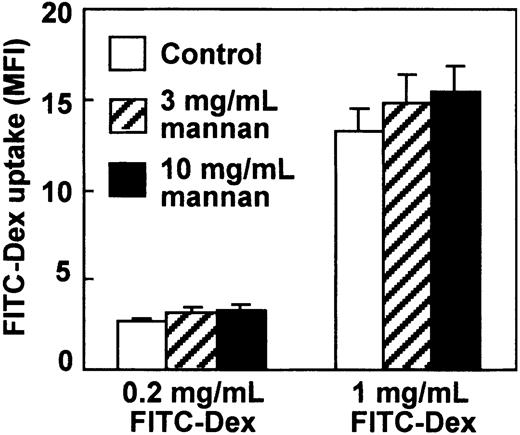
Dextrans are hydrophilic polysaccharides produced by bacteria such as Leuconostoc mesenteroides.18 Because it was reported that FITC-dextran was internalized via a mannose receptor–mediated pathway in human monocyte–derived DCs and this mannose receptor–mediated endocytosis was inhibited by mannan,4 we analyzed influence of mannan in CCR7 ligand–induced endocytosis by our mature DCs. It has been reported that mannan at 3 mg/mL almost completely inhibits endocytosis of FITC-dextran at 1 mg/mL by human monocyte–derived DCs.4 However, mannan up to 10 mg/mL failed to inhibit CCL19-induced endocytosis of FITC-dextran at 0.2 or 1 mg/mL by mature DCs (Figure 6).
The effect of mannan on CCL19-induced endocytosis by mature DCs. BC1 cells treated with LPS for 24 hours were used as mature DCs. Mature DCs were pretreated with mannan at 3 or 10 mg/mL for 10 minutes, then incubated with FITC-dextran (Dex) at 0.2 or 1 mg/mL in the presence of CCL19. The FITC-Dex uptake was determined 2 minutes after treatment. Each column represents the mean ± SE of 3 independent experiments.
The effect of mannan on CCL19-induced endocytosis by mature DCs. BC1 cells treated with LPS for 24 hours were used as mature DCs. Mature DCs were pretreated with mannan at 3 or 10 mg/mL for 10 minutes, then incubated with FITC-dextran (Dex) at 0.2 or 1 mg/mL in the presence of CCL19. The FITC-Dex uptake was determined 2 minutes after treatment. Each column represents the mean ± SE of 3 independent experiments.
Effects of C difficile toxin B, Y-27632, and PTX on CCL19-induced endocytosis of dextran by mature DCs
The constitutive endocytic activities of macrophages and DCs are abolished by treatment with C difficile toxin B, an inhibitor for the Rho family of GTPase proteins, including Rho, Rac, and Cdc42.19,20 We examined the effect of C difficile toxin B and Y-27632, a specific inhibitor of the Rho target Rho-associated kinase,21 on CCL19-induced endocytosis in mature DCs. Mature BC1 cells were pretreated with toxin B or Y-27632 for 3 hours, then incubated with FITC-dextran in the presence of CCL19 for 2 minutes at 37°C. Whereas CCL19 increased the endocytosis of FITC-dextran by nontreated mature DCs at 2 minutes (Figure 7A), toxin B strongly inhibited CCL19-induced endocytosis (Figure 7A-B). Toxin B treatment exerted no influences on cell viability (Figure 7C). Y-27632, however, did not inhibit CCL19-induced endocytosis (Figure 7A-B), although this treatment reduced cell viability slightly (Figure 7C) and induced a significant dendritic extension of mature BC1 cells (data not shown).12
The effects of C difficile toxin B, Y-27632, or PTX on CCL19-induced endocytosis by mature DCs. BC1 cells treated with LPS for 24 hours were used as mature DCs. Mature DCs were pretreated with C difficile toxin B, Y-27632, or PTX for 3 hours, then incubated with FITC-dextran (Dex) at 1 mg/mL. The amount of FITC-Dex endocytosis was determined 2 minutes after treatment. (A) Proportion of FITC-Dex–positive cells (left panel) and the MFI of the cells (right panel) following incubation; each column represents the mean ± SE of 4 independent experiments. (B) Representative fluorescence histograms from 4 independent experiments. (C) The effect of inhibitors on the viability of DCs. Following the treatment of mature BC1 cells with each inhibitor for 3 hours, the proportions of propidium iodide–negative (PI–) cells were determined; each column represents the mean ± SE of 4 independent experiments.
The effects of C difficile toxin B, Y-27632, or PTX on CCL19-induced endocytosis by mature DCs. BC1 cells treated with LPS for 24 hours were used as mature DCs. Mature DCs were pretreated with C difficile toxin B, Y-27632, or PTX for 3 hours, then incubated with FITC-dextran (Dex) at 1 mg/mL. The amount of FITC-Dex endocytosis was determined 2 minutes after treatment. (A) Proportion of FITC-Dex–positive cells (left panel) and the MFI of the cells (right panel) following incubation; each column represents the mean ± SE of 4 independent experiments. (B) Representative fluorescence histograms from 4 independent experiments. (C) The effect of inhibitors on the viability of DCs. Following the treatment of mature BC1 cells with each inhibitor for 3 hours, the proportions of propidium iodide–negative (PI–) cells were determined; each column represents the mean ± SE of 4 independent experiments.
PTX specifically binds to Gi proteins to inhibit chemokine-induced cell migration.22-24 We examined the involvement of Gi proteins in CCL19-induced endocytosis by pretreating mature BC1 cells with PTX for 3 hours. Cells were then incubated with FITC-dextran in the presence of CCL19 for 2 minutes at 37°C. PTX significantly inhibited CCL19-induced endocytosis (Figure 7A-B) without affecting cell viability (Figure 7C).
CCL19-induced Cdc42 and Rac activation in mature DCs
The inhibitor studies (Figure 7) suggested that Cdc42 and Rac, but not Rho, are involved in CCL19-induced endocytosis. No previous reports, however, have demonstrated the activation of Cdc42 and Rac by CCL19. We therefore examined the effect of CCL19 treatment on the activation of Cdc42 and Rac in mature DCs. Mature BC1 cells were treated at 37°C with CCL19 for a variable time period, ranging from 30 seconds to 5 minutes. Activated Cdc42 and Rac (GTP-bound form) were detected by affinity precipitation assay using an agarose bead–conjugated GTPase-binding domain derived from the p21-activated kinase-1, a typical substrate of Cdc42 and Rac.25 Low levels of constitutively active Cdc42 and Rac were detected in mature BC1 cells (Figure 8A-B). Thirty seconds after CCL19 treatment, the levels of activated Cdc42 and Rac increased significantly; these activities decreased from 1 to 5 minutes. The changes in activity of both Cdc42 and Rac followed similar time courses, although decreases in Rac activity appeared to be more rapid than those seen for Cdc42. In addition, PTX completely inhibited CCL19-induced activation of both Cdc42 and Rac (Figure 8C). The total amounts of Cdc42 and Rac protein, however, exhibited no significant changes after treatment with either CCL19 or PTX (Figure 8C).
Cdc42 and Rac activation in mature DCs after CCL19 stimulation. BC1 cells treated with LPS for 24 hours were used as mature DCs. The quantity of activated Cdc42 and Rac (GTP-bound form) in the cells was determined by an affinity precipitation assay. (A) Kinetics of Cdc42 and Rac activation following stimulation of mature BC1 cells with CCL19 for 0.5, 1, 2, and 5 minutes. (B) The mean relative intensity ± SE of 3 independent experiments. (C) The effect of PTX on Cdc42 and Rac activity. Mature BC1 cells were pretreated with PTX for 3 hours, then stimulated with CCL19 for 30 seconds. The amount of GTP-bound (GTP) and total (total) Cdc42 (left panel) and Rac (right panel) are each shown. These results are representative of 3 independent experiments with the same results.
Cdc42 and Rac activation in mature DCs after CCL19 stimulation. BC1 cells treated with LPS for 24 hours were used as mature DCs. The quantity of activated Cdc42 and Rac (GTP-bound form) in the cells was determined by an affinity precipitation assay. (A) Kinetics of Cdc42 and Rac activation following stimulation of mature BC1 cells with CCL19 for 0.5, 1, 2, and 5 minutes. (B) The mean relative intensity ± SE of 3 independent experiments. (C) The effect of PTX on Cdc42 and Rac activity. Mature BC1 cells were pretreated with PTX for 3 hours, then stimulated with CCL19 for 30 seconds. The amount of GTP-bound (GTP) and total (total) Cdc42 (left panel) and Rac (right panel) are each shown. These results are representative of 3 independent experiments with the same results.
Discussion
CCL19 and CCL21, selective agonists for CCR7, drive the migration of T cells and mature DCs into the LNs.6,7,26 Although the role of CCL19 and CCL21 in cellular trafficking into and within secondary lymphoid tissues has been well documented, additional functions of these chemokines have remained poorly understood. Recently, we demonstrated that CCR7 ligands induced the extension of dendrites in murine mature DCs.12 Thus, CCR7 ligands induce not only chemotaxis but also morphologic changes. We have demonstrated herein a novel function for the CCR7 ligands, CCL19 or CCL21, as rapid inducers of receptor-mediated endocytosis of dextran by mature, but not immature, DCs.
Immature DCs exhibit vigorous acquisition of fluid-phase molecules, whereas mature DCs captured only low levels of exogenous antigens because of the down-regulation of endocytic processes constitutive in immature DCs.4,5 Mature DCs are capable of presenting antigens taken up during the immature stage to T cells at the mature stage. Our findings, however, reveal that mature DCs can efficiently acquire antigens in the presence of CCR7 ligands.
DCs developmentally regulate the expression of chemokine receptors.6,7 Expressions of CCR1 and CCR5 on immature DCs are down-regulated on maturation, whereas the expression of CCR7 on mature DCs are selectively augmented.16,17 CCR7 ligands induce chemotaxis or Ca2+ influx in mature DCs but not immature DCs, whereas CCL3, a ligand for CCR1 and CCR5, is active on immature DCs but not mature DCs.16,17 In the present study, we found that CCR7 ligands markedly induced endocytosis of FITC-dextran by mature DCs but not immature DCs (Figure 1). On the other hand, CCL3 enhanced endocytosis by immature DCs in a few minutes but not mature DCs (Figure 3). Thus, chemokines may regulate rapid endocytic activity of DCs in a manner that corresponds to the expression pattern of chemokine receptors on these DCs. However, we consider that the chemokine-induced endocytosis of mature DCs is more impressive and physiologically important than that of immature DCs. Constitutive endocytic activity was considerably low in mature DCs compared with that in immature DCs (Figure 1).4 We are currently examining effects of various chemokines on endocytosis of immature and mature DCs.
Dextrans are hydrophilic polysaccharides produced by bacteria such as L mesenteroides.18 CCR7 ligands markedly induced endocytosis of FITC-dextran, while showing no effects on receptor independent endocytosis of FITC-albumin. It has been reported that FITC-dextran is internalized via a mannose receptor–mediated pathway in human monocyte–derived DCs.4 However, mannan, which potently inhibits mannose receptor–mediated endocytosis in human system, showed no influences on CCR7 ligand–induced endocytosis of FITC-dextran by mature DCs (Figure 6). Thus, CCL19-induced endocytosis of FITC-dextran may be mediated via a receptor(s) other than mannose receptors, which recognize bacterium-derived polysaccharides. Indeed, it has been reported that mouse peritoneal macrophages internalize HRP and FITC-dextran by constitutive endocytosis, and mannan significantly inhibits the HRP uptake but never affects the FITC-dextran uptake.27 Thus, unlike human mannose receptors, mouse mannose receptors may not be involved in FITC-dextran uptake. We are now pursuing receptor(s) involved in the chemokine-induced endocytosis of FITC-dextran by murine DCs.
In the present study, we used 3 DC culture systems, DC line (BC1), SDDCs, and splenic DCs. CCL19 induced considerable endocytosis in all DC culture systems. The endocytosis induced in mature splenic DCs was less impressive than the effects seen for mature BC1 cells and SDDCs. DCs freshly isolated from lymphoid organs undergo apoptosis during short-term culturing.28 In this study, freshly isolated DCs were cultured with LPS for 20 hours for use as mature splenic DCs. After this culture period, approximately one fifth of the DCs acquired propidium iodide positivity (Y.Y., unpublished data, October 2002). The proportions of propidium iodide–positive cells remained less than 2% in mature BC1 cell and SDDC cultures. Thus, the low level of CCL19-induced endocytosis observed in mature splenic DCs may result from low viability.
Rho GTPase proteins, including Rho, Rac, and Cdc42, belong to the Ras superfamily acting as molecular switches to control cellular processes by cycling between active (GTP-bound) and inactive (guanosine diphosphate [GDP]–bound) states.13 The Rho GTPase protein controls the organization of the actin cytoskeleton. Recently, it has been reported that Cdc42 and Rac regulate endocytosis of DCs.19,29 Whereas blockade of Cdc42 or Rac function using a dominant-negative inhibitor abrogated endocytosis in immature DCs, transfection of an active mutant of either Cdc42 or Rac increased the endocytic activity of mature DCs.19 Cdc42 activity also decreased with increasing DC maturation that corresponded to diminutions in endocytic activity.19 No report, however, has identified the molecule(s) regulating Cdc42 and Rac activity in DCs. We demonstrated that CCL19 activated both Cdc42 and Rac in mature DCs, corresponding to the increased endocytosis by these mature DCs. In addition, CCL19-induced endocytosis by mature DCs was inhibited by toxin B, a specific inhibitor of Rho GTPases, but not by Y-27632, a specific inhibitor of the Rho-mediated pathway. These observations indicate that CCL19 induces endocytosis via activation of Cdc42 and Rac in mature DCs.
We observed that Y-27632 (10 μM) induced a significant dendritic extension of DCs (data not shown).12 Similar observations were reported with human monocyte–derived DCs.30 Thus, the Rho-mediated pathway appeared to be involved in negative regulation of dendritic extension of DCs, although it was also reported that a high dose of Y-27632 (30 μM) led to the disappearance of dendrites of human monocyte-derived DCs.31
Chemokine receptors are coupled to Gi proteins. PTX, a specific inhibitor of Gi proteins, abrogates the cell migration induced by chemokines.22-24 PTX inhibits CCL19-induced migration of mature DCs.17 In this study, we demonstrated that PTX also inhibited CCL19-induced endocytosis of mature DCs. Thus, Gi proteins coupled to CCR7 are involved in signal pathways functioning not only in chemotaxis but also in the regulation of endocytosis in mature DCs. Gi proteins are known to be involved in the activation of Cdc42 and Rac in mouse fibroblasts.32 As the CCL19-induced activation of Cdc42 and Rac was inhibited by PTX, Gi proteins appeared to also be involved in Cdc42 and Rac activation by CCR7 ligands in mature DCs. Thus, we would like to hypothesize that CCL19 activates Cdc42 and Rac via Gi proteins coupled to CCR7, thereby inducing endocytosis in mature DCs. Toxin B and PTX failed to inhibit constitutive endocytosis of FITC-dextran by immature DCs (data not shown).
We demonstrated herein a novel role of CCR7 ligands in the regulation of endocytosis in mature DCs. Because the endocytosis of microorganic antigens by DCs is essential for antigen presentation to T cells and the induction of adaptive immunity against the microorganisms, it seems to us that severe immunodeficiency seen in CCR7 KO mice11 is attributed to not only malfunction of chemotaxis but also insufficient endocytosis by mature DCs. Elucidation of the complex pathways that control CCR7-induced endocytosis may lead to the development of clinical applications exploiting this new regulation system for the treatment of various infectious diseases and immune disorders.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-11-3474.
Supported by a Grant-in-Aid for Scientific Research (S) and a Grant-in-Aid for Scientific Research on a Priority Area (C) by Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, and by the Tomakomai East Hospital Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal