Abstract
Sphingosine 1-phosphate (S1P) is a pleiotropic lysosphingophospholipid stored and secreted by platelets. Using reverse transcription–polymerase chain reaction and flow cytometric analyses, we determined the expression of S1P receptors (S1P1, S1P3, S1P4, and S1P5) in peripheral blood T cells. T cells were induced to proliferate in the presence of phorbol 12-myristate 13-acetate (PMA) plus ionomycin, anti-CD3 plus anti-CD28, and allogeneic immature or mature dendritic cells. This activity was inhibited by the addition of S1P. Enhanced T-cell proliferation was observed when these cells were stimulated with the same stimuli, but were incubated in serum-free media (SFM). Addition of S1P to SFM inhibited the stimulation of T cells induced by T-cell stimuli, suggesting that S1P is an important inhibitory molecule present in the serum. T-cell proliferation was also inhibited by the addition of dihydrosphingosine 1-phosphate (DHS1P), sphingosine, and ceramide; however, the latter 2 sphingolipids required higher concentrations than S1P. Pretreatment of T cells with pertussis toxin (PTX) blocked the inhibitory effect of S1P on activation with PMA plus ionomycin, but not on activation with anti-CD3 plus anti-CD28. This is corroborated with the down-regulation of S1P1 in T cells stimulated with anti-CD3 plus anti-CD28. Similarly, PTX did not affect the inhibitory effect of S1P on T-cell proliferation when dendritic cells were used as stimuli. Further, S1P or DHS1P but not ceramide or sphingosine enhanced rather than decreased secretion of interleukin 2 and interferon γ by T cells stimulated with anti-CD3 plus anti-CD28. These results show differential effects of S1P on polyclonal T-cell proliferation and cytokine secretion.
Introduction
Sphingosine 1-phosphate (S1P), a lysosphingophospholipid, is generated by the conversion of sphingomyline into ceramide by sphingomylinase, ceramide into sphingosine by ceramidase, and sphingosine into S1P by sphingosine kinase.1 S1P acts exogenously by binding 7 transmembrane-spanning domain receptors that bind heterotrimeric G proteins.2,3 S1P1 couples to the pertussis toxin (PTX)–sensitive Gi and Go, whereas S1P2, S1P3, S1P4, and S1P5 bind to the PTX-sensitive as well as the PTX-insensitive Gq, G12, or G13.4-6
S1P is secreted by platelets, binds albumin, and represents a large percentage of bovine and human serum.7,8 The total S1P contents in human plasma and serum reach micromolar concentrations.7 Although platelets are considered to be the major source of S1P, other cell types such as erythrocytes, neutrophils, and mononuclear cells also secrete S1P.8 Goetzl et al9,10 described the presence of S1P and lysophosphatidic acid (LPA) receptors in T cells, with an emphasis on the expression of LPA receptors such as LPA1 and LPA2. LPA2 seems to play a major role in LPA-inhibition of apoptosis.9,10 Also, LPA has dual effects on the proliferation of various cell types. For example, it induces the proliferation of NIH 3T3 fibroblasts, whereas it inhibits the proliferation of cells of myeloid and lymphoid origin, such as YAC-1 lymphoma, EL4 lymphoma, or SP2 myeloma, among others.11 On the other hand, S1P either stimulates DNA synthesis in 3T3 fibroblasts12 or inhibits DNA synthesis in primary hepatic myofibroblasts.13
Although the effect of LPA has been described in T cells, where it blocks secretion of interleukin 2 (IL-2),9,10 little is known about the effect of S1P on T cells. Recently, Idzko and coworkers reported that S1P receptors are expressed on the surface of immature and mature dendritic cells (DCs), associated with favoring the polarization of Th2 cells by mature DCs generated in the presence of S1P.14 Also, recent results have shown that S1P and its derivatives induce lymphopenia in animals, associated with increased sequestration of lymphocytes in the lymph nodes and thoracic ducts.15 Here, we have examined the immunoregulatory action of S1P, with an emphasis on T-cell proliferation and cytokine secretion.
Materials and methods
Culture medium
Culture medium (CM) consisted of RPMI 1640 supplemented with 10% fetal calf serum, 10 U/mL penicillin, 100 μg/mL streptomycin, 1 mM l-glutamine, and 1% nonessential amino acids (Gibco BRL Life Technologies, Breda, the Netherlands) and 5 × 10–5 M 2-mercaptoethanol (Sigma-Aldrich, Oslo, Norway). Serum-free medium (AIM V) was purchased from Gibco BRL.
Reagents and antibodies
S1P, sphingosine (D-erythro-sphingosine), ceramide, phorbol 12-myristate 13-acetatae (PMA), ionomycin, mitomycin C, PTX, and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich. Dihydrosphingosine 1-phosphate (DHS1P) was purchased from Avanti Polar Lipid (Alabaster, AL). Recombinant human IL-4 was from Nordic Biosite (Propellervagen, Sweden), and recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) was from Gibco BRL. Antibodies to human CD3, or CD28, and fluorescein isothiocyanate (FITC)–conjugated antibodies to human CD80, CD83, CD86, or mouse IgG1 were purchased from Becton Dickinson Pharmingen (Laborel AS, Oslo, Norway). Rabbit polyclonal antibodies to the carboxy-terminal of S1P1, S1P4, and S1P5, monoclonal antibodies to the amino-terminal of S1P2 and S1P3, and peptides specific for anti-S1P1 or anti-S1P5 were purchased from Sigma-Aldrich. Monoclonal anti-S1P3 was purchased from Exalpha Biologicals (Boston, MA).
Semiquantitative RT-PCR
Peripheral blood T cells were isolated by depleting other leukocyte populations using a pan T-cell isolation kit (Miltenyi Biotech, Bergisch Gladbach, Germany). These cells were more than 98% CD3+ as determined by flow cytometric analysis. Jurkat, PEER, NK92, 221, U937, and RPMI 8866 (designated RPMI in Figure 1) cell lines were cultured in Iscove media supplemented with 10% fetal bovine serum (FBS). Preparation of RNA and the sequences of primer pairs for S1P1, S1P3, S1P4, and S1P5 have been recently described.16 The primers for glyceraldehyde-3-phosphate dehydrogenase (G3PDH) were: 5′-GGCATGGACTGTGGTCATGAG-3′ and 5′-TGCACCACCAACTGCTTAGC-3′. Reverse transcription–polymerase chain reaction (RT-PCR) products were prepared as described.16
Expression of S1P receptors in peripheral blood T cells. (A) mRNA for peripheral blood T cells and for T-cell lines (Jurkat and PEER), an NK-cell line (NK92), B-cell lines (RPMI and .221), and a monocytic cell line (U937) is shown. Shown is RT-PCR analysis of mRNA for S1P1, S1P3, S1P4, and S1P5, as well as the housekeeping gene for glyceraldehyde-3-phosphate dehydrogenase (G3PDH). (B) Flow cytometric analysis of the expression of S1P receptors in peripheral blood T cells is shown. Background controls in the presence of rabbit IgG (for S1P1, S1P4, and S1P5) or mouse IgG (for S1P2 and S1P3) and FITC-conjugated secondary antibodies are shown in black. In the panel designated S1P2/S1P3, white and black histograms represent the binding of monoclonal anti-S1P2 and anti-S1P3, respectively. Numbers indicate the percentage of positive cells. (C) Anti-S1P1 and anti-S1P5 were either left intact or were incubated with S1P1 or S1P5 peptide prior to binding the antibodies with T cells. Control is the binding of rabbit IgG plus secondary antibody.
Expression of S1P receptors in peripheral blood T cells. (A) mRNA for peripheral blood T cells and for T-cell lines (Jurkat and PEER), an NK-cell line (NK92), B-cell lines (RPMI and .221), and a monocytic cell line (U937) is shown. Shown is RT-PCR analysis of mRNA for S1P1, S1P3, S1P4, and S1P5, as well as the housekeeping gene for glyceraldehyde-3-phosphate dehydrogenase (G3PDH). (B) Flow cytometric analysis of the expression of S1P receptors in peripheral blood T cells is shown. Background controls in the presence of rabbit IgG (for S1P1, S1P4, and S1P5) or mouse IgG (for S1P2 and S1P3) and FITC-conjugated secondary antibodies are shown in black. In the panel designated S1P2/S1P3, white and black histograms represent the binding of monoclonal anti-S1P2 and anti-S1P3, respectively. Numbers indicate the percentage of positive cells. (C) Anti-S1P1 and anti-S1P5 were either left intact or were incubated with S1P1 or S1P5 peptide prior to binding the antibodies with T cells. Control is the binding of rabbit IgG plus secondary antibody.
Immunofluorescence analysis
Intracellular labeling of S1P receptors was done using polyclonal antibodies to the carboxy-terminal of S1P1, S1P4, and S1P5 or monoclonal antibodies to the amino-terminal of S1P2 and S1P3. T cells (1 × 105) were seeded in v-bottom 96-well plates and washed once with 150 μL washing buffer (phosphate-buffered saline [PBS] plus Ca++ and Mg++, 0.1% bovine serum albumin [BSA], and 10 mM sodium azide), and once with PBS alone. They were incubated with 100 μL fixative (4% paraformaldehyde in PBS, pH 7.4), and incubated for 15 minutes at room temperature. The cells were washed once with PBS and once with permeabilization buffer (0.1% saponin in washing buffer). They were resuspended in 100 μL permeabilization buffer, and incubated for 15 minutes at room temperature. After this, the cells were spun down and resuspended in 10 μL permeabilization buffer and appropriate dilutions of anti-S1P receptor antibodies (2 μg/10 μL = 1: 5 dilution, 1 μg/10 μL = 1:10 dilution, etc). Several dilutions of the antibodies (1:5-1:500) were used. Similar concentrations of rabbit IgG or mouse IgG (Sigma-Aldrich) were used as controls. The cells were incubated with the antibodies for 1 hour at 4°C. They were washed twice with permeabilization buffer, resuspended in 1:50 dilution of the secondary antibody, FITC-conjugated F(ab′)2 sheep antimouse (Sigma-Aldrich) or FITC-conjugated F(ab′)2 donkey antirabbit (Jackson Immunoresearch Laboratories, West Grove, PA), and incubated for 30 minutes at 4°C. They were washed twice with permeabilization buffer and resuspended in 400 μL washing buffer before examining in the flow cytometer (FACSCalibur, Becton Dickinson Biosciences, San Jose, CA). For the binding of antibodies to peptides, 50 molecules of the peptide (S1P1 or S1P5) were incubated with one molecule of the antibody (anti-S1P1 or anti-S1P5) for 20 minutes at 37°C, before examining these antibodies for their binding to T cells.
Pretreatment with PTX
T cells (1 × 106/mL) were either left intact or were treated with 100 ng/mL activated PTX overnight at 37°C. The cells were then washed 3 times with culture medium.
Preparation of DCs
Peripheral blood cells were collected from blood bank healthy volunteers (Ullevål Hospital, Oslo, Norway) and centrifuged over lymphoprep gradients (Nycomed, Oslo, Norway). Mononuclear cells were isolated and incubated (1 × 107/mL, total volume 10 mL) in 100-mm Petri dishes at 37°C for 2 hours, and the adherent cells were collected. These cells contained about 90% CD14+, about 10% CD19+ B cells, and no CD3+ T cells as determined by flow cytometric analysis. CD14+ cells were cultured at 5 × 106/mL with 6 ng/mL IL-4 and 25 ng/mL rhGM-CSF for 5 days at 37°C to generate immature (i) DCs. In the middle of the culture period (2.5 days later), the cells were supplemented with 1 mL fresh media containing IL-4 and GM-CSF. The phenotypes of both iDCs and mature (m) DCs were routinely checked by flow cytometric analysis. The iDCs showed low expression of CD80 and CD86, but not CD83 or CD14, whereas mDC highly expressed CD80, CD86, and CD83, but not CD14. In addition, the morphology of both cell types was routinely examined by cytospin analysis after each preparation, to ascertain that DCs are generated.
Proliferation assay
The 96-well plates were coated overnight at 4°C with 1 μg/mL (100 ng/100 μL) anti-CD3 and washed 3 times with PBS. T cells (1 × 105/100 μL) and 100 ng/100 μL anti-CD28 were added to each well. For activation with PMA plus ionomycin, 10 ng/mL (1 ng/100 μL) of each was added to the wells in the presence of 1 × 105 T cells/well. Three days later, 1 μCi/well (0.037 MBq) [3H]thymidine (Amersham Pharmacia Biotech, Oslo, Norway) was added to each well as previously described.17 Six hours later, the cells were harvested using a cell harvester (Skatron Instrument, Lier, Norway) on glass-fiber filters, and then counted in a beta counter. Each experiment was done 4 to 6 times; however, due to the variations in the proliferative response among individuals, only a single representative experiment is shown. All experiments show a similar response pattern. For DC-induced T-cell proliferation, iDCs were incubated with 1 μg/mL mitomycin C for 45 minutes at 37°C, and then washed 4 times to remove any residual mitomycin C. Mitomycin C was used to block DNA synthesis by these DCs. The iDCs (1 × 105) were incubated with T cells (5 × 105) in 96-well plates for 72 hours. These are considered iDC plus T cells. In other cultures, 1 μg/mL LPS was added to induce the generation of mDC. These are considered mDC plus T cells.
Measurement of IL-2 and IFN-γ secretion
The 24-well plates were coated with 1 μg/mL anti-CD3 overnight at 4°C. After washing the plates, T cells (1 × 106/mL) and 1 μg/mL anti-CD28 were added, in the presence or absence of S1P, DHS1P, ceramide, or sphingosine, incubated for 24 hours at 37°C, and the supernatants were collected from these cultures. The concentrations of IL-2 and interferon γ (IFN-γ) were measured by enzyme-linked immunosorbent assay (ELISA) using Quantikine ELISA kits (R & D Systems, Abingdon, United Kingdom). Each experiment was done 4 times; however, only representative experiments are shown due to the variation among individuals.
Statistical analysis
Significant values were determined by using the 2-tailed Student t test.
Results
Expression of S1P receptors in T cells
We recently reported that human natural killer (NK) cells express S1P1, S1P4, and S1P5, but not S1P2 or S1P3.16 Here, we examined the expression of these receptors in peripheral blood T cells using RT-PCR. The results show that peripheral blood T cells and the γ/δ T-cell line (PEER) expressed S1P1, S1P3, S1P4, and S1P5. However, the CD4+ cell line Jurkat expressed only S1P1 and S1P3, but not S1P4 and S1P5 (Figure 1A). No T cells or T-cell lines expressed mRNA for S1P2 (data not shown). For controls, we used NK (NK92), B (RPMI8866 and.221), and monocytic (U937) cell lines. A noticeable observation is the expression of mRNA for S1P5 in T cells and PEER cell line only. Also, S1P4 has relatively high transcript levels in these T cells when compared with other cells. No signal was observed in the absence of reverse transcriptase. Previous work showed weak expression of S1P3 but not S1P1 in human CD4+ T cells,9,10 whereas S1P4 was expressed in lymphoid tissues.18 Hence, the expression of S1P receptors described here is the first comprehensive effort to show the presence of S1P receptors in human T cells.
In addition, the expression of S1P receptors by flow cytometric analysis was examined. Results in Figure 1B show the binding of 1:10 to 1:50 dilutions of the antibodies. However, polyclonal anti-S1P1 and anti-S1P4 showed strong binding at the 1:200 dilution, whereas polyclonal anti-S1P5 and monoclonal anti-S1P3 bind at 1:10 to 1:50 dilutions. Between 83% and 98%, 1% and 15%, 30% and 50%, and 5% and 25% of T cells expressed S1P1, S1P3, S1P4, and S1P5, respectively. Similar to RT-PCR, T cells did not express S1P2. In fact, the binding of antibody to S1P2 was weaker than the binding of mouse IgG control. Although other donor T cells expressed between 10% and 15% S1P3,T cells from the donor shown in Figure 1 expressed only 1% of this receptor. However, when we used monoclonal anti-S1P2 rather than mouse IgG as a background control for monoclonal anti-S1P3, we observed that about 10% of T cells expressed S1P3 (S1P2/S1P3 in Figure 1B). Although anti-S1P3 is directed to the amino-terminal of the receptor, this antibody binds better to T cells when these cells are permeabilized. This could be due to conformational changes that may occur in the receptor on permeabilization, which allow the exposure of those epitopes that bind the antibody. Collectively, these results represent the first demonstration of a successful use of anti-S1P antibodies in the flow cytometric analysis, in any cell type. The only other report that showed the expression of S1P4 by flow cytometry used c-myc epitope-tagged S1P4 and anti–c-myc antibody.19
The level of mRNA for S1P1 and S1P5 is almost similar (Figure 1A). However, only 5% to 25% of T cells expressed S1P5, whereas more than 90% of the cells expressed S1P1 (Figure 1B). This could be due to a different mRNA stability between S1P1 and S1P5 as well as to differences in their translation efficacy. To determine the specificity of anti-S1P1 and anti-S1P5, we incubated these antibodies with specific as well as with nonspecific peptides prior to their examination by flow cytometry. Results in Figure 1C show that the binding of anti-S1P1 to T cells was completely blocked on its preincubation with S1P1 but not with S1P5 peptide. Reciprocally, the binding of anti-S1P5 to T cells was blocked on its preincubation with S1P5 but not with S1P1 peptide.
Effect of S1P on the proliferation of polyclonal T cells
To examine the function of S1P receptors in T cells, we used the combination of anti-CD3 plus anti-CD28, or PMA plus ionomycin. Anti-CD3 or PMA alone induced low proliferative responses, whereas ionomycin, anti-CD28, or control antibodies did not (data not shown). At 10 ng/mL, PMA plus ionomycin induced significant T-cell proliferation (P < .0006 compared with the control). Addition of 1 to 10 μM S1P alone did not affect T-cell proliferation; however, addition of 10 μM S1P inhibited T-cell proliferation triggered by PMA plus ionomycin (P < .01; Figure 2A). Similarly, 1 μg/mL each of anti-CD3 plus anti-CD28 induced T-cell proliferation (P < .0002). This activity was inhibited by the addition of 0.1, 1, or 10 μM S1P (P < .002). A higher T-cell proliferation was observed when the experiments were done in serum-free media (SFM). There was 2- to 3-fold enhancement of T-cell proliferation on activation with PMA plus ionomycin, and about 4- to 5-fold increase on activation with anti-CD3 plus anti-CD28 as compared with T-cell proliferation in media with serum (Figure 2B versus 2A), indicating that there are inhibitory factors present in the media supplemented with serum that regulate polyclonal T-cell proliferation. This was a consistent finding and was observed in more than 5 experiments conducted.
Effect of S1P on polyclonal T-cell proliferation. T cells were incubated with 10 ng/mL PMA plus ionomycin (IONO), or 1 μg/mL plated anti-CD3 plus 1 μg/mL anti-CD28, in the absence or the presence of 0.1 to 10 μM S1P for 3 days at 37°C. At this time, 1 μCi (0.037 MBq) [3H]thymidine ([3H]Tdr) was added to each well, and the cultures were incubated for an additional 6 hours before harvesting (A). The same experiment was conducted in serum-free media (SFM) (B). T cells (1 × 106/mL) were treated with 100 ng/mL PTX for 18 hours. These cells were washed 3 times, and then incubated with the various stimuli in the absence or presence of different concentrations of S1P (C). cpm indicates counts per minute.
Effect of S1P on polyclonal T-cell proliferation. T cells were incubated with 10 ng/mL PMA plus ionomycin (IONO), or 1 μg/mL plated anti-CD3 plus 1 μg/mL anti-CD28, in the absence or the presence of 0.1 to 10 μM S1P for 3 days at 37°C. At this time, 1 μCi (0.037 MBq) [3H]thymidine ([3H]Tdr) was added to each well, and the cultures were incubated for an additional 6 hours before harvesting (A). The same experiment was conducted in serum-free media (SFM) (B). T cells (1 × 106/mL) were treated with 100 ng/mL PTX for 18 hours. These cells were washed 3 times, and then incubated with the various stimuli in the absence or presence of different concentrations of S1P (C). cpm indicates counts per minute.
PMA plus ionomycin induced T-cell proliferation in SFM (P < .0001), and this proliferation was inhibited by the addition of 0.1, 1, or 10 μM S1P (P < .05, < .05, or < .01, respectively). Anti-CD3 plus anti-CD28 also enhanced T-cell proliferation in SFM (P < .0001), and this response was inhibited by the addition of 1 or 10 μM S1P (P < .01). There was T-cell proliferation even in the presence of high concentrations of S1P (Figure 2B), suggesting that although S1P is an important inhibitory molecule present in the serum, this molecule may not be the sole inhibitor. In fact, 0.1 μM S1P inhibited T-cell proliferation only in the presence of serum when anti-CD3 and anti-CD28 were used as stimuli, suggesting that S1P may synergize with other inhibitory molecules present in the serum. The approach of adding S1P to SFM is similar to the approach of Yanai et al,20 who observed that primitive hematopoietic THS119 did not invade the stromal cell layer in the absence of FBS. However, these cells restored their ability to invade on the inclusion of either FBS, S1P, or LPA in the media.20 Last, we did not observe differences in S1P receptor expression in T cells cultured in SFM, except for a low expression of S1P5 (data not shown), suggesting that the inhibition is related to the addition of the ligand.
Because receptors for S1P are coupled to PTX-sensitive as well as PTX-insensitive G proteins,1-6,16 we performed the experiments with T cells pretreated with PTX. Our results show that there was an increase in PTX-pretreated T-cell proliferation on stimulation with PMA plus ionomycin when compared with T-cell proliferation in the presence of the same molecules but without PTX treatment (Figure 2C versus 2A), suggesting that the inhibitory molecule in the serum functions through PTX-sensitive G proteins and that on blocking these G proteins with PTX, a higher proliferation of T cells is revealed. Hence, PTX-pretreated T cells significantly proliferated on stimulation with PMA plus ionomycin (P < .0001; Figure 2C). Addition of S1P failed to inhibit this proliferation, indicating that S1P acts through PTX-sensitive G protein–coupled receptors, on stimulating T cells with PMA plus ionomycin. In contrast, there was no difference in T-cell proliferation after activation with anti-CD3 plus anti-CD28 on pretreatment with PTX (Figure 2C versus 2A). These antibodies triggered significant proliferation of T cells pretreated with PTX (P < .001), and addition of 0.1 to 10 μM S1P inhibited this proliferation (P < .001; Figure 2C).
In addition to S1P, 0.1, 1, and 10 μM concentrations of DHS1P inhibited T-cell proliferation induced by anti-CD3 plus anti-CD28 (P < .05, < .01 and < .05, respectively; Figure 3). Because S1P may enter the cells and may be converted into sphingosine by sphingosine 1-phosphate phosphatase and to ceramide by ceramidase, which have antiproliferative or apoptotic activities in various cell types,21,22 we examined the effect of these metabolites on T-cell proliferation. Only 1 or 10 μM ceramide (P < .02, and < .005, respectively), and 10 μM sphingosine (P < .02) were inhibitory for T-cell proliferation (Figure 3). Hence, although ceramide and sphingosine are inhibitory, they required higher concentrations than S1P or DHS1P. It is difficult to attribute the inhibitory effect of 0.1 μM S1P to its conversion to 1 and 10 μM ceramide or 10 μM sphingosine, concentrations that are inhibitory for T-cell proliferation (Figure 3).
Sphingolipids inhibit T-cell proliferation with different dose responses. T cells were either left intact (□, left column) or were stimulated with 1 μg/mL plated anti-CD3 plus 1 μg/mL anti-CD28 (▪), for 3 days in the presence or absence of different concentrations of S1P, DHS1P, ceramide (CER), or sphingosine (SPH), in media supplemented with 10% serum. The incorporation of [3H]thymidine was done as in Figure 2. P values comparing T-cell proliferation in the presence of sphingolipids to the proliferation in their absence are placed on top of each column. Data are representative of 3 experiments performed. Error bars indicate means ± SD; NS, not significant.
Sphingolipids inhibit T-cell proliferation with different dose responses. T cells were either left intact (□, left column) or were stimulated with 1 μg/mL plated anti-CD3 plus 1 μg/mL anti-CD28 (▪), for 3 days in the presence or absence of different concentrations of S1P, DHS1P, ceramide (CER), or sphingosine (SPH), in media supplemented with 10% serum. The incorporation of [3H]thymidine was done as in Figure 2. P values comparing T-cell proliferation in the presence of sphingolipids to the proliferation in their absence are placed on top of each column. Data are representative of 3 experiments performed. Error bars indicate means ± SD; NS, not significant.
Regulation of S1P receptor expression by different T-cell stimuli
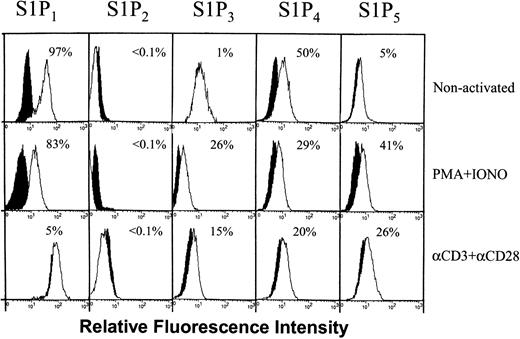
The differential effect of PTX on S1P activity in the presence of different stimuli suggests that perhaps there may be differential regulation of S1P receptor expression in T cells incubated with various stimuli. Consequently, we examined the expression of receptors for S1P after stimulation with PMA plus ionomycin, or anti-CD3 plus anti-CD28. Before stimulation, between 83% and 98% (mean ± SEM is 91 ± 3.1, n = 4) of T cells expressed S1P1. Stimulation with PMA plus ionomycin down-regulated to some extent the expression of S1P1 (Figure 4 shows a representative experiment, mean ± SEM is 73 ± 9, n = 3). However, a persistent down-regulation of this receptor was observed after stimulation with anti-CD3 plus anti-CD28; in the experiment shown in Figure 4, only 5% of the cells expressed this receptor 3 days after stimulation with these antibodies. In other experiments, anti-CD3 plus anti-CD28 reduced the expression of S1P1 with varying degrees depending on the donor (mean ± SEM is 38 ± 18, n = 3). No effect on the expression of S1P2 was observed after stimulation. On the other hand, stimulation with PMA plus ionomycin or anti-CD3 plus anti-CD28 up-regulated the expression of S1P3 on about 15% to 40% and 15% to 30% of the cells, respectively (Figure 4). Also, both stimuli up-regulated the expression of S1P5, but down-regulated the expression of S1P4 (Figure 4).
Differential regulation of S1P receptor expression on activation. T cells were either left intact (nonactivated) or were activated with PMA plus ionomycin or anti-CD3 plus anti-CD28 for 3 days. These cells were then labeled with antibodies to S1P1, S1P2, S1P3, S1P4, or S1P5. Background controls in the presence of rabbit IgG (for S1P1, S1P4, and S1P5) or mouse IgG (for S1P2 and S1P3) and FITC-conjugated secondary antibodies are shown in black. Numbers indicate the percentage of positive cells in one experiment. In 3 to 4 experiments performed, the percentage of positive cells for S1P1, S1P2, S1P3, S1P4, and S1P5 ranges between 83% and 98%, less than 0.1%, 1% and 15%, 25% and 50%, and 5% and 25% for nonactivated cells; 52% to 83%, less than 0.1%, 15% and 40%, 20% and 30%, and 23% and 41% for PMA plus ionomycin-treated cells; and 5% and 70%, less than 0.1%, 15% and 30%, 20% and 25%, and 12% and 26% for anti-CD3 plus anti-CD28 activated cells, respectively. White histograms represent the binding of the specific antibodies.
Differential regulation of S1P receptor expression on activation. T cells were either left intact (nonactivated) or were activated with PMA plus ionomycin or anti-CD3 plus anti-CD28 for 3 days. These cells were then labeled with antibodies to S1P1, S1P2, S1P3, S1P4, or S1P5. Background controls in the presence of rabbit IgG (for S1P1, S1P4, and S1P5) or mouse IgG (for S1P2 and S1P3) and FITC-conjugated secondary antibodies are shown in black. Numbers indicate the percentage of positive cells in one experiment. In 3 to 4 experiments performed, the percentage of positive cells for S1P1, S1P2, S1P3, S1P4, and S1P5 ranges between 83% and 98%, less than 0.1%, 1% and 15%, 25% and 50%, and 5% and 25% for nonactivated cells; 52% to 83%, less than 0.1%, 15% and 40%, 20% and 30%, and 23% and 41% for PMA plus ionomycin-treated cells; and 5% and 70%, less than 0.1%, 15% and 30%, 20% and 25%, and 12% and 26% for anti-CD3 plus anti-CD28 activated cells, respectively. White histograms represent the binding of the specific antibodies.
Effect of S1P on DC-induced T-cell proliferation
Because antigens are presented to T cells by professional antigen-presenting cells, such as DCs, we examined the ability of both iDCs and mDCs to affect allogeneic T-cell proliferation. Incubation of iDCs or mDCs with T cells induced potent T-cell proliferation (P < .0001 compared with T cells incubated with medium only; Figure 5A). To control for the effect of LPS, or the ability of DCs to proliferate, we examined whether T cells or iDCs proliferate in the presence of LPS. The results showed that none of these cells proliferated in the presence of LPS (Figure 5A). Addition of 0.01, 0.1, or 1 μM S1P inhibited the induction of T-cell proliferation induced by either iDCs or mDCs (P < .01-.001 depending on the S1P dose used, as compared with cells proliferated in the absence of the phospholipid). There was about a 2- to 3-fold increase in T-cell proliferation on performing these experiments in SFM (Figure 5B versus 5A), again suggesting that there must be inhibitory molecules present in the serum that block T-cell proliferation. Addition of 0.1 or 1 μM S1P significantly inhibited the effect of iDCs on T cells in SFM (P < .05). Also, addition of 0.01 to 1 μM S1P reduced T-cell proliferation when incubated with allogeneic mDCs in SFM (P < .03). These results suggest that S1P is one of the molecules present in the serum, which inhibits DC triggering of T-cell activation. PTX was also used to examine the signals activated on the interaction between DCs and T cells. PTX-pretreated cells proliferated similarly to PTX-untreated T cells (Figure 5C versus 5A). Also similar to PTX-untreated T cells, 0.01 to 1 μM S1P inhibited the proliferation of PTX-pretreated T cells induced by either iDCs (P < .005) or mDCs (P < .01), suggesting that PTX-insensitive G proteins are involved in DC/T-cell interaction.
Effect of S1P on T-cell proliferation induced by DCs. Allogeneic iDCs were incubated with T cells for 3 days in media supplemented with serum in the absence or presence of 1 μg/mL LPS (to generate mDCs). These cultures were either left intact or were incubated with 0.01 to 1 μM S1P for 3 days. At this time, 1 μCi (0.037 MBq) [3H]thymidine was added, and the cultures were incubated for an additional 6 hours before harvesting (A). The same experiment was conducted in SFM (B). T cells were pretreated with PTX and then incubated with DCs in the presence of serum (C). Control samples include T cells alone, iDCs alone, iDCs incubated with 1 μg/mL LPS, and T cells incubated with 1 μg/mL LPS in the presence or absence of S1P. Error bars indicate means ± SD.
Effect of S1P on T-cell proliferation induced by DCs. Allogeneic iDCs were incubated with T cells for 3 days in media supplemented with serum in the absence or presence of 1 μg/mL LPS (to generate mDCs). These cultures were either left intact or were incubated with 0.01 to 1 μM S1P for 3 days. At this time, 1 μCi (0.037 MBq) [3H]thymidine was added, and the cultures were incubated for an additional 6 hours before harvesting (A). The same experiment was conducted in SFM (B). T cells were pretreated with PTX and then incubated with DCs in the presence of serum (C). Control samples include T cells alone, iDCs alone, iDCs incubated with 1 μg/mL LPS, and T cells incubated with 1 μg/mL LPS in the presence or absence of S1P. Error bars indicate means ± SD.
Effect of S1P on the secretion of IL-2 and IFN-γ by activated T cells
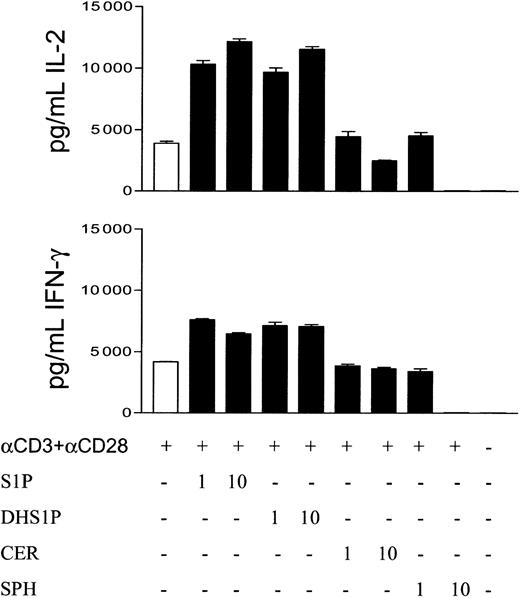
To examine other immunoregulatory effects of S1P, we collected supernatants from T cells stimulated with anti-CD3 plus anti-CD28. The levels of IL-2 or IFN-γ in these supernatants were measured by ELISA. T cells stimulated with PMA plus ionomycin did not secrete significant amounts of these cytokines (data not shown). However, T cells incubated with anti-CD3 plus anti-CD28 secreted significant amounts of IL-2 (P < .001 as compared with T cells incubated with medium only; Figure 6). Surprisingly, 1 or 10 μM S1P enhanced IL-2 secretion by T cells stimulated with anti-CD3 plus anti-CD28 (P < .005 as compared with cells activated with the antibodies but not with S1P). This activity was mimicked by DHS1P, which also stimulates the secretion of IL-2 when used at 1 or 10 μM (P < .005). In contrast, 10 μM of either ceramide or sphingosine inhibited this secretion (P < .01 and < .001, respectively, when compared with IL-2 secretion in the presence of anti-CD3 plus anti-CD28 only). Significant IFN-γ was also secreted by T cells stimulated with anti-CD3 plus anti-CD28 (P < .001 compared with T cells not stimulated with the antibodies). Similar to the results of IL-2 secretion, 1 or 10 μM concentration of S1P increased the level of IFN-γ secreted by T cells (P < .01 compared with cells stimulated with the antibodies, but in the absence of S1P). This enhancement occurred only on activation; that is, addition of S1P to T cells in the absence of T cell stimuli did not induce the secretion of IL-2 or IFN-γ (data not shown). One or 10 μM DHS1P also stimulated IFN-γ secretion (P < .01). Similar to their effects on IL-2 secretion, 10 μM ceramide or sphingosine inhibited IFN-γ secretion by activated T cells (P < .05 and < .0001, respectively).
S1P enhances IL-2 and IFN-γ secretion by T cells stimulated with anti-CD3 plus anti-CD28. T cells (1 × 106/mL) were stimulated with 1 μg/mL anti-CD3 plus anti-CD28 for 24 hours in the absence or presence of 1 or 10 μM S1P, DHS1P, ceramide (CER), or sphingosine (SPH). Supernatants were collected from these cultures. IL-2 and IFN-γ levels were measured by Quantikine ELISA. Error bars indicate means ± SD.
S1P enhances IL-2 and IFN-γ secretion by T cells stimulated with anti-CD3 plus anti-CD28. T cells (1 × 106/mL) were stimulated with 1 μg/mL anti-CD3 plus anti-CD28 for 24 hours in the absence or presence of 1 or 10 μM S1P, DHS1P, ceramide (CER), or sphingosine (SPH). Supernatants were collected from these cultures. IL-2 and IFN-γ levels were measured by Quantikine ELISA. Error bars indicate means ± SD.
Discussion
S1P is secreted by platelets and constitutes a high proportion of serum and plasma.7,8 It is also secreted by various cell types on stimulation of protein kinase C.23 However, its immunoregulatory role is not extensively studied. Here, we show for the first time the expression of S1P receptors in peripheral blood T cells by immunofluorescence analysis. We combined this technique with the expression of the receptor at the mRNA level by RT-PCR. Because S1P stimulates the chemotaxis of human NK cells,16 and because it is antiapoptotic and stimulates the proliferation of various cell types,1,12,22 we were surprised to observe that it inhibits the proliferation of polyclonal T cells. Addition of this phospholipid inhibits T-cell proliferation induced by several stimuli, including PMA plus ionomycin, anti-CD3 plus anti-CD28, and iDCs and mDCs. It was previously reported that S1P acts either extracellularly,24 intracellularly,25 or both.26 Our results favor receptor-mediated activity of S1P for the following reasons. (1) DHS1P, which acts on S1P plasma membrane receptors, and does not have intracellular activity, mimicked the inhibitory effect of S1P on T cell proliferation. (2) Sphingosine and ceramide, the metabolites that can be generated from the conversion of S1P, also inhibit T-cell proliferation. However, these sphingolipids inhibit T-cell proliferation with higher concentrations than did S1P or DHS1P (Figure 3). (3) The inhibitory effect of S1P on T-cell proliferation is observed only after stimulating T cells with anti-CD3 plus anti-CD28, iDCs or mDCs, which engage T-cell receptors, suggesting that S1P action is related to the expression of receptors for S1P after activation with these stimuli. (4) PTX blocked the inhibitory activity of S1P when PMA plus ionomycin were used as stimuli. (5) S1P as well as DHS1P enhanced the secretion of IL-2 and IFN-γ by T cells stimulated with anti-CD3 plus anti-CD28, an activity that cannot be attributed to sphingosine and ceramide. In fact, both ceramide and sphingosine when used at 10 μM concentration markedly inhibited the secretion of both cytokines.
PTX, which blocks Gi, reversed the inhibitory effect of S1P after stimulation with PMA plus ionomycin, but did not affect it when anti-CD3 and anti-CD28 were used. This activity resembles to some extent the effect of LPA in fibroblasts, where LPA enhances mitogen-activated protein kinase activation via PTX-sensitive G proteins, but activates p125FAK through PTX-insensitive G proteins.27 Here, we observed that triggering T-cell receptors (TCRs) with anti-CD3 plus anti-CD28 down-regulated the expression of S1P1, which couples to PTX-sensitive G proteins, and up-regulated the expression of S1P3, which is coupled to PTX-sensitive as well as PTX-insensitive G proteins.4,5 Although stimulation with PMA plus ionomycin also down-regulated the expression of S1P1, in all the experiments performed, a lower percentage of T cells expressing this receptor was observed after stimulation with anti-CD3 plus anti-CD28 than after stimulation with PMA plus ionomycin. These results may explain how PTX reversed the inhibitory effect of S1P on activating T cells with PMA plus ionomycin but not on activation with anti-CD3 plus anti-CD28. PTX also did not affect S1P inhibitory action on stimulating T cells with DCs. Unfortunately, we could not completely separate T cells from DCs to examine the expression of receptors for S1P in T cells after stimulation with DC. It is plausible that stimulation with DC may up- or down-regulate the expression of S1P receptors in T cells. Last, PMA plus ionomycin or anti-CD3 plus anti-CD28 down-regulated the expression of S1P4 and up-regulated the expression of S1P5 in T cells 3 days after stimulation.
Surprisingly, we observed that S1P enhances IL-2 and IFN-γ secretion on stimulation with anti-CD3 plus anti-CD28. The reason for increased cytokine secretion is not known, but it may be related to the up-regulation of S1P3 and S1P5 on T cells stimulated with anti-CD3 plus anti-CD28, although this has not been tested. These results indicate that although S1P inhibits the proliferation of T cells, it induces their terminal differentiation exemplified by cytokine secretion. The results are reminiscent of the effect of LPA on IL-2 secretion by phytohemagglutinin (PHA)–activated T cells, where LPA enhances rather than reduces the secretion of IL-2 in activated T cells.28
Recent studies suggest that a direct interaction between DCs and T cells29 or NK cells30-32 is necessary for regulating the effector cells. All the players, which include DC,14 NK cells,16 and T cells (this study), express receptors for S1P. It is plausible that S1P may play a role in bringing these cells together. In the case of T cells and mature DC cells, the presence of S1P results in inhibition of T-cell proliferation. On the other hand, incubating T cells in SFM results in higher T-cell activation, an activity that is reduced by the addition of S1P, suggesting that S1P is an important immunoregulatory molecule present in the serum. Our results may complement recent results showing that there is lymphopenia due to impairment of T-cell trafficking occurring in animals treated with S1P or with drugs that resemble its action.15 Here, we showed that in addition to inhibiting T-cell circulation, S1P inhibits polyclonal T-cell proliferation, resulting in lower number of these cells.
In summary, our results show for the first time that S1P, which is abundant in human serum and plasma, is an inhibitory molecule for polyclonal T-cell proliferation, which may represent an important mechanism for maintaining the homeostasis of the immune system after encountering foreign particles. Finally, it should be mentioned that S1P is not only present in serum but is also secreted by inflammatory cells.33 Consequently, it may regulate T-cell activation inside tissues and during inflammation. This, in addition to negatively regulating liver fibrogenesis,13 makes S1P a very important natural regulator of immune and nonimmune cells, and may function similarly to other immunoregulatory molecules such as transforming growth factor β (TGF-β). Unlike TGF-β, S1P is highly abundant in blood and in inflammatory tissues and is difficult to sequester or clear from these sites. Our method of detecting S1P receptors by flow cytometric analysis should facilitate the generation of additional knowledge regarding this important immunoregulatory molecule.
Prepublished online as Blood First Edition Paper, February 13, 2003; DOI 10.1182/blood-2002-09-2962.
Supported by grants from the European Community (grant QLRT-2000-02103 to A.A.M. and S.G.), Anders Jahres Foundation, and the Norwegian Cancer Society (grant 97025/002).
Y.J. and E.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 2. Effect of S1P on polyclonal T-cell proliferation. T cells were incubated with 10 ng/mL PMA plus ionomycin (IONO), or 1 μg/mL plated anti-CD3 plus 1 μg/mL anti-CD28, in the absence or the presence of 0.1 to 10 μM S1P for 3 days at 37°C. At this time, 1 μCi (0.037 MBq) [3H]thymidine ([3H]Tdr) was added to each well, and the cultures were incubated for an additional 6 hours before harvesting (A). The same experiment was conducted in serum-free media (SFM) (B). T cells (1 × 106/mL) were treated with 100 ng/mL PTX for 18 hours. These cells were washed 3 times, and then incubated with the various stimuli in the absence or presence of different concentrations of S1P (C). cpm indicates counts per minute.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/12/10.1182_blood-2002-09-2962/6/m_h81234468002.jpeg?Expires=1769111499&Signature=AYukVw~wb6jbeCpvfJt95PqknjElx-Yfw2RhvdBnQpfIjKWBsGrPZ1Dn0adYh2zs3hoSivoYSrJguIgzAsniE5iUE4S0ucNR4b-Po7Sfui3yuybTizE~BKY9nHFjZrQZE7A9lwgv~8us9dqk1aBohmh-dmI9CSiRJbWmsZ0927eNjy9oSk7aJ3vFBQcbyWhePsc3jBtSJsA910BY2yrieswMYA6wu9AVjSnmo05EhslmlA4Hu2liI9XjpEzVx-NKojdXHo-Gd6OR96p6Wph78e1dzCGJ-wAY2A2emMA6Gd~KpP4WUq3X8vusDhGnLU1eIC4GBaiR7gxDgmvTL7ao4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Sphingolipids inhibit T-cell proliferation with different dose responses. T cells were either left intact (□, left column) or were stimulated with 1 μg/mL plated anti-CD3 plus 1 μg/mL anti-CD28 (▪), for 3 days in the presence or absence of different concentrations of S1P, DHS1P, ceramide (CER), or sphingosine (SPH), in media supplemented with 10% serum. The incorporation of [3H]thymidine was done as in Figure 2. P values comparing T-cell proliferation in the presence of sphingolipids to the proliferation in their absence are placed on top of each column. Data are representative of 3 experiments performed. Error bars indicate means ± SD; NS, not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/12/10.1182_blood-2002-09-2962/6/m_h81234468003.jpeg?Expires=1769111499&Signature=wYGLGee1XKgVXcSgROw4h0K-bILeASBnnb7v5d2o5p-pe1~xO-kEbWELGPFN6dNX1NrWkxAJCEzStPB9WJf3tCqRFhZSoypjgvpnH3RsR9LxgIaZUicNcyqiqTvEw62rKcWDKs-XPkYWdnoIjqU2DFOItjw8WmAWElxea7MTHKnMYxLqXkOeWNp6QJ2-lv8Sp8IrjWXMirBvDQvsx-514IILa1~yELoxy~7Phzat5QhQmoMB0Z1FBx96NBRRzOn04P~a3WYjQUwI16jhRSe9KZdCLRNerdYK1h8arN1WJVcDzZAGxVPO-RSmSik4jEcW0GdYGYkSVZqMjZuj4dhyIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Effect of S1P on T-cell proliferation induced by DCs. Allogeneic iDCs were incubated with T cells for 3 days in media supplemented with serum in the absence or presence of 1 μg/mL LPS (to generate mDCs). These cultures were either left intact or were incubated with 0.01 to 1 μM S1P for 3 days. At this time, 1 μCi (0.037 MBq) [3H]thymidine was added, and the cultures were incubated for an additional 6 hours before harvesting (A). The same experiment was conducted in SFM (B). T cells were pretreated with PTX and then incubated with DCs in the presence of serum (C). Control samples include T cells alone, iDCs alone, iDCs incubated with 1 μg/mL LPS, and T cells incubated with 1 μg/mL LPS in the presence or absence of S1P. Error bars indicate means ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/12/10.1182_blood-2002-09-2962/6/m_h81234468005.jpeg?Expires=1769111499&Signature=lAnhwVRItjvgiqV1nLqhwK4AMpA1gbVB7ENEfdVwZ2KCgUhhj15BWvnUSXmMy1BBsn5djs5aRj51o6~KUszCHv4TfLg92x~0-myXwdp56n-zyaXNZJhCzl2Q5ww6fT9fqZzBstJmZWz5nFeAoioQwTLHTNUCb37KOBcJZ5XoqDjSLOWPPMefcjwTo7boKsvDVpRZhwc4ggETNWoWOlj1Oxs~R1ifVHy4m~S31pzP7NDx8MhS1CatdpWOv0RX4MwFVhKWp0pWWksPGKo4nWdYuPnRsj8JWt5lfIcejyn-tQpnsswMBNhHw8j-wYNmrBRnU0mhTBdwhWCvmo9F4xSuUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal